Abstract
Obliteration of the vascular lumen by endothelial cell growth is a hallmark of many forms of severe pulmonary arterial hypertension. Copper plays a significant role in the control of endothelial cell proliferation in cancer and wound-healing. We sought to determine whether angioproliferation in rats with experimental pulmonary arterial hypertension and pulmonary microvascular endothelial cell proliferation in humans depend on the proangiogenic action of copper. A copper-depleted diet prevented, and copper chelation with tetrathiomolybdate reversed, the development of severe experimental pulmonary arterial hypertension. The copper chelation–induced reopening of obliterated vessels was caused by caspase-independent apoptosis, reduced vessel wall cell proliferation, and a normalization of vessel wall structure. No evidence was found for a role of super oxide–1 inhibition or lysyl–oxidase–1 inhibition in the reversal of angioproliferation. Tetrathiomolybdate inhibited the proliferation of human pulmonary microvascular endothelial cells, isolated from explanted lungs from control subjects and patients with pulmonary arterial hypertension. These data suggest that the inhibition of endothelial cell proliferation by a copper-restricting strategy could be explored as a new therapeutic approach in pulmonary arterial hypertension. It remains to be determined, however, whether potential toxicity to the right ventricle is offset by the beneficial pulmonary vascular effects of antiangiogenic treatment in patients with pulmonary arterial hypertension.
Keywords: pulmonary hypertension, copper, angiogenesis, tetrathiomolybdate
Clinical Relevance
Severe pulmonary arterial hypertension is characterized by small pulmonary vessel lumen obliteration by the exuberant growth of endothelial cells. Angiogenesis and wound repair are critically dependent on the availability of copper. Pulmonary vascular remodeling in experimental pulmonary hypertension in rats and the in vitro proliferation of human pulmonary microvascular endothelial cells are copper-dependent. Copper chelation may be explored as a new therapeutic strategy in patients with pulmonary arterial hypertension.
Perhaps because pulmonary vasoconstriction is not an important disease component in most patients with chronic pulmonary arterial hypertension (PAH), more than a decade of treating PAH patients with vasodilator drugs has not had a decisive impact on outcomes (1). Based on the demonstration of endothelial cells with apoptosis-resistant and hyperproliferative characteristics in the lungs of patients with PAH, a conceptual model of quasimalignant cell growth in an “angiogenic niche” was proposed to explain the mechanism of complex vascular lesion formation in severe PAH (2–8). According to increasing evidence, copper plays a significant role in the control of normal endothelial cell growth (9, 10) and endothelial cell proliferation in wound-healing (11) and cancer (12). The copper chelator tetrathiomolybdate (TTM) was shown to inhibit tumor angiogenesis in animal models (13, 14), and is currently under investigation as an adjuvant cancer therapy in a number of clinical trials (clinicaltrials.gov). The mechanisms by which copper regulates endothelial cell growth have yet to be elucidated, but may involve the function of a number of copper-dependent enzymes and transcription factors, including super oxide dismutase (SOD)–1 (15), lysyl–oxidase (LOX)–1 (12), and hypoxia-inducible factor (HIF)–1α (16). Interestingly, increased tissue and serum concentrations of copper in pulmonary hypertensive patients and in animals with experimental pulmonary hypertension were already reported in the 1980s (17, 18). Moreover, pulmonary vascular remodeling in human and experimental PAH is associated with the increased expression of HIF-1α and of downstream vascular endothelial growth factor (VEGF) (19, 20). If pulmonary angioproliferation is to some degree attributable to “vascular wound-healing gone awry,” then copper-depleting strategies might affect the process of pulmonary vascular angioproliferation in severe PAH.
Here we test the hypothesis that angio-obliteration in experimental severe pulmonary hypertension is copper-dependent. We show that after exposure to the VEGF receptor tyrosine kinase inhibitor SU5416 and hypoxia (the SuHx model) (21–23), rats will only develop endothelial proliferation and severe pulmonary hypertension when they are provided with sufficient quantities of dietary copper. We also show that established pulmonary hypertension and angio-obliteration in SuHx rats can be reversed by chronic treatment with TTM, and that TTM inhibits the proliferation of cultured human lung endothelial cells from patients with PAH and control subjects. In the lung vessels of SuHx rats, the therapeutic effects of TTM are associated with the reduced expression of Survivin, the increased production of the proapoptotic sphingolipid metabolite ceramide, and the increased expression of apoptosis-inducing factor (AIF) and Bim, a B-cell leukemia/lymphoma (Bcl)–2 family member promoting apoptosis.
Materials and Methods
Animal Models
All experiments were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. Pulmonary hypertension was induced in male Sprague-Dawley rats through their exposure to the VEGF receptor tyrosine kinase inhibitor SU5416 and chronic hypoxia (SuHx model), as described previously (21–23), and in different sets of age-matched and gender-matched rats by exposure to 10% hypoxia for 8 weeks, or by administrating a single 60 mg/kg dose of monocrotaline (MCT). Animals received a diet with normal copper content (20–25 mg copper per kg diet, LM-485) or a copper-deficient diet (6 mg/kg copper, TD80388; both diets were comparable in trace elements other than copper, and were obtained from Harlan Teklad, Madison, WI). TTM (10 mg/kg; Sigma-Aldrich, St. Louis, MO) was dissolved in normal saline and administered intraperitoneally every other day for 10 days. Other rats received intraperitoneal injections of β-aminopropionitrite (100 mg/kg in normal saline, 5 days a week for 4 weeks; Sigma-Aldrich) or the pan-caspase inhibitor Z-Asp (3 mg/kg in DMSO and saline, daily for 10 days; Bachem, Buckinghamshire, United Kingdom). TTM, β-aminopropionitrite, and Z-Asp treatments were initiated either directly upon return to normoxic air breathing (4 weeks after the initial exposure to SU5416), or 2 weeks later. A low dose of TTM was chosen after determination of the lowest effective dose that caused no weight loss or other phenotypic changes. Doppler echocardiography, using the Vevo770 imaging system (VisualSonics, Toronto, ON, Canada), and hemodynamic measurements, using a 4.5-mm conductance catheter (Millar Instruments, Houston, TX) and the Powerlab data acquisition system (AD Instruments, Colorado Springs, CO), were performed as described previously (22, 23).
