Abstract
Asthma affects an estimated 300 million people worldwide and accounts for 1 of 250 deaths and 15 million disability-adjusted life years lost annually. Plastic-adherent bone marrow–derived cell (BMC) administration holds therapeutic promise in regenerative medicine. However, given the low cell engraftment in target organs, including the lung, cell replacement cannot solely account for the reported therapeutic benefits. This suggests that BMCs may act by secreting soluble factors. BMCs also possess antiinflammatory and immunomodulatory properties and may therefore be beneficial for asthma. Our objective was to investigate the therapeutic potential of BMC-secreted factors in murine asthma. In a model of acute and chronic asthma, intranasal instillation of BMC conditioned medium (CdM) prevented airway hyperresponsiveness (AHR) and inflammation. In the chronic asthma model, CdM prevented airway smooth muscle thickening and peribronchial inflammation while restoring blunted salbutamol-induced bronchodilation. CdM reduced lung levels of the TH2 inflammatory cytokines IL-4 and IL-13 and increased levels of IL-10. CdM up-regulated an IL-10–induced and IL-10–secreting subset of T regulatory lymphocytes and promoted IL-10 expression by lung macrophages. Adiponectin (APN), an antiinflammatory adipokine found in CdM, prevented AHR, airway smooth muscle thickening, and peribronchial inflammation, whereas the effect of CdM in which APN was neutralized or from APN knock-out mice was attenuated compared with wild-type CdM. Our study provides evidence that BMC-derived soluble factors prevent murine asthma and suggests APN as one of the protective factors. Further identification of BMC-derived factors may hold promise for novel approaches in the treatment of asthma.
Keywords: cellular therapy, bone marrow stromal cells, hypersensitivity, paracrine
Allergic disorders, such as anaphylaxis, hay fever, eczema, and asthma, afflict roughly 20 to 30% of people in the developed world (1). Asthma is characterized by airway hyperresponsiveness (AHR), inflammation, and remodeling with thickening of the airway smooth muscle (ASM) cell layer refractory to bronchodilator therapy (2, 3). Despite the progress achieved in the past decades in the management of asthma, refractory asthma accounts for more than 250,000 deaths worldwide every year (4).
Recent studies have explored the therapeutic potential of stem cells for regenerative medicine in multiple clinical disorders, including myocardial infarction, diabetes, sepsis, and hepatic and acute renal failure (5). Bone marrow (BM)-derived cells (BMCs) also prevent various experimental lung diseases, including pulmonary fibrosis (6), acute LPS-induced lung injury (7, 8), and chronic oxygen-induced neonatal lung disease (9, 10). Over the past decade, numerous reviews have summarized the increasing amount of evidence in support of the therapeutic benefits exerted by BMCs (11–16). However, cell engraftment is consistently disproportionately low for cell replacement to account for the therapeutic benefit. An alternate hypothesis proposed for mesenchymal stem cells (MSCs) is the release of soluble factors with exertion of their therapeutic benefit through a paracrine-mediated mechanism (5).
BMCs display immunosuppressive and antiinflammatory properties (16–21), suggesting their potential benefit in allergic and inflammatory disease such as asthma. Thus, we hypothesized that airway delivery of plastic adherent BMC-conditioned medium (CdM) prevents AHR, inflammation, and remodeling in experimental murine asthma.
Materials and Methods
Expanded methods are available in the online supplement.
Cell Culture and CdM Preparation
BMCs from adult male wild-type (WT) BALB/c (Charles River Laboratories, Wilmington, MA) and adiponectin (APN) knock-out (KO) mice (Dr. Kenneth Walsh, Boston University) were isolated based on plastic adherence and differentiated along mesenchymal lineages (Figure E1A–EF) (10, 22). CD45− and CD45+ BMCs were purified using streptavidin-coated magnetic beads (Dynabeads, Invitrogen, Burlington, ON, Canada) and biotinylated CD45 antibody (BD Biosciences, Missisauga, ON, Canada). Fibroblasts were isolated from adult mouse lungs (23). Approximately 80% confluent cells (passage 2) were serum deprived for 24 hours; the supernatant was concentrated (25×) using centrifugal filters (Millipore, Billerica, MA). Individual experiments were run using different CdM batches, and similar results were observed with different batches.
Fluorescence-Activated Cell Sorting
BMCs were characterized (8, 22, 24) using Sca-1, CD11b, CD29, CD31, CD34, CD44, CD45, CD73, and CD105 antibodies (Biolegend, San Diego, CA) (see Figures E2 and E3 in the online supplement).
Lung lymphocytes were isolated by collagenase III digestion (Worthington Biochemical Corp., Lakewood, NJ) (25) and stained using IL-10 antibody and a commercially available kit (Mouse Treg Flow Kit; Biolegend) or CD11b antibody (BD Biosciences) and analyzed using BD FACScan or FACSCanto flow cytometers (BD Biosciences).
Bronchoalveolar Lavage Fluid Analysis
Lungs of nonventilated animals were instilled with five successive aliquots of 1 ml sterile, ice-cold PBS (recovery consistently > 80%). Cells were counted, cytospun (Thermo Shandon, Pittsburgh, PA) onto slides, and differentially stained (Hema 3; Thermo Fisher Scientific, Nepean, ON, Canada) (26).
Animal Model
Adult male BALB/c mice were housed in specific pathogen-free conditions. All procedures were approved by the University of Alberta Animal Policy and Welfare Committee.
In the acute asthma model (26), mice received two intraperitoneal sensitizations and two intranasal ovalbumin (OVA) challenges. In the chronic model (adapted from Reference 27), mice were sensitized four times and challenged six times (Figure 1). Control animals received saline. Concentrated, desalted CdM (25 μl) or recombinant APN (rAPN) (5 μg/g body weight) (R&D Systems, Minneapolis, MN) was administered intranasally after each OVA challenge.
