Abstract
Dendritic cells (DCs) are essential for innate and adaptive immunity, but are purported to exhibit variable radiosensitivity in response to irradiation in various bone marrow transplantation (BMT) protocols. To address this controversy, we analyzed the magnitude of depletion and repopulation of both lung CD11bpos DC and CD103pos DC subsets in response to irradiation and BMT in a murine model. In our study, CD45.2pos donor bone marrow cells were transplanted into irradiated CD45.1pos recipient mice to examine the depletion of recipient DC subsets and the repopulation of donor DC subsets. We observed an apoptosis-mediated and necrosis-mediated depletion (> 90%) of the recipient CD103pos DC subset, and only a 50–60% depletion of recipient CD11bpos DCs from lung parenchymal tissue on Days 3 and 5, whereas recipient alveolar and lung macrophages were much less radiosensitive, showing an approximately 50% depletion by Days 14–21 after treatment. A repopulation of lung tissue with donor DC subsets had occurred by Days 10 and 28 for CD11bpos DCs and CD103pos DCs, whereas alveolar and lung macrophages were repopulated by 6 and 10 weeks after treatment. Furthermore, the infection of mice with Streptococcus pneumoniae further accelerated the turnover of lung DCs and lung macrophage subsets. Our data illustrate the vulnerability of lung CD103pos DCs and CD11bpos DCs to irradiation, and indicate that an accelerated turnover of lung DC subsets occurs, relative to pulmonary and lung macrophages. Our findings may have important implications in the development of adjuvant immune-stimulatory protocols that could reduce the risk of opportunistic infections in patients undergoing BMT.
Keywords: dendritic cell, macrophage, pneumonia, Streptococcus pneumoniae, CD103
Dendritic cells (DCs) are professional phagocytes that form a “cellular bridge” between the innate and adaptive immune system. In the lung, DCs are strategically located between the alveolar epithelium and the lung capillary system, where they are involved in sampling inhaled noninfectious and infectious particles, and in their processing and transportation into lung-draining lymph nodes to trigger immune responses or tolerance (1). The classical “myeloid” DC population of the lung was refined to consist of (at least) two subsets of DCs, immunophenotypically characterized as CD11bhigh and major histocompatibility complex (MHC) Class IIpos DCs (CD11bpos DCs), and CD103pos DCs, which are characterized by their CD11blow, MHC Class IIpos, and CD103pos expression profiles. Both of these lung DC subsets were recently suggested to originate in noninflammatory and inflammatory monocyte precursors, respectively (2–4). More recent reports, however, suggest that in nonlymphoid organs such as the lung, CD103pos DCs originate in bone marrow–derived pre-DCs, whereas CD11bpos DCs are more heterogeneous (5).
DCs require both a fully functional state of differentiation and activation, and a tightly controlled pool size to contribute to the immunologic integrity of the lung (6, 7). Various DC subsets of extrapulmonary organ systems such as thymic and lymph-node DCs, as well as splenic DCs and epidermal Langerhans cells, were reported to exhibit significant radiosensitivity in response to whole-body irradiation and bone marrow transplantation (BMT) (8). For the lung, only limited information is available on the radiosensitivity of both major myeloid DC subsets. Irradiation/BMT therapeutic regimens are used in the treatment of certain hematologic malignancies, and are frequently accompanied by severe opportunistic lung infections, possibly attributable to the impact of these treatment protocols on pulmonary DC and macrophage subset homeostasis. A better understanding of the impact of these treatment regimens on DC homeostasis could lead to novel adjuvant/immune therapeutic interventions that would minimize the risk of developing opportunistic infections of the lung that often accompany irradiation/BMT protocols. We examined the effects of whole-body irradiation and subsequent BMT on lung CD11bpos DC and CD103pos DC subset pool sizes, and we determined detailed time response analyses of both CD11bpos DC and CD103pos DC turnover in the lung. To address this, we irradiated CD45.1 alloantigen-expressing recipient mice, and reconstituted their hematopoietic system with bone marrow cells from CD45.2 alloantigen-expressing donor mice. We then performed a detailed time response analysis of the depletion and repopulation characteristics, as well as turnover characteristics, of lung CD11bpos DC and CD103pos DC subsets, both at baseline and in response to bacterial pneumonia attributable to Streptococcus pneumoniae.
Materials and Methods
Animals
Donor C57BL/6 mice were purchased from Charles River (Sulzfeld, Germany) and expressed the CD45.2 alloantigen on the cell surface of circulating leukocytes. Recipient B6.SJL-Ptprca mice (C57BL/6) expressing the CD45.1 alloantigen were purchased from Jackson Laboratories (Sacramento, CA), and were housed under conventional conditions and used in experiments at ages 7–16 weeks.
Reagents
The reagents used in this study are outlined in the online supplement.
Generation of Chimeric Mice
Chimeric mice were generated as previously described (9–11). For details, please see the online supplement. Animal experiments were approved by our local government authorities.
