Abstract
Intracranial hemorrhage is a life-threatening condition, the outcome of which can be improved by intensive care. Intracranial hemorrhage may be spontaneous, precipitated by an underlying vascular malformation, induced by trauma, or related to therapeutic anticoagulation. The goals of critical care are to assess the proximate cause, minimize the risks of hemorrhage expansion through blood pressure control and correction of coagulopathy, and obliterate vascular lesions with a high risk of acute rebleeding. Simple bedside scales and interpretation of computed tomography scans assess the severity of neurological injury. Myocardial stunning and pulmonary edema related to neurological injury should be anticipated, and can usually be managed. Fever (often not from infection) is common and can be effectively treated, although therapeutic cooling has not been shown to improve outcomes after intracranial hemorrhage. Most functional and cognitive recovery takes place weeks to months after discharge; expected levels of functional independence (no disability, disability but independence with a device, dependence) may guide conversations with patient representatives. Goals of care impact mortality, with do-not-resuscitate status increasing the predicted mortality for any level of severity of intraparenchymal hemorrhage. Future directions include refining the use of bedside neuromonitoring (electroencephalogram, invasive monitors), novel approaches to reduce intracranial hemorrhage expansion, minimizing vasospasm, and refining the assessment of quality of life to guide rehabilitation and therapy.
Keywords: intracranial hemorrhage, cerebral hemorrhage, subarachnoid hemorrhage, outcomes
Overview of Types of Intracranial Hemorrhage
Anatomical Compartments of Intracranial Hemorrhage
Intracranial hemorrhage is diagnosed by its anatomical location. Intraparenchymal hemorrhage (IPH; Figure 1) refers to nontraumatic bleeding into the brain parenchyma. (Intracerebral hemorrhage, often abbreviated ICH, is used more often in the clinical literature.) Subarachnoid hemorrhage (SAH) refers to bleeding into the space between the pia and the arachnoid membranes. Nontraumatic causes of rupture include cerebral aneurysms (Figure 2), bleeding from arteriovenous malformations or tumors, cerebral amyloid angiopathy, and vasculopathies (such as vasculitis). A subdural hematoma (Figure 3) is due to bleeding between the dura and the arachnoid, whereas an epidural hematoma involves bleeding between the dura and the bone. Subdural and epidural hematomas are usually traumatic injuries.
Figure 1.
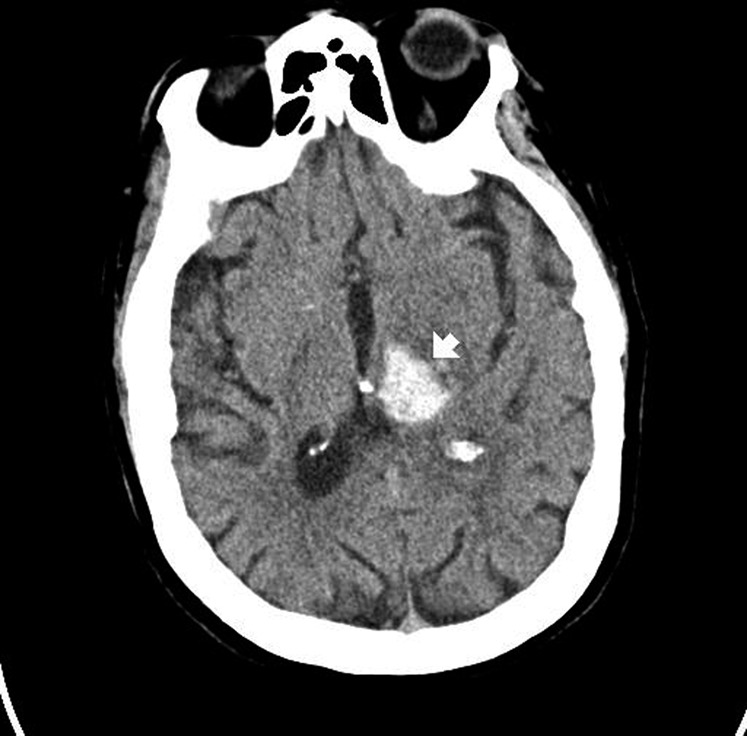
Intraparenchymal hemorrhage on computerized tomography scan. The hyperdense (bright) area represents acute bleeding (arrow). This location is suggestive of chronic hypertension, not compatible with an aneurysm, and would be highly atypical in trauma.
Figure 2.
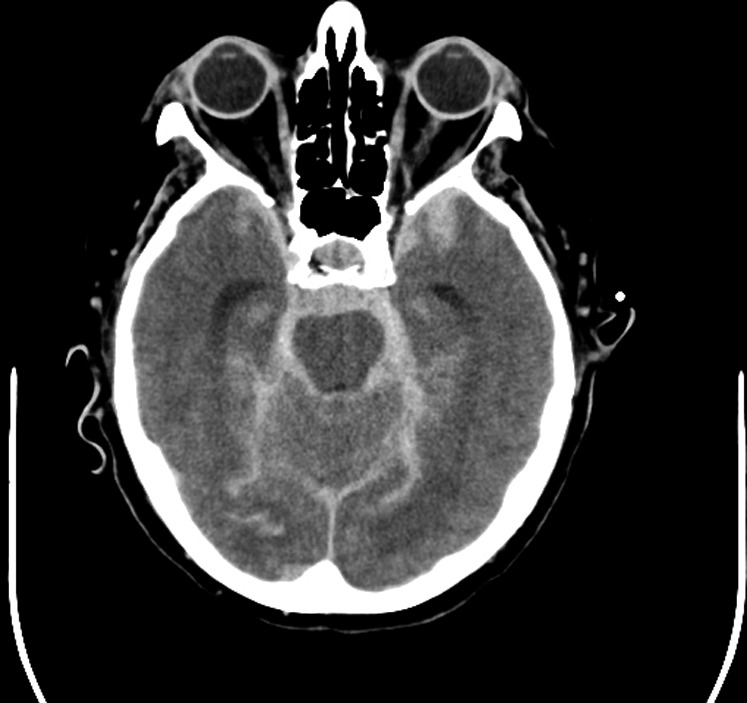
Subarachnoid hemorrhage on computerized tomography scan. Hyperdense (bright) signal, indicating blood, surrounds the brainstem and fills the subarachnoid space. This appearance is typical of a ruptured aneurysm.
Figure 3.
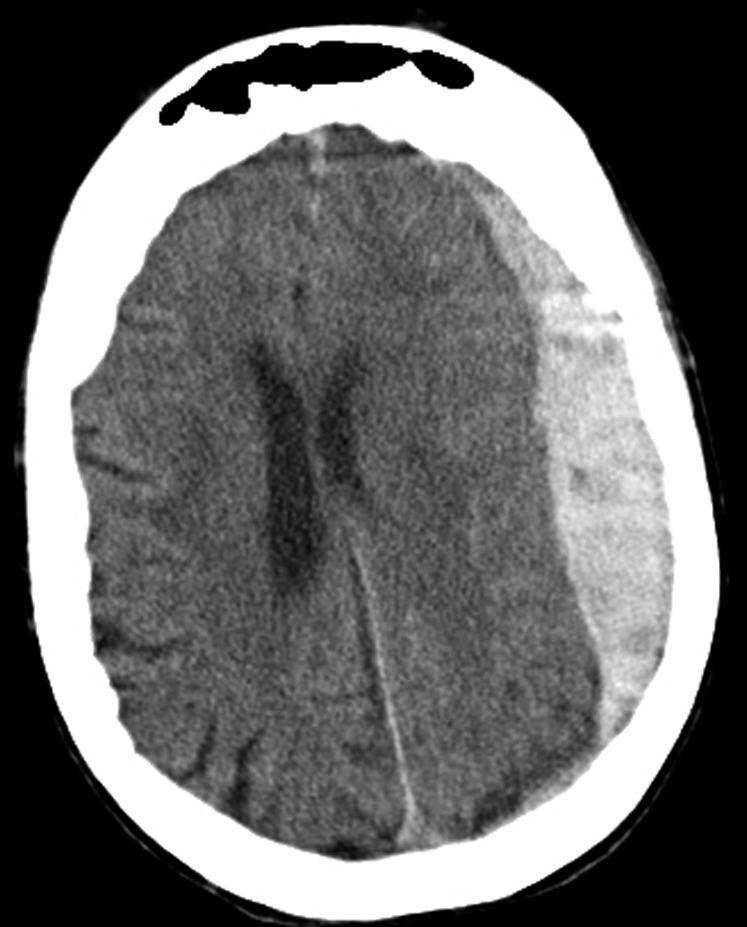
Subdural hemorrhage on computerized tomography scan. Hyperdense (bright) signal, indicating blood, is seen on the patient's left. There is a midline shift to the patient's right.