Cell Culture
Human endothelial lung cells from four patients with idiopathic PAH and four control subjects were isolated from lung specimens obtained at time of transplantation (PAH) or lobectomy or pneumonectomy for localized lung cancer (control subjects), and were cultured according to previous protocols (24). The mean age (± SD) was 42 ± 9 years in patients with idiopathic PAH, and 48 ± 11 years in control subjects. The mean pulmonary arterial pressure (± SD) in patients with idiopathic PAH was 60 ± 13 mm Hg. Preoperative echocardiography was performed in all control subjects to rule out pulmonary hypertension, and lung specimens were collected at a distance from the tumor foci. This study was approved by the local ethics committee (Comite de Protection des Persones Ile-de-France VII, Le Kremlin-Bicêtre, France). All patients gave informed consent before the study. Endothelial cell proliferation was measured by the incorporation of 5-bromo-2-deoxyuridine and staining using the DELFIA Cell Proliferation Kit (PerkinElmer, Courtaboeuf, France) and a time-resolved fluorometer EnVision Multilabel Reader (PerkinElmer). Cells were counted using a Neubauer hemocytometer (Hausser Scientific, Horsham, PA) with trypan blue dye exclusion.
Protein Expression, Histology, and Immunohistochemistry
See the online supplement for details.
Statistical Analysis
Differences between groups were assessed with ANOVA (parametric) and the Kruskall-Wallis test (nonparametric). Bonferroni (parametric) and Dunn (nonparametric) post hoc tests were used to assess significant differences between pairs of groups. P < 0.05 was considered significant. For reasons of clarity, all data are reported as means ± SEM, unless specified otherwise, even if differences between groups were tested with a nonparametric test that makes no use of means and standard deviations. We used 6 to 8 rats per group, unless otherwise specified.
Results
Dietary Copper Restriction Inhibits the Development of Severe Pulmonary Hypertension Induced by SU5416/Hypoxia, but Exerts No Effect on Pulmonary Hypertension Induced by Chronic Hypoxia or MCT
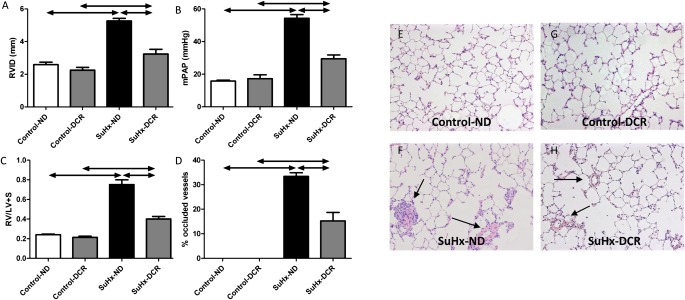
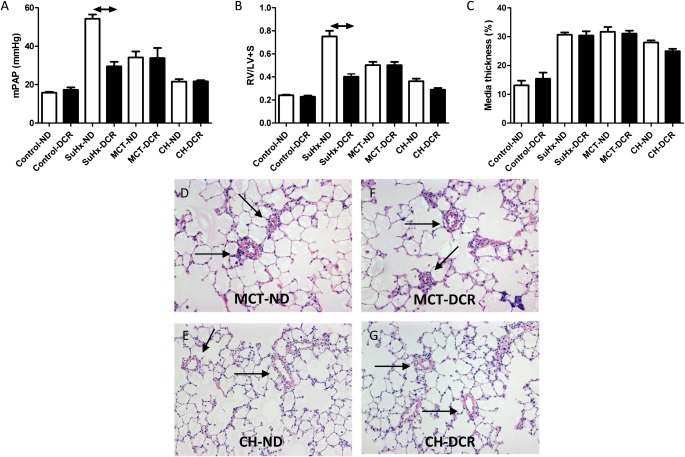
Rats receiving a normal diet and exposed to SU5416 plus 4 weeks of hypoxia (the SuHx model) (21–23) all developed severe angioproliferative PAH and right ventricular (RV) dilatation and hypertrophy (Figures 1A–1C). As described previously (21, 25), the increased pulmonary vascular resistance in this model is attributable to a combination of small pulmonary artery medial thickening and luminal obstruction, in part by proliferating, apoptosis-resistant endothelial cells (Figures 1D and 1F). Dietary copper restriction (DCR) commencing 2 weeks before initiating the SU5416/hypoxia protocol and continuing throughout the experiment completely prevented pulmonary vascular occlusion (Figures 1D and 1H), which resulted in a lower mean pulmonary artery pressure (mPAP; Figure 1B) and in a reduction of RV hypertrophy (Figure 1C), dilatation (Figure 1A), and fibrotic remodeling (data not shown). DCR did not prevent the development of mild pulmonary hypertension and RV hypertrophy in rats exposed to chronic hypoxia or MCT (Figures 2A and 2B). In contrast to its effect on vascular luminal occlusion in SuHx rats, DCR did not affect the degree of pulmonary artery media thickening in any of the three pulmonary hypertension models, including the SuHx model (Figures 1H, 2C, 2F, and 2G). Thus, the experimentally generated pulmonary arteriolar muscularization was not dependent on dietary copper.
Figure 1.
Angioproliferative pulmonary hypertension develops in rats receiving a normal diet (ND), but not in rats on a restricted copper diet (DCR). This is reflected by less right ventricular (RV) dilatation (A) (RVID, RV inner diameter in diastole), less RV hypertrophy (C) (LV + S, left ventricular plus septal weight), a lower mean pulmonary artery pressure (mPAP) (B), and a lower percentage of small pulmonary vessels with complete luminal obliteration (D). (E–H) Representative lung-tissue sections (high and low magnifications; hematoxylin and eosin stains). Arrows indicate remodeled small pulmonary vessels. SuHx, model involving exposure to the vascular endothelial growth factor receptor tyrosine kinase inhibitor SU5416 and hypoxia.