Collection of Blood Leukocytes and Mononuclear Phagocyte Subsets from Lung Parenchymal Tissue and the Bronchoalveolar Space, and Determination of Their Turnover Kinetics
Mice were killed with an overdose of isoflurane (Baxter, Unterschleissheim, Germany) and their peripheral blood was immediately collected from the vena cava into EDTA-containing tubes, followed by the lysis of red blood cells with ammonium chloride buffer. Bone marrow engraftment in chimeric mice was assessed by a FACS-based immunophenotypic analysis of CD45 alloantigen expression profiles of peripheral blood leukocytes, as described in detail elsewhere (9, 12). Bronchoalveolar lavage (BAL) was performed as outlined in the online supplement. The analysis of lung macrophages was performed as described in detail elsewhere (7, 9), and is further outlined in the online supplement. We here define alveolar macrophages as those accessible by bronchoalveolar lavage, and lung macrophages as those retained in the lungs after lavage.
Immunophenotypic Analysis of CD45.1 and CD45.2 Cell Surface Antigen Expression of Lung Mononuclear Phagocyte Subsets
The turnover kinetics of lung CD11bpos DCs and CD103pos DCs, both of which express CD11c, were determined in lung parenchymal tissue of chimeric mice. The purification of CD11c-positive lung mononuclear phagocytes was performed using CD11c-labeled magnetic beads. The identification of CD11bpos DCs and CD103pos DCs was performed according to their forward-scatter/side-scatter characteristics and their low green autofluorescence properties, in conjunction with their CD11cpos, CD11bhigh, MHC Class IIhigh, CD103neg (CD11bpos DCs), CD11cpos, CD11blow, MHC Class IIhigh, and CD103pos (CD103pos DCs) immunophenotype, and their respective CD45.1 (recipient) or CD45.2 (donor) alloantigen expression profiles. The turnover kinetics of alveolar and lung macrophages were determined essentially as described elsewhere (9).
Analysis of Apoptosis and Necrosis
Irradiation-induced apoptosis and necrosis in lung mononuclear phagocyte subsets of chimeric mice were analyzed as outlined in the online supplement.
Infection Experiments with S. pneumoniae
In selected experiments, we determined the turnover kinetics of lung DCs and macrophages in response to acute inflammation triggered by “low-level” infection of mice with the prototype Gram-positive lung pathogen, S. pneumoniae (9, 11, 14–16). Chimeric CD45.1 mice were infected with S. pneumoniae on Day 10 or Day 35 after irradiation/BMT for the determination of infection-induced turnover of DCs and macrophages, respectively. For details, please see the online data supplement.
Statistical Analysis
All data are expressed as mean ± SEM. Significant differences between groups were analyzed by ANOVA, followed by the post hoc Dunnett test, using SPSS software (SPSS, Inc., Chicago, IL). Statistically significant differences between groups were assumed when P < 0.05.
Results
Efficiency of Bone Marrow Engraftment in Chimeric CD45.1 Mice Assessed by CD45.2 Cell Surface Expression in Peripheral Blood Leukocytes
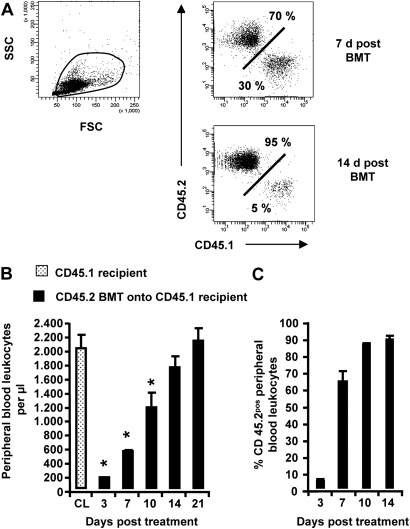
We evaluated the efficiency of bone marrow engraftment in irradiated CD45.1 recipient mice transplanted with CD45.2 bone marrow cells. Figure 1A illustrates the FACS-based immunophenotypic approach to discriminate between recipient (CD45.1) and donor-type (CD45.2) peripheral blood leukocytes (Figure 1A). Chimeric CD45.1 mice developed profound leukopenia as early as 3 days after treatment, which recovered to baseline levels by Day 14 after treatment (Figure 1B). CD45.2 bone marrow engraftment reached nearly 70% by Day 7 after treatment, and reached an engraftment efficiency of 80–90% between Days 14–21 after treatment (Figure 1C). Consistent with previous reports (9), these data illustrate an exceedingly efficient engraftment process by which CD45.1pos recipient-type peripheral blood leukocytes are replaced by CD45.2pos donor-type cells in chimeric CD45.1 mice.
Figure 1.