The anatomical location of intracranial hemorrhage specifies the source. In IPH, a ruptured arteriole is the likely culprit, underscored by contrast extravasation into the hemorrhage on computed tomography (CT) (1). Aneurysmal SAH is due to rupture of an aneurysm in the subarachnoid space, usually near the circle of Willis. “Perimesencephalic” SAH refers to scant subarachnoid blood around the brainstem (2) and is related to venous bleeding (3). Subdural hematomas are generally due to tearing of cortical veins, whereas epidural hematomas are typically due to arterial lacerations. Although neurotrauma is common, this review focuses on nontraumatic hemorrhage. A fistulous or malformed connection between the cerebral arteries and veins, intracranial aneurysm, or trauma may produce hemorrhage in more than one anatomical compartment.
Risk Factors for Intracranial Hemorrhage
The most common risk factor for IPH is hypertension (4). Antiplatelet (5) and anticoagulant medications also increase the risk of spontaneous IPH. Cerebral amyloid angiopathy is related to build-up of amyloid proteins in arterial walls, making them more susceptible to rupture. Hemorrhages due to cerebral amyloid angiopathy are typically lobar (near the cortex), multiple, and occur in patients at least 55 years of age (6).
The rupture of an intracranial aneurysm (SAH) may be spontaneous, precipitated by exertion, or from hypertension. About 1–2% of the adult population harbors an unruptured intracranial aneurysm (7), but only about 1% of these rupture, and how best to select patients for prophylactic aneurysm obliteration is unsettled (8). The most important modifiable risk factors are tobacco use, hypertension, and cocaine use (9); nonmodifiable risk factors include a personal history of SAH, familial history of SAH (10), larger aneurysm size (11, 12), female sex (13), connective tissue disease, and older age. Arteriovenous malformations are often congenital and may become symptomatic later in life.
Intracranial Hemorrhage During Medical Treatment
Anticoagulant-, Antiplatelet-, and Fibrinolytic-related Hemorrhage
Antiplatelet medication is associated with a lower rate of bleeding compared with warfarin (14), although IPH can still occur. Combination clopidogrel–aspirin therapy has a higher rate of IPH compared with clopidogrel alone (15).
Anticoagulation with heparin (unfractionated or low molecular weight heparin, LMWH), warfarin, fibrinolytic agents (e.g., alteplase), and thrombin inhibitors (dabigatran) may all lead to IPH as a complication. Adding antiplatelet therapy to warfarin increases the risk of IPH (16). The risks of each medication depend on the underlying reason for therapy, dosage, and the control in its use. Minidose heparin (5,000 units subcutaneously twice to three times daily) and LMWH may be safe for chemoprophylaxis after cerebral hemorrhage, a few days after symptom onset (17).
As the population ages and atrial fibrillation becomes more frequent, the use of warfarin and other agents to prevent ischemic stroke will likely increase (18). There is a potential for severe bleeding (including IPH) with therapeutic anticoagulation, even with the international normalized ratio (INR) in the goal range. An increased risk for microbleeds is seen with warfarin, and may presage an increased risk for symptomatic IPH (19). As the intensity of anticoagulation increases, so does the risk for IPH (18, 20). Dabigatran may have a lower rate of IPH compared with warfarin when used for stroke prevention in atrial fibrillation (21).
Alteplase (tissue plasminogen activator) is approved for the treatment of acute ischemic stroke, and its use reduces 90-day disability when given up to 4.5 hours after symptom onset (22–24). The risk for IPH after alteplase use is increased by more severe stroke symptoms (25), older age in some (26) but not all (27) data sets, and (particularly) higher blood pressure shortly after treatment (25, 28, 29). Combined aspirin and clopidogrel is associated with a higher risk of IPH after alteplase, but the increased risk does not offset the benefits of therapy (30). Whereas failure to follow the protocol is associated with a higher rate of IPH and less benefit, strictly following the protocol is associated with low risk (31). Not surprisingly, symptomatic IPH after alteplase is associated with a poor prognosis; there are no standardized treatment protocols, and this represents an opportunity for further research (32). Fresh frozen plasma is typically given, and cryoprecipitate is typically given for hypofibrinogenemia in this setting.
Liver Failure and Coagulopathy Related
A variety of conditions that lead to severe hepatic damage (acetaminophen intoxication, chronic or acute hepatitis, septic shock, etc.) will lead to coagulopathy. Although the INR is typically elevated with severe hepatic injury, such patients are more accurately termed “coagulopathic” rather than “endogenously anticoagulated.” Risk of intracranial hemorrhage generally rises with the INR. Clinical bleeding is often a problem, and normalizing laboratory parameters of clotting (INR < 1.5 and platelets > 100,000/μl) is often impossible.
Patients with liver failure are often comatose or have altered mental status, cerebral edema on neuroimaging, and may be candidates for transplant. Intracranial pressure (ICP) monitors are often placed to exclude elevated ICP (33). Device-related hemorrhage is a significant risk (34), and therefore placement requires minimization of the coagulopathy before the procedure, while the monitor is in place, and until several hours after it is discontinued.
Mycotic Aneurysms and Sepsis
The brain is a common site of septic emboli that may lead to mycotic aneurysms and SAH (35). Such aneurysms tend to be more distal (away from the circle of Willis) than spontaneous cerebral aneurysms or those due to meningitis (36).
Assessing the Severity of Intracranial Hemorrhage
Assessing the severity of neurological injury is important for prioritizing diagnostic studies, guiding resuscitation, and planning interventions (e.g., a patient with a worsening examination may benefit from interventions to reduce ICP before proceeding to the angiography suite). Commonly used and well-validated scoring systems are available. Examinations should be performed off sedation: medications with short half-lives (e.g., propofol) should be discontinued (often the start of rounds is long enough), but longer lasting sedatives (e.g., benzodiazepines, barbiturates by continuous infusion) can cloud the examination for hours or days.
General Scoring Systems
Glasgow Coma Scale.
The Glasgow Coma Scale (GCS) (37) is a standard assessment for the level of consciousness. The three axes are eye opening (1–4 points), motor response (1–6 points), and verbal response (1–5 points). Intubated patients typically receive 1 point for verbal with a “T” noted (e.g., a score of 8T). The best possible GCS of 15 (eyes open spontaneously, follows commands, and oriented) is compatible with weakness on one side and trouble speaking, whereas the worst possible score of 3 (no eye opening, no motor response, no verbal response to pain) does not mean the patient is brain dead. The FOUR score (38) is a more recent scale that assesses pupil response to light and triggering of a mechanical ventilator, important findings in deeply comatose patients that are not part of the GCS.
NIH Stroke Scale.
Competently performing the neurological examination is often frustrating for physicians who do not have comprehensive training. Designed to be a reliable, reproducible examination for trials in acute ischemic stroke, the NIH Stroke Scale (39) is a simplified neurological examination that covers most acute neurological symptoms: speaking and comprehension, weakness, sensory abnormalities, visual deficit, neglect, and ataxia. A normal examination has zero points, with increasing scores indicating a worse neurological deficit. Training and certification are available online at learn.heart.org/nihss (with regular renewals), and the examination has a high interrater reliability between healthcare providers of different specialties. The examination is sensitive for mild to moderate deficits.
