Abstract
Integrating current evidence with fundamental concepts from decision analysis suggests that management of patients with pulmonary nodules should begin with estimating the pretest probability of cancer from the patient's clinical risk factors and computed tomography characteristics. Then, the consequences of treatment should be considered, by comparing the benefits of surgery if the patient has lung cancer with the potential harm if the patient does not have cancer. This analysis determines the “treatment threshold,” which is the point around which the decision centers. This varies widely among patients depending on their cardiopulmonary reserve, comorbidities, and individual preferences. For patients with a very low probability of cancer, careful observation with serial computed tomography is warranted. For those with a high probability of cancer, surgical diagnosis is warranted. For patients in the intermediate range of probabilities, either computed tomography–guided fine-needle aspiration biopsy or positron emission tomography, possibly followed by computed tomography–guided fine-needle aspiration biopsy, is best. Patient preferences should be considered because the absolute difference in outcome between strategies may be small. The optimal approach to the management of patients with pulmonary nodules is evolving as technologies develop. Areas of uncertainty include quantifying the hazard of delayed diagnosis; determining the optimal duration of follow-up for ground-glass and semisolid opacities; establishing the roles of volumetric imaging, advanced bronchoscopic technologies, and limited surgical resections; and calculating the cost-effectiveness of different strategies.
Keywords: lung cancer, solitary pulmonary nodule, lung cancer screening, positron emission tomography, lung nodule
Lung nodules are a common problem in pulmonary practice. Estimates of their frequency range from 0.2% in older studies with chest radiographs to approximately 40–60% in lung cancer screening trials using low-dose computed tomography (CT) (1–7). The widespread use of CT has increased the incidental detection of pulmonary nodules that would not have been identified previously. If lung cancer screening with low-dose CT becomes commonplace in clinical practice, as seems likely given the findings from the National Lung Screening Trial (8–10), the frequency of detection of lung nodules will probably increase dramatically. Possible causes of pulmonary nodules include many benign diseases, but the primary concern is bronchogenic carcinoma. Because large tumor size and advanced stage are associated with worse prognosis, the goal is to rapidly identify and resect malignant lesions while avoiding unnecessary surgery in patients with benign disease, and to do so in a cost-effective manner.
This concise clinical review (1) updates the definition of pulmonary nodules; (2) reviews methods for estimating the probability of cancer; (3) compares benefits and harms of available management strategies; and (4) integrates definitions, risk stratification, and management alternatives into a practical clinical algorithm.
Updating the Definition of Pulmonary Nodules
The definition of a classical solitary pulmonary nodule is a single, spherical, well-circumscribed, radiographic opacity less than or equal to 30 mm in diameter that is completely surrounded by aerated lung and is not associated with atelectasis, hilar enlargement, or pleural effusion (1, 11). The differential diagnosis includes malignancies, such as bronchogenic carcinoma, carcinoid tumors, lymphoma, and solitary pulmonary metastasis, and a variety of benign causes, including nonspecific granulomas, granulomatous infections, and hamartomas.
The term “solitary pulmonary nodule” was coined when most nodules were detected incidentally by chest radiography and were solitary. Today, most nodules are detected by CT, which greatly enhances nodule detection and characterization. Thus, the classical definition of pulmonary nodules now needs to be updated to integrate data from CT studies.
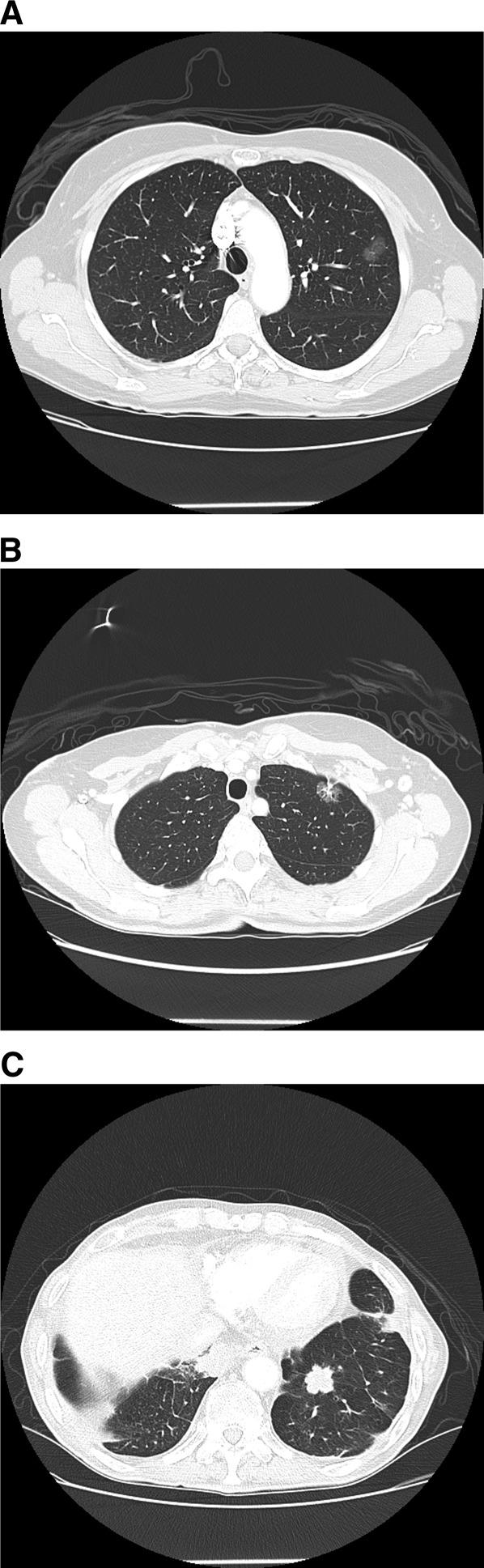
Pulmonary nodules should be characterized on the basis of number, size, and density. The modifying term “solitary” should not be used for nodules accompanied by additional nodules or associated findings, or for nodules not completely surrounded by aerated lung. An increasingly important subset of nodules are subcentimeter nodules, which we define as those less than or equal to 8 mm in diameter. Subcentimeter nodules may be spherical or nonspherical, and malignant nodules may have either shape (12). Lesions greater than 30 mm in diameter should be called masses rather than nodules and are presumed to be malignant until proved otherwise (13). For masses, a tissue diagnosis should be made by the least invasive means (14). Finally, CT has also led to a more precise and nuanced classification of nodules according to whether ground-glass opacification is present. Nodules may have a pure ground-glass appearance; a pure solid appearance; or a mixed ground-glass and solid appearance (also called semisolid) (Figure 1). These characteristics can be used to help estimate the probability of cancer in the nodule.
Figure 1.
(A) Ground-glass opacity. (B) Mixed ground-glass and solid nodule, also called a semisolid nodule. (C) Solid lung nodule.
Estimating the Probability of Cancer
Risk stratification entails estimating the probability of cancer in the nodule. This pretest probability of cancer must first be estimated using the available information, including the patient's clinical risk factors and CT characteristics.