Figure 1.
Experimental protocols for the acute (A) and chronic (B) ovalbumin (OVA)-induced allergic asthma models. i.p. = intraperitioneal.
Lung Function Testing
Lung function was assessed using a small animal ventilator (flexiVent; Scireq, Montreal, PQ, Canada) (28). Saline (to establish baseline responses), methacholine (29) (2–32 mg/ml), and salbutamol (0.5 mg/ml) solutions were aerosolized intratracheally, and functional parameters were recorded for 3 minutes after each dose.
Cytokine Quantification
Cytokine concentrations in nonlavaged, nonventilated lungs and CdM were determined using commercially available ELISA kits for detection of IL-4, IL-10, IL-13 (eBioscience, San Diego, CA), and APN (Millipore).
Lung Histology
Nonlavaged lungs were fixed as previously described (10). Quantitative assessments of ASM thickness and peribronchial inflammation were performed on hematoxylin and eosin–stained lung sections using a computer-controlled light microscope (Leica Microsystems Canada, Richmond Hill, ON, Canada) and the Openlab imaging software (Improvision, Coventry, UK) (30).
Statistics
Statistical analysis was performed using StatView 5.0 software (SAS Institute, Cary, NC). Means were compared using one-way or repeated measures (lung function testing) ANOVA followed by Fisher's probable least significant difference post hoc test. All values are expressed as mean (± SEM). A value of P < 0.05 was considered significant.
Results
BMC CdM Prevents Airway Inflammation in Experimental Asthma
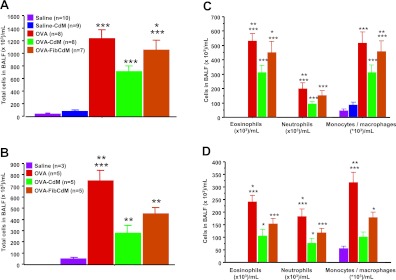
BMC CdM significantly reduced the total number of cells in the brochoalveolar lavage fluid (BALF) in OVA-CdM animals compared with untreated OVA mice in the acute (n = 8; P < 0.0001 [Figure 2A]) and the chronic (n = 6; P = 0.0001 [Figure 2B]) asthma models. Polymorphonuclear cells (PMNs), predominantly eosinophils (∼ 50% of total BALF cells), were significantly increased in the BALF of OVA mice compared with control animals, whereas BMC CdM significantly attenuated lung PMN influx (acute model: P < 0.0001 [Figure 2C]; chronic model: P = 0.0006 [Figure 2D]). BMC CdM had no effect on control saline animals in the acute model (Figure 2A). Control CdM from mouse lung fibroblasts (Fib CdM) had no effect when compared with untreated OVA animals in the acute model (Figure 2B). Fib CdM significantly lowered the total number of cells and PMNs in the BALF in the chronic model (Figure 2D), suggesting some antiinflammatory properties of Fib CdM.
Figure 2.
Bronchoalveolar lavage fluid (BALF) cell count. Bone marrow cell (BMC) conditioned medium (CdM) significantly decreased the total cell number and lung polymorphonuclear cell influx (in BALF). (A) Total cells in BALF (acute). *P < 0.05, OVA–Fib CdM versus OVA-CdM. ***P < 0.001, OVA, OVA–mouse lung fibroblast (Fib) CdM and OVA-CdM versus saline and saline-CdM, OVA versus OVA-CdM. (B) Total cells in BALF (chronic). *P < 0.05, OVA-CdM versus saline. **P < 0.01, OVA versus OVA–Fib CdM and OVA–Fib CdM versus saline. ***P < 0.001, OVA versus saline, OVA-CdM. (C) Differential cell counts in BALF (acute). *P < 0.05, OVA–Fib CdM versus OVA-CdM (eosinophils only). **P < 0.01, OVA versus OVA-CdM. ***P < 0.001, OVA, OVA–Fib CdM and OVA-CdM versus saline and saline-CdM. (D) Differential cell counts in BALF (chronic). *P < 0.05, OVA versus OVA–Fib CdM and OVA-CdM versus saline; ***P < 0.001, OVA–Fib CdM versus saline (eosinophils and neutrophils). *P < 0.05, OVA–Fib CdM versus saline; **P < 0.01, OVA versus OVA–Fib CdM; ***P < 0.001, OVA versus OVA-CdM (monocytes/macrophages). ***P < 0.001, OVA versus saline. The values are expressed as means ± SEM. Data are representative of a series of five separate experiments.
BMC CdM Prevents AHR in Experimental Asthma
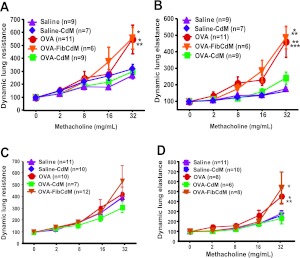
In the acute model, the methacholine dose–dependent increases in dynamic whole-lung resistance (Figure 3A) and elastance (Figure 3B) were exacerbated in untreated OVA mice (n = 11) compared with the control saline-treated (n = 9). BMC CdM treatment attenuated the dose-dependent increase in both parameters—dynamic whole-lung resistance (Figure 3A) and elastance (Figure 3B)—by 50%. In the chronic model, there were no differences between groups in the dynamic lung resistance (Figure 3C). BMC CdM attenuated the increase in dynamic lung elastance in the chronic model by over 50% (Figure 3D). Conversely, control Fib CdM had no beneficial effect compared with untreated OVA mice (Figures 3A–3D), suggesting that the benefit was specific to the CdM of BMCs. BMC CdM had no deleterious effect in the control saline-treated mice (Figures 3A–3D).
Figure 3.
