Abstract
Acute lung injury (ALI) is due to an uncontrolled systemic inflammatory response resulting from direct injury to the lung or indirect injury in the setting of a systemic process. Such insults lead to the systemic inflammatory response syndrome (SIRS), which includes activation of leukocytes—alveolar macrophages and sequestered neutrophils—in the lung. Although systemic inflammatory response syndrome is a physiologic response to an insult, systemic leukocyte activation, if excessive, can lead to end organ injury, such as ALI. Excessive recruitment of leukocytes is critical to the pathogenesis of ALI, and the magnitude and duration of the inflammatory process may ultimately determine the outcome in patients with ALI. Leukocyte recruitment is a well orchestrated process that depends on the function of chemokines and their receptors. Understanding the mechanisms that contribute to leukocyte recruitment in ALI may ultimately lead to the development of effective therapeutic strategies.
Keywords: acute lung injury, inflammation, leukocytes, experimental, clinical
Acute lung injury (ALI) or the acute respiratory distress syndrome (ARDS) is a devastating syndrome, with an incidence of approximately 200,000 in the United States and a mortality approaching 40% (1). ALI is defined by the presence of bilateral pulmonary infiltrates, severe hypoxemia, and the absence of left atrial hypertension (2). Causes of ALI may be direct (pneumonia, aspiration, inhalational injury, etc.) or indirect (sepsis, pancreatitis, blood transfusion, etc.). The pathogenesis of ALI involves inflammatory injury to the alveolo–capillary membrane, resulting in lung permeability and the accumulation of protein-rich pulmonary edema fluid in the airspaces, which, in turn, leads to the pulmonary infiltrates and hypoxemia that define clinical ALI (3). In ALI, resident lung cells are stimulated to release chemoattractants, which recruit inflammatory cells to migrate from the intravascular space across the endothelium and epithelium into the airspaces. Chemokines, which are small (8–10 kD) heparin-binding proteins, are secreted by resident lung cells in response to bacterial products and early response inflammatory mediators, and are retained by matrix heparin sulfate proteoglycans at the site of inflammation, establishing a chemokine gradient toward the inflammatory focus (4). Chemokines are classified into four subfamilies based on the positioning of specific amino acid (aa) residues in the NH2 terminus of the protein (described subsequently here). Each subfamily binds to specific receptors on inflammatory cells, resulting in the recruitment of that cell type.
In ALI, neutrophils, followed by mononuclear cells, are recruited to the lung by chemokine gradients. There is extensive experimental evidence demonstrating the critical role of these inflammatory cells and their chemokines in the pathogenesis ALI (5–9). In this review, we discuss the major classes of chemokines and their receptors, as well as the evidence from both animal and clinical studies supporting the role that these chemokines play in the pathogenesis of ALI. Pharmacologic chemokine blockade, if it could prevent massive, dysregulated inflammatory injury to the lung without compromising host defense, may be a potential therapeutic strategy for ALI (10), a syndrome for which there are few specific treatments (3).
Pathogenesis of Ali
The role of inflammation in the pathogenesis of ALI is well supported by clinical and animal studies. Bronchoalveolar lavage (BAL) (11) and lung histologic samples (12) from patients with ALI reveal the accumulation of neutrophils as well as macrophages within the airspaces, and, indeed, the degree of neutrophilic influx correlates with mortality in ALI (11). In animal models, neutrophils (13) and their products (14) cause lung injury. During ALI, resident lung cells are stimulated to release chemoattractants, which recruit neutrophils to migrate from the intravascular space across the endothelium and epithelium into the airspaces. In the presence of chemokines and other inflammatory mediators, neutrophils, which usually deform to pass through the narrow capillary lumen, become stiff, likely due to actin polymerization, and therefore become sequestered in the microvasculature (15). After sequestration, ligation of neutrophil cell surface molecules to their cognate receptors on the endothelial and epithelial cells results in tethering, rolling, firm adhesion, and crawling along the endothelium, followed by transendothelial and transepithelial migration (5, 16). During migration, large numbers of activated neutrophils release toxic mediators, including proteases, oxidants, and peptides (17, 18), in close proximity to the endothelium and epithelium. Neutrophils and their products cause endothelial and epithelial permeability, in part due to apoptotic and necrotic cell death (12, 19, 20), although, in some cases, ALI may occur in the absence of neutrophils (21), and neutrophils may migrate into the airspaces without causing permeability (22, 23). Inflammatory injury to the lung epithelium also impairs its capacity for active fluid transport out of the airspaces (24) and for surfactant production, further contributing to the severity of the pulmonary edema and decreased lung compliance that characterize ALI (25). Macrophages are also recruited to the lung by chemokine gradients in ALI, and play a role in the pathogenesis and resolution of lung injury. Undoubtedly, given the critical role of inflammatory cells and their mediators in causing the endothelial and epithelial injury that characterize ALI, the chemokines that recruit these inflammatory cells to the lung are of pivotal importance in the pathogenesis of ALI (26, 27). Chemokines, including CXCL-8, CXCL-1, CXCL-5, and CCL-2, are elevated in the BAL of patients with ALI (6, 7), and levels may correlate with outcome (28). In animal models of ALI, chemokine levels are elevated in the lungs, and neutralization of chemokines (26, 27, 29, 30) or their receptors (31) can attenuate injury. Chemokine gradients are established and regulated via complex mechanisms, including proteolytic processing (32) and scavenging (33, 34).