Clinical Risk Factors
The clinical assessment includes the patient's history and physical examination. Clinical risk factors associated with a higher probability of malignancy are shown in Table 1 (1, 11, 13, 15). The physician should estimate the pretest probability of cancer by evaluating these risk factors and using clinical judgment.
TABLE 1.
RISK FACTORS FOR LUNG CANCER IN PATIENTS WITH SOLITARY PULMONARY NODULES
| Cancer Risk |
|||
| Variable | Low | Intermediate | High |
| Nodule size, diameter in mm | <8 | 8–20 | >20 |
| Age, yr | <45 | 45–60 | >60 |
| Prior cancer history | No prior cancer | Prior cancer history | |
| Tobacco use | Never smoked | Current smoker <1 pack per day | Current smoker ≥ 1 pack per day |
| Smoking cessation | Quit ≥ 7 yr ago | Quit <7 yr ago | Never quit |
| Chronic obstructive lung disease | No COPD | COPD | |
| Asbestos exposure | No exposure | Exposed | |
| Nodule characteristics | Smooth | Lobulated | Spiculated |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Alternatively, models using logistic regression (16–18) can be used to estimate this probability. The logistic model of Swensen and coworkers (16) was developed using data from patients with newly discovered solitary pulmonary nodules that were 4–30 mm in diameter and radiologically indeterminate. The model uses age, smoking history, history of cancer greater than or equal to 5 years before the nodule was discovered, diameter, spiculation, and upper lobe location to estimate the probability of malignancy. No significant difference was found between results from the logistic model and the predictions of physicians (17). Free Internet-based and mobile device applications are now available that facilitate such calculations. A similar model was developed and subsequently validated in a population with a higher prevalence of malignancy (18, 19).
CT Characteristics
The variables to assess with CT are the nodule's size, border characteristics, and density.
The probability of malignancy varies with size. For subcentimeter pulmonary nodules, the overall prevalence of malignancy is relatively low. In seven studies of nodules detected in lung cancer screening trials, the prevalence of malignancy was 0–1% in patients with nodules less than 5 mm in diameter, 6–28% for 5- to 10-mm nodules, 33–64% for 11- to 20-mm nodules, and 64–82% for nodules measuring greater than 20 mm (20).
Border characteristics can also be used to help estimate the probability of malignancy. Nodules with irregular, lobulated, or spiculated borders are associated with a progressively higher probability of malignancy than those with a smooth border. Similarly, nodules with a pure ground-glass or semisolid appearance have a higher probability of malignancy than pure solid lesions (20).
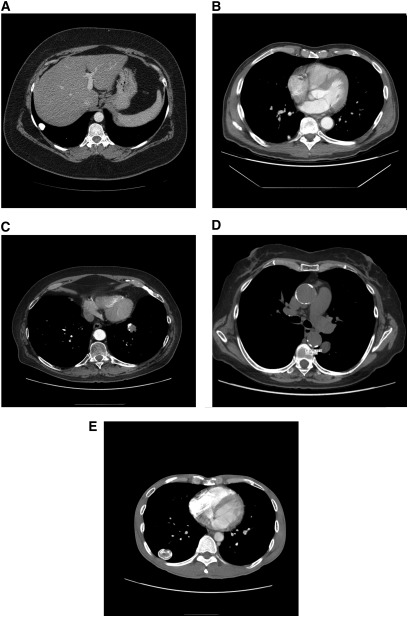
The density of nodules is also useful to discriminate between benign and malignant nodules. Benign calcification patterns (diffuse, central, laminated, or popcorn patterns) and intranodular fat density (i.e., hamartoma) are associated with an extremely low probability of malignancy, and nodules with these characteristics warrant careful (or even no) observation rather than additional diagnostic testing (Figure 2) (21). Stippled and eccentric calcification patterns do not exclude malignancy, and further work-up is required.
Figure 2.
(A) Diffuse calcified granuloma. (B) Granuloma with central calcification. (C) Hamartoma with popcorn pattern of calcifications. (D) Hamartoma with fat density areas (−31.25 HU). (E) Laminated calcification pattern indicative of benign disease.
Ground Glass Opacities (subsolid nodules)
Over the last 20 years, studies of screening-detected and incidentally detected peripheral adenocarcinomas have clarified associations between CT characteristics, histopathology, growth rates, and clinical outcomes (22–25). In general, the prevalence of malignancy is especially high in nodules with pure ground-glass attenuation (Figure 1A). Small, ground-glass lesions typically represent adenocarcinoma in situ, previously referred to as bronchioloalveolar cell carcinoma (BAC), or its putative precursor lesion, atypical adenomatous hyperplasia. These lesions tend to grow slowly and are associated with a very favorable prognosis, even when resection is delayed by a period of observation (26, 27). Accelerated growth or development of a solid component (Figure 1B) is strongly associated with transition to invasive adenocarcinoma, so either of these findings should prompt surgical consultation.
Pre-test Probability and Post-test Probability
The pretest probability of cancer can be estimated from the clinical risk factors and the CT characteristics, as described previously. The posttest probability of cancer can be determined by combining the pretest probability with test results, provided that the test characteristics (sensitivity and specificity) are known, by using Bayes’ theorem (see online supplement). However, even if one can determine the posttest probability of cancer, one then has to ask the following questions: Is this probability high enough to warrant surgery? Is it low enough to warrant careful observation?
Conceptual Framework for Decision Making
To answer these questions and evaluate management strategies, a conceptual framework is needed that facilitates comparison of options. When selecting and interpreting tests for pulmonary nodule evaluation, it is important to consider not only the likelihood of false-positive and false-negative test results, but also the consequences of these findings.
Decision Thresholds
For patients with pulmonary nodules, three management strategies exist: (1) careful observation with serial CT; (2) additional diagnostic testing (imaging, biopsy, or combinations); and (3) surgical resection. Clearly, if the probability of cancer is close to 0, careful observation is best. Conversely, if the probability of cancer is very close to 1, proceeding directly to surgery (after an appropriate staging work-up) is best. For patients with an intermediate probability of cancer, additional diagnostic testing is best.
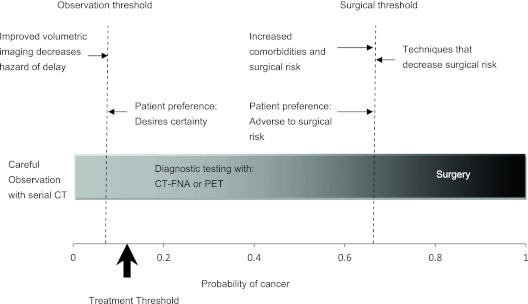
Note that there must be some probability of cancer at which the optimal strategy changes from one option to the next, from careful observation to diagnostic testing and from diagnostic testing to surgery; these are decision thresholds (Figure 3). We will call the lower decision threshold the “observation threshold”; a probability of cancer below this warrants careful observation with serial CT. We will call the upper decision threshold the “surgical threshold”; a probability of cancer above this warrants surgery with frozen section, usually followed by lobectomy if malignancy is identified. What factors affect the decision thresholds, and where do those thresholds lie for a particular patient? To answer these questions, the consequences of treatment need to be considered.