Invasive lung function testing. BMC CdM significantly reduced the methacholine-induced increase in dynamic lung resistance (acute) and elastance (acute and chronic models). (A) Dynamic lung resistance (acute). *P < 0.05, OVA versus OVA-CdM, OVA-Fib versus saline and OVA-CdM. **P < 0.01, OVA versus saline. (B) Dynamic lung elastance (acute). *P < 0.05, OVA-Fib versus OVA-CdM. **P < 0.01, OVA versus OVA-CdM, OVA-Fib versus saline and saline-CdM. ***OVA versus saline and saline-CdM. Data for each methacholine dose are expressed as means ± SEM of the peak percent increase compared with baseline response to aerosolized saline (n = 4 separate experiments). “n” indicates the number of animals in combined experiments. (C) Dynamic lung resistance (chronic). (D) Dynamic lung elastance (chronic). *P < 0.05, OVA and OVA–Fib CdM versus OVA-CdM, saline-CdM; OVA–Fib CdM versus saline. **P < 0.01, OVA versus saline. Data for each methacholine dose are expressed as means ± SEM of the peak percent increase compared with baseline response to aerosolized saline (n = 5 separate experiments). “n” indicates the number of animals in combined experiments.
BMC CdM Improves Bronchial Responsiveness to Salbutamol and Prevents Chronic ASM Thickening and Peribronchial Inflammation
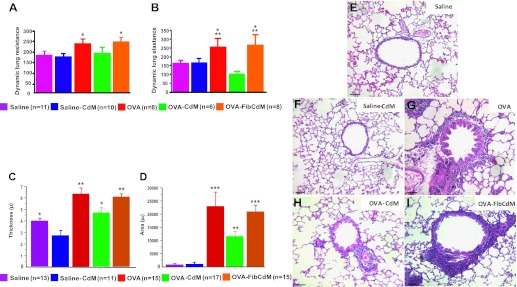
The bronchodilator response to salbutamol was significantly blunted in the chronic OVA model as documented by a persistent increase in dynamic lung resistance (Figure 4A) and elastance (Figure 4B). The response to salbutamol remained blunted in OVA-Fib CdM–treated animals as quantified by the persistent increase in dynamic lung resistance (Figure 4A) and elastance (Figure 4B). Conversely, BMC CdM restored the bronchodilator response to salbutamol similar to the control groups (Figures 4A and 4B).
Figure 4.
(A, B) Bronchodilator response to salbutamol. Bronchodilation in response to salbutamol was blunted in the chronic asthma model. BMC CdM restored the bronchodilator response to salbutamol. (A) Dynamic lung resistance. *P < 0.05, OVA and OVA–Fib CdM versus saline and saline-CdM. (B) Dynamic lung elastance. *P < 0.05, OVA versus saline, OVA–Fib CdM versus saline and saline-CdM. **P < 0.01, OVA and OVA–Fib CdM versus OVA-CdM. The values are expressed as means ± SEM (n = 5 separate experiments). “n” indicates the number of animals in combined experiments. (C–I) Airway remodeling in chronic asthma. Hematoxylin and eosin staining showing the characteristic features of airway remodeling of chronic asthma including increased airway smooth muscle layer thickening and peribronchial inflammatory infiltrate in chronic OVA-exposed mice. BMC CdM significantly reduced both. (C) Bronchial smooth muscle layer thickness. *P < 0.05, saline versus saline-CdM, OVA-CdM versus OVA–Fib CdM. **P < 0.01, OVA and OVA–Fib CdM versus saline and saline-CdM, OVA-CdM versus OVA and saline-CdM. (D) Peribronchial inflammatory infiltrate area. **P < 0.01, OVA-CdM versus saline, saline-CdM, OVA, OVA–Fib CdM. ***P < 0.001, OVA and OVA-Fib CdM versus saline, saline-CdM. Scale bar: 65 μm. Data are representative of a series of five separate experiments. Data expressed as means ± SEM. “n” indicates the number of airways analyzed in combined experiments.
Medium-sized bronchi had a significant thickening of the ASM layer and an increase in peribronchial inflammatory infiltrate in OVA-sensitized animals compared with control mice as quantified by ASM thickening (Figure 4C) and increased peribronchial inflammatory infiltrate (Figure 4D) and shown in representative H&E sections for each group (Figures 4E–4I). Increased ASM thickening (Figure 4C) and peribronchial inflammatory infiltrate (Figure 4D) were significantly reduced by 20 and 40%, respectively, in BMC CdM–treated OVA mice compared with untreated OVA mice. Fib CdM had no beneficial effect on these parameters.
BMC CdM Attenuates T helper-2 Lymphocytes Cytokine Response
The T helper-2 (TH2) cytokines IL-4 and IL-13 were significantly lower in the lungs of OVA-CdM mice compared with untreated OVA mice and OVA-Fib CdM mice in the acute (Table 1) and the chronic (Table 2) models. The levels of the antiinflammatory cytokine IL-10 were significantly increased in the lungs of OVA BMC CdM mice compared with untreated OVA mice. IL-10 was present in BMC CdM (29.4 ± 3.26 ng/ml) but not detectable in Fib CdM by ELISA.
TABLE 1.
CYTOKINE PROFILE IN THE ACUTE ASTHMA MODEL
| Group | IL-4 (pg/mg protein) | IL-13 (pg/mg protein) | IL-10 (pg/mg protein) |
| Saline (n = 11) | 39.39 ± 5.26* | 152.37 ± 8.48 | 652.75 ± 88.64 |
| Saline-CdM (n = 9) | 27.87 ± 1.96 | 147.19 ± 6.32 | 654.13 ± 88.02 |
| OVA (n = 17) | 66.37 ± 4.81†‡ | 207.04 ± 12.99§ | 753.63 ± 55.65 |
| OVA-CdM (n = 18) | 51.62 ± 4.16‡ | 126.44 ± 5.08 | 1,156.50 ± 95.78‖ |
| OVA-Fib CdM (n = 7) | 63.21 ± 3.27‡ | 190.44 ± 18.18¶§ | 992.91 ± 60.40** |
Definition of abbreviations: CdM = conditioned medium; Fib = mouse lung fibroblasts; OVA = ovalbumin.