Resolution of ALI depends on several factors. To prevent ongoing inflammatory injury to the lungs, the recruitment of inflammatory cells to the lung must be halted, and this occurs by chemokine scavenging (33, 34), followed by apoptosis (35) and clearance (36) of inflammatory cells. Resident and recruited macrophages play an important role in clearance of both injured tissue debris and apoptotic cells, and are therefore important for the resolution of inflammation. Recently, it has been demonstrated that T lymphocytes also play a critical role in the resolution of inflammatory lung injury via transforming growth factor-β–dependent mechanisms (37). As ongoing inflammation is limited, edema fluid must be cleared (25), and the injured lung must repair. Repair of the denuded lung epithelium, which correlates with prognosis (24, 38), depends on surviving alveolar type II cells, which proliferate and differentiate into alveolar type I cells. Epithelial repair in ALI depends on several pathways, including keratinocyte growth factor, hepatocyte growth factor (39, 40), and β-catenin signaling (41). Local and bone marrow–derived stem cell populations also contribute to repair of the injured lungs (42–45), and there is evidence that bone marrow–derived stems cells are recruited to the injured lung by the release of soluble factors (46). Although the mechanisms by which bone marrow–derived stem cells are recruited to the lungs have not been completely elucidated, there is significant evidence that the injured lung expresses stromal-derived factor-1 (CXCL12), which interacts with the CXCR4 receptor on the surface of bone marrow–derived stem cells. Stem cells can also express other chemokine and cytokine receptors and migrate toward other mediators, including IL-8, TNF-α, macrophage migration inhibitor factor, transforming growth factor-β, and others (47–50). The recruitment of bone marrow–derived endothelial progenitor cells to the injured lung depends on CXCL1 and CXCL2 chemokines via CXCR2 (51). Resolution of inflammation, followed by physiological repair of the lung, is required to prevent the development of fibrotic changes, which leads to increased mortality or permanent restrictive ventilatory defects (52).
Chemokines
Leukocyte recruitment during inflammation requires intercellular communication between infiltrating leukocytes and the endothelium, resident stromal cells, and parenchymal cells. These events are mediated via the generation of early-response cytokines, the expression of cell surface adhesion molecules, and the production of chemotactic molecules, chemokines, which are a specific class of inflammatory mediators that play a key role in the pathogenesis of ALI (Figure 1). Chemokines are a large family of small proteins (8–10 kD) that are distinguished from other cytokines by being the only members of the cytokine family that act on the superfamily of G protein–coupled serpentine receptors. Chemokines can be classified as constitutive (developmentally regulated) or inducible (inflammatory), the latter of which contribute to ALI. Inducible chemokines were identified early in the chemokine biology field, probably for the obvious reason that they were produced at high levels in the tissues or tissue cultures in which they were identified. Chemokines have chemotactic and activating effects on leukocyte subsets, and provide a key stimulus for directing leukocytes to areas of injury (4, 53). Based on cysteine residue positioning, chemokines are classified into four subfamilies: CXC (α); CC (β); C (γ); and CX3C (δ). The CXC (α) subfamily has the first two NH2-terminal cysteines separated by one nonconserved aa residue, the CXC cysteine motif (4). This group could be further subdivided based on the presence or absence of a glu-leu-arg (ELR) aa motif immediately preceding the first cysteine residue.
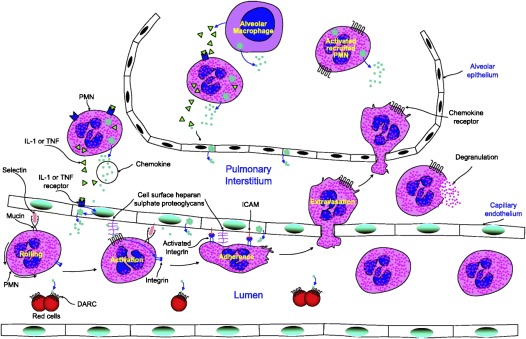
Figure 1.
Polymorphonuclear cell (PMN) chemotaxis into the lung in acute lung injury (ALI). Chemokines are secreted at the site of inflammation by resident tissue cells, leukocytes, and cytokine-activated endothelial and epithelial cells. Chemokines are locally retained by matrix heparan sulfate proteoglycans establishing a chemokine gradient surrounding the inflammatory stimulus. PMNs roll over the endothelium in a selectin-mediated process. Chemokine signaling activates leukocyte integrins, leading to firm adherence and extravasation. The PMNs then pass out of the blood vessel and move up the concentration gradient of the chemotactic peptides toward the site of inflammation. The Duffy antigen receptors for chemokines (DARC) functions as a sink, removing the chemokines from circulation and thus helping to maintain the tissue blood stream gradient. In ALI, the capillary endothelium and alveolar epithelium have separate injuries. Macrophages are recruited to the lung by chemokine gradients in ALI and play a role in the pathogenesis and resolution of lung injury. Activated alveolar macrophages release TNF-α and IL-1β and, in response to this, other cells of the alveolar environment produce CC and CXC chemokines, which, in turn, activate the inflammatory cascade, resulting in PMN migration into the lungs (reproduced by permission from Ref. 2).
Importantly, ELR+ CXC chemokines exert potent neutrophil chemotactic and activating properties both in vitro and in vivo. CXCL-1 (54, 55), CXCL-2 (56, 57), and CXCL-15 (58, 59) have been discovered as important neutrophil chemoattractants in mice during lung inflammation. Most chemokines that are associated with neutrophil trafficking in the lung are secreted in enormous quantities by myeloid cells, such as macrophages and neutrophils (54–57). However, CXCL15 is secreted exclusively by bronchial epithelial cells (58, 59), and CXCL5, which plays a role in neutrophil trafficking during LPS-induced lung inflammation (60), is primarily produced by alveolar type II epithelial cells (61).
The ELR− CXC chemokines consist of three members, CXCL-9, CXCL-10, and CXCL-11. In contrast to ELR+ CXC chemokines, ELR− CXC chemokines exert no chemotactic effects on neutrophils, but are strongly chemotactic for activated/memory T cells and natural killer cells (18–21). The ELR− chemokines are believed to act primarily on mononuclear leukocytes (4).