Figure 3.
Decision thresholds and the probability of cancer. The observation threshold is the probability of cancer below which careful observation with serial CT is warranted. The surgical threshold is the probability of cancer above which surgery is warranted. Diagnostic testing with CT-FNA or PET is warranted for probabilities of cancer between these two extremes. Different factors that may alter these decision thresholds are shown. For example, increased comorbidities and surgical risk increase the surgical threshold. Improved detection of nodule growth with volumetric CT, thereby decreasing the hazard of delay, increases the observation threshold. The observation and surgical thresholds vary depending on individual patient comorbidities and surgical risk factors, patient preferences, and the availability of diagnostic tests and treatments. CT = computed tomography; CT-FNA = CT-guided fine-needle aspiration; PET = positron emission tomography.
Integrating Probability and Consequences
Decision thresholds depend on treatment consequences—the relative potential for benefit and harm (28). The benefit of a treatment is defined as the difference in outcome between patients with the disease who receive the treatment and similar patients who do not receive the treatment. The harm of a treatment is defined as the difference in outcome between patients without the disease who receive no treatment and similar patients who receive the treatment. The treatment threshold probability is defined as the probability of disease at which the expected outcome of treatment and no treatment are exactly equal, so that neither option is clearly better. As applied to pulmonary nodules, treatment usually means surgery. However, if surgery was not a viable alternative but radiation therapy was possible, the same logic would apply, although a different treatment threshold probability would result. Given the benefit and the harm, the treatment threshold probability is as follows (28):
For a more detailed discussion, formulas, and proofs, see the online supplement.
If no diagnostic tests were available and the pretest probability of disease was greater than the treatment threshold, empiric treatment would be warranted. A low treatment threshold is warranted when the benefit of treatment for diseased individuals is high and the harm of accidentally treating nondiseased individuals is low. An example is the use of antibiotics in suspected meningitis. In other situations, a higher treatment threshold is appropriate; an example is chemotherapy for possible cancer. Treatment threshold varies not only among diseases but also among patients with the same disease, because benefit and harm vary depending on comorbidities, clinical context, and patient preferences.
To decide whether further diagnostic testing is advisable for a patient, one first needs to estimate the pretest probability and determine whether test results could change the management of that patient. For patients with an intermediate probability of malignancy that lies above the treatment threshold, one possible strategy is to select a test (or tests) that, if negative, would result in a posttest probability of malignancy below the treatment threshold, so that careful observation is warranted. Alternatively, one could select a test that, if positive, results in a posttest probability of malignancy above the treatment threshold, so that surgery is warranted. Note that the difference between the pretest probability and the posttest probability, the “distance moved,” is just a function of the test characteristics (sensitivity and specificity) as determined by Bayes’ theorem (see online supplement). The better is the test, the more the probability changes.
Based on this, one can imagine that in some instances the pretest probability is so high that even if all tests were negative, the resulting posttest probability would be greater than the treatment threshold. As the pretest probability decreases, it must reach some point at which a negative test results in a posttest probability less than the treatment threshold. As applied to pulmonary nodules, this is the surgical threshold. This probability is also affected by the risk of the tests involved. The converse scenario is when the pretest probability is extremely low, such that even if test results were positive, the resulting posttest probability would be less than the treatment threshold. As the pretest probability increases, there must be some point at which a positive test will result in a posttest probability above the treatment threshold. By definition the treatment threshold must lie between the observation and surgical thresholds. The distance between the decision thresholds (observation to treatment to surgery) is merely a function of the quality of the available tests. When tests have poor sensitivity and specificity or are very risky, then the distance between thresholds is small. Conversely, if tests carry no risk and are highly sensitive this range is wide. Additional details about the value of testing are provided in the online supplement, but a useful rule of thumb is that the value of testing is greatest when the pretest probability of malignancy is closest to the treatment threshold probability.
Based on this formulation, it is apparent that both probabilities and potential consequences of treatment must be considered when selecting an optimal management strategy. Thinking of treatment outcomes before finalizing a management strategy may seem counterintuitive, but this “thinking in reverse” is needed when making decisions.
Management Strategies
We will now apply this conceptual framework to evaluate the three management alternatives: (1) careful observation, (2) diagnostic testing, and (3) surgery. For each it is suggested when the strategy should be used, the evidence in support of the strategy, its limitations, and areas of uncertainty.
Careful Observation
Most malignant lesions double in volume every 20 to 300 days (29–33), leading to the clinical axiom that radiographic stability for 2 years suggests benign etiology (1, 11). Careful observation usually involves radiologic surveillance with serial CT and is most appropriate when the pretest probability of malignancy is relatively low (<5–10%) (1, 11). This is predicated on the assumption that growth rates, measured radiographically, can be used to distinguish benign from malignant nodules (7, 20, 31). For most nodules detected by CT during lung cancer screening trials, this has proved to be a good strategy, because the probability of malignancy is appropriately low (6, 7, 10).
The primary weakness of this strategy is the hazard of delay; specifically, the probability that a previously curable lesion would metastasize during the period of observation. Although the optimal schedule for imaging is not known, the Fleischner Society has provided consensus recommendations on imaging small nodules (11, 34, 35). The recommended frequency of imaging depends on the size of the nodule and the presence of risk factors for lung cancer. These recommendations are summarized in Table 2 (11, 35).
TABLE 2.
FREQUENCY OF CT IMAGING FOR SURVEILLANCE OF SUBCENTIMETER NODULES (<8 mm)
| Nodule Size | No Lung Cancer Risk Factors | Lung Cancer Risk Factors Present |
| ≤ 4 mm | Follow-up optional* | Follow-up at 12 mo, no additional follow-up if stable |
| >4–6 mm | Follow-up at 12 mo, no additional follow-up if stable | Follow-up at 6–12 mo, if stable follow-up at 18–24 mo |
| >6–8 mm | Follow-up at 6–12 mo, if stable follow-up at 18–24 mo | Follow-up at 3–6 mo, 9–12 mo, and 24 mo if stable |
Definition of abbreviation: CT = computed tomography.
The risk of malignancy in this category is substantially less than that in a baseline CT scan of asymptomatic smokers. If follow-up is elected then it is reasonable to do so in 12 months.
This strategy has substantial limitations. First, few prospective studies in patients with lung nodules have compared outcomes in patients managed by careful observation with those who were more aggressively evaluated. Second, older studies of tumor growth rates tended to enroll patients who had benign-appearing nodules or nodules that had previously been missed, potentially biasing the results in favor of longer doubling times (36). In addition, older studies typically did not include patients with ground-glass opacities or semisolid nodules. Third, some malignant nodules, in particular those with nonsolid or partly solid density, may be associated with very long doubling times, so that the 2-year stability rule may prove insufficient (30, 33). In one study, volume doubling times for BAC (now considered adenocarcinoma in situ) were 42–1,486 days, whereas those for invasive adenocarcinoma were 124–402 days. As mentioned previously, adenocarcinoma in situ and other slow-growing adenocarcinomas may first manifest as ground-glass opacities that grow slowly, only later taking on a more aggressive phenotype. In a study of small tumors, the average doubling times were 813 days for pure ground-glass opacities, 457 days for semisolid lesions, and 149 days for solid lesions (32). Accordingly, some argue that healthy patients with pure ground-glass opacities should be followed radiographically for more than 2 years. However, no studies have prospectively evaluated whether extended follow-up improves outcome.