Figure 2.
Dietary copper content (ND, normal diet; DCR, dietary copper restriction) does not affect the development of pulmonary hypertension (mean pulmonary artery pressure, mPAP) (A) or right ventricular hypertrophy (B) (RV/LV + S, right ventricular over left ventricular + septal weight) after exposure to monocrotaline (MCT) or chronic hypoxia (CH). Likewise, dietary copper content does not affect the degree of media thickening in any of the three models (SuHx, MCT, or CH) (C; see also Figure 1H). DCR-induced changes in right ventricular hypertrophy and media thickening in the CH model did not reach statistical significance. (D–G) Representative lung tissue sections (high and low magnifications; hematoxylin-and-eosin stains). Remodeled (muscularized) small pulmonary vessels are denoted by arrows.
Reversal of Pulmonary Hypertension in SuHx Rats by Administration of the Copper Chelator Tetrathiomolybdate Is Associated with Reduced Cell Proliferation and Increased Rates of Cell Death; Pulmonary Hypertension Returns upon Cessation of Treatment
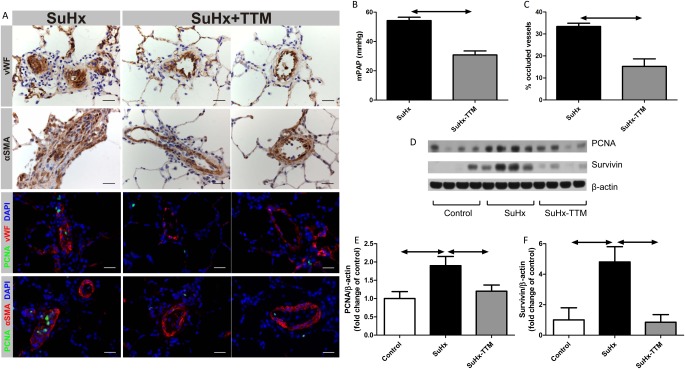
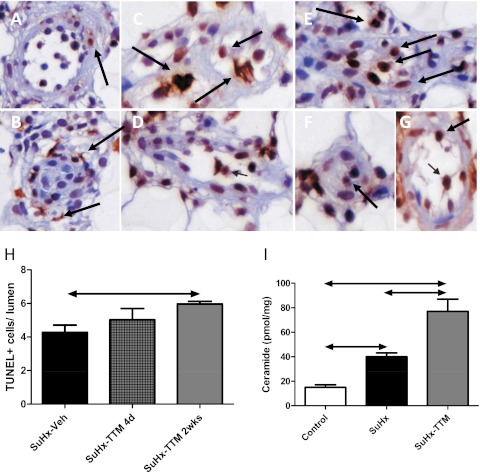
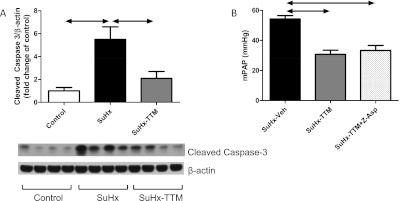
In SuHx rats with established severe PAH (either 4 weeks after the initial exposure to SU5417, i.e., upon return to normal air breathing, or 6 weeks after SU5416, i.e., after 2 weeks of normal air breathing), a switch from a normal diet to DCR after the establishment of angioproliferative pulmonary vascular lesions did not result in a reopening of occluded pulmonary vessels (data not shown). In contrast, treatment with the copper chelator TTM (10 mg/kg every other day), whether or not combined with DCR, resulted in a reopening of pulmonary vessels in established SuHx lungs (Figures 3A and 3C; see also Figure E1 in the online supplement; the effect was evident after the first dose of TTM, and reached a plateau after 10 days), a lowering of pulmonary artery pressure (Figure 3B), and a reduction in RV remodeling (Figure E2). The lungs of untreated SuHx rats showed evidence of increased rates of cell proliferation (increased expression of proliferating cell nuclear antigen [PCNA] was demonstrated by immunohistochemistry and Western blotting; Figures 3A, 3D, and 3E) and the emergence of resistance to apoptosis (increases in whole-lung expression of Survivin; Figures 3A, 3D, and 3F). TTM treatment was associated with a normalization of PCNA and Survivin expression (Figures 3D and 3F). Lungs in SuHx rats showed evidence of increased cell turnover, because exuberant cell proliferation was found along with high rates of cell death (as reflected by positive terminal deoxynucledotidyl transferase dUTP nick end labeling (TUNEL) staining; Figures 4 and E3). TTM treatment was not only associated with a further increase in overall rates of TUNEL positivity, but also with a change in the localization of TUNEL positivity: the copper chelator induced the death of cells within (and obstructing) the lumen of small pulmonary vessels, whereas in untreated SuHx lungs, evidence of cell death primarily existed around obstructive vascular lesions. Increased rates of cell death in SuHx lungs were confirmed by the finding of elevated concentrations of the proapoptotic sphingolipid metabolite ceramide (Figure 4I). In accordance with the TUNEL data, TTM treatment resulted in a further increase in ceramide concentrations. With the cessation of TTM treatment, RV pressure overload, RV hypertrophy, and luminal occlusion all reappeared (Figure E4),indicating that temporary TTM treatment does not forever eliminate the angioproliferative potential within the SuHx lung. Ten days of TTM treatment in control rats did not result in phenotypic or hemodynamic changes, and did not affect the protein expression of PCNA or cleaved caspase-3 in comparison with vehicle-treated control rats (data not shown).
Figure 3.