Values are mean ± SEM.
P < 0.05 OVA versus OVA-CdM.
P < 0.01 OVA and OVA-Fib CdM versus saline, saline-CdM; OVA-CdM versus saline-CdM.
P < 0.01 OVA versus saline, saline-CdM, OVA-CdM; OVA-Fib CdM versus OVA-CdM.
P < 0.01 OVA-CdM vs. saline, saline-CdM, OVA.
P < 0.05 OVA-Fib CdM vs. saline, saline-CdM.
P < 0.05 OVA-Fib CdM vs. saline, saline-CdM.
TABLE 2.
CYTOKINE PROFILE IN THE CHRONIC ASTHMA MODEL
| Group | IL-4 (pg/mg protein) | IL-13 (pg/mg protein) | IL-10 (pg/mg protein) |
| Saline (n = 5) | 19.18 ± 3.12* | 99.42 ± 4.90 | 662.09 ± 22.87 |
| OVA (n = 7) | 131.93 ± 12.67† | 165.60 ± 11.39‡ | 840.54 ± 60.94 |
| OVA-CdM (n = 7) | 87.78 ± 9.71†§ | 118.56 ± 5.56‡ | 1,365.95 ± 100.24¶ |
| OVA-Fib CdM (n = 7) | 121.57 ± 10.97† | 140.07 ± 9.16‡‖ | 862.35 ± 35.13 |
Definition of abbreviations: CdM = conditioned medium; Fib = mouse lung fibroblasts; OVA = ovalbumin.
Values are mean ± SEM.
P < 0.01 OVA, OVA-CdM, OVA-Fib CdM versus saline; OVA-CdM versus OVA.
P < 0.01 OVA and OVA-Fib CdM versus saline; OVA-CdM versus OVA.
P < 0.05 OVA-CdM versus OVA-Fib CdM.
P < 0.01 OVA-Fib CdM versus OVA.
P < 0.01 OVA-CdM vs. saline, OVA, OVA-Fib CdM.
APN Mediates Protective Effects of BMC CdM on AHR
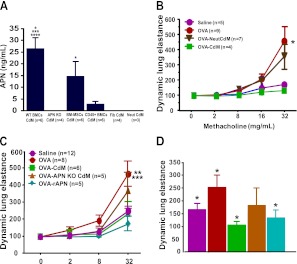
We also found APN, an antiinflammatory adipokine, to be expressed in BMC CdM but not in Fib CdM (Figure 5A), suggesting that some of the beneficial effects of BMC CdM could be mediated by APN. APN was produced predominantly by BM-MSCs (5-fold compared with CD45+ BMCs) (Figure 5A).
Figure 5.
(A) Adiponectin (APN) concentration in CdM preparations. *P < 0.05, wild-type (WT) CdM versus CD45− CdM; CD45− versus APN knock-out (KO) CdM, CD45+ CdM, Fib CdM, NeutCdM. ***P < 0.001, WT CdM versus NeutCdM. ****P < 0.0001, WT CdM versus APN KO CdM, CD45− CdM, Fib CdM. Results are from two separate experiments. Data are expressed as means ± SEM. “n” indicates the number of independent CdM batches. (B) Invasive lung function testing in the acute asthma model. NeutCdM failed to prevent acute OVA-induced AHR. *P < 0.05, OVA versus saline, OVA-CdM. (C) Invasive lung function testing in the chronic asthma model. BMC CdM and rAPN significantly reduced the increase in dynamic lung elastance. **P < 0.01, OVA versus OVA-CdM, saline. ***P < 0.001, OVA versus OVA-rAPN. Data for each methacholine dose are expressed as means ± SEM of the peak percent increase compared with baseline response to aerosolized saline (n = 5 separate experiments). “n” indicates the number of animals in combined experiments. (D) Bronchodilator response to salbutamol. Bronchodilation in response to salbutamol was blunted in the chronic asthma model. BMC CdM and recombinant APN (rAPN) restored the bronchodilator response to salbutamol. *P < 0.05, OVA versus OVA-CdM, OVA-rAPN, and saline. Values are expressed as means ± SEM (n = 5 separate experiments). “n” indicates the number of animals in combined experiments.
To test for the contribution of APN to the beneficial effects of CdM, we tested the effects of APN absence in BMC CdM using a neutralizing antibody and BMCs from APN KO mice. APN inhibition with a neutralizing antibody efficiently decreased APN in BMC CdM (NeutCdM) (Figure 5A). APN inhibition significantly attenuated the beneficial effect of BMC CdM on dynamic lung elastance in the acute asthma model (Figure 5B).
Likewise, APN was undetectable in BMCs of APN KO mice (Figure 5A). In addition, APN KO BMC CdM was unable to prevent AHR (Figure 5C) and bronchodilator responsiveness (Figure 5D). Conversely, rAPN treatment alone significantly improved AHR (Figure 5C) and bronchodilator responsiveness to salbutamol (Figure 5D).