The superior resolution of CT compared with chest radiography enables more precise measurement and better growth detection, thereby limiting the hazard of delay. Volumetric CT may allow the detection of growing lesions earlier than conventional transverse CT. Preliminary studies demonstrated that three-dimensional analysis enabled tumor growth to be detected in 5-mm diameter nodules 30 days after CT (37, 38). Subsequently, volumetric CT was successfully used to determine volume doubling time and to guide evaluation of small lung nodules in the NELSON trial (7, 39). In that trial, a CT volume doubling time of less than 400 days or a new solid component in a previously nonsolid nodule was defined as positive (7). Follow-up imaging could detect growth only 6 weeks after imaging of an indeterminate nodule. This represents a significant improvement because earlier growth detection is likely to minimize the hazard of delay. However, further studies are needed to validate those results, and interscan variability in CT measurements of size and location remains an issue (7, 40, 41).
Given the abovementioned limitations, it is currently reasonable to use 2-year radiographic stability as indicating a benign etiology for most lesions, with the caveat that longer follow-up should be considered in select patients with ground-glass or semisolid lesions. Lesions that demonstrate growth on serial imaging or that develop a new solid component in a previously nonsolid nodule should have a tissue diagnosis established, usually by CT-guided fine-needle aspiration (CT-FNA) or surgery (11).
Diagnostic Testing
When the probability of malignancy is intermediate (∼10–60%), a diagnostic test is usually warranted. The main options are positron emission tomography (PET), CT-FNA, and bronchoscopy.
PET.
The sensitivity and specificity of PET for identifying malignancy are approximately 87% and 83%, respectively (11, 20). Accordingly, PET has a high negative predictive value when the pretest probability of cancer is low, and such patients can be subsequently managed with careful observation. PET does have important limitations. First, PET is less sensitive for nodules less than 8–10 mm in diameter (13, 15, 42–46). Also, false-negative PET scans can be seen in patients with adenocarcinoma in situ, carcinoid tumors, and mucinous adenocarcinomas. False-positive PET scans can be seen in patients with inflammatory conditions (sarcoidosis or rheumatoid nodules) or infectious processes (endemic mycosis or mycobacterial infection). However, occasionally PET demonstrates evidence of lymph node involvement or extrapulmonary disease that might not otherwise have been detected (47–49). In addition, even false-positive PET results have some value because they alert the clinician to the presence of an active infectious or inflammatory process that requires additional evaluation. Today integrated PET-CT has largely replaced dedicated PET, although it is not at all clear that integrated PET-CT improves characterization of lung nodules.
CT-guided FNA biopsy.
CT-FNA has been shown to have reasonable sensitivity for identifying malignant lung nodules. In 11 studies, counting nondiagnostic results as false-negatives, the median sensitivity was 90% (20). As with other techniques, the reported sensitivity varied widely among studies (range, 65–94%) (20). The risk of pneumothorax also varied, ranging from 15–43% (median, 27%). However, not all of the pneumothoraces required chest tubes. The risk of pneumothorax requiring a chest tube ranged from 4–18% (median, 5%) (20). Risk factors for pneumothorax include smaller lesions, deeper locations, emphysema, lateral puncture site, proximity to fissures, and low entry angle to the pleura (50–52).
Conventional bronchoscopy.
Although bronchoscopy is useful for central lesions, it has proved less accurate for peripheral pulmonary nodules. Studies using traditional techniques, such as conventional fluoroscopic guidance, demonstrated diagnostic yields of 10–50% overall and approximately 33% for peripheral lesions less than 20 mm in diameter (53). The presence on CT of an air bronchogram within the nodule is associated with a substantially higher diagnostic yield of approximately 70%, especially if the CT reveals a bronchus leading to the lesion (13, 54, 55).
Radial endobronchial ultrasound.
Recently, advanced diagnostic bronchoscopy techniques have been developed. A metaanalysis of studies using bronchoscopy with radial endobronchial ultrasound (EBUS) for peripheral lesions identified 16 studies and found a pooled sensitivity and specificity of 73% and 100%, respectively (56). Of the 16 studies, 7 had sufficient data to stratify the results on the basis of nodule size. In patients with nodules less than 25 mm in diameter, the pooled sensitivity was 71% (56).
Electromagnetic navigation.
Electromagnetic navigation combines bronchoscopy with CT imaging by using an electromagnetic field (57, 58). Uncontrolled studies in carefully selected patients demonstrated a diagnostic yield of 63–74% (57, 59–63). Diagnostic yield is higher when a CT bronchus sign is present (55, 63), when divergence is low (60), and with middle lobe (61) or non–lower lobe locations (62, 63). In one randomized trial, the combination of EBUS and electromagnetic navigation was found to be superior to either method alone (62). No randomized studies have compared electromagnetic navigation with conventional bronchoscopy.
From the limited data available, both radial EBUS and electromagnetic navigation seem to be useful techniques in centers with the appropriate expertise. However, the diagnostic sensitivity even for the combination does not approach that of CT-FNA. Except in patients with an air bronchus sign and a pathway to the lesion, CT-FNA should be considered the method of choice when the lesion is accessible (11).
Surgery
Video-assisted thoracic surgery, traditional thoracotomy, and sometimes a combination may be warranted when the probability of cancer is high (>60–70%), both to establish a diagnosis and for definitive treatment. The risk of surgery depends on whether the nodule resected is malignant. If the nodule is found to be benign at frozen section, then only a wedge resection is required, and operative mortality is typically low (∼0.5%) (11, 64–67). Conversely, if the nodule is found to be malignant, then a lobectomy with systematic lymph node dissection is preferred. Lobectomy mortality has been reported to be 1–4% (64–67).
Currently, lobectomy with systematic mediastinal lymph node dissection is the standard of care in patients who are good surgical candidates (11). Video-assisted thoracic surgery lobectomy is becoming more widely available and, in experienced hands, offers the potential benefits of decreased perioperative morbidity and a shorter hospital stay. Even with an experienced surgeon, conversion to thoracotomy may be required in approximately 12% of cases (11, 67–70).
Areas of uncertainty include the role of limited resections in patients with small tumors. Previous studies have demonstrated the superiority of lobectomy over limited resections for tumors less than 30 mm in diameter (71–73). However, questions remain about whether specific subgroups of patients might benefit from limited resection. The original randomized trials of lobectomy versus limited resection used both wedge resection and segmentectomy (72). However, new data suggest that segmentectomy is superior to wedge resection for tumors less than 20 mm in diameter (74–77), probably because segmentectomy produces better margins and wider resection of lymphatics and intralobar lymph nodes (76, 78–80). The original trials may have been flawed by combining wedge resection and segmentectomy into one group. In addition, subsequent retrospective and case-control studies have suggested that patients with small tumors (<20 mm in diameter), especially those with BAC, have acceptable survival times after segmentectomy (75, 79, 81). Current work focuses on whether segmentectomy in patients with small tumors can achieve comparable results to lobectomy; randomized trials are now under way.