Copper chelation with tetrathiomolybdate (TTM; 10 mg/kg every other day for 10 days) reverses pulmonary hypertension in SuHx rats, as shown by a reduction in mean pulmonary artery pressure (mPAP) (B) and a reduced percentage of fully occluded blood vessels (C). The intraluminal cells in characteristic occlusive lesions in the SuHx lung stain partly positive for von Willebrand Factor (VWF), and partly positive for α–smooth muscle actin (SMA) (A). Photographs were taken using a ×40 objective, at ×400 magnification. Scale bar = 25 μm. TTM removes most intraluminal cells, thereby restoring a monolayer of cells that maintain vWF and α-SMA positivity. Staining with proliferating cell nuclear antigen (PCNA) suggests an increased proliferation rate of intraluminal cells in vehicle-treated SuHx rats, whereas PCNA positivity diminishes after TTM treatment (A). These findings are confirmed by PCNA protein expression in whole-lung lysates (D and E). Consistent with the emergence of resistance to apoptosis, the protein expression of Survivin is increased in the SuHx lung, and normalizes after TTM treatment (D and F).
Figure 4.
(A and B) Untreated SuHx lungs exhibit high rates of death of vascular lesions cells, with preferential terminal deoxynucledotidyl transferase dUTP nick end labeling (TUNEL) staining of cells outside the lumen of occluded vessels and little positive TUNEL staining of lumen cells within fully occluded vessels. (C and D) Four days after a single dose of TTM, TUNEL-positive cells begin to appear within the lumen of reopened small pulmonary arteries (arrows indicate examples of positive cells). (E–G) After 2 weeks of tetrathiomolybdate (TTM) treatment, the TUNEL positivity of lumen cells of small pulmonary vessels is increased further. (H) Quantification of TUNEL images. (I) Increased rates of cell death in the SuHx lung are also reflected by an increased expression of ceramide in whole-lung lysates, whereas TTM treatment leads to a further increase in ceramide. d, days; Veh, vehicle; wks, weeks.
TTM Inhibits the Proliferation of Primary Lung Endothelial Cells Derived from Patients with Idiopathic PAH
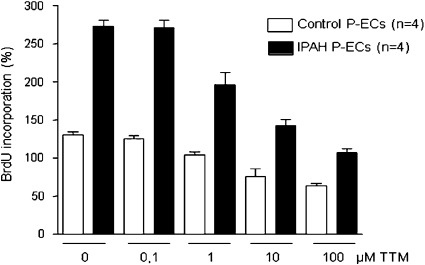
To explore more fully the potential for copper chelation as a therapeutic strategy in PAH, we cultured human primary lung endothelial cells derived from patients with idiopathic PAH in the presence of incremental concentrations of the copper chelator TTM. TTM significantly inhibited the proliferation of endothelial cells derived from both normal control subjects and patients with PAH (Figure 5). Growth inhibition occurred in PAH endothelial cells at a lower concentration of TTM, but no concentration of TTM led to the normalization of cell proliferation to control levels.
Figure 5.
Tetrathiomolybdate (TTM) inhibits the proliferation of human primary endothelial cells (P-ECs) derived from healthy control subjects and patients with idiopathic pulmonary arterial hypertension (iPAH). At all concentrations of TTM, cell proliferation is significantly higher in iPAH endothelial cells than in control endothelial cells. A significant inhibition of cell proliferation occurs at concentrations of 1 μM and higher in both types of endothelial cells. BrdU, 5-bromo-2-deoxyuridine.
Copper Chelation Is Associated with the Activation of Caspase-Independent Pathways of Cell Death
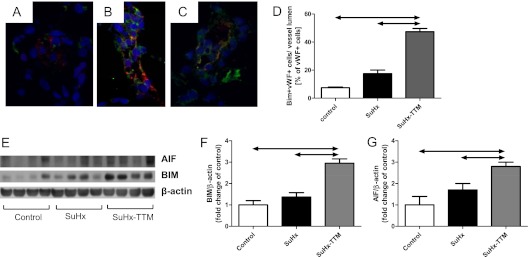
Both preventing the proliferation of lumen-obstructing cells and inducing the death of these cells likely contributed to the therapeutic effect of TTM. Surprisingly, whole-lung lysates from TTM-treated SuHx rats exhibited a decreased expression of cleaved caspase-3 (Figure 6A). The concurrent administration of the pan-caspase inhibitor Z-Asp did not prevent the reopening of small pulmonary vessels by TTM, as shown by a persistent drop in mPAP after TTM + Z-Asp cotreatment (Figure 6B). Because ceramide was implicated in both caspase-dependent and caspase-independent cell death pathways (26–28), we explored in TTM-treated SuHx rats the expression of two markers of caspase-independent cell death: AIF and the proapoptotic Bcl-2 family member Bim (29, 30). Lungs from SuHx rats not treated with TTM did not exhibit any Bim-positive vascular luminal cells (Figure 7A), whereas the TTM treatment of SuHx rats resulted in the appearance of von Willebrand factor (vWF) and Bim coexpressing cells in pulmonary vessels (Figures 7B and 7C; quantified in Figure 7D). Furthermore, the whole-lung protein expression of AIF and BIM in SuHx rats was elevated with TTM treatment (Figures 7E–7G).
Figure 6.
The reopening of small vessels and the reduction of pulmonary hypertension by tetrathiomolybdate (TTM) is associated with reduced cleaved caspase-3 expression (A), and cannot be prevented by concurrent treatment with the pan-caspase inhibitor Z-Asp (B), which suggests that the induction of cell-death by TTM is caspase-independent.
Figure 7.
The administration of tetrathiomolybdate (TTM) in SuHx rats is associated with the appearance of a B-cell leukemia/lymphoma–2 family member promoting apoptosis (Bim)/von Willebrand factor (vWF) coexpressing cells in TTM-treated SuHx lungs. (A) SuHx lung. (B and C) TTM-treated SuHx lungs. Blue, nuclei (4′,6-diamidino-2-phenylindole [DAPI] stain); red, vWF-positive cells; green, Bim-positive cells (DAPI stain). (D) A quantification of immunofluorescence data. (E–G) The increased protein expression of Bim and apoptosis-inducing factor (AIF) in whole-lung lysates of TTM-treated SuHx rats is consistent with the induction of caspase-independent pathways of cell death.