Disease-specific scores for SAH.
Disease-specific scoring systems for SAH include the classic Hunt and Hess Scale (40). This qualitative scale is scored from 1 (no symptoms) to 5 (deeply comatose, extensor posturing). Broadly, the Hunt and Hess Scale is predictive of a variety of medical complications, vasospasm, cerebral infarction, and worse outcomes. The World Federation of Neurosurgical Societies (WFNS) Scale is similar to the Hunt and Hess Scale, but derived from the GCS, which increases interrater reliability. The Hunt and Hess, WFNS, and GCS have similar prognostic significance (41).
Predicting vasospasm is important after SAH because vasospasm may lead to cerebral infarction. Vasospasm is generally predictable from the admission CT scan (42), with thick blood in the subarachnoid space and hemorrhage in both lateral ventricles broadly predictive of an increased risk. “Vasospasm” may refer to a narrowed vessel lumen on angiography, elevated flow velocities on transcranial Doppler (which implies a narrowed vessel lumen), new neurological symptoms (typically aphasia or weakness, usually 3–14 d after SAH onset), or cerebral ischemia on neuroimaging referable to a narrowed vessel. Infarction on neuroimaging often does not correspond to an area of known vessel narrowing (43), however, making vasospasm an incomplete explanation for cerebral infarction after SAH.
Disease-specific scores for IPH.
Although coma, a severe neurological deficit, and old age are predictive of mortality after both SAH and IPH, mortality after IPH often has different causes. The most widely used predictive scale is the ICH score (44), a composite of 0 (best) to 6 (worst) of hemorrhage size greater than 30 ml, age at least 80 years, intraventricular hemorrhage, cerebellar or brainstem location, and GCS. There are modified versions of the ICH score (45), and they are more similar than different. The functional outcome risk stratification scale score (46) also accounts for prehemorrhage cognitive impairment (Table 1). The NIH Stroke Scale and its components assessing level of consciousness can also be used (along with age) to predict outcome after IPH. These scores do not include physiological derangement or events after the initial assessment.
TABLE 1.
EXAMPLES OF DISEASE-SPECIFIC SCORES FOR INTRAPARENCHYMAL HEMORRHAGE
| Variable | ICH Score (points)* | FUNC Score (points)* |
| Hematoma volume | ||
| <30 ml | 0 | 4 |
| 30–60 ml | 1 | 2 |
| >60 ml | 1 | 0 |
| Age | ||
| <70 yr | 0 | 2 |
| 70–79 yr | 0 | 1 |
| ≥80 yr | 1 | 0 |
| Glasgow Coma Scale | ||
| 3–4 | 2 | 0 |
| 5–8 | 1 | 0 |
| 9–12 | 1 | 2 |
| 13–15 | 0 | 2 |
| Hematoma location | ||
| Lobar | 0 | 2 |
| Deep | 0 | 1 |
| Infratentorial | 1 | 0 |
| Intraventricular hemorrhage | 1 if present | Not scored |
| Pre-IPH cognitive impairment | Not scored | 0 if present |
Definition of abbreviations: FUNC = functional outcome risk stratification scale; ICH = intracerebral hemorrhage; IPH = intraparenchymal hemorrhage.
The ICH score ranges from 0 (best) to 6 (worst possible); the FUNC score ranges from 0 (worst possible) to 11 (best).
Treatment and Medical Complications of Intracranial Hemorrhage
Common Principles of Management
Intracranial pressure monitoring.
Emergent neurosurgical consultation should be obtained regarding the potential utility of placing a device to measure ICP. Whether such a device should be placed depends on the nature, severity, and location of the injury. If hydrocephalus is a concern, a ventricular drain will allow both measurement of ICP and drainage of cerebrospinal fluid. A parenchymal monitor permits pressure measurement of the ICP at the tip of the device. Some devices also permit measurement of brain temperature and brain oxygen tension; it is not known whether optimization of parameters other than ICP and cerebral perfusion pressure (mean arterial pressure minus intracranial pressure) leads to improved outcomes.
Elevated ICP (generally defined as >20 mm Hg) may lead to herniation and death. Interventions to reduce elevated ICP include sedation to a calm and motionless state, external ventricular drainage of cerebrospinal fluid, optimizing cerebral perfusion pressure (generally 60 to 80 mm Hg), mannitol or hypertonic saline (usually with a goal osmolality of 315–325 mOsm/L), hyperventilation, hypothermia, paralysis, and induced coma with medication (generally with barbiturates) (47).
Cerebral edema.
Cerebral edema may result from cerebral ischemia, infarction, or contusion. Mannitol is commonly given for cerebral edema and signs of herniation, although repeated doses may lead to hypovolemia because mannitol is an osmotic diuretic. Hypertonic saline may be slightly more effective (48), and a bolus dose often reverses clinical signs of brain herniation and reduces ICP (49). Administration of 3% saline as a continuous infusion (0.5–1 ml/kg/h to start) is common, but there are few prospective data on which to base protocols.
Fever.
Fever is common after intracranial hemorrhage. Risk factors include intraventricular blood, blood near the pituitary, damage to the anterior hypothalamus (rupture of an aneurysm of the anterior communicating artery notoriously leads to high and recurrent fever), and a ventricular drainage catheter (often placed for intraventricular hemorrhage) (50). Often, an infectious source is not found. Greater burden of fever is related to worse outcomes after IPH (51) and SAH (52, 53).
The routine use of acetaminophen for fever control does not improve outcomes (54). There are a variety of devices that ostensibly reduce fever, but no device has improved outcomes in a clinical trial. A circulating cool-air blanket was not effective in one study of critically ill patients in a neurological intensive care unit (ICU) (55), but an external cool-water blanket (56) and invasive catheter reduced core temperatures (57).
Enforced normothermia may lead to shivering. Increased metabolic demand from shivering can be simply and reliably measured at the bedside (58). Shivering can be ablated with sedation, but this confounds the bedside examination and may delay weaning of ventilatory support. Although some data suggest aggressive temperature control will eventually lead to improved outcomes and the expense of more ICU complications (59), it has not yet been done prospectively.
Specific Management of Intraparenchymal Hemorrhage
Severe hypertension is associated with hematoma growth after the diagnostic CT scan and worse outcomes. This implies that increased arterial pressure translates into more blood extravasation into the brain; conversely, lowering blood pressure (BP) might reduce hematoma growth. A prospective randomized trial of “aggressive” versus conventional BP control found that aggressive control was associated with reduced hematoma growth (60). This study was not powered to detect a difference in clinical outcomes, but such a trial (INTERACT [Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial] 2) is now underway. The major risk (especially in patients with chronic hypertension) is that acute BP lowering might lead to reduced cerebral perfusion, cerebral ischemia, and infarction. Decreased diffusion on magnetic resonance imaging (MRI) (consistent with acute ischemia) is associated with more acute BP reduction in patients with IPH (61), and is in turn associated with increased odds of death or dependence at 3 months in multivariate models (62). The most recent guidelines on management of IPH (63) reflect the uncertainty of how best to acutely manage hypertension in this setting.
A hematoma may compress brain tissue or the outflow of cerebrospinal fluid, leading to herniation and death. Emergent neurosurgical consultation is advisable to assess the suitability of decompression. Generally agreed-on indications for surgical decompression include brainstem compression, hydrocephalus, or cerebellar hemorrhage with neurological deterioration (63). Relative indications include a cortical (as opposed to deep) or cerebellar location, a steadily worsening clinical examination, and larger hemorrhage size. When the suitability of emergent surgical decompression was not clear, a randomized trial found no benefit to emergent decompression over expectant management (64). A subpopulation that may be especially helped by surgery (cortical location and lack of ventricular hemorrhage) is eligible for enrollment in a follow-up trial (Surgical Trial in Intracerebral Haemorrhage [STICH] 2).