Clinical Algorithm
Management algorithms need to incorporate the probability of cancer, the potential benefits and harms of surgery, the accuracy of the available diagnostic tests, and patient preferences. Given the complexity of the problem, most decisions can be guided by asking two questions: What is the pretest probability of cancer? What is the patient's surgical risk?
The decision-making process begins with a history and physical, focusing on estimating the pretest probability of cancer and assessing surgical risk (Figure 4) (82). Assessing surgical risk is critical because it affects the potential benefits and harms of surgery, which in turn determine the treatment threshold and hence the surgical threshold and the observation threshold (Figure 3). Patients with severe comorbidities (e.g., advanced chronic obstructive pulmonary disease) may not be able to tolerate some treatments and other treatments may be of limited benefit in those with poor performance status. If these comorbidities eliminate treatment options, then the optimal management strategy must also change.
Figure 4.
Clinical algorithm for decision making in patients with pulmonary nodules. Probabilities are estimates, because true probability thresholds vary depending on patient comorbidities and preferences. CT = computed tomography; CT-FNA = CT-guided fine-needle aspiration; PET = positron emission tomography.
For patients with an intermediate probability of malignancy, there are two main diagnostic strategies: CT-FNA alone or PET, possibly followed by CT-FNA. If the pretest probability is just above the observation threshold, then a strategy of CT-FNA is warranted. If the CT-FNA result is nondiagnostic, then careful observation is warranted. The finding of a specific benign diagnosis (e.g., endemic mycosis) should be followed by appropriate treatment. PET is not as useful as CT-FNA at this pretest probability because the specificity of CT-FNA is close to 100%, resulting in a positive likelihood ratio that approaches infinity (11, 20). In contrast, PET has an estimated specificity of 83%, resulting in a positive likelihood ratio of approximately 5 (20). Thus, a positive PET scan does not necessarily change the management of a patient with a low pretest probability that lies just above the observation threshold, because the posttest probability of cancer is insufficient to make a definitive treatment decision. A useful way to conceptualize this is to use Figure 3 and plot the pretest probability. Imagine a test result comes back positive. This moves the probability to the right. How far it moves is determined by the likelihood ratio positive (Bayes’ theorem). A positive CT-FNA moves the probability across the surgical threshold and all the way to the right (posttest probability approaches 1). A positive PET moves the probability modestly to the right, and in this case the distance moved is not sufficient to cross the surgical threshold.
However, if the pretest probability of malignancy is somewhat higher but still in the intermediate range, then a strategy of PET possibly followed by CT-FNA is better. As the pretest probability increases, it eventually reaches a point when even a modest move to the right moves it across the surgical threshold. A positive PET results in a posttest probability above the surgical threshold and is sufficient to change management.
One can use the same thought experiment to think about the results of negative tests. Imagine that one gets a negative test. This moves the probability to the left. How far it moves is determined by the likelihood ratio negative (Bayes’ theorem). If the pretest probability is just below the surgical threshold, then a strategy of PET, possibly followed by CT-FNA, is still best. The likelihood ratio negative is approximately 0.16 for PET and approximately 0.1 for CT-FNA (20). The pretest probability is so high in this case that a single negative test results in a posttest probability that is not below the observation threshold. Thus, a second negative test is needed to lower the probability below the observation threshold. The reason for doing PET first in such instances is that it is less risky. If CT-FNA is done before PET, all patients are exposed to some risk, albeit small, and some patients avoid PET (when CT-FNA is positive). If PET is done first, some fraction of patients (those with positive PET scans) avoid the small, but real, risk associated with CT-FNA.
Note that the decision thresholds vary for different patients because the surgical risk, and hence the potential benefit and harm of surgery, vary. However, the order of the preferred strategies is the same when going from lowest to highest pretest probability of cancer: careful observation, then CT-FNA, then PET with possible CT-FNA, and then surgery.
Areas of uncertainty include optimizing the cost-effectiveness of clinical algorithms (11, 82). PET seems to be most cost-effective when the clinical probability of malignancy is discordant with what CT suggests, for instance when the CT suggests malignancy but the clinical probability of cancer is low, or if the CT suggests a benign diagnosis but the clinical probability of cancer is high. It is also important to recognize that the absolute difference in survival between alternative strategies may not be great (83). Therefore, it is important to consider patient preferences. Patient values and risk-taking attitudes can affect cost-effectiveness and should be taken into account (11, 84).
Another area of uncertainty is the impact that lung cancer screening will have on the diagnostic approach to pulmonary nodules. In the National Lung Screening Trial, the risk of death from lung cancer was 20% lower among patients who had annual low-dose CT screening than among those who had annual chest radiography (10). How generalizable the results are to practice remains to be seen. The cost-effectiveness of screening will in part be determined by how effectively identified nodules are evaluated. Economic evaluations of CT screening for lung cancer have also varied widely in cost-effectiveness ratios reported (85–91). No doubt the National Lung Screening Trial results will help clarify some of these issues.
Summary
Providing effective, cost-effective, patient-centered care for patients with pulmonary nodules can be challenging because many factors must be considered simultaneously and technologies are constantly developing. One approach to decision making is to apply the fundamental principles of pretest probability, treatment thresholds, and Bayes’ theorem and to take patient preferences into account. These concepts provide a useful conceptual framework. Although the specific algorithms may change as technologies develop, the underlying concepts remain the same. Mastery of these concepts makes rote memorization of lengthy guidelines and complex algorithms unnecessary and facilitates effective integration of new technologies (15). Because no set of guidelines can encompass all possible scenarios that occur in day-to-day clinical practice, applying these first principles will be useful for physicians, especially in challenging cases. Understanding the concepts of decision analysis allows physicians to view the patient management process as a unified whole: a system of principles rather than a multitude of isolated facts.
Integrating current evidence with these fundamental concepts suggests that management of pulmonary nodules should begin with estimating the pretest probability of cancer. Then, the consequences of treatment should be considered by comparing the benefits of surgery if the patient has cancer with the potential harm if the patient does not have cancer. This analysis determines the treatment threshold, which is the point around which the decision centers. These thresholds vary widely among patients depending on their cardiopulmonary reserve, comorbidities, and individual preferences. For patients with a very low probability of cancer, careful observation with serial CT is warranted. For those with a very high probability of cancer, surgery is preferred. For patients with an intermediate probability of cancer, either CT-FNA or PET possibly followed by CT-FNA is best. Patient preferences should be considered because the absolute difference in outcomes between strategies may not be large (84).
The optimal approach to the management of patients with pulmonary nodules is evolving as technologies develop. Areas of uncertainty include quantifying the hazard of delayed diagnosis; determining the optimal duration of follow-up for ground-glass and semisolid opacities; establishing the roles of volumetric imaging, advanced bronchoscopic technologies, and limited surgical resections; and identifying the most cost-effective strategies.