Immunophenotypic analysis of bone marrow engraftment in chimeric CD45.1 mice. CD45.1pos recipients were irradiated and transplanted with bone marrow cells from CD45.2pos donors (1 × 107 bone marrow cells/mouse). Peripheral blood was collected from control and chimeric CD45.1 mice. (A–C) Numbers of peripheral blood leukocytes were counted at different time points after irradiation and bone marrow transplantation (BMT), and were subsequently subjected to FACS analysis of CD45.1 versus CD45.2 alloantigen expression. The data are presented as mean ± SEM of n ≥ 4 mice per time point. d, days; FSC, forward scatter; SSC, side scatter; CL, control. *Significant decrease (P < 0.05) compared with CD45.1 recipient mice (CL) at indicated time points.
Effects of Irradiation and BMT on Pool Sizes and Turnover Kinetics of CD103pos and CD11bpos DCs in Lung Parenchymal Tissue of Chimeric CD45.1 Mice
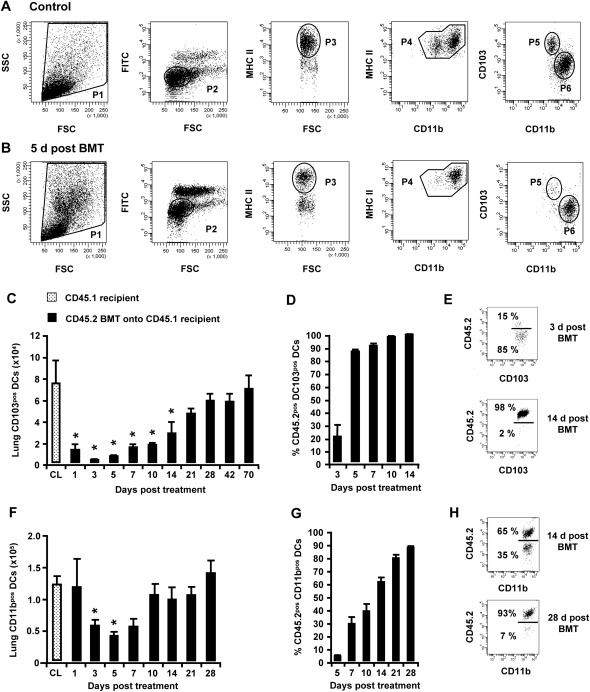
We next aimed to determine the effects of irradiation and BMT on the numbers of CD103pos DCs and CD11bpos DCs in the lung tissue of chimeric CD45.1 mice. Figures 2A and 2B illustrate the gating strategy used to discriminate lung CD11bpos DCs from CD103pos DCs in purified CD11cpos mononuclear phagocytes collected from the lung tissues of control mice and chimeric mice. Interestingly, we found a massive, nearly complete (>90%) depletion of CD103pos DCs from the lung parenchymal tissue of mice undergoing irradiation/BMT, with lowest CD103pos DC counts observed on Day 3 (Figure 2C), with a repopulation toward baseline CD103pos DC numbers by Days 21–28 after treatment. Consequently, the turnover rate of recipient versus donor-type CD45.2pos lung CD103pos DCs was found to be greater than 90% as early as Days 7–10 after irradiation (Figures 2D and 2E), demonstrating that lung CD103pos DCs are extremely radiosensitive and are repopulated quickly. Lung CD11bpos DCs were depleted to a lesser extent after irradiation/BMT in the lung tissue of chimeric CD45.1 mice (Figures 2F–2H), with the greatest depletion of lung CD11bpos DCs at only approximately 60% by Day 5 after treatment, followed by repopulation of lung CD11bpos DC numbers by Day 10. Relative to CD103pos DCs, delayed turnover kinetics reached an 80–90% exchange of recipient versus donor-type lung CD11bpos DCs by Days 21–28 after treatment (Figures 2F–2H). Notably, the turnover of CD103pos DCs and CD11bpos DCs in the lung-draining lymph nodes reached more than 80% for CD103pos DCs and CD11bpos DCs by Day 10 after irradiation and BMT, again demonstrating a brisk turnover of these two lung DC subsets (data not shown).
Figure 2.
Turnover kinetics of lung CD103pos dendritic cells (DCs) and lung CD11bpos DCs in chimeric CD45.1 mice. Lungs of control mice and chimeric CD45.1 mice were initially subjected to bronchoalveolar lavage (BAL) to remove alveolar macrophages, followed by the enrichment of lung mononuclear phagocyte subsets (including CD11bpos DCs and CD103pos DCs), using CD11c-labeled magnetic beads. (A, B, E, and H) Representative FACS analyses of the baseline turnover of lung CD103pos DCs and lung CD11bpos DCs, gated according to their FSC versus SSC characteristics (P1), followed by hierarchical subgating according to their FSC versus low green autofluorescence (P2) and CD11c cell surface expression. Lung DC subsets were further subgated according to FSC versus major histocompatibility complex (MHC) Class II (P3), CD11b versus MHC Class II (P4), and CD11b versus CD103 (P5, CD103pos DCs; P6, CD11bpos DCs), followed by analyses of their CD45.1 or CD45.2 cell-surface expression (E and H). We also performed the quantification of total numbers of (C) CD103pos DCs and (F) CD11bpos DCs purified from lung parenchymal tissue digests. We further performed the quantification of (C and D) CD45.2pos donor-type lung CD103pos DCs and (F and G) lung CD11bpos DCs. The data are presented as mean ± SEM of n ≥ 4–12 mice per time point. *Significant decrease (P < 0.05) compared with CD45.1 recipient mice (CL) at the indicated time points.