Warfarin use and an elevated INR are associated with hematoma growth and worse outcome in patients who present with nontraumatic IPH (65). The greater the INR, the greater the risk of hematoma growth on serial CT scans; the longer the time to normalization of the INR, the worse the clinical outcome is likely to be. It is generally agreed that anticoagulant therapy should be reversed, but the best way to do so is not known. Fresh frozen plasma (FFP) is most commonly used because of its availability and relatively low cost, and may be given in repeated doses until the INR is 1.4 or less. Prothrombin complex concentrates (PCCs) may be superior to FFP for warfarin-related IPH, chiefly because of faster normalization of the INR (66). A prospective, randomized trial of FFP versus PCC (INR Normalization in Coumadin-associated ICH, or INCH) for acute treatment is currently underway. PCCs are not a single product, but a variety of related products; before selecting one for routine use, consultation with a hematologist for the best available product and dose is advisable.
Antiplatelet therapies (67, 68) and reduced platelet activity (69) are associated with a higher risk of hematoma growth and death. Platelet transfusion for IPH is considered experimental (63), not standard of care, and two clinical trials of platelet transfusion for acute IPH are underway (70, 71). Desmopressin (DDAVP) reverses the effect of aspirin and improves platelet activity in other conditions (72–74) but has not been formally studied in patients with IPH.
Procoagulant medication is another potentially effective method to reduce hemorrhage growth and improve outcomes. One pilot study evaluated the use of aminocaproic acid for acute IPH, but early results were not promising (75). Recombinant activated factor VII led to reduced hematoma growth and better 90-day outcomes in a phase II trial (76). The phase III trial also showed reduced hematoma growth, but there was no effect on 90-day outcomes (77), and the drug was not approved by the U.S. Food and Drug Administration. There is an increased risk of thrombotic complications with factor VII compared with placebo. Procoagulant therapy is not generally used for the reversal of warfarin-related IPH, and blood products (typically plasma) would be subsequently required (63).
Seizures and anticonvulsant medication.
Seizures after IPH usually occur within a few days of IPH symptom onset and are associated with lobar location of hemorrhage, larger hemorrhage size, depressed mental status, and a history of epilepsy. Patients with IPH due to hepatic failure may be at particularly high risk (78, 79). Clinical seizures should be treated with anticonvulsant medication. When subclinical seizures are suspected or a decreased level of consciousness is unexplained, continuous electroencephalography should be considered (63). Prophylactic anticonvulsant therapy is associated with more fever and worse functional outcomes (80, 81), however, so it should not be used routinely.
Anemia and packed red blood cell transfusion.
The brain is dependent on a continuous supply of nutrients, especially oxygen. Almost all oxygen in blood is bound to hemoglobin (HGB). It follows that progressive anemia, a state of reduced HGB concentration in blood, will eventually lead to reduced oxygen delivery to the brain. Anemia is common in patients with IPH, and is likely to be multifactorial. Anemia is associated with worse outcomes, but the relationship is confounded by larger hematoma volume (82). Packed red blood cell transfusion is associated with reduced mortality after IPH (83), although the proximate mechanism is not clear.
Goals of care and self-fulfilling prophecies.
Perhaps because of the lack of a specific approved treatment for IPH, older patient age, and high mortality, patients with IPH are particularly likely to have a do-not-resuscitate (DNR) order placed. DNR policies typically specify only what to do in the case of cardiac or respiratory failure, but may be associated with fewer interventions overall (e.g., antibiotics for pneumonia, volume resuscitation, etc.). DNR status is associated with a markedly higher mortality for any level of IPH severity (84).
Specific Management of Aneurysmal Subarachnoid Hemorrhage
After aneurysm rupture, a temporary plug forms. Emergent aneurysm obliteration with surgical clipping or endovascular coiling is indicated to prevent aneurysm rebleeding, which makes a good outcome unlikely (85). When aneurysm obliteration is unavoidably delayed, procoagulant therapy reduces the risk of aneurysm rebleeding. A prospective trial found that up to 72 hours of tranexamic acid substantially reduced the risk of aneurysm rebleeding with minimal thrombotic side effects (86). Aminocaproic acid use is also associated with a reduced risk of rebleeding (87).
Severe hypertension is associated with an increased risk of aneurysm rebleeding (88). BP should be controlled to reduce this risk while maintaining cerebral perfusion (85). After aneurysm obliteration, BP management is dictated by the perceived risk of cerebral ischemia and vasospasm. Precisely how high a BP is tolerable is not well defined. An autonomous increase in BP may be an early signal of increased oxygen demand by ischemic brain tissue, and reducing BP may lead to cerebral ischemia. A reliable neurological examination is helpful: If a patient has a reproducible, sensitive examination (e.g., no or minimal aphasia, following commands, arousal to voice or minimal stimulation), then a change from baseline can be interpreted as potentially due to vasospasm and lead to more invasive evaluation (angiography, MRI, a trial of increased BP, etc.). Without a sensitive and reproducible bedside examination, routine imaging or invasive studies are more often obtained to detect cerebral ischemia. A variety of studies are used, including screening with transcranial Doppler, screening angiography, or imaging-based perfusion studies, but there are no validated protocols.
If vasospasm or cerebral ischemia is diagnosed, BP augmentation is a mainstay of treatment. “Triple-H” (hypertension, hypervolemia, hemodilution) is commonly employed as a group, although hemodilution turns out to be difficult (89) and anemia is associated with worse outcomes. (90) “Hyperdynamic” therapy may better describe the intent. Induced hypertension leads to increased cerebral blood flow and cerebral oxygenation (91), and usually reverses clinical symptoms of vasospasm. It is not clear how long such therapy should be continued—usually, a patient with a stable examination will have slowly decreased BP goals or vasopressor infusion rates with close monitoring. Any recurrence of symptoms typically leads to reinstitution of the previous (higher) BP goal for another 48–72 hours. Measurements of cerebral perfusion, oxygen extraction fraction, and cerebral blood flow are attractive physiologic goals, but have not been well verified.
Nimodipine (a cerebroselective calcium channel blocker) reduces poor outcome after SAH, and should be given to all patients with aneurysmal SAH (85, 92). Some clinicians also routinely use pravastatin, based on a phase II study (93); a phase III study is underway.
Anemia and packed red blood cell transfusion.
After SAH anemia is common, due to triple-H therapy, routine measurement of serum electrolytes, and other causes. In observational data sets of patients with SAH, anemia is associated with an increased risk of cerebral ischemia, cerebral infarction, and worse outcomes (90). In patients with brain oxygen tension monitors in place, anemia is associated with reduced brain oxygen tension, and a packed red blood cell (PRBC) transfusion generally increases brain oxygen tension (94). In anemic patients with vasospasm, a PRBC transfusion increases cerebral oxygen delivery and reduces oxygen extraction fraction on positron emission tomography scanning (95), implying less neuronal distress.
If anemia is harmful, it does not necessarily follow that PRBC transfusion is the appropriate treatment. PRBC transfusion may increase the risk of acute lung injury (96). In unselected patients, PRBC transfusion is associated with harm, but not in patients with vasospasm (97), and these are the patients most likely to benefit from PRBC transfusion. In a prospective, randomized pilot trial, higher goal hemoglobin in patients with SAH generally required only one or two more PRBC units through 14 days and was well tolerated (98). PRBC age does not seem to correlate with acute adverse events, vasospasm, or outcomes (99). A phase III study of two different HGB goals after SAH has been proposed.
