Abstract
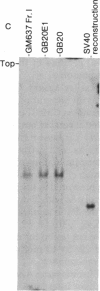
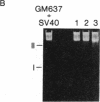
Somatic cell hybrids derived from fusion of GM637, a human cell line transformed by simian virus 40, and mouse B82 cells were examined for simian virus 40 T antigen, V antigen, and viral DNA. All hybrid cell lines that contained viral DNA were T-antigen positive. Cells that did not have viral DNA were T-antigen negative. We determined that there is a single viral insertion in these hybrid cells. Correlation of T-antigen expression and viral DNA with the partial complements of the human genome retained in the hybrids shwed that the inserted viral genome is in human chromosome 8. The integrated viral DNA is stable; free viral DNA found in GM637 does not insert at other potential sites in the human genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Aden D., Koprowski H. Somatic cell hybrids between mouse peritoneal macrophages and simian-virus-40-transformed human cells: II. Presence of human chromosome 7 carrying simin virus 40 genome in cells of tumors induced by hybrid cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1397–1400. doi: 10.1073/pnas.72.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M. Assignment of the integration site for simian virus 40 to chromosome 17 in GM54VA, a human cell line transformed by simian virus 40. Proc Natl Acad Sci U S A. 1977 Jan;74(1):315–318. doi: 10.1073/pnas.74.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Girardi A. J., Koprowski H. Assignment of the T-antigen gene of simian virus 40 to human chromosome C-7. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3617–3620. doi: 10.1073/pnas.70.12.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Knowles B. B., Koprowski H. Preferential retention of the human chromosome C-7 in human-(thymidine kinase deficient) mouse hybrid cells. Exp Cell Res. 1973 Dec;82(2):457–461. doi: 10.1016/0014-4827(73)90366-2. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Koprowski H. Assignment of gene(s) for cell transformation to human chromosome 7 carrying the simian virus 40 genome. Proc Natl Acad Sci U S A. 1975 May;72(5):1658–1660. doi: 10.1073/pnas.72.5.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Koprowski H. Concordant segregation of the expression of SV40 T antigen and human chromosome 7 in mouse-human hybrid subclones. J Exp Med. 1974 May 1;139(5):1350–1353. doi: 10.1084/jem.139.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Koprowski H. Somatic cell hybrids between mouse peritoneal macrophages and SV40-transformed human cells. I. Positive control of the transformed phenotype by the human chromosome 7 carrying the SV40 genome. J Exp Med. 1974 Nov 1;140(5):1221–1229. doi: 10.1084/jem.140.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., O'Malley K. A., Wheeler T. B. Polyethylene glycol-induced mammalian cell hybridization: effect of polyethylene glycol molecular weight and concentration. Somatic Cell Genet. 1976 May;2(3):271–280. doi: 10.1007/BF01538965. [DOI] [PubMed] [Google Scholar]
- Gelinas R. E., Myers P. A., Weiss G. H., Roberts R. J., Murray K. A specific endonuclease from Brevibacterium albidum. J Mol Biol. 1977 Aug 15;114(3):433–440. doi: 10.1016/0022-2836(77)90260-1. [DOI] [PubMed] [Google Scholar]
- Gerber P. Studies on the transfer of subviral infectivity from SV40-induced hamster tumor cells to indicator cells. Virology. 1966 Apr;28(4):501–509. doi: 10.1016/0042-6822(66)90234-0. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols E. A., Ruddle F. H. A review of enzyme polymorphism, linkage and electrophoretic conditions for mouse and somatic cell hybrids in starch gels. J Histochem Cytochem. 1973 Dec;21(12):1066–1081. doi: 10.1177/21.12.1066. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Giles R. E., Kucherlapati R. S., Shimizu Y., Ruddle F. H. Somatic cell genetic assignment of the human gene for mitochondrial NADP-linked isocitrate dehydrogenase to the long arm of chromosome 15. Somatic Cell Genet. 1977 Jan;3(1):47–60. doi: 10.1007/BF01550986. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinberg B., Pollack R., Topp W., Botchan M. Isolation and characterization of T antigen-negative revertants from a line of transformed rat cells containing one copy of the SV40 genome. Cell. 1978 Jan;13(1):19–32. doi: 10.1016/0092-8674(78)90134-4. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. F., Dulbecco R. Production of SV40 virus in heterokaryons of transformed and susceptible cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1396–1403. doi: 10.1073/pnas.58.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. C. Further studies on loss of T-antigen from somatic hybrids between mouse cells and SV40-transformed human cells. Proc Natl Acad Sci U S A. 1970 May;66(1):79–86. doi: 10.1073/pnas.66.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heyningen V., Bobrow M., Bodmer W. F., Gardiner S. E., Povey S., Hopkinson D. A. Chromosome assignment of some human enzyme loci: mitochondrial malate dehydrogenase to 7, mannosephosphate isomerase and pyruvate kinase to 15 and probably, esterase D to 13. Ann Hum Genet. 1975 Jan;38(3):295–303. doi: 10.1111/j.1469-1809.1975.tb00613.x. [DOI] [PubMed] [Google Scholar]