The CC chemokine subfamily (β-subfamily) has the first two NH2-terminal cysteines adjacent to one another with no intervening aa, the CC cysteine motif (4). Target cells for CC chemokines are believed to be eosinophils, T cells, and monocytes, although recent studies have shown that these chemokines also contribute to neutrophil infiltration (62–67). The C chemokines (γ-subfamily) lack the first and third cysteines, and have a lone NH2-terminal cysteine aa, the C cysteine motif. Two C chemokines, XCL1 and XCL2, have been described. They are predominantly expressed in the thymus, and appear to recruit immature T cells from the bone marrow (4).
The CX3C chemokines (δ-subfamily) have the first two NH2-terminal cysteines separated by three nonconserved aa residues. CX3CL-1, the only member of this subfamily, can exist either as a membrane-anchored or a shed glycoprotein. CX3CL-1 is believed to act as a potent adhesion molecule for T cells or a chemoattractant for monocytes (4), and has recently been shown to modulate the inflammatory response in sepsis (68).
Chemokine Receptors
Chemokines produce their biological effects by interacting with specific receptors on the cell surface of their target cells. Chemokine receptors have a seven-transmembrane structure and couple to G protein for signal transduction, making them members of a large protein family of G protein–coupled receptors. Chemokine receptors are divided into different families, CXC chemokine receptors, CC chemokine receptors, CX3C chemokine receptors, and XC chemokine receptors, which correspond to the four subfamilies of chemokines described previously here. There are a few receptors that bind a single ligand, and several chemokines can bind to more than one receptor, giving rise to an apparent “redundancy.” It appears that the same receptor can display differential effects depending on the ligand to which it binds (69, 70). Table 1 summarizes our current understanding of chemokines and their receptors.
TABLE 1.
CHEMOKINES AND CHEMOKINES RECEPTORS
| Systemic Name | Other Name(s) | Receptor(s) |
| CCL1 | I-309 | CCR8 |
| CCL2 | MCP-1 | CCR2 |
| CCL3 | MIP-1α | CCR1,CCR5 |
| CCL4 | MIP-1β | CCR5 |
| CCL5 | RANTES | CCR1,CCR3, CCR5 |
| CCL6 | — | — |
| CCL7 | MCP-3 | CCR-1, CCR-2, CCR-3 |
| CCL8 | MCP-2 | CCR-3 |
| CCL9 | — | — |
| CCL10 | — | — |
| CCL11 | Eotaxin | CCR-3 |
| CCL12 | — | CCR-2 |
| CCL13 | MCP-4 | CCR-2, CCR-3 |
| CCL14 | HCC-1 | CCR-1 |
| CCL15 | HCC-2 | CCR-1, CCR-3 |
| CCL16 | HCC-4 | CCR-1 |
| CCL17 | TARC | CCR-4 |
| CCL18 | DC-CK1 | — |
| CCL19 | MIP-3β | CCR-7 |
| CCL20 | MIP-3α | CCR-6 |
| CCL21 | SLC | CCR-7 |
| CCL22 | MDC | CCR-4 |
| CCL23 | MPIF-1 | CCR-1, CCR-12 |
| CCL24 | MPIF-2 | CCR-3 |
| CCL25 | TECK | CCR-9 |
| CCL26 | Eotaxin-3 | CCR-3 |
| CCL27 | CTACK | CCR-10 |
| CCL28 | MEC | CCR-10 |
| XCL1 | Lymphotactin | XCR1 |
| XCL2 | SCM1-α | XCR1 |
| CXCL1 | GROα, CINC, KC | CXCR2 |
| CXCL2 | GROβ, MIP-2 | CXCR2 |
| CXCL3 | GROγ | CXCR2 |
| CXCL4 | PF4 | — |
| CXCL5 | ENA-78 | CXCR2 |
| CXCL6 | GCP-2 | CXCR1, CXCR2 |
| CXCL7 | NAP-2 | CXCR2 |
| CXCL8 | IL-8 | CXCR1, CXCR2 |
| CXCL9 | MIG | CXCR3 |
| CXCL10 | IP-10 | CXCR3 |
| CXCL11 | I-TAC | CXCR3, CXCR7 |
| CXCL12 | SDF-1α, SDF-1β | CXCR4, CXCR7 |
| CXCL13 | BCA-1 | CXCR5 |
| CXCL14 | BRAK | — |
| CXCL15 | Lungkine | — |
| CXCL16 | — | CXCR6 |
| CXCL17 | VCC-1 | — |
| CX3CL1 | Fractalkine | CX3CR1 |
Definition of abbreviations: BCA-1, B cell–attracting chemokine-1; BRAK, breast and kidney-expressed chemokine; CINC, cytokine-induced neutrophil chemoattractant; CTACK, cutaneous T cell–attracting chemokine; DC-CK1, dendritic cell chemokine 1; ENA, epithelial-derived neutrophil-activating peptide; GCP, granulocyte chemotactic protein; GRO, growth-related oncogene; HCC, human CC chemokine; IP, IFN-γ–induced protein; I-TAC, interferon-inducible T cell alpha chemoattractant; KC, keratinocyte chemoattractant; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; MEC, mucosa-associated epithelial chemokine; MIG, monokine induced by IFN-γ; MIP, macrophage inflammatory protein; MPIF, myeloid progenitor inhibitory factors; NAP, neutrophil-activating peptide; PF4, platelet factor-4; RANTES, regulated upon activation, normal T cell expressed and secreted; SCM, single C motif; SDF, stromal-derived factor; SLC, secondary lymphoid-tissue chemokine; TARC, T cell–directed CC chemokine; TECK, thymus expressed chemokine; VCC, vascular endothelial growth factor–coregulated chemokine.