PRBC transfusion may not be the only effective treatment for preventing cerebral hypoxia and anemia. Recombinant erythropoietin leads to increased HGB in critical care, and has some neuroprotective properties. In a phase II study of patients with SAH, erythropoietin use led to reduced cerebral ischemia (100). This follows similar promising studies, but ultimately negative pivotal trials, in ischemic stroke (101) and critical care (102).
Neurogenic stunned myocardium, or myocardial stunning.
Intracranial hemorrhage, especially SAH, leads to a surge in catecholamines. Depressed cardiac output may lead to clinical heart failure and loss of consciousness. Similar symptoms may be seen in severe emotional distress (103) or Tako-Tsubo syndrome (104). Cardiac troponin I (cTI) predicts the magnitude of the decrease in ejection fraction on echocardiography, the risk of vasospasm and cerebral ischemia, and the odds of dependence or death at 3 months (105). Cardiac adrenoreceptors mediate the severity of the myocardial dysfunction (106). Depressed ejection fraction on echocardiography is not typically due to ischemic myocardium and improves over the following 1 to 2 weeks. Electrocardiographic abnormalities (especially ST-T wave changes) are also common, but are not as predictive of poor outcome as cTI. Neurogenic stunned myocardium and vasospasm may coexist because the risk factors for both conditions are similar (worse neurological injury, more subarachnoid blood); however, the increased afterload from vasopressors (largely catecholamines or their derivatives) may lead to further depression of the ejection fraction, pulmonary edema, and hypotension. Patients with a depressed ejection fraction, elevated cTI, and vasospasm may be better served by cerebral angioplasty or intraarterial vasodilators than vasopressors.
Seizures and anticonvulsant medication.
After SAH, prophylactic phenytoin therapy might reasonably be given to prevent a seizure and the potential attendant increase in BP and risk of aneurysm rebleeding. Subarachnoid hemorrhage may be complicated by nonconvulsive seizures or status epilepticus (107), and routine EEG monitoring is often obtained in patients with a depressed level of consciousness. Like IPH, however, routine anticonvulsant medication use is probably not advisable. Phenytoin exposure (based on serum levels) through 14 days is associated with a dose-dependent increase in functional disability and cognitive deficits at 14 days and 3 months of follow-up (108). Given that 3 days of therapy seems as effective as 14 days (109), a short course, if given at all, is preferable. Levetiracetam may have fewer side effects with similar efficacy (110).
Outcomes After Intracranial Hemorrhage
Mortality is generally not an appropriate sole clinical outcome for neurological disease because many potential patients consider “alive but dependent” a worse outcome than death (111). Functional outcome scales are common in neurological studies (the modified Rankin Scale, Glasgow Outcome Scale, etc.) and reliably grade dependence. The patient-perceived quality of life (QOL) for any level of physical ability is another matter. Most patients who survive intracranial hemorrhage have a markedly lower QOL than the general population (112, 113), even if independent for daily living. (A modified Rankin Scale score of 3 or less, independent with a device or better, is generally considered a “good” outcome after intracranial hemorrhage.)
Outcomes at ICU or hospital discharge are problematic for neurological disease because the period of greatest functional recovery is between the initial injury and 3 months, usually with smaller degrees of improvement later. Patients who are dependent or comatose at hospital discharge may regain consciousness and functional independence in a delayed fashion. Unfortunately, we remain better at reliably predicting a poor outcome than good recovery. Fever and sedatives (e.g., benzodiazepines and barbiturates) cloud the clinical assessment. As general principles, a smaller size of brain injury on neuroimaging, lack of brainstem involvement, and younger age are associated with greater degrees of improvement.
Goals of care are particularly important for the planning of extended life support or subacute care for comatose patients (e.g., tracheostomy and placement in a nursing facility). These discussions may provoke substantial anxiety. A comatose patient nearing ICU discharge will have at least some disability for a period of time, and families may not realize that popular depictions of recovery from coma are unrealistic (114). Consultation with specialists in palliative care may be appropriate to describe protocols for comfort and end-of-life planning. During family conferences, the most commonly missed opportunities are those to listen (115). If a meaningful recovery is possible but uncertain, it is reasonable to pursue supportive measures and reassess the situation in a few months, taking the patient's progress into account. Later emergence from a vegetative state is possible, but rare (116).
In summary, intracranial hemorrhage can be quickly assessed by symptoms, a standardized physical examination, and a CT scan. Major goals of early intensive care (BP control, correction of coagulopathy) can be quickly planned, and intensive care can improve outcomes. Future work will refine therapeutic options, and increase our prognostic and therapeutic armamentarium.
Supplementary Material
Footnotes
Author contributions: A.M.N. performed the literature search and wrote the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201103-0475CI on June 23, 2011
Author Disclosure: A.M.N.’s institution, Northwestern University, has received grants from Gaymar and the Northwestern Memorial Foundation; his institution has filed a use patent on desmopressin for acute intracerebral hemorrhage. A.M.N. has received unrelated research funding from Gaymar and Astellas Pharma US, and serves as a safety monitor for an NIH-supported study in patients with subarachnoid hemorrhage.
References
- 1.Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, Oleinik A, Lev MH, Gonzalez RG, Romero JM. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke 2009;40:2994–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer SA, Bernardini GL, Solomon RA. Subarachnoid hemorrhage. In: Rowland LP, Pedley TA, editors Merritt's neurology, 12th ed Philadelphia: Lippincott Williams & Wilkins; 2010 [Google Scholar]
- 3.van der Schaaf IC, Velthuis BK, Gouw A, Rinkel GJE. Venous drainage in perimesencephalic hemorrhage. Stroke 2004;35:1614–1618 [DOI] [PubMed] [Google Scholar]
- 4.Josephson CB, Frantzias J, Samarasekera N, Al-Shahi Salman R. The persisting burden of intracerebral haemorrhage: can effective treatments be found? PLoS Med 2010;7:e1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, Whelton PK, Vu B, Klag M. Aspirin and the risk of hemorrhage stroke: a meta-analysis of randomized controlled trials. JAMA 1998;280:1930–1935 [DOI] [PubMed] [Google Scholar]
- 6.Knudsen KARJ, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–539 [DOI] [PubMed] [Google Scholar]
- 7.Wermer MJH, van der Schaaf IC, Algra A, Rinkel GJE. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke 2007;38:1404–1410 [DOI] [PubMed] [Google Scholar]
- 8.Greving JP, Rinkel GJE, Buskens E, Algra A. Cost-effectiveness of preventive treatment of intracranial aneurysms. Neurology 2009;73:258–265 [DOI] [PubMed] [Google Scholar]