Copper Chelation Does Not Reverse Angioproliferation via the Inhibition of Cu/Zn SOD-1 or LOX-1
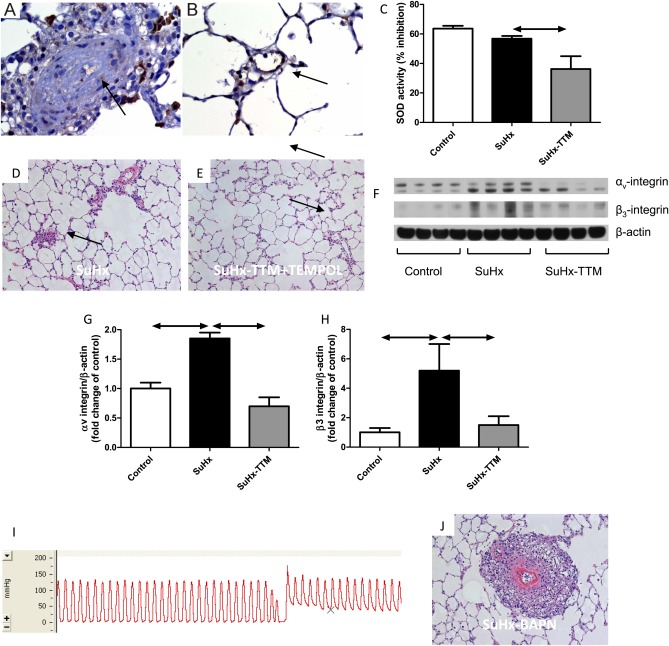
Copper is an essential cofactor for the antioxidant enzyme SOD-1, and TTM treatment is associated with reduced SOD-1 activity in cultured cancer cells and endothelial cells (15). Because TTM reduced cytosolic SOD-1 activity in SuHx and control lungs (Figure 8C), we looked for evidence supporting the hypothesis that TTM exerted its therapeutic effect via the induction of oxidative stress. However, concurrent treatment with N-acetylcysteine (1% in drinking water) or with the cell-permeable SOD mimetic 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (1 mmol/L in drinking water) did not prevent the treatment effect of TTM in SuHx rats (Figure 8E). Strikingly, there was complete absence of SOD-1 expression within the occluded pulmonary vessels of SuHx rats, whereas control endothelial cells showed abundant expression of SOD-1 (Figures 8A and 8B). This makes SOD-1 a very unlikely treatment target of TTM in the SuHx model. Alternatively, TTM could have exerted its anti-angioprolifierative effect via the inhibition of LOX-1, which is another copper-dependent enzyme. LOX-1 is involved in extracellular matrix protein cross-linking. Consistent with alterations in extracellular matrix remodeling, we found increased expression of αvβ3 integrin in SuHx lung tissue, which was reversed by TTM treatment (Figures 8F–8H). Rather than mimicking the effect of TTM, treatment with the LOX-1 inhibitor β-aminopropionitrile (100 mg/kg) worsened angioproliferation in SuHx rats, and led to the development of very severe pulmonary hypertension (Figure 8I) with evidence of fibrinoid necrosis of the vascular wall (Figure 8J).
Figure 8.
Super oxide dismutase (SOD)–1 expression is absent in the obliterated lumen of SuHx rats (A), whereas extensive SOD-1 expression can be found in normal lung endothelial cells (B, arrow). Whole-lung SOD activity tends to be lower in SuHx rats, whereas 2 weeks of treatment with the copper chelator tetrathiomolybdate (TTM; 10 mg/kg every other day) resulted in a further significant decrease in SOD activity (C). The reversal of angioproliferation by treatment with TTM is not prohibited by concurrent treatment with the scavenger N-acetylcysteine (not shown) or with the SOD mimetic 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPOL) (D, hematoxylin-and-eosin stain of angioproliferative lesions in the lung of a vehicle-treated SuHx rats; E, hematoxylin-and-eosin stain of reopened lung vessels in a TTM + TEMPOL–treated SuHx rat). (F–H) Lung vascular remodeling in the SuHx model is associated with an up-regulation of αvβ3 integrin, which is consistent with remodeling of the extracellular matrix. The inhibition of lysyl–oxidase (LOX)–1 by β-aminopropionitrile (BAPN) does not mimic the treatment effect of TTM, but leads instead to very severe pulmonary hypertension (I depicts a representative pressure tracing) and a remarkable degree of lung-vessel remodeling, with fibrinoid necrosis of the media and a large adventitial cell infiltrate (J, hematoxylin-and-eosin stain).
Discussion
Patients with severe and progressive forms of PAH frequently manifest complex angio-obliterative vascular lesions, and are refractory to vasodilator treatment. According to one concept of the pathogenesis of angio-obliterative PAH, pulmonary arteriolar lumens are filled by proliferating, phenotypically altered and apoptosis-resistant cells (2–8). Antiproliferative effects were described for prostacyclins, endothelin receptor antagonists, and phosphodiesterase inhibitors, but because these currently used PAH medications predominantly act to decrease vascular tone, the use of new antiproliferative and antiangiogenic drug treatments may require further exploration in PAH (31). The trace metal copper has long been recognized for its proangiogenic and wound-healing properties (9–11), and the copper chelator TTM was shown experimentally to inhibit tumor growth in murine models (12). The availability of this drug allowed us to conduct proof-of-principle studies and to examine the question of whether established pulmonary angio-obliterative lesions can be resolved, resulting in a drop in pulmonary artery pressure.