In addition to these chemokine receptor subtypes, several virus-encoded proteins have been identified that have sequence homology and share the serpentine structure of the cloned chemokine receptors, and have therefore been termed “virocepters.” For example, Duffy antigen receptor for chemokines (DARC), a protein first identified in human erythrocytes as a CXCL-8–binding protein, has been shown to bind both CC and CXC chemokines with high affinity. DARC is identical to the Duffy blood group antigen, a receptor for the malarial parasite Plasmodium vivax. This receptor has no signal-transducing activity, and may act as a sink, mopping up excess free chemokines and thereby preventing inappropriate activation of circulating leukocytes. DARC selectively binds certain proinflammatory chemokines and down-regulates their activities upon binding (71). Other receptors with a similar action are D6 (72) and CCX/CKR (73). Finally, there is also evidence that CXCR7 can function as a sink for CXCL12 (74).
Chemokines in Ali—Experimental
To investigate the role of chemokines in animal models of ALI, experimental tools include receptor-neutralizing antibodies, modified chemokines that act as receptor antagonists, and small molecule receptor antagonists. In addition, approaches that inhibit chemokine synthesis and mice deficient for selected chemokines or their receptors can also be used.
Studies employing these approaches have contributed substantially to our understanding of chemokine function in ALI. Plasma levels of both CCL-2 and CXCL-1 are increased in ALI associated with acute pancreatitis (75). Treatment with an anti–CXCL-1–neutralizing antibody reduces the inflammatory response in this model of ALI (29). In addition, treatment with antileukinate, an antagonist of the CXCR2 receptor (that binds CXCL-1), protects mice against ALI associated with acute pancreatitis (76). In septic peritonitis, the absence of CXCR2 has been reported to protect mice from septic injury, potentially by delaying inflammatory cell recruitment and enhancing CXCL10 expression in the peritoneum (77). Regarding chemokine ligands, CXCL1, CXCL2, and CXCL5/LIX are important CXC chemokines in ALI (34, 60, 61, 78). CXCL-2 has been shown to play an important role in murine models of Klebsiella pneumonia and septic peritonitis (79, 80). The absence of CXCL-2 was deleterious to the clearance of infection due to decreased neutrophil responses. In addition, CCL-3 mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia (81). Lung inflammation in hyperoxia has been shown to be prevented by antichemokine treatment in newborn rats (82). Moreover, ELR− CXC chemokines have been shown to contribute to inflammation in hemorrhage-associated ALI (83–85). Deletion of CCR1 is associated with protection from pulmonary inflammation secondary to acute pancreatitis in the mouse (62). Treatment with Met-RANTES (methionine–regulated upon activation, normal T cell expressed and secreted), a CCR1 antagonist, protects mice against acute pancreatitis–associated lung injury (64). Furthermore, treatment with BX471, a small-molecule CCR1 antagonist, protects mice against lung injury associated with acute pancreatitis and sepsis (65, 66). Studies with transgenic mice that overexpress CCL-2 in type II alveolar epithelial cells have shown that CCL-2 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice, but requires an additional stimulus for inflammatory activation (86). CCL-2 acts as an efficient neutrophil chemoattractant in mice in the context of acute and chronic inflammation (87, 88). Intratracheal instillation of CCL-2 in mice has been shown to cause increased alveolar monocyte accumulation in the absence of lung inflammation, whereas combined CCL-2/LPS challenge provoked acute lung inflammation with early alveolar neutrophil and delayed alveolar monocyte influx (89). In one article, however, CCL-2 was reported to protect mice in lethal endotoxemia (90). Recently, CCL-2 has been shown to regulate pulmonary host defense via neutrophil recruitment during Escherichia coli infection (88). Treatment with bindarit, an inhibitor of CCL-2 synthesis, both prophylactically and therapeutically significantly reduced CCL-2 levels in the liver and lungs in cecal ligation, puncture-induced sepsis, and LPS-induced endotoxemia. In addition, prophylactic and therapeutic treatment with bindarit significantly protected mice against sepsis and endotoxemia, as evidenced by the attenuation in lung and liver myeloperoxidase activity, an indicator of neutrophil recruitment. The protective effect of bindarit was further confirmed by histological examination of lung and liver sections (91). In addition, we have recently shown that administration of CX3CL-1 modulates inflammatory ALI in a murine model of sepsis (68). In recent years, hydrogen sulfide and substance P have been identified as mediators of inflammation in ALI associated with acute pancreatitis, sepsis, and severe burns. Both hydrogen sulfide and substance P contribute to inflammation in these conditions via activation of chemokines (92–103).
In summation, over 30 different chemokines and over 20 different receptors, with overlapping functions, have been identified, and it is likely that additional chemokines and chemokine receptors exist that are as yet undiscovered. Despite the complexity and apparent redundancy of this system, we believe that specific chemokine receptor antagonists that interfere with leukocyte migration and activation could be therapeutically useful in ALI.
Chemokines in Ali—Clinical
Evidence from clinical studies confirms the key role for chemokines in the pathogenesis of ALI. An early study showed significantly higher CXCL-8 levels in patients with ARDS plus pneumonia than in those with ARDS or pneumonia alone (104). In subsequent studies, plasma and BAL levels of chemokines, such as CXCL-8, CXCL-1, CXCL-5, and CCL-2, have all been found to be elevated in patients at risk for, and with established, ARDS (6, 105). Early enhanced neutrophil migratory activity due to elevated pulmonary concentrations of CXCL-8 may be critical to the neutrophil infiltration that is characteristic of ARDS in patients who later develop ARDS (106). Circulating levels of CXCL-8, CXCL-1, and CXCL-5 are elevated in severe acute pancreatitis, and are predictors of disease severity (107). In a recent study, a panel of seven plasma biomarkers (CXCL-8, receptor for advanced glycation end products, procollagen peptide III, brain natriuretic peptide, angiopoietin-2, IL-10, and TNF-α) could accurately differentiate patients with trauma-induced ALI from patients who have undergone trauma without ALI (108). In another recent multicenter study, it has been shown that, when combined with clinical data, plasma biomarkers (CXCL-8, intercellular adhesion molecule-1, von Willebrand factor, soluble TNF receptor-1, and surfactant protein-D) measured at the onset of ALI can improve the accuracy of risk prediction. A reduced set of three biomarkers (CXCL-8, soluble TNF receptor-1, and surfactant protein-D) had nearly equivalent prognostic value. In summation, biomarkers including chemokine levels may be useful for identifying a population at high risk of ALI (109). Moreover, the results of these studies and preclinical research with experimental animal models of ALI suggest that chemokines can act as therapeutic targets for ALI of different etiologies.