APN Mediates Protective Effects of BMC CdM on Airway Remodeling
Representative H&E-stained lung sections showed that, compared with the control saline-treated mice (Figure 6A), the chronic OVA model had significant airway remodeling (Figure 6B). Airway remodeling was prevented in OVA-CdM (Figure 6C) but not by APN KO BMC CdM (Figure 6D). Treatment with rAPN in turn prevented airway remodeling (Figure 6E). These data were confirmed by quantitative airway morphometry showing attenuated ASM thickness (Figure 6F) and peribronchial inflammation (Figure 6G) in OVA-CdM and rAPN-treated mice but not in APN KO BMC CdM–treated mice, further suggesting that APN contributes to the beneficial effects of BMCs CdM.
Figure 6.
(A–E) Airway remodeling parameters in chronic asthma. Representative hematoxylin and eosin–stained lung sections showing the characteristic feature of airway remodeling in the chronic asthma model, including increased airway smooth muscle layer thickening and peribronchial inflammatory infiltrate in chronic OVA-exposed mice. BMC CdM and rAPN significantly reduced both. (F) Bronchial smooth muscle layer thickness. *P < 0.05, OVA versus OVA-CdM, OVA-rAPN. **P < 0.01, OVA versus saline, OVA–KO CdM versus OVA-CdM, OVA-rAPN. ***P < 0.001, OVA–KO CdM versus saline. (G) Peribronchial inflammatory infiltrate area. *P < 0.05, OVA and OVA–KO CdM versus OVA-CdM, OVA-CdM versus saline. **P < 0.01, OVA and OVA–KO CdM versus OVA-rAPN. ****P < 0.0001, OVA and OVA–KO CdM versus saline. Scale bar: 65 μm. Data are representative of a series of five separate experiments. The values are expressed as means ± SEM. “n” indicates the number of airways analyzed in combined experiments.
BMC CdM Induces Subsets of IL-10–Producing T Regulatory Cells and Macrophages
FACS revealed a subpopulation of lung lymphocytes coexpressing the surface markers CD25 (Figure 7A) and CD4 (Figure 7B) and intracellular IL-10 but lacking expression of the transcription factor Foxp3 (Figures 7C–7F). This population was significantly up-regulated in OVA-CdM mice compared with OVA and control mice (Figure 7G). FACS also revealed a subpopulation of IL-10–expressing CD11b+ macrophages (Figures 7H–7K). This population was significantly up-regulated in BMC CdM– and rAPN-treated mice (Figure 7L).
Figure 7.
Fluorescence-activated cell sorting of lung lymphocytes. (A) Gating for activated CD4+ lymphocytes (CD4+CD25+) followed by (B) gating for Foxp3− within this cell population. (C–F) Representative scatterplots for CD4+CD25+Foxp3−IL-10+ cells from each experimental group. (G) BMC CdM increased the proportion of lung CD4+CD25+Foxp3−IL-10 cells. ***P < 0.001, saline and OVA-CdM versus OVA and OVA-rAPN. (H–K) Representative scatterplots for CD11b+IL-10+ cells from each experimental group. (L) BMC CdM increased the proportion of lung CD11b+IL-10+ cells. *P < 0.05, OVA versus OVA-CdM and saline. ***P < 0.001, OVA-rAPN versus saline. ****P < 0.0001, OVA-CdM versus saline. Data are representative of two experiments. The values are expressed as means ± SEM.
Discussion
We show that airway delivery of CdM obtained from BMCs prevents the development of AHR, airway inflammation, and airway remodeling while restoring airway responsiveness to salbutamol in a murine model of acute and chronic asthma. BMC CdM also attenuated TH2-associated cytokine release, induced a subset of antiinflammatory IL-10–secreting T regulatory (Treg) cells, and promoted IL-10 production by macrophages. Our data support the potential for APN as a target for asthma therapy by showing that APN contributed significantly to prevent AHR, inflammation, and the histological features of airway remodeling. We propose APN as a candidate for asthma therapy. BMC-derived soluble factors may be a viable alternative to whole-cell delivery and could therefore serve as a novel approach for the treatment of asthma.
Recent evidence suggests that stromal stem cells prevent organ damage through a paracrine effect rather than cell replacement (5). In addition, these cells display antiinflammatory and immunomodulatory properties (17–21). These properties render stromal stem cells particularly appealing and amenable to the treatment of inflammatory diseases. In vitro, these cells have been shown to inhibit the proliferation of immune effector cells such as B lymphocytes and T lymphocytes (20). In humans, a phase II clinical trial suggested that stromal stem cells attenuate steroid-resistant graft-versus-host disease after hemopoietic stem cell transplantation (19). Inhaled and systemic steroids have been successfully used in asthma since the recognition of the contribution of airway inflammation to asthma pathogenesis, but refractory asthma is still responsible for 250,000 deaths worldwide every year (4). Refractory asthma is a pathological entity characterized by reduced (or a lack of) responsiveness to conventional bronchodilator therapy (2, 3). Here we show that BMC-derived CdM exerts potent antiinflammatory effects in acute and chronic murine asthma, preventing airway inflammation (Figure 2) and AHR in both models (Figure 3). In chronic asthma, BMC CdM restored the response to salbutamol, a commonly prescribed bronchodilator (Figures 4A and 4B), and prevented ASM layer thickening and peribronchial inflammation (Figures 4C–4I). Meanwhile, Fib CdM failed to significantly affect AHR, bronchodilator responsiveness, and airway remodeling. This suggests that the benefits were specific to BMC CdM.
Asthma is an inflammatory disease caused by a complex set of interactions between multiple effector cell types. The presence of the antigen triggers TH2 lymphocyte activation, leading to an acute cascade of events, which include recruitment of PMNs to the lung parenchyma (typically eosinophils) (31) and secretion of TH2 cytokines such as IL-4 and IL-13 (32–34). In our study, PMN recruitment was inhibited by BMC CdM treatment, as indicated by BALF analysis in acute and chronic asthma models (Figure 2). Fib CdM also decreased total BALF cellularity and PMN numbers. This effect was limited to the chronic model and was reduced compared with BMC CdM. A similar partial reduction in BALF cellularity and eosinophilia after skin fibroblast administration has been reported (35). These observations highlight the importance of using appropriate cell controls to further our understanding about the mechanism of action of MSCs and CdM.