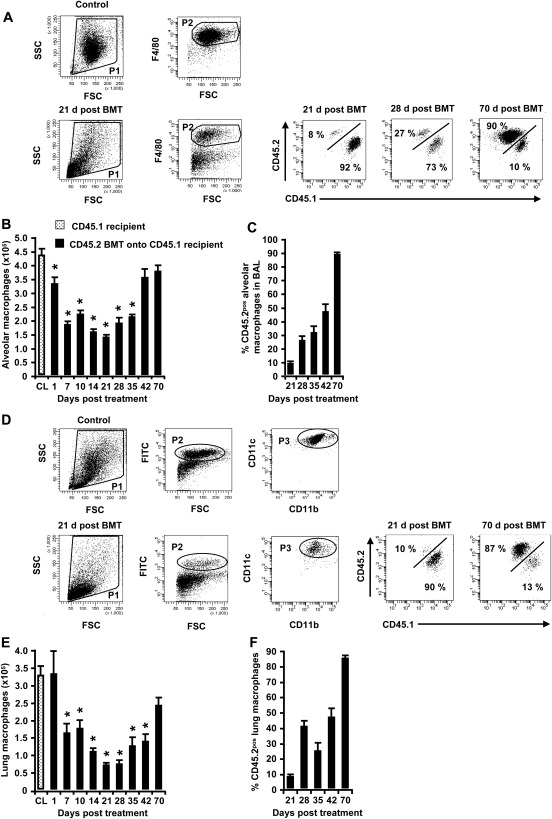
We also performed a comparative analysis of the turnover kinetics of alveolar and lung macrophages in the same mice under the same experimental conditions. Similar to the results for lung CD11bpos DCs and CD103pos DCs, irradiation/BMT also triggered a transient depletion of both alveolar macrophages (Figures 3A–3C) and lung macrophages (Figures 3D–3F) in the lungs of chimeric CD45.1 mice, but in contrast to lung CD11bpos DCs and CD103pos DCs, macrophages reached their maximal depletion on Day 21 after treatment, which is considerably delayed compared with lung CD103pos DCs (peak of depletion, Day 3; Figure 2C) and lung CD11bpos DCs (peak of depletion, Day 5; Figure 2F). This delay in macrophage depletion in response to irradiation/BMT was accompanied by a delayed reconstitution of both alveolar and lung macrophages, reaching an approximately 50% exchange of recipient versus donor-type macrophages by Day 42 after treatment (Figures 3C and 3F), and an overall turnover of approximately 90% by 10 weeks after treatment (Figures 3C and 3F), similar to the findings in previous reports (9, 12, 17).
Figure 3.
Depletion and repopulation and turnover kinetics of alveolar macrophages and lung macrophages in chimeric CD45.1 mice. (A and C) Representative FACS analysis of the baseline turnover of alveolar macrophages, gated according to their FSC versus SSC characteristics (P1), followed by hierarchical subgating according to their FSC versus F4/80 antigen expression characteristics (P2), followed by a determination of their turnover profiles according to CD45.2 versus CD45.1 alloantigen expression. (B and C) At the indicated time points, chimeric CD45.1 mice were killed, and the numbers and CD45 phenotypes of alveolar macrophages were determined in whole-lung washes. (D–F) Lung macrophages were collected from the lung tissue of chimeric CD45.1 mice at different time points after irradiation/BMT, and the quantification of total numbers of green autofluorescent/CD11cpos cells was performed according to the FACS-based identification of CD45.2pos donor-type cells. Data are presented as mean ± SEM of n = 4–12 mice per time point. *Significant decrease (P < 0.05) compared with CD45.1 recipient mice (CL).
Thus, our data show remarkably different replacement kinetics for the various mononuclear phagocyte subsets in the lungs of mice, with the fastest depletion and repopulation rates observed for lung CD103pos DCs, followed by lung CD11bpos DCs, and finally alveolar and lung macrophages on Days 21 and 42–70 after irradiation/BMT, respectively.