Conclusions
ALI is a devastating clinical syndrome. Although there are no specific therapies for ALI, there has been progress in the management of patients with ALI using lung-protective ventilation methods and careful fluid management. Chemokines are critical for neutrophil and macrophage recruitment to the lungs in ALI. An ideal therapeutic strategy for ALI would attenuate the extensive tissue-destructive potential of recruited leukocytes without impairing host defense mechanisms. Based on the findings from experimental and clinical studies of ALI, our understanding of the molecular and cellular mechanisms that regulate the pathogenesis of ALI has improved substantially over recent years. Development of unique tools, such as gene-deficient mice and blocking antibodies for chemokines and/or their receptors, along with biomarker discoveries in ALI, have contributed to these new insights. The future challenge will be to apply our current understanding of chemokine function to develop therapeutic methods to modulate the destructive potential of inflammatory lung injury. Specific pharmacological inhibitors of chemokines and/or their receptors may be useful therapies for ALI, although the timing and specificity of such therapies must be exquisite so as not to limit the critical host defense functions of inflammatory cells (110).
Supplementary Material
Footnotes
This work was supported by in part by a University of Otago Establishment grant (M.B.), National Institutes of Health (NIH) grant K08-HL103772, a Flight Attendant Medical Research Institute (FAMRI) Young Clinical Scientist Award, a Parker B. Francis Fellowship (R.L.Z.), and by NIH grant R01-HL091958 (S.J.) and FAMRI grant YCSA_062466 (S.J.).
Originally Published in Press as DOI: 10.1165/rcmb.2011-0392TR on February 9, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693 [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American–European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824 [DOI] [PubMed] [Google Scholar]
- 3.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 2011;6:147–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 2005;288:L3–L15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol 2009;40:519–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1996;154:602–611 [DOI] [PubMed] [Google Scholar]
- 7.Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, Grant IS, Pollok AJ, Haslett C. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet 1993;341:643–647 [DOI] [PubMed] [Google Scholar]
- 8.Abraham E. Neutrophils and acute lung injury. Crit Care Med 2003;31(4, Suppl):S195–S199 [DOI] [PubMed] [Google Scholar]
- 9.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med 2008;358:716–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manicone AM. Role of the pulmonary epithelium and inflammatory signals in acute lung injury. Expert Rev Clin Immunol 2009;5:63–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;150:113–122 [DOI] [PubMed] [Google Scholar]
- 12.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 1982;3:35–56 [PubMed] [Google Scholar]
- 13.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest 1999;103:1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakamaki F, Ishizaka A, Urano T, Sayama K, Nakamura H, Terashima T, Waki Y, Tasaka S, Hasegawa N, Sato K, et al. Effect of a specific neutrophil elastase inhibitor, ONO-5046, on endotoxin-induced acute lung injury. Am J Respir Crit Care Med 1996;153:391–397 [DOI] [PubMed] [Google Scholar]
- 15.Worthen GS, Schwab B, III, Elson EL, Downey GP. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science 1989;245:183–186 [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Doerschuk CM. The signaling pathways induced by neutrophil–endothelial cell adhesion. Antioxid Redox Signal 2002;4:39–47 [DOI] [PubMed] [Google Scholar]
- 17.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med 2011;17:293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moraes TJ, Plumb J, Martin R, Vachon E, Cherepanov V, Koh A, Loeve C, Jongstra-Bilen J, Zurawska JH, Kus JV, et al. Abnormalities in the pulmonary innate immune system in cystic fibrosis. Am J Respir Cell Mol Biol 2006;34:364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J Immunol 1999;163:2217–2225 [PubMed] [Google Scholar]
- 20.Smedly LA, Tonnesen MG, Sandhaus RA, Haslett C, Guthrie LA, Johnston RB, Jr, Henson PM, Worthen GS. Neutrophil-mediated injury to endothelial cells: enhancement by endotoxin and essential role of neutrophil elastase. J Clin Invest 1986;77:1233–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laufe MD, Simon RH, Flint A, Keller JB. Adult respiratory distress syndrome in neutropenic patients. Am J Med 1986;80:1022–1026 [DOI] [PubMed] [Google Scholar]
- 22.Martin TR. Neutrophils and lung injury: getting it right. J Clin Invest 2002;110:1603–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin TR, Pistorese BP, Chi EY, Goodman RB, Matthay MA. Effects of leukotriene B4 in the human lung: recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest 1989;84:1609–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1376–1383 [DOI] [PubMed] [Google Scholar]
- 25.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 1990;142:1250–1257 [DOI] [PubMed] [Google Scholar]