Supplementary Material
Acknowledgments
The authors thank Dr. Myrna C.B. Godoy for the computed tomography images.
Footnotes
Supported in part by the National Institutes of Health through Cancer Center Core Support Grants CA016672 (D.E.O.) and CA014089 (M.K.G.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201104-0679CI on October 6, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ost D, Fein AM, Feinsilver SH. Clinical practice: the solitary pulmonary nodule. N Engl J Med 2003;348:2535–2542 [DOI] [PubMed] [Google Scholar]
- 2.Swensen SJ, Morin RL, Schueler BA, Brown LR, Cortese DA, Pairolero PC, Brutinel WM. Solitary pulmonary nodule: CT evaluation of enhancement with iodinated contrast material—a preliminary report. Radiology 1992;182:343–347 [DOI] [PubMed] [Google Scholar]
- 3.Swensen SJ, Jett JR, Payne WS, Viggiano RW, Pairolero PC, Trastek VF. An integrated approach to evaluation of the solitary pulmonary nodule. Mayo Clin Proc 1990;65:173–186 [DOI] [PubMed] [Google Scholar]
- 4.Diederich S, Wormanns D, Semik M, Thomas M, Lenzen H, Roos N, Heindel W. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology 2002;222:773–781 [DOI] [PubMed] [Google Scholar]
- 5.McWilliams A, Mayo J, MacDonald S, leRiche JC, Palcic B, Szabo E, Lam S. Lung cancer screening: a different paradigm. Am J Respir Crit Care Med 2003;168:1167–1173 [DOI] [PubMed] [Google Scholar]
- 6.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, Sykes AM, Aughenbaugh GL, Bungum AO, Allen KL. CT screening for lung cancer: five-year prospective experience. Radiology 2005;235:259–265 [DOI] [PubMed] [Google Scholar]
- 7.van Klaveren RJ, Oudkerk M, Prokop M, Scholten ET, Nackaerts K, Vernhout R, van Iersel CA, van den Bergh KA, van 't Westeinde S, van der Aalst C, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221–2229 [DOI] [PubMed] [Google Scholar]
- 8.National Lung Screening Trial Research Team, Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, Galen B, Gareen IF, Gatsonis C, Goldin J, et al. The national lung screening trial: overview and study design. Radiology 2011;258:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Statement concerning the national lung screening trial. [accessed November 14, 2011]. Available from: http://www.cancer.gov/images/dsmb-nlst.pdf. [Google Scholar]
- 10.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gould MK, Fletcher J, Iannettoni MD, Lynch WR, Midthun DE, Naidich DP, Ost DE. Evaluation of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:108S–130S [DOI] [PubMed] [Google Scholar]
- 12.Xu DM, van der Zaag-Loonen HJ, Oudkerk M, Wang Y, Vliegenthart R, Scholten ET, Verschakelen J, Prokop M, de Koning HJ, van Klaveren RJ. Smooth or attached solid indeterminate nodules detected at baseline CT screening in the nelson study: cancer risk during 1 year of follow-up. Radiology 2009;250:264–272 [DOI] [PubMed] [Google Scholar]
- 13.Ost D, Fein A. Evaluation and management of the solitary pulmonary nodule. Am J Respir Crit Care Med 2000;162:782–787 [DOI] [PubMed] [Google Scholar]
- 14.Almeida FA, Uzbeck M, Ost D. Initial evaluation of the nonsmall cell lung cancer patient: diagnosis and staging. Curr Opin Pulm Med 2010;16:307–314 [DOI] [PubMed] [Google Scholar]
- 15.Ost D, Fein A. Management strategies for the solitary pulmonary nodule. Curr Opin Pulm Med 2004;10:272–278 [DOI] [PubMed] [Google Scholar]
- 16.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849–855 [PubMed] [Google Scholar]
- 17.Swensen SJ, Silverstein MD, Edell ES, Trastek VF, Aughenbaugh GL, Ilstrup DM, Schleck CD. Solitary pulmonary nodules: clinical prediction model versus physicians. Mayo Clin Proc 1999;74:319–329 [DOI] [PubMed] [Google Scholar]
- 18.Gould MK, Ananth L, Barnett PG. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 2007;131:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz EM, Sanders GD, Trotter PR, Patz EF, Jr, Silvestri GA, Owens DK, Gould MK. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax 2008;63:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94S–107S [DOI] [PubMed] [Google Scholar]
- 21.Siegelman SS, Khouri NF, Scott WW, Jr, Leo FP, Hamper UM, Fishman EK, Zerhouni EA. Pulmonary hamartoma: CT findings. Radiology 1986;160:313–317 [DOI] [PubMed] [Google Scholar]
- 22.Noguchi M, Morikawa A, Kawasaki M, Matsuno Y, Yamada T, Hirohashi S, Kondo H, Shimosato Y. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844–2852 [DOI] [PubMed] [Google Scholar]
- 23.Noguchi M, Shimosato Y. The development and progression of adenocarcinoma of the lung. Cancer Treat Res 1995;72:131–142 [DOI] [PubMed] [Google Scholar]
- 24.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology 2009;253:606–622 [DOI] [PubMed] [Google Scholar]
- 26.Watanabe S, Watanabe T, Arai K, Kasai T, Haratake J, Urayama H. Results of wedge resection for focal bronchioloalveolar carcinoma showing pure ground-glass attenuation on computed tomography. Ann Thorac Surg 2002;73:1071–1075 [DOI] [PubMed] [Google Scholar]
- 27.Suzuki K, Asamura H, Kusumoto M, Kondo H, Tsuchiya R. Early peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg 2002;74:1635–1639 [DOI] [PubMed] [Google Scholar]
- 28.Hunink M, Glasziou P, Siegel J, Weeks J, Pliskin J, Elstein A, Weinstein M. Decision making in health and medicine. Cambridge: Cambridge University Press; 2001 [Google Scholar]
- 29.Nathan MH, Collins VP, Adams RA. Differentiation of benign and malignant pulmonary nodules by growth rate. Radiology 1962;79:221–232 [DOI] [PubMed] [Google Scholar]
- 30.Yankelevitz DF, Henschke CI. Does 2-year stability imply that pulmonary nodules are benign? AJR Am J Roentgenol 1997;168:325–328 [DOI] [PubMed] [Google Scholar]
- 31.Takashima S, Sone S, Li F, Maruyama Y, Hasegawa M, Matsushita T, Takayama F, Kadoya M. Small solitary pulmonary nodules (< or =1 cm) detected at population-based CT screening for lung cancer: reliable high-resolution CT features of benign lesions. AJR Am J Roentgenol 2003;180:955–964 [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa M, Sone S, Takashima S, Li F, Yang ZG, Maruyama Y, Watanabe T. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 2000;73:1252–1259 [DOI] [PubMed] [Google Scholar]
- 33.Aoki T, Nakata H, Watanabe H, Nakamura K, Kasai T, Hashimoto H, Yasumoto K, Kido M. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time. AJR Am J Roentgenol 2000;174:763–768 [DOI] [PubMed] [Google Scholar]