In humans, chronic airway remodeling is characterized by airway wall thickening, mainly due to smooth muscle hypertrophy and hyperplasia, accompanied by the narrowing of the airway lumen with goblet cell hyperplasia and peribronchial and perivascular fibrosis (30). These structural changes in chronic asthmatic airways may be a consequence of prolonged inflammation (36). These changes may contribute to fatal asthma refractory to bronchodilator therapy. We evaluated the impact of BMC CdM administration on airway remodeling by quantifying the degree of ASM layer thickening and peribronchial inflammation in the chronic asthma model, where remodeling was expected to occur. Both parameters were reduced in OVA-CdM animals compared with the OVA and OVA-Fib CdM groups (Figures 4C and 4D), suggesting that BMC CdM prevents the development of airway remodeling. This histological improvement was accompanied by restoration of the bronchial responsiveness to salbutamol, a conventional bronchodilator (Figures 4A and 4B). Administration of a single dose of salbutamol on maximally constricted airways led to a significant decrease in dynamic resistance and elastance in the OVA-CdM similar to controls, whereas this response was blunted in untreated OVA mice and OVA-Fib CdM mice, a major feature in the occurrence of refractory asthma.
We sought to determine the mechanisms through which CdM prevents the development of asthma features. We found that the levels of IL-4 and IL-13, two TH2 cytokines generally elevated in asthma (34), were attenuated in the lungs of OVA-CdM animals compared with untreated OVA mice (Tables 1 and 2). Our findings are consistent with recent reports showing that BMC administration decreases lung inflammation and BALF levels of TH2 cytokines in an acute, ragweed-induced model of allergic asthma (35) and in OVA-induced mouse models of AHR (37–39). Moreover, in our study the levels of the antiinflammatory IL-10 were significantly increased in the OVA-CdM lungs, suggesting that BMC CdM attenuates allergic TH2 inflammation through an IL-10–dependent mechanism. IL-10 has been shown to contribute to the reduction of airway inflammation (40). BMC CdM itself acted as an exogenous source of IL-10; however, we did not find a significant difference between the levels of IL-10 in the lungs of BMC CdM–treated control animals and untreated controls, suggesting that endogenous production of IL-10 in the allergic inflammatory environment may be an important component of elevated IL-10 levels found only in OVA-CdM lungs. We therefore sought to identify cellular effectors that would mediate the actions of BMC-secreted factors and also potential local IL-10 sources. Treg cells have recently been shown to diminish inflammation in asthma (41), and BMCs are able to induce IL-10 secretion by Treg cells (42) and macrophages (43, 44). Naturally occurring Treg cells are most commonly defined as cells that express the surface markers CD4 and CD25 and the intracellular marker Foxp3 upon activation (45). We did not find significant changes in the proportion of naturally occurring CD4+CD25+Foxp3+ regulatory Treg cells in the draining lymph nodes or lung lymphocytes (Fig. E4), suggesting that local BMC CdM administration does not act on a systemic cellular immunity level in the acute model. This is consistent with reports indicating that short-term exposure to ovalbumin may lead to decreased lung CD4+CD25+Foxp3+ cells (46) or spleen CD4+Foxp3+ cells (47). A recent study suggests up-regulation of CD4+CD25+Foxp3+ in the lungs of BALB/c mice after allogeneic BMC treatment in an OVA-induced model of AHR (38). However, in this report the allogeneic BMCs were also administered at the time of sensitization to OVA, not only upon antigen challenges. We did not attempt to prevent antigen sensitization because we sought to mimic the clinical conditions in which the patient would have already been hypersensitive to specific triggers. Instead, we found that BMC CdM prevented the OVA-induced down-regulation of Treg cells expressing CD4, CD25, and IL-10 and lacking expression of Foxp3 (Figures 7A–7G), a subset of inducible Treg cells that have been described to be IL-10–induced and IL-10–secreting Treg cells (Tr1) (48–50). This finding correlates with our finding that IL-10 is present in BMC CdM and is consistent with other reports indicating the presence of IL-10 in the human stromal stem cell secretome (51). Simultaneously, using CD11b as a macrophage marker, we found a significant up-regulation of CD11b+ coexpressing IL-10 in OVA-CdM lungs (Figure 7H–7L). It has been proposed that BMCs may contribute to the reduction of allergic airway inflammation by promoting a switch from TH2 to TH1 phenotype in an OVA-induced murine model of AHR (39). Recently, a population of CD11b+Gr1+F4/80+ immunoregulatory myeloid-derived suppressor cells has been shown to contribute to blunted TH2 responses after preallergen LPS exposure in a house dust mite murine model of AHR. Myeloid-derived suppressor cells were also able to prevent airway eosinophilia and IL-13 production when transplanted in vivo after OVA exposure. The hypothesis that IL-10 has a direct role in reducing TH2 inflammation is supported by the finding that TH2 cytokine levels were restored with neutralization of IL-10 (52).
BMCs have also been shown to act via TGF-β production to alleviate airway inflammation in a ragweed-induced model of murine asthma (35).