- 26.Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. Chemokine expression during hepatic ischemia/reperfusion–induced lung injury in the rat: the role of epithelial neutrophil activating protein. J Clin Invest 1995;95:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folkesson HG, Matthay MA, Hebert CA, Broaddus VC. Acid aspiration–induced lung injury in rabbits is mediated by interleukin-8–dependent mechanisms. J Clin Invest 1995;96:107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller EJ, Cohen AB, Nagao S, Griffith D, Maunder RJ, Martin TR, Weiner-Kronish JP, Sticherling M, Christophers E, Matthay MA. Elevated levels of NAP-1/interleukin-8 are present in the airspaces of patients with the adult respiratory distress syndrome and are associated with increased mortality. Am Rev Respir Dis 1992;146:427–432 [DOI] [PubMed] [Google Scholar]
- 29.Bhatia M, Brady M, Zagorski J, Christmas SE, Campbell F, Neoptolemos JP, Slavin J. Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut 2000;47:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest 2002;110:1703–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sue RD, Belperio JA, Burdick MD, Murray LA, Xue YY, Dy MC, Kwon JJ, Keane MP, Strieter RM. CXCR2 is critical to hyperoxia-induced lung injury. J Immunol 2004;172:3860–3868 [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002;111:635–646 [DOI] [PubMed] [Google Scholar]
- 33.Lee JS, Frevert CW, Wurfel MM, Peiper SC, Wong VA, Ballman KK, Ruzinski JT, Rhim JS, Martin TR, Goodman RB. Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo. J Immunol 2003;170:5244–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mei J, Liu Y, Dai N, Favara M, Greene T, Jeyaseelan S, Poncz M, Lee JS, Worthen GS. CXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity 2010;33:106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matute-Bello G, Liles WC, Radella F, II, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med 1997;156:1969–1977 [DOI] [PubMed] [Google Scholar]
- 36.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation: programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest 1989;83:865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, et al. CD4+CD25+FOXP3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 2009;119:2898–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ware LB, Matthay MA. Maximal alveolar epithelial fluid clearance in clinical acute lung injury: an excellent predictor of survival and the duration of mechanical ventilation [abstract]. Am J Respir Crit Care Med 1999;159:A694 [Google Scholar]
- 39.Panos RJ, Rubin JS, Csaky KG, Aaronson SA, Mason RJ. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest 1993;92:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verghese GM, McCormick-Shannon K, Mason RJ, Matthay MA. Hepatocyte growth factor and keratinocyte growth factor in the pulmonary edema fluid of patients with acute lung injury: biologic and clinical significance. Am J Respir Crit Care Med 1998;158:386–394 [DOI] [PubMed] [Google Scholar]
- 41.Zemans RL, Briones N, Campbell M, McClendon J, Young SK, Suzuki T, Yang IV, De Langhe S, Reynolds SD, Mason RJ, et al. Neutrophil transmigration triggers repair of the lung epithelium via β-catenin signaling. Proc Natl Acad Sci U S A 2011;108:15990–15995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823–835 [DOI] [PubMed] [Google Scholar]
- 43.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med 2005;172:854–860 [DOI] [PubMed] [Google Scholar]
- 44.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin–induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 2009;106:16357–16362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 2011;147:525–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow–derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005;33:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hannoush EJ, Sifri ZC, Elhassan IO, Mohr AM, Alzate WD, Offin M, Livingston DH. Impact of enhanced mobilization of bone marrow derived cells to site of injury. J Trauma 2011;71:283–289; discussion 289–291 [DOI] [PubMed] [Google Scholar]
- 48.Weiss DJ, Bertoncello I, Borok Z, Kim C, Panoskaltsis-Mortari A, Reynolds S, Rojas M, Stripp B, Warburton D, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc 2011;8:223–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, Prockop DJ. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation Workshop. Proc Am Thorac Soc 2006;3:193–207 [DOI] [PubMed] [Google Scholar]
- 50.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol 2002;30:973–981 [DOI] [PubMed] [Google Scholar]
- 51.Jones CP, Pitchford SC, Lloyd CM, Rankin SM. CXCR2 mediates the recruitment of endothelial progenitor cells during allergic airways remodeling. Stem Cells 2009;27:3074–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zapol WM, Trelstad RL, Coffey JW, Tsai I, Salvador RA. Pulmonary fibrosis in severe acute respiratory failure. Am Rev Respir Dis 1979;119:547–554 [DOI] [PubMed] [Google Scholar]
- 53.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol 2004;202:145–156 [DOI] [PubMed] [Google Scholar]
- 54.Bozic CR, Kolakowski LF, Jr, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol 1995;154:6048–6057 [PubMed] [Google Scholar]
- 55.Frevert CW, Huang S, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol 1995;154:335–344 [PubMed] [Google Scholar]
- 56.Driscoll KE, Hassenbein DG, Howard BW, Isfort RJ, Cody D, Tindal MH, Suchanek M, Carter JM. Cloning, expression, and functional characterization of rat MIP-2: a neutrophil chemoattractant and epithelial cell mitogen. J Leukoc Biol 1995;58:359–364 [DOI] [PubMed] [Google Scholar]
- 57.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA 1989;86:612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen SC, Mehrad B, Deng JC, Vassileva G, Manfra DJ, Cook DN, Wiekowski MT, Zlotnik A, Standiford TJ, Lira SA. Impaired pulmonary host defense in mice lacking expression of the CXC chemokine lungkine. J Immunol 2001;166:3362–3368 [DOI] [PubMed] [Google Scholar]