Pattern of Inducing Apoptosis and Necrosis in Chimeric Mice Subsequent to Irradiation and BMT
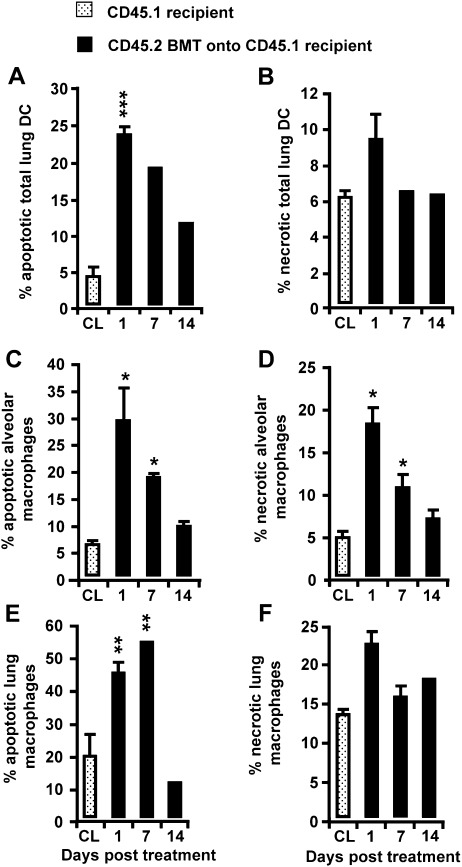
To determine the mechanism by which a regimen of irradiation/BMT triggers the brisk depletion of recipient-type DC subsets in the lungs of mice, we examined the induction of apoptosis and necrosis in CD45.1 cells. Figure 4 shows that the reduction of CD45.1 recipient-type lung DC numbers, observed as early as Days 3 and 5 after irradiation/BMT, was accompanied by a dramatically increased induction of apoptosis and necrosis in this phagocyte subset, with a peak of approximately 23% apoptotic and approximately 9% necrotic total lung DCs, respectively, observed only on Day 1 after treatment (Figures 4A and 4B), and declining toward lower values thereafter, just as the process of depletion subsided. A similar situation was evident for both CD45.1 recipient-type alveolar and lung macrophages, that is, a transient depletion in cell numbers was accompanied by a significantly increased induction of apoptosis and necrosis in these phagocytes, particularly at early time points (on Day 1 after treatment), when the depletion process was found to be initiated (Figures 4C–4F).
Figure 4.
Determination of apoptosis and necrosis induced in CD45.1 recipient-type lung DCs and alveolar and lung macrophages of chimeric CD45.1 mice. Chimeric CD45.1 mice were subjected to BAL, followed by lung tissue digestion, as outlined in Materials and Methods. Subsequently, total lung DCs (A and B) and alveolar macrophages (C and D), as well as lung macrophages (E and F), were stained with annexin V and 7-Aminoactinomycin D (7-AAD) for a FACS analysis of the induction of apoptosis and necrosis, as indicated. Because of the low numbers of lung DC subsets observed on Days 1–14 after irradiation/BMT, the induction of apoptosis and necrosis shown in A and B was analyzed in total lung DCs. Data are presented as mean ± SEM of at least 3–4 mice per time point. A significant increase (*P < 0.05, **P < 0.01) was evident, compared with CD45.1 recipient mice (CL).
The Lung DC Turnover in Chimeric CD45.1 Mice Is Accelerated in Response to Low-Dose Infection with S. pneumoniae
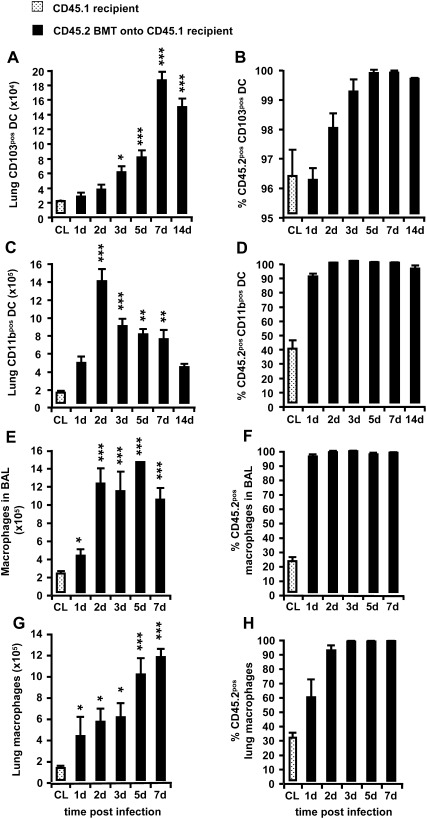
Finally, we examined whether the pneumonia triggered by “low-dose” infection with S. pneumoniae would accelerate the repopulation and turnover kinetics of DC subsets in the lungs of chimeric CD45.1 mice. The infection of chimeric CD45.1 mice with S. pneumoniae (performed on Day 10 after irradiation/BMT) induced a strong recruitment of CD103pos lung DCs within 7 days after infection (Figures 5A and 5B). Under these conditions, numbers of lung CD103pos DCs were increased by 9-fold relative to control values (Figure 5A). Moreover, the numbers of lung CD11bpos DCs were increased 14-fold on Day 2 after infection, relative to the numbers of CD11bpos DCs observed before infection (Figure 5C). A complete turnover of recipient versus donor-type CD11bpos DCs was evident on Days 1–2 after infection (Figure 5D).
Figure 5.