In our quest to identify candidate factors mediating the beneficial effects of BMCs, we found APN, an antiinflammatory adipokine, to be present in BMC CdM. APN is a white adipose tissue–derived antiinflammatory cytokine that is found in lower levels in obese patients. Obesity is associated with increased incidence and worse outcomes of asthma, suggesting that APN levels may serve as a biomarker in asthma (53). It has been shown that APN KO mice develop more severe features of chronic allergic asthma, including airway inflammation and remodeling, compared with WT animals (54). In addition, rAPN decreased AHR and neutrophil and eosinophil counts as well as BALF IL-13 and IL-5 levels in acute murine OVA-induced asthma (55). Consistent with these observations, we found that administration of CdM from WT BMCs in which APN was neutralized (acute asthma model) or CdM from BMCs of APN KO mice (chronic model) was less effective than WT CdM in attenuating AHR (Figure 5) and remodeling (Figure 6) in chronic OVA-induced asthma. Conversely, administration of rAPN alone restored airway responsiveness to normal levels (Figures 5C and 5D) and prevented ASM thickening (Figure 6F) and peribronchial inflammation (Figure 6G) in the chronic model.
Given the relative scarcity of reports regarding the phenotype of BMCs from the BALB/c mouse strain (22, 56) and the heterogeneity of the BMCs used in this study (Fig. E2), we sought to determine the cellular source of APN. We separated the BM-MSCs by negative CD45-based selection. BM-MSCs displayed a CD45−Sca1+CD29+ phenotype, whereas the positively selected population (CD45+ BMCs) displayed a CD45+Sca1loCD29+ phenotype (Fig. E4). The BM-MSCs were found to be the major contributor to APN production compared with CD45+ BMCs (Figure 5A).
To clarify the role of APN in our study, we tested whether APN alone could contribute to the up-regulation of IL-10–expressing cellular subtypes. rAPN administration did not affect Tr1 levels when compared with untreated OVA animals (Figures 7A–7G). APN has been shown to induce IL-10 secretion by macrophages (57, 58), another cellular source potentially responsible for the increased IL-10 found in the OVA-CdM lungs compared with the untreated OVA animals. In the chronic asthma model, rAPN induced the up-regulation of IL-10–expressing macrophages (Figures 7H–7L), similar to BMC CdM administration. It is therefore possible that administration of BMC CdM would induce the Tr1 through IL-10 found in the CdM with a similar, simultaneous up-regulation of IL-10 production by lung macrophages through APN. This would lead to a sustained production of endogenous IL-10 that may contribute to attenuation of inflammatory events (40).
We aimed to determine the effects of CdM alone to come as close as possible to the clinical setting: a relatively quick delivery of a cell-free preparation that can be administered via the airways. The former paradigm for the benefit of BMC administration focused on cell replacement; however, this concept encompassed the concerns related to cell tumorigenic potential and allogeneic transplantation (59, 60). We believed that the delivery of a cell-free preparation would alleviate concerns related to tumorigenesis. A recent report (61) has compared the effects of BM-MSCs and BM-MSC CdM in endotoxin-induced lung injury (an inflammatory model) and did not find significant differences between the effects of the CdM and the administration of cells themselves.
In summary, this is the first study to investigate the therapeutic potential of MSC CdM and to propose APN as a MSC-derived protective factor in experimental asthma. Overall, our data highlight the therapeutic benefit of BMCs in asthma and suggest the use of BMC-derived soluble factors as a novel, practical, and clinically relevant approach for the treatment of asthma and other inflammatory disorders, thereby alleviating the potential risks of whole-cell therapy. Further identification of BMC-derived factors may hold promise for novel approaches in the treatment of asthma.
Supplementary Material
Acknowledgments
The authors thank Dr. Valentin Duta, Dr. Gaia Weissmann, Dr. Arul Vadivel, and the Alberta Diabetes Institute Histology Core (Lynette Elder, Alana Eshpeter) for their collaboration in this study.
Footnotes
This work was supported by the Canadian Institutes for Health Research (CIHR), Alberta Innovates – Health Solutions (AIHS), Canadian Stem Cell Network, Canada Research Chair Program, Canada Foundation for Innovation (CFI), and the Stollery Children's Hospital Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0391OC on September 8, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Grammatikos AP. The genetic and environmental basis of atopic diseases. Ann Med 2008;40:482–495 [DOI] [PubMed] [Google Scholar]
- 2.Kenyon NJ, Jarjour NN. Severe asthma. Clin Rev Allergy Immunol 2003;25:131–149 [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society Proceedings of the ATS Workshop on Refractory Asthma. Current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med 2000;162:2341–2351 [DOI] [PubMed] [Google Scholar]
- 4.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59:469–478 [DOI] [PubMed] [Google Scholar]
- 5.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 2009;17:939–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 2003;100:8407–8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 2007;179:1855–1863 [DOI] [PubMed] [Google Scholar]
- 8.Mei SHJ, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med 2007;4:e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med 2009;180:1122–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med 2009;180:1131–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005;33:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keating A. Mesenchymal stromal cells. Curr Opin Hematol 2006;13:419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, Prockop DJ. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation Workshop. Proc Am Thorac Soc 2006;3:193–207 [DOI] [PubMed] [Google Scholar]
- 14.Weiss DJ, Kolls JK, Ortiz LA, Panoskaltsis-Mortari A, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc 2008;5:637–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthay MA. Treatment of acute lung injury: clinical and experimental studies. Proc Am Thorac Soc 2008;5:297–299 [DOI] [PubMed] [Google Scholar]
- 16.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007;110:3499–3506 [DOI] [PubMed] [Google Scholar]
- 17.Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med 2007;262:509–525 [DOI] [PubMed] [Google Scholar]