- 59.Rossi DL, Hurst SD, Xu Y, Wang W, Menon S, Coffman RL, Zlotnik A. Lungkine, a novel CXC chemokine, specifically expressed by lung bronchoepithelial cells. J Immunol 1999;162:5490–5497 [PubMed] [Google Scholar]
- 60.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun 2004;72:7247–7256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol 2005;32:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gerard C, Frossard JL, Bhatia M, Saluja A, Gerard NP, Lu B, Steer M. Targeted disruption of the beta-chemokine receptor CCR1 protects against pancreatitis-associated lung injury. J Clin Invest 1997;100:2022–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhatia M, Ramnath RD, Chevali L, Guglielmotti A. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 2005;288:G1259–G1265 [DOI] [PubMed] [Google Scholar]
- 64.Bhatia M, Proudfoot AE, Wells TN, Christmas S, Neoptolemos JP, Slavin J. Treatment with Met-RANTES reduces lung injury in caerulein-induced pancreatitis. Br J Surg 2003;90:698–704 [DOI] [PubMed] [Google Scholar]
- 65.He M, Horuk R, Bhatia M. Treatment with BX471, a nonpeptide CCR1 antagonist, protects mice against acute pancreatitis–associated lung injury by modulating neutrophil recruitment. Pancreas 2007;34:233–241 [DOI] [PubMed] [Google Scholar]
- 66.He M, Horuk R, Moochhala SM, Bhatia M. Treatment with BX471, a CC chemokine receptor 1 antagonist, attenuates systemic inflammatory response during sepsis. Am J Physiol Gastrointest Liver Physiol 2007;292:G1173–G1180 [DOI] [PubMed] [Google Scholar]
- 67.Pan ZZ, Parkyn L, Ray A, Ray P. Inducible lung-specific expression of RANTES: preferential recruitment of neutrophils. Am J Physiol Lung Cell Mol Physiol 2000;279:L658–L666 [DOI] [PubMed] [Google Scholar]
- 68.He M, Moochhala SM, Adhikari S, Bhatia M. Administration of exogenous fractalkine, a CX3C chemokine, is capable of modulating inflammatory response in cecal ligation and puncture-induced sepsis. Shock 2009;31:33–39 [DOI] [PubMed] [Google Scholar]
- 69.Schall TJ, Proudfoot AE. Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat Rev Immunol 2011;11:355–363 [DOI] [PubMed] [Google Scholar]
- 70.Horuk R, Proudfoot AE. Drug discovery targeting the chemokine system—where are we? Front Biosci (Elite Ed) 2009;1:209–219 [DOI] [PubMed] [Google Scholar]
- 71.Kashiwazaki M, Tanaka T, Kanda H, Ebisuno Y, Izawa D, Fukuma N, Akimitsu N, Sekimizu K, Monden M, Miyasaka M. A high endothelial venule-expressing promiscuous chemokine receptor DARC can bind inflammatory, but not lymphoid, chemokines and is dispensable for lymphocyte homing under physiological conditions. Int Immunol 2003;15:1219–1227 [DOI] [PubMed] [Google Scholar]
- 72.Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, Alcami A, Lira SA, Wiekowski M, Graham GJ. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol 2005;6:403–411 [DOI] [PubMed] [Google Scholar]
- 73.Comerford I, Milasta S, Morrow V, Milligan G, Nibbs R. The chemokine receptor CCX–CKR mediates effective scavenging of CCL19 in vitro. Eur J Immunol 2006;36:1904–1916 [DOI] [PubMed] [Google Scholar]
- 74.Wang H, Beaty N, Chen S, Qi CF, Masiuk M, Shin DM, Morse HC., III The CXCR7 chemokine receptor promotes B-cell retention in the splenic marginal zone and serves as a sink for CXCL12. Blood 2012;119:465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brady M, Bhatia M, Christmas S, Boyd MT, Neoptolemos JP, Slavin J. Expression of the chemokines MCP-1/JE and cytokine-induced neutrophil chemoattractant in early acute pancreatitis. Pancreas 2002;25:260–269 [DOI] [PubMed] [Google Scholar]
- 76.Bhatia M, Hegde A. Treatment with antileukinate, a CXCR2 chemokine receptor antagonist, protects mice against acute pancreatitis and associated lung injury. Regul Pept 2007;138:40–48 [DOI] [PubMed] [Google Scholar]
- 77.Ness TL, Hogaboam CM, Strieter RM, Kunkel SL. Immunomodulatory role of CXCR2 during experimental septic peritonitis. J Immunol 2003;171:3775–3784 [DOI] [PubMed] [Google Scholar]
- 78.Cai S, Batra S, Lira SA, Kolls JK, Jeyaseelan S. CXCL1 regulates pulmonary host defense to Klebsiella infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. J Immunol 2010;185:6214–6225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Laichalk LL, McGillicuddy DC, Standiford TJ. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine klebsiella pneumonia. J Infect Dis 1996;173:159–165 [DOI] [PubMed] [Google Scholar]
- 80.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect Immun 1997;65:3847–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Standiford TJ, Kunkel SL, Lukacs NW, Greenberger MJ, Danforth JM, Kunkel RG, Strieter RM. Macrophage inflammatory protein-1 alpha mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol 1995;155:1515–1524 [PubMed] [Google Scholar]
- 82.Deng H, Mason SN, Auten RL., Jr Lung inflammation in hyperoxia can be prevented by antichemokine treatment in newborn rats. Am J Respir Crit Care Med 2000;162:2316–2323 [DOI] [PubMed] [Google Scholar]
- 83.Lomas JL, Chung CS, Grutkoski PS, LeBlanc BW, Lavigne L, Reichner J, Gregory SH, Doughty LA, Cioffi WG, Ayala A. Differential effects of macrophage inflammatory chemokine-2 and keratinocyte-derived chemokine on hemorrhage-induced neutrophil priming for lung inflammation: assessment by adoptive cells transfer in mice. Shock 2003;19:358–365 [DOI] [PubMed] [Google Scholar]
- 84.Lomas-Neira JL, Chung CS, Grutkoski PS, Miller EJ, Ayala A. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol 2004;76:58–64 [DOI] [PubMed] [Google Scholar]