- 9.Broderick JP, Viscoli CM, Brott T, Kernan WN, Brass LM, Feldmann E, Morgenstern LB, Wilterdink JL, Horwitz RI. Major risk factors for aneurysmal subarachnoid hemorrhage in the young are modifiable. Stroke 2003;34:1375–1381 [DOI] [PubMed] [Google Scholar]
- 10.Broderick JP, Brown RD Jr, Sauerbeck L, Hornung R, Huston J III, Woo D, Anderson C, Rouleau G, Kleindorfer D, Flaherty ML, et al. ; FIA Study Investigators Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke 2009;40:1952–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishibashi T, Murayama Y, Urashima M, Saguchi T, Ebara M, Arakawa H, Irie K, Takao H, Abe T. Unruptured intracranial aneurysms: incidence of rupture and risk factors. Stroke 2009;40:313–316 [DOI] [PubMed] [Google Scholar]
- 12.Burns JD, Huston J III, Layton KF, Piepgras DG, Brown RD Jr. Intracranial aneurysm enlargement on serial magnetic resonance angiography: frequency and risk factors. Stroke 2009;40:406–411 [DOI] [PubMed] [Google Scholar]
- 13.de Rooij NK, Linn FHH, van der Plas JA, Algra A, Rinkel GJE. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 2007;78:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, Kistler JP, Albers GW, Pettigrew LC, Adams HP, Jr, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med 2001;345:1444–1451 [DOI] [PubMed] [Google Scholar]
- 15.CAPRIE steering committee. A randomised, blinded, trial of Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE). Lancet 1996;348:1329–1339 [DOI] [PubMed] [Google Scholar]
- 16.Toyoda K, Yasaka M, Iwade K, Nagata K, Koretsune Y, Sakamoto T, Uchiyama S, Gotoh J, Nagao T, Yamamoto M, et al. Bleeding with Antithrombotic Therapy Study Group Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: a prospective, multicenter, observational study. Stroke 2008;39:1740–1745 [DOI] [PubMed] [Google Scholar]
- 17.Wu TC, Kasam M, Harun N, Hallevi H, Bektas H, Acosta I, Misra V, Barreto AD, Gonzales NR, Lopez GA, et al. Pharmacological deep vein thrombosis prophylaxis does not lead to hematoma expansion in intracerebral hemorrhage with intraventricular extension. Stroke 2011;42:705–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steiner T, Rosand J, Diringer M. Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions. Stroke 2006;37:256–262 [DOI] [PubMed] [Google Scholar]
- 19.Lovelock CE, Cordonnier C, Naka H, Al-Shahi Salman R, Sudlow CLM, Edinburgh Stroke Study Group, Sorimachi T, Werring DJ, Gregoire SM, Imaizumi T, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke 2010;41:1222–1228 [DOI] [PubMed] [Google Scholar]
- 20.Algra A, De Schryver EL, van Gijn J, Kapelle LJ, Koudstaal PJ. Oral anticoagulants versus antiplatelet therapy for preventing further vascular events after transient ischaemic attack or minor stroke of presumed arterial origin. Cochrane Database Syst Rev [serial on the Internet]. 2006 [accessed July 2011];3:CD001342. Available from http://www.cochrane.org/reviews/clibintro.htm [DOI] [PubMed] [Google Scholar]
- 21.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151 [DOI] [PubMed] [Google Scholar]
- 22.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695–1703 [DOI] [PubMed] [Google Scholar]
- 23.Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, Wardlaw J, Hacke W. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol 2009;8:1095–1102 [DOI] [PubMed] [Google Scholar]
- 24.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329 [DOI] [PubMed] [Google Scholar]
- 25.Derex L, Hermier M, Adeleine P, Pialat J.-B, Wiart M, Berthezène Y, Philippeau F, Honnorat J, Froment J-C, Trouillas P, et al. Clinical and imaging predictors of intracerebral haemorrhage in stroke patients treated with intravenous tissue plasminogen activator. J Neurol Neurosurg Psychiatry 2005;76:70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alshekhlee A, Mohammadi A, Mehta S, Edgell RC, Vora N, Feen E, Kale S, Shakir ZA, Cruz-Flores S. Is thrombolysis safe in the elderly?: Analysis of a national database. Stroke 2010;41:2259–2264 [DOI] [PubMed] [Google Scholar]
- 27.Mateen FJ, Buchan AM, Hill MD; CASES Investigators Outcomes of thrombolysis for acute ischemic stroke in octogenarians versus nonagenarians. Stroke 2010;41:1833–1835 [DOI] [PubMed] [Google Scholar]
- 28.Yong M, Kaste M. Association of characteristics of blood pressure profiles and stroke outcomes in the ECASS-II trial. Stroke 2008;39:366–372 [DOI] [PubMed] [Google Scholar]
- 29.Ahmed N, Wahlgren N, Brainin M, Castillo J, Ford GA, Kaste M, Lees KR, Toni D; SITS Investigators Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR). Stroke 2009;40:2442–2449 [DOI] [PubMed] [Google Scholar]
- 30.Diedler J, Ahmed N, Sykora M, Uyttenboogaart M, Overgaard K, Luijckx G-J, Soinne L, Ford GA, Lees KR, Wahlgren N, et al. Safety of intravenous thrombolysis for acute ischemic stroke in patients receiving antiplatelet therapy at stroke onset. Stroke 2010;41:288–294 [DOI] [PubMed] [Google Scholar]
- 31.Scott PA, Frederiksen SM, Kalbfleisch JD, Xu Z, Meurer WJ, Caveney AF, Sandretto A, Holden AB, Haan MN, Hoeffner EG, et al. Safety of intravenous thrombolytic use in four emergency departments without acute stroke teams. Acad Emerg Med 2010;17:1062–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein JN, Marrero M, Masrur S, Pervez M, Barrocas AM, Abdullah A, Oleinik A, Rosand J, Smith EE, Dzik WH, et al. Management of thrombolysis-associated symptomatic intracerebral hemorrhage. Arch Neurol 2010;67:965–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stravitz RT, Kramer AH, Davern T, Shaikh AO, Caldwell SH, Mehta RL, Blei AT, Fontana RJ, McGuire BM, Rossaro L, et al. ; Acute Liver Failure Study Group. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med 2007;35:2498–2508 [DOI] [PubMed] [Google Scholar]
- 34.Blei AT, Olafsson S, Webster S, Levy R. Complications of intracranial pressure monitoring in fulminant hepatic failure. Lancet 1993;341:157–158 [DOI] [PubMed] [Google Scholar]
- 35.Kannoth S, Thomas SV, Nair S, Sarma PS. Proposed diagnostic criteria for intracranial infectious aneurysms. J Neurol Neurosurg Psychiatry 2008;79:943–946 [DOI] [PubMed] [Google Scholar]
- 36.Kannoth S, Iyer R, Thomas SV, Furtado SV, Rajesh BJ, Kesavadas C, Radhakrishnan VV, Sarma PS. Intracranial infectious aneurysm: presentation, management and outcome. J Neurol Sci 2007;256:3–9 [DOI] [PubMed] [Google Scholar]
- 37.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet 1974;2:81–84 [DOI] [PubMed] [Google Scholar]
- 38.Wijdicks EFM, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: the FOUR score. Ann Neurol 2005;58:585–593 [DOI] [PubMed] [Google Scholar]
- 39.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870 [DOI] [PubMed] [Google Scholar]
- 40.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14–20 [DOI] [PubMed] [Google Scholar]
- 41.Julien JS, Bandeen-Roche K, Tamargo RJ. Validation of an aneurysmal subarachnoid hemorrhage grading scale in 1532 consecutive patients. Neurosurgery 2008;63:204–211 [DOI] [PubMed] [Google Scholar]
- 42.Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D, Connolly ES, Mayer SA. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher Scale revisited. Stroke 2001;32:2012–2020 [DOI] [PubMed] [Google Scholar]
- 43.Rabinstein AA, Friedman JA, Weigand SD, McClelland RL, Fulgham JR, Manno EM, Atkinson JL, Wijdicks EF. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 2004;35:1862–1866 [DOI] [PubMed] [Google Scholar]