- 18.Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB, Pinsky DJ, Peters-Golden M, Lama VN. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 2008;181:4389–4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008;371:1579–1586 [DOI] [PubMed] [Google Scholar]
- 20.Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther 2008;8:569–581 [DOI] [PubMed] [Google Scholar]
- 21.Knight DA, Rossi FM, Hackett TL. Mesenchymal stem cells for repair of the airway epithelium in asthma. Expert Rev Respir Med 2010;4:747–758 [DOI] [PubMed] [Google Scholar]
- 22.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 2004;103:1662–1668 [DOI] [PubMed] [Google Scholar]
- 23.Sugiura H, Liu X, Duan F, Kawasaki S, Togo S, Kamio K, Wang XQ, Mao L, Ahn Y, Ertl RF, et al. Cultured lung fibroblasts from ovalbumin-challenged “asthmatic” mice differ functionally from normal. Am J Respir Cell Mol Biol 2007;37:424–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sung JH, Yang HM, Park JB, Choi GS, Joh JW, Kwon CH, Chun JM, Lee SK, Kim SJ. Isolation and characterization of mouse mesenchymal stem cells. Transplant Proc 2008;40:2649–2654 [DOI] [PubMed] [Google Scholar]
- 25.Stämpfli MR, Wiley RE, Neigh GS, Gajewska BU, Lei XF, Snider DP, Xing Z, Jordana M. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J Clin Invest 1998;102:1704–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebeling C, Forsythe P, Ng J, Gordon JR, Hollenberg M, Vliagoftis H. Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. J Allergy Clin Immunol 2005;115:623–630 [DOI] [PubMed] [Google Scholar]
- 27.Vieira RP, Claudino RC, Duarte AC, Santos AB, Perini A, Faria Neto HC, Mauad T, Martins MA, Dolhnikoff M, Carvalho CR. Aerobic exercise decreases chronic allergic lung inflammation and airway remodeling in mice. Am J Respir Crit Care Med 2007;176:871–877 [DOI] [PubMed] [Google Scholar]
- 28.Vanoirbeek JAJ, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PHM, Verbeken E, Decramer M, Nemery B, et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol 2010;42:96–104 [DOI] [PubMed] [Google Scholar]
- 29.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, et al. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med 2000;161:309–329 [DOI] [PubMed] [Google Scholar]
- 30.Hirota JA, Ellis R, Inman MD. Regional differences in the pattern of airway remodeling following chronic allergen exposure in mice. Respir Res 2006;7:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy 2008;38:709–750 [DOI] [PubMed] [Google Scholar]
- 32.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie ANJ, Dent LA, et al. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med 2002;195:1433–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev 2004;202:175–190 [DOI] [PubMed] [Google Scholar]
- 35.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Gorham JD, Bundoc VG, Bundoc VG, Hodges MG, Jelinek I, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA 2010;107:5652–5657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tagaya E, Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol Int 2007;56:331–340 [DOI] [PubMed] [Google Scholar]
- 37.Bonfield TL, Koloze M, Lennon DP, Zuchowski B, Yang SE, Caplan AI. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol 2010;299:L760–L770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy 2011;66:523–531 [DOI] [PubMed] [Google Scholar]
- 39.Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, Leclair L, Poynter ME, Steele C, Rincon M, Weiss DJ. Bone marrow derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells 2011;29:1137–1148 10.1002/stem.656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa Y, Duru EA, Ameredes BT. Role of IL-10 in the resolution of airway inflammation. Curr Mol Med 2008;8:437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: the ins and outs. Annu Rev Immunol 2008;26:205–232 [DOI] [PubMed] [Google Scholar]
- 42.Ghannam S, Pène J, Torcy-Moquet G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol 2010;185:302–312 [DOI] [PubMed] [Google Scholar]
- 43.Németh K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009;15:42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 2009;37:1445–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGee HS, Agrawal DK. TH2 cells in the pathogenesis of airway remodeling: regulatory T cells a plausible panacea for asthma. Immunol Res 2006;35:219–232 [DOI] [PubMed] [Google Scholar]
- 46.Burchell JT, Wikstrom ME, Stumbles PA, Sly PD, Turner DJ. Attenuation of allergen-induced airway hyperresponsiveness is mediated by airway regulatory T cells. Am J Physiol Lung Cell Mol Physiol 2009;296:L307–L319 [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Bai C, Wang G, Wang D, Cheng X, Huang J, Jiang D, Qian G, Wang X. Protection against the allergic airway inflammation depends on the modulation of spleen dendritic cell function and induction of regulatory T cells in mice. Genet Vaccines Ther 2010;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O'Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol 2004;172:5986–5993 [DOI] [PubMed] [Google Scholar]
- 49.Hawrylowicz CM. Regulatory T cells and IL-10 in allergic inflammation. J Exp Med 2005;202:1459–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol 2005;5:271–283 [DOI] [PubMed] [Google Scholar]
- 51.Wagner W, Roderburg C, Wein F, Diehlmann A, Frankhauser M, Schubert R, Eckstein V, Ho AD. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells 2007;25:2638–2647 [DOI] [PubMed] [Google Scholar]
- 52.Arora M, Poe SL, Oriss TB, Krishnamoorthy N, Yarlagadda M, Wenzel SE, Billiar TR, Ray A, Ray P. TLR4/MyD88-induced CD11b+Gr-1intF4/80+ non-migratory myeloid cells suppress Th2 effector function in the lung. Mucosal Immunol 2010;3:578–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol 2005;115:925–927 [DOI] [PubMed] [Google Scholar]
- 54.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol 2009;41:397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 2006;118:389–395 [DOI] [PubMed] [Google Scholar]
- 56.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007;25:2739–2749 [DOI] [PubMed] [Google Scholar]
- 57.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 2004;109:2046–2049 [DOI] [PubMed] [Google Scholar]
- 58.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 2010;285:6153–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lepperdinger G, Brunauer R, Jamnig A, Laschober G, Kassem M. Controversial issue: is it safe to employ mesenchymal stem cells in cell-based therapies? Exp Gerontol 2008;43:1018–1023 [DOI] [PubMed] [Google Scholar]
- 60.Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 2009;6:e1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 2009;106:16357–16362 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.