Here we show that the angio-obliterative process in the SuHx model is associated with the simultaneous elevated expression of markers of cell death, proliferation, and resistance to apoptosis. The net result of this elevated rate of cell turnover is a conglomerate of heterogenous lumen-obstructing cells that stain partly positive for vWF and for α–smooth muscle actin. The administration of the VEGF-receptor (R) inhibitor SU5416 was shown to cause apoptosis of lung endothelial cells and a subsequent loss of alveoli (32), an observation that led to the recognition of a VEGF-dependent lung vascular maintenance program. Because SU5416 is administered to SuHx rats only once (at the onset of hypoxic exposure), the death of lung endothelial cells in later stages of the disease is probably independent from the inhibition of VEGF-R, and depends instead on other sources of injury, including elevated shear stress and inflammatory responses. This hypothesis is consistent with the fact that the SuHx model is progressive even after reexposure to normoxic air, and also with the fact that in addition to hypoxia, the absence of regulatory T cells is also sufficient as a second hit to induce angioproliferation after SU5416 administration (33). In addition to its association with high rates of cell death, the pulmonary vascular remodeling observed in the lungs of SuHx rats was characterized by high rates of cellular proliferation. One possible explanation for this high cell turnover involves a phenomenon that has been termed “apoptosis-induced compensatory proliferation” (34), which relates to the strong stimulus for cell proliferation provided by the presence of apoptotic cells. Our study did not address the question of whether the high rates of cell proliferation in the SuHx model require the continued presence of apoptotic cells, or whether the process of remodeling is associated with a permanent phenotypic switch of the remaining endothelial cells, providing them with a self-sustaining, permanent quasi-malignant phenotype (35).
We also show that a copper-depleted diet initiated before the commencement of the SuHx protocol prevented the development of angioproliferative lesions and severe PAH (Figures 1B, 1D, and 1H). Importantly, the TTM treatment of SuHx rats reduced the degree of pulmonary hypertension without affecting media thickening. In fact, TTM exerted no effect on the media in any of our three experimental models of pulmonary hypertension (i.e., SuHx, MCT, and chronic hypoxia). This suggests that angio-obliteration is an important component of pulmonary vascular resistance in the SuHx model, and is likely to be similarly important in pulmonary vascular remodeling in patients with PAH. The striking resemblance of vascular lesions in SuHx rats and patients with PAH was recently demonstrated (36), and it is of importance to underline the fact that PAH-like vascular obstructions are not found in other frequently used pulmonary hypertension models, including the MCT and chronic hypoxia models. Our findings demonstrate that an antiangiogenic treatment strategy is fundamentally different from current treatment strategies in patients with PAH, which are all predominantly directed toward vasodilatation and pulmonary artery smooth muscle hypertrophy, but not toward reopening obliterated small pulmonary arteries. The similarities between cancer angiogenesis and vascular remodeling in PAH (2) may suggest that, as in cancer treatments, PAH may be appropriately treated with a multimodality approach, perhaps using copper chelation in conjunction with tyrosine kinase inhibitors, which are also being explored in PAH (37).
Consistent with the notion that copper is required for the formation of angioproliferative lesions, the cessation of copper restriction and chelation led to the recurrence of lesions and of PAH (Figure 4). Although a unifying mechanism of action of TTM in the SuHx model has yet to be elucidated, our data indicate that the copper chelator not only slows the growth of pulmonary endothelial cells (including cells cultured from patients with idiopathic PAH; Figure 5), confirming earlier reports of the antiangiogenic action of the molybdate (13, 14), but also induces the apoptosis of intraluminal vascular lesion cells. With TTM treatment, nonadherent, TUNEL-positive cells were seen in the pulmonary vascular lumen, and many of these cells expressed Bim, which is a marker of apoptosis of cells induced by inadequate or inappropriate cell–matrix interactions, or anoikis (38). After 2 weeks of TTM treatment, the total lung tissue expression of the antiapoptotic protein Survivin had decreased, and the amount of tissue ceramide had increased beyond the amount measured in SuHx lung tissue. TTM treatment was also associated with the increased expression of AIF, whereas the pan-caspase inhibitor Z-Asp did not prevent the reopening of arterioles and the reduction of pulmonary hypertension by TTM. These findings, taken together, are consistent with the induction of caspase-independent pathways of cell death (27, 29, 30), rather than with vessel luminal reopening by means of caspase-dependent apoptosis. We lack information regarding whether ceramide can contribute to the caspase-independent death of lung cells, although endothelial cell apoptosis in the lung after the stimulation of ceramide production has been reported (39), similar to the death of cancer cells (26–28).
The intracellular actions of TTM are a subject of intense investigation. In our experiments, dietary copper restriction completely prevented the development of vascular lesions, which suggests that the elimination of copper by TTM is an important mode of action. Recent studies indicate that the extraordinary affinity of molybdenum sulfate for copper is responsible for the ability of TTM to block metal transfer functions between copper-trafficking proteins (40). In this way, TTM effectively eliminates the function of copper without physically removing the trace metal from the cell. By this mechanism, TTM may affect the phenotype of lumen-obliterating cells in SuHx rats. Copper serves as a critically important cofactor of enzymes that regulate cellular respiration, antioxidant defenses, and iron metabolism (9). Hyperproliferative lesions in cancer and atherosclerosis have higher cell nuclear copper levels than normal tissue cells (41). Our choice of a TTM dose of 10 mg/kg was based on the literature concerning safe and effective antiangiogenic treatments in rodents. Because the effect of TTM does not depend on the prevention of intestinal absorption or on the physical removal of copper from tissues, we did not consider it critical to titrate toward an effective minimal dose, or to add an experimental group of SuHx rats treated with TTM and fed a high copper diet.