Turnover kinetics of lung CD103pos DCs and lung CD11bpos DCs versus macrophages in chimeric CD45.1 mice after low-dose infection with S. pneumoniae. Chimeric CD45.1 mice were infected with S. pneumoniae on Days 10 or 35 after irradiation/BMT, followed by a determination of repopulation and turnover profiles of the various mononuclear phagocyte subsets at the indicated time points. (A–D) Total numbers and percentages of CD45.2pos CD103pos DCs (A and B) and CD11bpos DCs (C and D). (E–H) Total numbers and percentages of CD45.2pos alveolar macrophages (E and F) and lung macrophages (G and H). The data are presented as the mean ± SEM of n = 3–9 mice per time point. A significant increase (*P < 0.05, **P < 0.01, and ***P < 0.001) was evident, compared with CL.
We next analyzed the repopulation and turnover kinetics of alveolar and lung macrophages of chimeric CD45.1 mice. Mice were infected with a low dose of S. pneumoniae on Day 35 after irradiation/BMT, that is, when the pool of both alveolar and lung mononuclear phagocyte subsets was composed of 20–30% donor-type cells (CD45.2pos) and 70–80% recipient-type cells (CD45.1pos). We found that a low-dose infection of mice with S. pneumoniae triggered a 7-fold increased pool size of mononuclear phagocytes, both in the alveolar and lung parenchymal compartment relative to preinfection conditions, which remained elevated for 7 days after infection (Figures 5E and 5G). Consistent with recent reports (9), at 1–2 days after infection with S. pneumoniae, approximately 90% of BAL fluid mononuclear phagocytes and lung macrophages expressed the CD45.2 donor-type alloantigen, representing newly recruited cells (Figures 5F and 5H). These data clearly demonstrate that the infection of chimeric CD45.1 mice with S. pneumoniae triggers a rapid recruitment of immune cells and thus a rapid replacement of resident CD45.1pos recipient-type cells by newly recruited CD45.2pos donor-type cells.
Discussion
The main goal of our study was to determine the impact of whole-body irradiation and BMT on the repopulation profile and turnover characteristics of lung DC subsets, compared with alveolar and lung macrophages under baseline and inflammatory conditions, using S. pneumoniae as an inflammatory stimulus. To the best of our knowledge, we show for the first time that irradiation/BMT triggers a rapid, greater than 90% depletion of CD103pos DCs from the lung parenchymal tissue of mice by Day 3 after irradiation, whereas CD11bpos DCs are only depleted by approximately 50–60% on Day 5 after treatment. In contrast to these data, alveolar and lung macrophages are depleted much later, by Day 21 after treatment, reaching a maximal depletion of only 50–60%. Moreover, we observed a differentially regulated repopulation of lung tissue within the respective lung DC and macrophage subsets. Whereas lung CD11bpos DCs and lung CD103pos DCs reached their original pool size by Days 10 and 21 after treatment, respectively, the repopulation of alveolar and lung macrophages reached baseline pool sizes on Days 42 and 70 after treatment, respectively. Furthermore, the infection of chimeric mice with low levels of S. pneumoniae triggered a brisk acceleration of the repopulation of all myeloid-derived DC and macrophage subsets in the lung. Altogether, our study provides a comprehensive analysis of the effects of irradiation/BMT on the depletion and repopulation and turnover kinetics of the major myeloid-derived lung DC and macrophage subsets, under both baseline and infection conditions. This information could be particularly important in the development of adjuvant immune enhancement strategies to overcome the immunosuppression of whole-body–irradiated BMT recipients.
Our report contains several novel aspects. First, to the best of our knowledge, we are the first to provide a detailed subset-specific analysis of the depletion and repopulation and turnover kinetics of both CD103pos and CD11bpos DC subsets in a chimeric irradiation/BMT murine model system. Second, we demonstrate that CD103pos DCs in the lung are more sensitive than CD11bpos DCs and lung macrophages to irradiation, resulting in an overall transient depletion of more than 90% in chimeric mice. Third, we show a rapid repopulation of the lungs with CD103pos DC and CD11bpos DC subsets subsequent to irradiation/BMT within 10 days (CD11bpos DCs) and 21 days (CD103pos DCs) after treatment, respectively, whereas alveolar and lung macrophages exhibit a more delayed depletion and repopulation, by Days 21 and 70 after treatment, respectively. Finally, we provide evidence that all myeloid-derived phagocyte subsets examined in the present study, including lung CD103pos DCs, lung CD11bpos DCs, and alveolar and lung macrophages, are eliminated from the lung parenchymal and bronchoalveolar compartment subsequent to irradiation by apoptosis-mediated and necrosis-mediated processes. Previous investigators observed a decline in rat airway MHC Class IIpos cells shortly after irradiation/BMT, but they thought this decline was attributable to the emigration of mature DCs to regional lymph nodes (18). Our data indicate, however, that elevated levels of apoptosis and necrosis occur in all lung mononuclear phagocyte subsets after irradiation/BMT, indicating that this is the major mechanism underlying the process of depletion. Furthermore, we observed that the draining lymph nodes of the lungs of chimeric mice also contain reduced numbers of CD103pos DCs and CD11bpos DCs (data not shown).