- 34.Henschke CI, Yankelevitz D, Westcott J, Davis SD, Fleishon H, Gefter WB, McLoud TC, Pugatch RD, Sostman HD, Tocino I, et al. Work-up of the solitary pulmonary nodule. American College of Radiology appropriateness criteria. Radiology 2000;215:607–609 [PubMed] [Google Scholar]
- 35.MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, Patz EF, Jr, Swensen SJ. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400 [DOI] [PubMed] [Google Scholar]
- 36.Bach PB, Silvestri GA, Hanger M, Jett JR. Screening for lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:69S–77S [DOI] [PubMed] [Google Scholar]
- 37.Tsubamoto M, Johkoh T, Kozuka T, Honda O, Koyama M, Murai S, Inoue A, Sumikawa H, Tomiyama N, Hamada S, et al. Coronal multiplanar reconstruction view from whole lung thin-section CT by multidetector-row CT: determination of malignant or benign lesions and differential diagnosis in 68 cases of solitary pulmonary nodule. Radiat Med 2003;21:267–271 [PubMed] [Google Scholar]
- 38.Kostis WJ, Reeves AP, Yankelevitz DF, Henschke CI. Three-dimensional segmentation and growth-rate estimation of small pulmonary nodules in helical CT images. IEEE Trans Med Imaging 2003;22:1259–1274 [DOI] [PubMed] [Google Scholar]
- 39.Xu DM, Gietema H, de Koning H, Vernhout R, Nackaerts K, Prokop M, Weenink C, Lammers JW, Groen H, Oudkerk M, et al. Nodule management protocol of the nelson randomised lung cancer screening trial. Lung Cancer 2006;54:177–184 [DOI] [PubMed] [Google Scholar]
- 40.Gietema HA, Schaefer-Prokop CM, Mali WP, Groenewegen G, Prokop M. Pulmonary nodules: interscan variability of semiautomated volume measurements with multisection CT–influence of inspiration level, nodule size, and segmentation performance. Radiology 2007;245:888–894 [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, van Klaveren RJ, van der Zaag-Loonen HJ, de Bock GH, Gietema HA, Xu DM, Leusveld AL, de Koning HJ, Scholten ET, Verschakelen J, et al. Effect of nodule characteristics on variability of semiautomated volume measurements in pulmonary nodules detected in a lung cancer screening program. Radiology 2008;248:625–631 [DOI] [PubMed] [Google Scholar]
- 42.Herder GJ, Golding RP, Hoekstra OS, Comans EF, Teule GJ, Postmus PE, Smit EF. The performance of(18)f-fluorodeoxyglucose positron emission tomography in small solitary pulmonary nodules. Eur J Nucl Med Mol Imaging 2004;31:1231–1236 [DOI] [PubMed] [Google Scholar]
- 43.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914–924 [DOI] [PubMed] [Google Scholar]
- 44.Pastorino U, Bellomi M, Landoni C, De Fiori E, Arnaldi P, Picchio M, Pelosi G, Boyle P, Fazio F. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet 2003;362:593–597 [DOI] [PubMed] [Google Scholar]
- 45.Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K. Evaluation of f-18 fluorodeoxyglucose (FDG) pet scanning for pulmonary nodules less than 3 cm in diameter, with special reference to the CT images. Lung Cancer 2004;45:19–27 [DOI] [PubMed] [Google Scholar]
- 46.Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Nathan MA, Lowe VJ. Lung cancer screening experience: a retrospective review of PET in 22 non-small cell lung carcinomas detected on screening chest CT in a high-risk population. AJR Am J Roentgenol 2005;185:126–131 [DOI] [PubMed] [Google Scholar]
- 47.Weder W, Schmid RA, Bruchhaus H, Hillinger S, von Schulthess GK, Steinert HC. Detection of extrathoracic metastases by positron emission tomography in lung cancer. Ann Thorac Surg 1998;66:886–892; discussion 892–883 [DOI] [PubMed] [Google Scholar]
- 48.Valk PE, Pounds TR, Hopkins DM, Haseman MK, Hofer GA, Greiss HB, Myers RW, Lutrin CL. Staging non-small cell lung cancer by whole-body positron emission tomographic imaging. Ann Thorac Surg 1995;60:1573–1581; discussion 1581–1572 [DOI] [PubMed] [Google Scholar]
- 49.Niho S, Fujii H, Murakami K, Nagase S, Yoh K, Goto K, Ohmatsu H, Kubota K, Sekiguchi R, Nawano S, et al. Detection of unsuspected distant metastases and/or regional nodes by FDG-PET (corrected) scan in apparent limited-disease small-cell lung cancer. Lung Cancer 2007;57:328–333 [DOI] [PubMed] [Google Scholar]
- 50.Cox JE, Chiles C, McManus CM, Aquino SL, Choplin RH. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology 1999;212:165–168 [DOI] [PubMed] [Google Scholar]
- 51.Laurent F, Michel P, Latrabe V, Tunon de Lara M, Marthan R. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: incidence and risk factors. AJR Am J Roentgenol 1999;172:1049–1053 [DOI] [PubMed] [Google Scholar]
- 52.Ko JP, Shepard JO, Drucker EA, Aquino SL, Sharma A, Sabloff B, Halpern E, McLoud TC. Factors influencing pneumothorax rate at lung biopsy: are dwell time and angle of pleural puncture contributing factors? Radiology 2001;218:491–496 [DOI] [PubMed] [Google Scholar]
- 53.Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123:115S–128S [DOI] [PubMed] [Google Scholar]
- 54.Henschke CI, Davis SD, Auh Y, Romano P, Westcott J, Berkmen YM, Kazam E. Detection of bronchial abnormalities: comparison of CT and bronchoscopy. J Comput Assist Tomogr 1987;11:432–435 [DOI] [PubMed] [Google Scholar]
- 55.Naidich DP, Sussman R, Kutcher WL, Aranda CP, Garay SM, Ettenger NA. Solitary pulmonary nodules. CT-bronchoscopic correlation. Chest 1988;93:595–598 [DOI] [PubMed] [Google Scholar]
- 56.Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902–910 [DOI] [PubMed] [Google Scholar]
- 57.Gildea TR, Mazzone PJ, Karnak D, Meziane M, Mehta AC. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med 2006;174:982–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shulman L, Ost D. Advances in bronchoscopic diagnosis of lung cancer. Curr Opin Pulm Med 2007;13:271–277 [DOI] [PubMed] [Google Scholar]
- 59.Schwarz Y, Greif J, Becker HD, Ernst A, Mehta A. Real-time electromagnetic navigation bronchoscopy to peripheral lung lesions using overlaid CT images: the first human study. Chest 2006;129:988–994 [DOI] [PubMed] [Google Scholar]
- 60.Makris D, Scherpereel A, Leroy S, Bouchindhomme B, Faivre JB, Remy J, Ramon P, Marquette CH. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesions. Eur Respir J 2007;29:1187–1192 [DOI] [PubMed] [Google Scholar]