In fully occluded pulmonary arterioles, the expression of the antioxidant enzyme SOD-1, surprisingly, was lost, as shown by immunohistochemistry, and TTM treatment for 2 weeks reduced SOD activity in lung tissue below the control activity level. The latter finding was expected, because TTM affects the expression and action of the CuZn SOD (15). Although the combination of elevated ceramide concentrations and reduced SOD activity may have exposed pulmonary arteriolar lesion cells to intensive oxidant stress, we were unable to block the lumen-opening actions of TTM by treating SuHx animals with high doses of N-actelycysteine or a SOD mimetic, suggesting that reduced SOD activity alone does not explain the mechanism of TTM in this model of PAH. Lastly, we considered that TTM might resolve pulmonary vascular lesion cells by inhibiting the copper-dependent elastin and collagen cross-linking enzyme LOX-1, but we found that an established inhibitor of this enzyme (β-aminopropionitrile) only worsened the pulmonary vascular remodeling and pulmonary hypertension in Su/Hx animals. In our experiments, we did not address the possibility of immune modulation by copper restriction and TTM, although there is work suggesting that such mechanisms could be involved (42).
We conclude that in the SuHx model, proliferative lumen-obliterating pulmonary vasculopathy is copper-dependent. Both the cell growth and the phenotypic switch of cells to apoptosis-resistant status can be prevented by copper deficiency, whereas the vascular muscularization in the chronic hypoxia and MCT models cannot. The mechanism of luminal reopening in our model of severe PAH appears to be complex, depending on a form of caspase-independent apoptosis, a reduction of cell proliferation, and a return to a more normal vessel wall cell phenotype. The increased expression of the αvβ3 integrin was described as characteristic of activated endothelial cells and of endothelial cell growth (43–47). In this context, it is of interest that the treatment of SuHx animals with TTM for 2 weeks normalized the tissue expression of this integrin.
Based on our data, exploring the possibility of TTM treatment in patients with PAH seems justified. After establishing the feasibility and safety of TTM treatment in cancer patients (48, 49), the efficacy of the drug as an anticancer treatment is being tested. Although the drug may appear safe in oncologic patients, inherent dangers to antiangiogenic treatment may arise in patients with pulmonary hypertension. We recently reported that RV adaptation to mechanical stress induced by pulmonary artery banding is impeded by dietary copper restriction (23). For a given degree of RV pressure overload, copper restriction is associated with excessive fibrogenesis and capillary rarefaction. The preclinical data presented here suggest that TTM treatment may be safe as long as it can induce a substantial decrease in pulmonary vascular resistance. This concept will need to be rigorously tested before TTM can be proposed as a novel therapeutic agent in PAH.
Supplementary Material
Footnotes
This study was supported by the Victoria Johnson Center for Obstructive Lung Disease Research at the Virginia Commonwealth University, Richmond, VA.
H.J.B., S.M., C.G., M.H., A.V.N., S.S., and N.F.V. contributed to the study design. H.J.B., S.M., C.G., A.A.A.H., D.F., G.R., D.K., E.F., J.C.A., and L.F. contributed to the collection of data. H.J.B., S.M., C.G., A.A.A.H., D.F., G.F., J.C.A., and L.F. contributed to the analysis of data. H.J.B., S.M., C.G., A.A.A.H., D.F., S.S., and N.F.V. contributed to the preparation of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0296OC on December 28, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156–163 [DOI] [PubMed] [Google Scholar]
- 2.Rai PR, Cool CD, King JA, Stevens T, Burns N, Winn RA, Kasper M, Voelkel NF. The cancer paradigm of severe angioproliferative pulmonary hypertension. Am J Respir Crit Care Med 2008;178:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA 2007;104:11418–11423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michelakis ED. Spatio-temporal diversity of apoptosis within the vascular wall in pulmonary arterial hypertension: heterogeneous BMP signaling may have therapeutic implications. Circ Res 2006;98:172–175 [DOI] [PubMed] [Google Scholar]
- 5.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 2005;115:1479–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cool CD, Kennedy D, Voelkel NF, Tuder RM. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum Pathol 1997;28:434–442 [DOI] [PubMed] [Google Scholar]
- 7.Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest 1998;101:927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994;144:275–285 [PMC free article] [PubMed] [Google Scholar]
- 9.Pena MM, Lee J, Thiele DJ. A delicate balance: homeostatic control of copper uptake and distribution. J Nutr 1999;129:1251–1260 [DOI] [PubMed] [Google Scholar]
- 10.Hu GF. Copper stimulates proliferation of human endothelial cells under culture. J Cell Biochem 1998;69:326–335 [DOI] [PubMed] [Google Scholar]
- 11.Mandinov L, Mandinova A, Kyurkchiev S, Kyurkchiev D, Kehayov I, Kolev V, Soldi R, Bagala C, de Muinck ED, Lindner V, et al. Copper chelation represses the vascular response to injury. Proc Natl Acad Sci USA 2003;100:6700–6705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowndes SA, Harris AL. The role of copper in tumour angiogenesis. J Mammary Gland Biol Neoplasia 2005;10:299–310 [DOI] [PubMed] [Google Scholar]
- 13.Khan MK, Miller MW, Taylor J, Gill NK, Dick RD, Van GK, Brewer GJ, Merajver SD. Radiotherapy and antiangiogenic TM in lung cancer. Neoplasia 2002;4:164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, De CM, Mesri EA, Robins DM, Dick RD, et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res 2002;62:4854–4859 [PubMed] [Google Scholar]
- 15.Juarez JC, Betancourt O, Jr, Pirie-Shepherd SR, Guan X, Price ML, Shaw DE, Mazar AP, Donate F. Copper binding by tetrathiomolybdate attenuates angiogenesis and tumor cell proliferation through the inhibition of superoxide dismutase 1. Clin Cancer Res 2006;12:4974–4982 [DOI] [PubMed] [Google Scholar]