One major difficulty when comparing the published turnover kinetics of mononuclear phagocyte subsets in the lungs or in extrapulmonary organ systems is related to the different experimental protocols used in the various reported studies. Here, we used a linear accelerator (also used in the therapeutic whole-body irradiation of BMT recipients) and an irradiation/BMT protocol, resulting in an overall engraftment efficacy of greater than 90%. In a recent study by Ginhoux and colleagues, where parabiosis and separation experiments were performed in BL/6 mice and BL/6 CD45.1pos mice, relatively short half-lives were also reported for both lung DC subsets (5). However, comparing these two model systems is difficult, particularly because of the limited clinical relevance of parabiosis model systems. Another study using a different irradiation protocol reported that numbers of macrophages in the alveolar compartment were restored within 30 days after irradiation (17), whereas in our study, the time needed to restore numbers of macrophages in the alveolar compartment and lung parenchymal tissue comprised approximately 42–70 days. These data clearly indicate that different experimental protocols may affect the comparative analyses of DCs and of macrophage turnover kinetics between different studies.
The origin of various lung DC subsets in the bone marrow and their developmental stages are still subject to ongoing debate. In this regard, a bone marrow precursor of macrophages and DCs, termed the macrophage and DC progenitor, and a common DC precursor were proposed to have the potential to differentiate into monocytes, macrophages, and different DC subsets (19, 20). Moreover, recent data indicate that two distinct murine blood monocyte populations can serve as DC precursors (21). Given the nearly complete depletion of CD103pos DCs in lung parenchymal tissue reported in this study, and furthermore, given that in the present chimeric murine model, bone marrow cells from normal BL/6 mice (characterized by their CD45.2 alloantigen expression) were used as donor bone marrow, the present irradiation and transplantation protocol may also prove useful in identifying candidate molecules that may regulate the developmental pathways of progenitor cells giving rise to CD103pos lung DCs, as already proven for CCR2 (22).
The repopulation and turnover kinetics of lung CD11bpos DCs and CD103pos DCs upon infection with S. pneumoniae constitute another aspect of the present report. We infected mice with a clinical isolate of serotype 19 S. pneumoniae, using a “low-level” inoculum size of 106 CFUs/mouse. This inoculum size is known to cause mild lobar pneumonia in mice, without causing severe lung tissue destruction or invasive disease progression (15). Despite this relatively weak bacterial challenge, we found that the rapid depletion of DC subsets was followed by a large recruitment of donor-type CD11bpos DCs and CD103pos DCs. These data illustrate that the lung host defense machinery responds to infection not only with a transient increase of lung macrophage pool size, as recently reported (9, 12), but also with an increased recruitment of lung CD11bpos DCs and CD103pos DCs. Interestingly, this transient “overshooting” mobilization of donor-type DCs was particularly evident in the later phase of pneumococcal pneumonia, when both the process of bacterial pathogen elimination and the recruitment of alveolar neutrophils had already returned to baseline (data not shown). However, the relevance of an increased lung DC pool size to the resolution/repair process in pneumococcal pneumonia is not yet clear, as opposed to the relevance of lung macrophages, which we previously showed to play a prominent role in the resolution/repair process subsequent to lung infection with S. pneumoniae, in terms of regaining lung homeostasis (13, 16, 23), However, we speculate that lung DC subsets, which are located beneath the alveolar epithelial barrier, may be involved in sensing apoptotic/necrotic neutrophils or responding to inflammatory mediators, thereby possibly contributing to host-derived inflammatory cytokine/chemokine responses subsequent to infection.
In conclusion, this study, to the best of our knowledge, is the first to provide a detailed comparative analysis of the turnover kinetics of lung DC and macrophage subsets under noninfectious conditions, as well as in response to infection with S. pneumoniae. We show that under noninfectious conditions, CD103pos lung DCs were almost completely depleted from lung tissue after irradiation/BMT, indicating their strong radiosensitivity. Moreover, the turnover of lung CD103pos DCs was clearly accelerated compared with lung CD11bpos DCs, and both DC subsets exhibited a strongly accelerated turnover profile relative to alveolar and lung macrophages. Moreover, we demonstrated a brisk repopulation and turnover of all mononuclear phagocyte subsets after low-dose infection with S. pneumoniae. We believe that our newly provided information on the irradiation-induced effects of baseline and inflammation-induced lung DC and macrophage turnover profiles will be of major clinical importance in the development of immune-supportive therapeutic or prophylactic treatment regimens, to lower the risk of both bacterial and viral infections in irradiated BMT recipients.