- 44.Hemphill JC, III, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897 [DOI] [PubMed] [Google Scholar]
- 45.Weimar C, Benemann J, Diener H-C. Development and validation of the Essen Intracerebral Haemorrhage Score. J Neurol Neurosurg Psychiatry 2006;77:601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, FitzMaurice E, Wendell L, Goldstein JN, Greenberg SM, et al. ; Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 2008;39:2304–2309 [DOI] [PubMed] [Google Scholar]
- 47.Dennis LJ, Mayer SA. Diagnosis and management of increased intracranial pressure. Neurol India 2001;49:S37–S50 [PubMed] [Google Scholar]
- 48.Kamel H, Navi BB, Nakagawa K, Hemphill JC, III, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med 2011;39:554–559 [DOI] [PubMed] [Google Scholar]
- 49.Koenig MA, Bryan M, Lewin JL, III, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology 2008;70:1023–1029 [DOI] [PubMed] [Google Scholar]
- 50.Commichau C, Scarmeas N, Mayer SA. Risk factors for fever in the neurologic intensive care unit. Neurology 2003;60:837–841 [DOI] [PubMed] [Google Scholar]
- 51.Schwarz S, Hafner K, Aschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology 2000;54:354–361 [DOI] [PubMed] [Google Scholar]
- 52.Todd MM, Hindman BJ, Clarke WR, Torner JC, Weeks JB, Bayman EO, Shi Q, Spofford CM. Perioperative fever and outcome in surgical patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 2009;64:897–908, discussion 908 [DOI] [PubMed] [Google Scholar]
- 53.Fernandez A, Schmidt JM, Claassen J, Pavlicova M, Huddleston D, Kreiter KT, Ostapkovich ND, Kowalski RG, Parra A, Connolly ES, et al. Fever after subarachnoid hemorrhage: risk factors and impact on outcome. Neurology 2007;68:1013–1019 [DOI] [PubMed] [Google Scholar]
- 54.den Hertog HM, van der Worp HB, van Gemert HM, Algra A, Kappelle LJ, van Gijn J, Koudstaal PJ, Dippel DW. The Paracetamol (Acetaminophen) in Stroke (PAIS) trial: a multicentre, randomised, placebo-controlled, phase III trial. Lancet Neurol 2009;8:434–440 [DOI] [PubMed] [Google Scholar]
- 55.Mayer S, Commichau C, Scarmeas N, Presciutti M, Bates J, Copeland D. Clinical trial of an air-circulating cooling blanket for fever control in critically ill neurologic patients. Neurology 2001;56:292–298 [DOI] [PubMed] [Google Scholar]
- 56.Mayer SA, Kowalski RG, Presciutti M, Ostapkovich ND, McGann E, Fitzsimmons BF, Yavagal DR, Du YE, Naidech AM, Janjua NA, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med 2004;32:2508–2515 [DOI] [PubMed] [Google Scholar]
- 57.Diringer MN. Treatment of fever in the neurologic intensive care unit with a catheter-based heat exchange system. Crit Care Med 2004;32:559–564 [DOI] [PubMed] [Google Scholar]
- 58.Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, Buitrago M, Schmidt JM, Ostapkovich ND, Mayer SA. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke 2008;39:3242–3247 [DOI] [PubMed] [Google Scholar]
- 59.Badjatia N, Fernandez L, Schmidt JM, Lee K, Claassen J, Connolly ES, Mayer SA. Impact of induced normothermia on outcome after subarachnoid hemorrhage: a case–control study. Neurosurgery 2010;66:696–700; discussion 700–691 [DOI] [PubMed] [Google Scholar]
- 60.Anderson C, Huang Y, Wang J, Arima H, Neal B, Peng B, Heeley E, Skulina C, Parsons M, Kim J, et al. Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT): a randomised pilot trial. Lancet Neurol 2008;7:391–399 [DOI] [PubMed] [Google Scholar]
- 61.Prabhakaran S, Gupta R, Ouyang B, John S, Temes RE, Mohammad Y, Lee VH, Bleck TP. Acute brain infarcts after spontaneous intracerebral hemorrhage: a diffusion-weighted imaging study. Stroke 2010;41:89–94 [DOI] [PubMed] [Google Scholar]
- 62.Garg RK, Liebling SM, Maas MB, Nemeth AJ, Russell EJ, Naidech AM. Blood pressure reduction, decreased diffusion on MRI, and outcomes after intracerebral hemorrhage. Stroke (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgenstern LB, Hemphill JC, III, Anderson C, Becker K, Broderick JP, Connolly ES, Jr, Greenberg SM, Huang JN, Macdonald RL, Messe SR, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010;41:2108–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD, Barer DH; STICH Investigators Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005;365:387–397 [DOI] [PubMed] [Google Scholar]
- 65.Flibotte J, Hagan N, O'Donnell J, Greenberg S, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004;63:1059–1064 [DOI] [PubMed] [Google Scholar]
- 66.Huttner HB, Schellinger PD, Hartmann M, Kohrmann M, Juettler E, Wikner J, Mueller S, Meyding-Lamade U, Strobl R, Mansmann U, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke 2006;37:1465–1470 [DOI] [PubMed] [Google Scholar]
- 67.Saloheimo P, Ahonen M, Juvela S, Pyhtinen J, Savolainen E, Hillborm M. Regular aspirin use preceding the onset of primary intracerebral hemorrhage is an independent predictor for death. Stroke 2006;37:129–133 [DOI] [PubMed] [Google Scholar]
- 68.Thompson BB, Bejot Y, Caso V, Castillo J, Christensen H, Flaherty ML, Foerch C, Ghandehari K, Giroud M, Greenberg SM, et al. Prior antiplatelet therapy and outcome following intracerebral hemorrhage: a systematic review. Neurology 2010;75:1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naidech AM, Jovanovic B, Liebling S, Garg RK, Bassin SL, Bendok BR, Bernstein RA, Alberts MJ, Batjer HH. Reduced platelet activity is associated with early clot growth and worse 3 month outcome after intracerebral hemorrhage. Stroke 2009;40:2398–2401 [DOI] [PubMed] [Google Scholar]
- 70.Hillbom M, Huhtakangas J. Platelet transfusion in acute intracerebral hemorrhage [clinical trial no. NCT00699621]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00699621?term=platelet+transfusion+intracerebral+hemorrhage&recr=Open&rank=1
- 71.de Gans K, de Haan RJ, Majoie CB, Koopman MM, Brand A, Dijkgraaf MG, Vermeulen M, Roos YB. PATCH: platelet transfusion in cerebral haemorrhage: study protocol for a multicentre, randomised, controlled trial. BMC Neurol 2010;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Franchini M. The use of desmopressin as a hemostatic agent: a concise review. Am J Hematol 2007;82:731–735 [DOI] [PubMed] [Google Scholar]
- 73.Flordal PA, Sahlin S. Use of desmopressin to prevent bleeding complications in patients treated with aspirin. Br J Surg 1993;80:723–724 [DOI] [PubMed] [Google Scholar]
- 74.Beck KH, Mohr P, Bleckmann U, Schweer H, Kretschmer V. Desmopressin effect on acetylsalicylic acid impaired platelet function. Semin Thromb Hemost 1995;21:32–39 [DOI] [PubMed] [Google Scholar]
- 75.Piriyawat P, Morgenstern LB, Yawn DH, Hall CE, Grotta JC. Treatment of acute intracerebral hemorrhage with ε-aminocaproic acid: a pilot study. Neurocrit Care 2004;1:47–51 [DOI] [PubMed] [Google Scholar]
- 76.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer M, Skolnick BE, Steiner T. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–785 [DOI] [PubMed] [Google Scholar]
- 77.Mayer SA, Brun NC, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–2137 [DOI] [PubMed] [Google Scholar]
- 78.Ellis AJ, Wendon JA, Williams R. Subclinical seizure activity and prophylactic phenytoin infusion in acute liver failure: a controlled clinical trial. Hepatology 2000;32:536–541 [DOI] [PubMed] [Google Scholar]
- 79.Bhatia V, Batra Y, Acharya SK. Prophylactic phenytoin does not improve cerebral edema or survival in acute liver failure—a controlled clinical trial. J Hepatol 2004;41:89–96 [DOI] [PubMed] [Google Scholar]