- 16.Martin F, Linden T, Katschinski DM, Oehme F, Flamme I, Mukhopadhyay CK, Eckhardt K, Troger J, Barth S, Camenisch G, et al. Copper-dependent activation of hypoxia-inducible factor (HIF)–1: implications for ceruloplasmin regulation. Blood 2005;105:4613–4619 [DOI] [PubMed] [Google Scholar]
- 17.Ahmed T, Sackner MA. Increased serum copper in primary pulmonary hypertension: a possible pathogenic link? Respiration 1985;47:243–246 [DOI] [PubMed] [Google Scholar]
- 18.Ward WF, Molteni A, Ts'ao C, Ischiropoulos H. Serum copper concentration as an index of experimental lung injury. Adv Exp Med Biol 1989;258:287–302 [DOI] [PubMed] [Google Scholar]
- 19.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, et al. An abnormal mitochondrial-hypoxia inducible factor–1{alpha}–Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 2006;113:2630–2641 [DOI] [PubMed] [Google Scholar]
- 20.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 2001;195:367–374 [DOI] [PubMed] [Google Scholar]
- 21.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, McMahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death–dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 2001;15:427–438 [DOI] [PubMed] [Google Scholar]
- 22.Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang P, Chau V, Hoke N, Kraskauskas D, Kasper M, Salloum F, et al. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med 2010;182:652–660 [DOI] [PubMed] [Google Scholar]
- 23.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 2009;120:1951–1960 [DOI] [PubMed] [Google Scholar]
- 24.Tu L, Dewachter L, Gore B, Fadel E, Dartevelle P, Simonneau G, Humbert M, Eddahibi S, Guignabert C. Autocrine FGF2 signaling contributes to altered endothelial phenotype in pulmonary hypertension. Am J Respir Cell Mol Biol 2001;45:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 2005;2005:1178–1180 [DOI] [PubMed] [Google Scholar]
- 26.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science 1996;274:1855–1859 [DOI] [PubMed] [Google Scholar]
- 27.Park JY, Kim MJ, Kim YK, Woo JS. Ceramide induces apoptosis via caspase-dependent and caspase-independent pathways in mesenchymal stem cells derived from human adipose tissue. Arch Toxicol 2011;85:1057–1065 [DOI] [PubMed] [Google Scholar]
- 28.Ruvolo PP. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res 2003;47:383–392 [DOI] [PubMed] [Google Scholar]
- 29.Schneiders UM, Schyschka L, Rudy A, Vollmar AM. BH3-only proteins Mcl-1 and Bim as well as endonuclease G are targeted in spongistatin 1–induced apoptosis in breast cancer cells. Mol Cancer Ther 2009;8:2914–2925 [DOI] [PubMed] [Google Scholar]
- 30.Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res 2007;13:4934–4942 [DOI] [PubMed] [Google Scholar]
- 31.Erzurum S, Rounds SI, Stevens T, Aldred M, Aliotta J, Archer SL, Asosingh K, Balaban R, Bauer N, Bhattacharya J, et al. Strategic plan for lung vascular research: an NHLBI-ORDR workshop report. Am J Respir Crit Care Med 2010;182:1554–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, Cool C, Wood K, Parr JE, Boackle SA, Voelkel NF. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med 2007;175:1280–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation: the cell is dead. Long live the cell! Trends Cell Biol 2008;18:467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurasz P, Courtman D, Babaie S, Stewart DJ. Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Ther 2010;126:1–8 [DOI] [PubMed] [Google Scholar]
- 36.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 2010;121:2747–2754 [DOI] [PubMed] [Google Scholar]
- 37.Grimminger F, Schermuly RT, Ghofrani HA. Targeting non-malignant disorders with tyrosine kinase inhibitors. Nat Rev Drug Discov 2010;9:956–970 [DOI] [PubMed] [Google Scholar]
- 38.Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK, Brugge JS. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol 2003;5:733–740 [DOI] [PubMed] [Google Scholar]
- 39.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez HM, Xue Y, Robinson CD, Canalizo-Hernandez MA, Marvin RG, Kelly RA, Mondragon A, Penner-Hahn JE, O'Halloran TV. Tetrathiomolybdate inhibits copper trafficking proteins through metal cluster formation. Science 2010;327:331–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itoh S, Kim HW, Nakagawa O, Ozumi K, Lessner SM, Aoki H, Akram K, McKinney RD, Ushio-Fukai M, Fukai T. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem 2008;283:9157–9167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei H, Frei B, Beckman JS, Zhang WJ. Copper chelation by tetrathiomolybdate inhibits lipopolysacharide-induced inflammatory responses in vivo. Am J Physiol Heart Circ Physiol 2011;301:H712–H720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hood JD, Frausto R, Kiosses WB, Schwartz MA, Cheresh DA. Differential alphav integrin–mediated Ras-ERK signaling during two pathways of angiogenesis. J Cell Biol 2003;162:933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sajid M, Stouffer GA. The role of alpha(v)beta3 integrins in vascular healing. Thromb Haemost 2002;87:187–193 [PubMed] [Google Scholar]
- 45.Silletti S, Kessler T, Goldberg J, Boger DL, Cheresh DA. Disruption of matrix metalloproteinase 2 binding to integrin alpha vbeta 3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc Natl Acad Sci USA 2001;98:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erdreich-Epstein A, Shimada H, Groshen S, Liu M, Metelitsa LS, Kim KS, Stins MF, Seeger RC, Durden DL. Integrins alpha(v)beta3 and alpha(v)beta5 are expressed by endothelium of high-risk neuroblastoma and their inhibition is associated with increased endogenous ceramide. Cancer Res 2000;60:712–721 [PubMed] [Google Scholar]
- 47.Eliceiri BP, Klemke R, Stromblad S, Cheresh DA. Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol 1998;140:1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowndes SA, Adams A, Timms A, Fisher N, Smythe J, Watt SM, Joel S, Donate F, Hayward C, Reich S, et al. Phase I study of copper-binding agent ATN-224 in patients with advanced solid tumors. Clin Cancer Res 2008;14:7526–7534 [DOI] [PubMed] [Google Scholar]
- 49.Gartner EM, Griffith KA, Pan Q, Brewer GJ, Henja GF, Merajver SD, Zalupski MM. A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-flurouracil, and leucovorin for metastatic colorectal cancer. Invest New Drugs 2009;27:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.