- 85.Lomas-Neira JL, Chung CS, Wesche DE, Perl M, Ayala A. In vivo gene silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage-induced, neutrophil-mediated septic acute lung injury. J Leukoc Biol 2005;77:846–853 [DOI] [PubMed] [Google Scholar]
- 86.Gunn MD, Nelken NA, Liao X, Williams LT. Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J Immunol 1997;158:376–383 [PubMed] [Google Scholar]
- 87.Johnston B, Burns AR, Suematsu M, Issekutz TB, Woodman RC, Kubes P. Chronic inflammation upregulates chemokine receptors and induces neutrophil migration to monocyte chemoattractant protein-1. J Clin Invest 1999;103:1269–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during Escherichia coli infection. Infect Immun 2011;79:2567–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tzeng HP, Ho FM, Chao KF, Kuo ML, Lin-Shiau SY, Liu SH. β-lapachone reduces endotoxin-induced macrophage activation and lung edema and mortality. Am J Respir Crit Care Med 2003;168:85–91 [DOI] [PubMed] [Google Scholar]
- 90.Zisman DA, Kunkel SL, Strieter RM, Tsai WC, Bucknell K, Wilkowski J, Standiford TJ. MCP-1 protects mice in lethal endotoxemia. J Clin Invest 1997;99:2832–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramnath RD, Ng SW, Guglielmotti A, Bhatia M. Role of MCP-1 in endotoxemia and sepsis. Int Immunopharmacol 2008;8:810–818 [DOI] [PubMed] [Google Scholar]
- 92.Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C, Steer ML. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci U S A 1998;95:4760–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J 2005;19:623–625 [DOI] [PubMed] [Google Scholar]
- 94.Puneet P, Hegde A, Ng SW, Lau HY, Lu J, Moochhala SM, Bhatia M. Preprotachykinin-A gene products are key mediators of lung injury in polymicrobial sepsis. J Immunol 2006;176:3813–3820 [DOI] [PubMed] [Google Scholar]
- 95.Zhang H, Zhi L, Moore PK, Bhatia M. Role of hydrogen sulfide in cecal ligation and puncture-induced sepsis in the mouse. Am J Physiol Lung Cell Mol Physiol 2006;290:L1193–L1201 [DOI] [PubMed] [Google Scholar]
- 96.Sun J, Bhatia M. Blockade of neurokinin-1 receptor attenuates CC and CXC chemokine production in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol 2007;292:G143–G153 [DOI] [PubMed] [Google Scholar]
- 97.Zhang H, Zhi L, Moochhala S, Moore PK, Bhatia M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am J Physiol Lung Cell Mol Physiol 2007;292:L960–L971 [DOI] [PubMed] [Google Scholar]
- 98.Sio SW, Puthia MK, Lu J, Moochhala S, Bhatia M. The neuropeptide substance P is a critical mediator of burn-induced acute lung injury. J Immunol 2008;180:8333–8341 [DOI] [PubMed] [Google Scholar]
- 99.Sio SW, Ang SF, Lu J, Moochhala S, Bhatia M. Substance P upregulates cyclooxygenase-2 and prostaglandin E metabolite by activating ERK1/2 and NF-kappaB in a mouse model of burn-induced remote acute lung injury. J Immunol 2010;185:6265–6276 [DOI] [PubMed] [Google Scholar]
- 100.Zhang J, Sio SW, Moochhala S, Bhatia M. Role of hydrogen sulfide in severe burn injury-induced inflammation in mice. Mol Med 2010;16:417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ang SF, Moochhala SM, Macary PA, Bhatia M. Hydrogen sulfide and neurogenic inflammation in polymicrobial sepsis: involvement of substance P and ERK–NF-kappaB signaling. PLoS ONE 2011;6:e24535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ang AL, Linn YC. Treatment of severe neutropenic sepsis with granulocyte transfusion in the current era—experience from an adult haematology unit in Singapore. Transfus Med 2011;21:13–24 [DOI] [PubMed] [Google Scholar]
- 103.Ang SF, Sio SW, Moochhala SM, Macary PA, Bhatia M. Hydrogen sulfide upregulates cyclooxygenase-2 and prostaglandin E metabolite in sepsis-evoked acute lung injury via transient receptor potential vanilloid type 1 channel activation. J Immunol 2011;187:4778–4787 [DOI] [PubMed] [Google Scholar]
- 104.Chollet-Martin S, Montravers P, Gibert C, Elbim C, Desmonts JM, Fagon JY, Gougerot-Pocidalo MA. High levels of interleukin-8 in the blood and alveolar spaces of patients with pneumonia and adult respiratory distress syndrome. Infect Immun 1993;61:4553–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Villard J, Dayer-Pastore F, Hamacher J, Aubert JD, Schlegel-Haueter S, Nicod LP. GRO alpha and interleukin-8 in pneumocystis carinii or bacterial pneumonia and adult respiratory distress syndrome. Am J Respir Crit Care Med 1995;152:1549–1554 [DOI] [PubMed] [Google Scholar]
- 106.Pallister I, Dent C, Topley N. Increased neutrophil migratory activity after major trauma: a factor in the etiology of acute respiratory distress syndrome? Crit Care Med 2002;30:1717–1721 [DOI] [PubMed] [Google Scholar]
- 107.Shokuhi S, Bhatia M, Christmas S, Sutton R, Neoptolemos JP, Slavin J. Levels of the chemokines growth-related oncogene alpha and epithelial neutrophil-activating protein 78 are raised in patients with severe acute pancreatitis. Br J Surg 2002;89:566–572 [DOI] [PubMed] [Google Scholar]
- 108.Fremont RD, Koyama T, Calfee CS, Wu W, Dossett LA, Bossert FR, Mitchell D, Wickersham N, Bernard GR, Matthay MA, et al. Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma 2010;68:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Calfee CS, Ware LB, Glidden DV, Eisner MD, Parsons PE, Thompson BT, Matthay MA. Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury. Crit Care Med 2011;39:711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol 1994;56:672–686 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.