- 61.Eberhardt R, Anantham D, Herth F, Feller-Kopman D, Ernst A. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest 2007;131:1800–1805 [DOI] [PubMed] [Google Scholar]
- 62.Eberhardt R, Anantham D, Ernst A, Feller-Kopman D, Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36–41 [DOI] [PubMed] [Google Scholar]
- 63.Seijo LM, de Torres JP, Lozano MD, Bastarrika G, Alcaide AB, Lacunza MM, Zulueta JJ. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: results from a prospective study. Chest 2010;138:1316–1321 [DOI] [PubMed] [Google Scholar]
- 64.Freixinet JL, Varela G, Molins L, Rivas JJ, Rodríguez-Paniagua JM, de Castro PL, Izquierdo JM, Torres J. Benchmarking in thoracic surgery. Eur J Cardiothorac Surg 2011;40:124–129 [DOI] [PubMed] [Google Scholar]
- 65.Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, Tsubota N. Evolution of surgical outcomes for nonsmall cell lung cancer: time trends in 1465 consecutive patients undergoing complete resection. Ann Thorac Surg 2004;77:1926–1930; discussion 1931 [DOI] [PubMed] [Google Scholar]
- 66.Matsubara Y, Takeda S, Mashimo T. Risk stratification for lung cancer surgery: impact of induction therapy and extended resection. Chest 2005;128:3519–3525 [DOI] [PubMed] [Google Scholar]
- 67.Walker WS. Video-assisted thoracic surgery (VATS) lobectomy: the Edinburgh experience. Semin Thorac Cardiovasc Surg 1998;10:291–299 [DOI] [PubMed] [Google Scholar]
- 68.Lewis RJ, Caccavale RJ, Bocage JP, Widmann MD. Video-assisted thoracic surgical non-rib spreading simultaneously stapled lobectomy: a more patient-friendly oncologic resection. Chest 1999;116:1119–1124 [DOI] [PubMed] [Google Scholar]
- 69.Lewis RJ, Caccavale RJ. Video-assisted thoracic surgical non-rib spreading simultaneously stapled lobectomy (VATS(N)SSL). Semin Thorac Cardiovasc Surg 1998;10:332–339 [DOI] [PubMed] [Google Scholar]
- 70.McKenna RJ, Jr, Fischel RJ, Wolf R, Wurnig P. Video-assisted thoracic surgery (VATS) lobectomy for bronchogenic carcinoma. Semin Thorac Cardiovasc Surg 1998;10:321–325 [DOI] [PubMed] [Google Scholar]
- 71.Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 1994;107:1087–1093; discussion 1093–1084 [PubMed] [Google Scholar]
- 72.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615–622; discussion 622–613 [DOI] [PubMed] [Google Scholar]
- 73.Lederle FA. Lobectomy versus limited resection in T1 N0 lung cancer. Ann Thorac Surg 1996;62:1249–1250 [DOI] [PubMed] [Google Scholar]
- 74.Miller DL, Rowland CM, Deschamps C, Allen MS, Trastek VF, Pairolero PC. Surgical treatment of non-small cell lung cancer 1 cm or less in diameter. Ann Thorac Surg 2002;73:1545–1550; discussion 1550–1541 [DOI] [PubMed] [Google Scholar]
- 75.Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, Tsubota N. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87–93 [DOI] [PubMed] [Google Scholar]
- 76.El-Sherif A, Fernando HC, Santos R, Pettiford B, Luketich JD, Close JM, Landreneau RJ. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007;14:2400–2405 [DOI] [PubMed] [Google Scholar]
- 77.Sienel W, Dango S, Kirschbaum A, Cucuruz B, Horth W, Stremmel C, Passlick B. Sublobar resections in stage Ia non-small cell lung cancer: segmentectomies result in significantly better cancer-related survival than wedge resections. Eur J Cardiothorac Surg 2008;33:728–734 [DOI] [PubMed] [Google Scholar]
- 78.Watanabe A, Ohori S, Nakashima S, Mawatari T, Inoue N, Kurimoto Y, Higami T. Feasibility of video-assisted thoracoscopic surgery segmentectomy for selected peripheral lung carcinomas. Eur J Cardiothorac Surg 2009;35:775–780, discussion 780 [DOI] [PubMed] [Google Scholar]
- 79.Bando T, Miyahara R, Sakai H, Shoji T, Sonobe M, Sato K, Fujinaga T, Chen F, Okubo K, Hirata T, et al. A follow-up report on a new method of segmental resection for small-sized early lung cancer. Lung Cancer 2009;63:58–62 [DOI] [PubMed] [Google Scholar]
- 80.Nomori H, Ohba Y, Shibata H, Shiraishi K, Mori T, Shiraishi S. Required area of lymph node sampling during segmentectomy for clinical stage Ia non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;139:38–42 [DOI] [PubMed] [Google Scholar]
- 81.Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer <= 1cm in size: a review of seer data. Chest 2011;139:491–496 [DOI] [PubMed] [Google Scholar]
- 82.Gould MK, Sanders GD, Barnett PG, Rydzak CE, Maclean CC, McClellan MB, Owens DK. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules. Ann Intern Med 2003;138:724–735 [DOI] [PubMed] [Google Scholar]
- 83.Cummings SR, Lillington GA, Richard RJ. Managing solitary pulmonary nodules. The choice of strategy is a close call. Am Rev Respir Dis 1986;134:453–460 [DOI] [PubMed] [Google Scholar]
- 84.Raab SS, Hornberger J. The effect of a patient's risk-taking attitude on the cost effectiveness of testing strategies in the evaluation of pulmonary lesions. Chest 1997;111:1583–1590 [DOI] [PubMed] [Google Scholar]
- 85.Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, Ayres J, Bain L, Thomas S, Godden D, et al. The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews. Health Technol Assess 2006;10:iii–iv, ix–x, 1–90 [DOI] [PubMed] [Google Scholar]
- 86.Manser R, Dalton A, Carter R, Byrnes G, Elwood M, Campbell DA. Cost-effectiveness analysis of screening for lung cancer with low dose spiral CT (computed tomography) in the Australian setting. Lung Cancer 2005;48:171–185 [DOI] [PubMed] [Google Scholar]
- 87.Klittich WS, Caro JJ. Lung cancer screening: will the controversy extend to its cost-effectiveness? Am J Respir Med 2002;1:393–401 [DOI] [PubMed] [Google Scholar]
- 88.Wisnivesky JP, Mushlin AI, Sicherman N, Henschke C. The cost-effectiveness of low-dose CT screening for lung cancer: preliminary results of baseline screening. Chest 2003;124:614–621 [DOI] [PubMed] [Google Scholar]
- 89.Mahadevia PJ, Fleisher LA, Frick KD, Eng J, Goodman SN, Powe NR. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA 2003;289:313–322 [DOI] [PubMed] [Google Scholar]
- 90.Chirikos TN, Hazelton T, Tockman M, Clark R. Screening for lung cancer with CT: a preliminary cost-effectiveness analysis. Chest 2002;121:1507–1514 [DOI] [PubMed] [Google Scholar]
- 91.Marshall D, Simpson KN, Earle CC, Chu C. Potential cost-effectiveness of one-time screening for lung cancer (LC) in a high risk cohort. Lung Cancer 2001;32:227–236 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.