Supplementary Material
Footnotes
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0279OC on December 22, 2010
Author Disclosure: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol 2008;8:142–152 [DOI] [PubMed] [Google Scholar]
- 2.Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)–beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol 2006;176:2161–2172 [DOI] [PubMed] [Google Scholar]
- 3.Beaty SR, Rose CE, Jr, Sung SS. Diverse and potent chemokine production by lung CD11b high dendritic cells in homeostasis and in allergic lung inflammation. J Immunol 2007;178:1882–1895 [DOI] [PubMed] [Google Scholar]
- 4.del Rio ML, Rodriguez-Barbosa JI, Bolter J, Ballmaier M, Dittrich-Breiholz O, Kracht M, Jung S, Forster R. CX3CR1+ c-kit+ bone marrow cells give rise to CD103+ and CD103− dendritic cells with distinct functional properties. J Immunol 2008;181:6178–6188 [DOI] [PubMed] [Google Scholar]
- 5.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med 2009;206:3115–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Wulffen W, Steinmueller M, Herold S, Marsh LM, Bulau P, Seeger W, Welte T, Lohmeyer J, Maus UA. Lung dendritic cells elicited by FMS-like tyrosine 3–kinase ligand amplify the lung inflammatory response to lipopolysaccharide. Am J Respir Crit Care Med 2007;176:892–901 [DOI] [PubMed] [Google Scholar]
- 7.Winter C, Taut K, Langer F, Mack M, Briles DE, Paton JC, Maus R, Srivastava M, Welte T, Maus UA. FMS-like tyrosine kinase 3 ligand aggravates the lung inflammatory response to Streptococcus pneumoniae infection in mice: role of dendritic cells. J Immunol 2007;179:3099–3108 [DOI] [PubMed] [Google Scholar]
- 8.Merad M, Manz MG. Dendritic cell homeostasis. Blood 2009;113:3418–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taut K, Winter C, Briles DE, Paton JC, Christman JW, Maus R, Baumann R, Welte T, Maus UA. Macrophage turnover kinetics in the lungs of mice infected with Streptococcus pneumoniae. Am J Respir Cell Mol Biol 2008;38:105–113 [DOI] [PubMed] [Google Scholar]
- 10.Maus UA, Waelsch K, Kuziel WA, Delbeck T, Mack M, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2–CCR2 axis. J Immunol 2003;170:3273–3278 [DOI] [PubMed] [Google Scholar]
- 11.Herbold W, Maus R, Hahn I, Ding N, Srivastava M, Christman JW, Mack M, Reutershan J, Briles DE, Paton JC, et al. Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect Immun 2010;78:2620–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, Seeger W, Welte T, Lohmeyer J. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol 2006;35:227–235 [DOI] [PubMed] [Google Scholar]
- 13.Winter C, Taut K, Srivastava M, Langer F, Mack M, Briles DE, Paton JC, Maus R, Welte T, Gunn MD, et al. Lung-specific overexpression of CC chemokine ligand (CCL) 2 enhances the host defense to Streptococcus pneumoniae infection in mice: role of the CCL2–CCR2 axis. J Immunol 2007;178:5828–5838 [DOI] [PubMed] [Google Scholar]
- 14.Henken S, Bohling J, Martens-Lobenhoffer J, Paton JC, Ogunniyi AD, Briles DE, Salisbury VC, Wedekind D, Bode-Boger SM, Welsh T, et al. Efficacy profiles of daptomycin for treatment of invasive and noninvasive pulmonary infections with Streptococcus pneumoniae. Antimicrob Agents Chemother 2010;54:707–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun 1992;60:111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maus UA, Backi M, Winter C, Srivastava M, Schwarz MK, Ruckle T, Paton JC, Briles D, Mack M, Welte T, et al. Importance of phosphoinositide 3–kinase gamma in the host defense against pneumococcal infection. Am J Respir Crit Care Med 2007;175:958–966 [DOI] [PubMed] [Google Scholar]
- 17.Matute-Bello G, Lee JS, Frevert CW, Liles WC, Sutlief S, Ballman K, Wong V, Selk A, Martin TR. Optimal timing to repopulation of resident alveolar macrophages with donor cells following total body irradiation and bone marrow transplantation in mice. J Immunol Methods 2004;292:25–34 [DOI] [PubMed] [Google Scholar]
- 18.Holt PG, Stumbles PA. Characterization of dendritic cell populations in the respiratory tract. J Aerosol Med 2000;13:361–367 [DOI] [PubMed] [Google Scholar]
- 19.del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, Forster R. Development and functional specialization of CD103+ dendritic cells. Immunol Rev 2010;234:268–281 [DOI] [PubMed] [Google Scholar]
- 20.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev 2010;234:45–54 [DOI] [PubMed] [Google Scholar]
- 21.Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood monocyte subsets differentially give rise to CD103+ and CD103− pulmonary dendritic cell populations. J Immunol 2008;180:3019–3027 [DOI] [PubMed] [Google Scholar]
- 22.Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte–derived dendritic cells in steady state and inflammation. Immunol Rev 2010;234:90–104 [DOI] [PubMed] [Google Scholar]
- 23.Winter C, Herbold W, Maus R, Langer F, Briles DE, Paton JC, Welte T, Maus UA. Important role for CC chemokine ligand 2–dependent lung mononuclear phagocyte recruitment to inhibit sepsis in mice infected with Streptococcus pneumoniae. J Immunol 2009;182:4931–4937 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.