- 80.Naidech AM, Garg RK, Liebling S, Levasseur K, Macken MP, Schuele SU, Batjer HH. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke 2009;40:3810–3815 [DOI] [PubMed] [Google Scholar]
- 81.Messe SR, Sansing LH, Cucchiara BL, Herman ST, Lyden PD, Kasner SE. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care 2009;11:38–44 [DOI] [PubMed] [Google Scholar]
- 82.Kumar MA, Rost NS, Snider RW, Chanderraj R, Greenberg SM, Smith EE, Rosand J. Anemia and hematoma volume in acute intracerebral hemorrhage. Crit Care Med 2009;37:1442–1447 [DOI] [PubMed] [Google Scholar]
- 83.Sheth KN, Gilson AJ, Chang Y, Kumar MA, Rahman RM, Rost NS, Schwab K, Cortellini L, Goldstein JN, Smith EE, Greenberg SM, Rosand J. Packed red blood cell transfusion and decreased mortality in intracerebral hemorrhage. Neurosurgery 2011;68:1286–1292 [DOI] [PubMed] [Google Scholar]
- 84.Zahuranec DB, Morgenstern LB, Sanchez BN, Resnicow K, White DB, Hemphill JC., III Do-not-resuscitate orders and predictive models after intracerebral hemorrhage. Neurology 2010;75:626–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bederson JB, Connolly ES, Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE, Jr, Harbaugh RE, Patel AB, Rosenwasser RH. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 2009;40:994–1025 [DOI] [PubMed] [Google Scholar]
- 86.Hillman J, Fridriksson S, Nilsson O, Yu Z, Saveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg 2002;97:771–778 [DOI] [PubMed] [Google Scholar]
- 87.Starke RM, Kim GH, Fernandez A, Komotar RJ, Hickman ZL, Otten ML, Ducruet AF, Kellner CP, Hahn DK, Chwajol M, et al. Impact of a protocol for acute antifibrinolytic therapy on aneurysm rebleeding after subarachnoid hemorrhage. Stroke 2008;39:2617–2621 [DOI] [PubMed] [Google Scholar]
- 88.Ohkuma H, Tsurutani H, Suzuki S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke 2001;32:1176–1180 [DOI] [PubMed] [Google Scholar]
- 89.Lennihan L, Mayer SA, Fink ME, Beckford A, Paik MC, Zhang H, Wu YC, Klebanoff LM, Raps EC, Solomon RA. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke 2000;31:383–391 [DOI] [PubMed] [Google Scholar]
- 90.Naidech AM, Jovanovic B, Wartenberg KE, Parra A, Ostapkovich N, Connolly ES, Mayer SA, Commichau C. Higher hemoglobin is associated with improved outcome after subarachnoid hemorrhage. Crit Care Med 2007;35:2383–2389 [DOI] [PubMed] [Google Scholar]
- 91.Muench E, Horn P, Bauhuf C, Roth H, Philipps M, Hermann P, Quintel M, Schmiedek P, Vajkoczy P. Effects of hypervolemia and hypertension on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation after subarachnoid hemorrhage. Crit Care Med 2007;35:1844–1851; quiz 1852 [DOI] [PubMed] [Google Scholar]
- 92.Dorhout Mees SM, Rinkel GJ, Feigin VL, Algra A, van den Bergh WM, Vermeulen M, van Gijn J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2007;CD000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke 2005;36:1627–1632 [DOI] [PubMed] [Google Scholar]
- 94.Smith MJ, Stiefel MF, Magge S, Frangos S, Bloom S, Gracias V, Le Roux PD. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med 2005;33:1104–1108 [DOI] [PubMed] [Google Scholar]
- 95.Dhar R, Zazulia AR, Videen TO, Zipfel GJ, Derdeyn CP, Diringer MN. Red blood cell transfusion increases cerebral oxygen delivery in anemic patients with subarachnoid hemorrhage. Stroke 2009;40:3039–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med 2006;34:196–202 [DOI] [PubMed] [Google Scholar]
- 97.Kramer AH, Gurka MJ, Nathan B, Dumont AS, Kassell NF, Bleck TP. Complications associated with anemia and blood transfusion in patients with aneurysmal subarachnoid hemorrhage. Crit Care Med 2008;36:2070–2075 [DOI] [PubMed] [Google Scholar]
- 98.Naidech AM, Shaibani A, Garg RK, Duran IM, Liebling SM, Bassin SL, Bendok BR, Bernstein RA, Batjer HH, Alberts MJ. Prospective, randomized trial of higher goal hemoglobin after subarachnoid hemorrhage. Neurocrit Care 2010;13:313–320 [DOI] [PubMed] [Google Scholar]
- 99.Naidech AM, Liebling SM, Duran IM, Ault ML. Packed red blood cell age does not impact adverse events or outcomes after subarachnoid haemorrhage. Transfus Med 2011;21:130–133 [DOI] [PubMed] [Google Scholar]
- 100.Tseng MY, Hutchinson PJ, Richards HK, Czosnyka M, Pickard JD, Erber WN, Brown S, Kirkpatrick PJ. Acute systemic erythropoietin therapy to reduce delayed ischemic deficits following aneurysmal subarachnoid hemorrhage: a phase II randomized, double-blind, placebo-controlled trial. J Neurosurg 2009;111:171–180 [DOI] [PubMed] [Google Scholar]
- 101.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 2009;40:e647–e656 [DOI] [PubMed] [Google Scholar]
- 102.Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, An R, Bowers PJ, Burton P, Klausner MA, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med 2007;357:965–976 [DOI] [PubMed] [Google Scholar]
- 103.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–548 [DOI] [PubMed] [Google Scholar]
- 104.Lee VH, Connolly HM, Fulgham JR, Manno EM, Brown RD, Jr, Wijdicks EF. Tako-Tsubo cardiomyopathy in aneurysmal subarachnoid hemorrhage: an underappreciated ventricular dysfunction. J Neurosurg 2006;105:264–270 [DOI] [PubMed] [Google Scholar]
- 105.Naidech AM, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C, Fitzsimmons BF, Connolly ES, Mayer SA. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation 2005;112:2851–2856 [DOI] [PubMed] [Google Scholar]
- 106.Zaroff JG, Pawlikowska L, Miss JC, Yarlagadda S, Ha C, Achrol A, Kwok PY, McCulloch CE, Lawton MT, Ko N, et al. Adrenoceptor polymorphisms and the risk of cardiac injury and dysfunction after subarachnoid hemorrhage. Stroke 2006;37:1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Claassen J, Hirsch LJ, Frontera JA, Fernandez A, Schmidt M, Kapinos G, Wittman J, Connolly ES, Emerson RG, Mayer SA. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care 2006;4:103–112 [DOI] [PubMed] [Google Scholar]
- 108.Naidech AM, Kreiter KT, Janjua N, Ostapkovich N, Parra A, Commichau C, Connolly ES, Mayer SA, Fitzsimmons BF. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke 2005;36:583–587 [DOI] [PubMed] [Google Scholar]
- 109.Chumnanvej S, Dunn IF, Kim DH. Three-day phenytoin prophylaxis is adequate after subarachnoid hemorrhage. Neurosurgery 2007;60:99–102, discussion 102–103 [DOI] [PubMed] [Google Scholar]
- 110.Szaflarski JP, Sangha KS, Lindsell CJ, Shutter LA. Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care 2010;12:165–172 [DOI] [PubMed] [Google Scholar]
- 111.Solomon N, Glick H, Russo C, Lee J, Schulman K. Patient preferences for stroke outcomes. Stroke 1994;25:1721–1725 [DOI] [PubMed] [Google Scholar]
- 112.Christensen MC, Mayer S, Ferran J-M. Quality of life after intracerebral hemorrhage: results of the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke 2009;40:1677–1682 [DOI] [PubMed] [Google Scholar]
- 113.Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke 2010;41:e519–e536 [DOI] [PubMed] [Google Scholar]
- 114.Casarett D, Fishman JM, MacMoran HJ, Pickard A, Asch DA. Epidemiology and prognosis of coma in daytime television dramas. BMJ 2005;331:1537–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Curtis JR, Engelberg RA, Wenrich MD, Shannon SE, Treece PD, Rubenfeld GD. Missed opportunities during family conferences about end-of-life care in the intensive care unit. Am J Respir Crit Care Med 2005;171:844–849 [DOI] [PubMed] [Google Scholar]
- 116.Andrews K. Recovery of patients after four months or more in the persistent vegetative state. BMJ 1993;306:1597–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


