Abstract
The scavenger decapping enzyme Dcs1 has been shown to facilitate the activity of the cytoplasmic 5′-3′ exoribonuclease Xrn1 in eukaryotes. Dcs1 has also been shown to be required for growth in glycerol medium. We therefore wondered whether the capacity to activate RNA degradation could account for its requirement for growth on this carbon source. Indeed, a catalytic mutant of Xrn1 is also unable to grow in glycerol medium, and removal of the nuclear localization signal of Rat1, the nuclear homolog of Xrn1, restores glycerol growth. A cytoplasmic 5′-3′ exoribonuclease activity is therefore essential for yeast growth on glycerol, suggesting that Xrn1 activation by Dcs1 is physiologically important. In fact, Xrn1 is essentially inactive in the absence of Dcs1 in vivo. We analyzed the role of Dcs1 in the control of exoribonuclease activity in vitro and propose that Dcs1 is a specific cofactor of Xrn1. Dcs1 does not stimulate the activity of other 5′-3′ exoribonucleases, such as Rat1, in vitro. We demonstrate that Dcs1 improves the apparent affinity of Xrn1 for RNA and that Xrn1 and Dcs1 can form a complex in vitro. We examined the biological significance of this regulation by performing 2D protein gel analysis. We observed that a set of proteins showing decreased levels in a DCS deletion strain, some essential for respiration, are also systematically decreased in an XRN1 deletion mutant. Therefore, we propose that the activation of Xrn1 by Dcs1 is important for respiration.
Keywords: RNA turnover, ribonuclease, post-transcriptional control, porin
Turnover of messenger RNA (mRNA) is a regulated process and a key step in the control of gene expression (1). In eukaryotic cells, most cytoplasmic mRNAs are degraded through two alternative pathways, each of which is initiated by the removal of the poly(A) tail by deadenylases. Subsequently, the cap (5′-m7GpppN) structure is removed by the decapping complex Dcp1/Dcp2, and the mRNA is degraded in the 5′ to 3′ direction by the major cytoplasmic enzyme Xrn1. Alternatively, deadenylated mRNAs can be degraded from their 3′-ends by the exosome, a multimeric complex possessing 3′ to 5′ exoribonuclease activity (2). The cap structure resulting from 3′-end decay is hydrolyzed by conserved scavenger decapping enzyme Dcs1 (3). Dcs1 is also necessary for the 5′ to 3′ exonucleolytic activity of Xrn1, a requirement that functionally links these two alternative degradation pathways (4). The 5′-3′ exoribonuclease Xrn1 is highly conserved in eukaryotes and has been extensively described for its role in the degradation of cytoplasmic mRNAs (5, 6). Xrn1 also participates in the degradation of nonfunctional mRNAs (7) and noncoding RNAs (8, 9).
In addition to causing direct defects in RNA turnover, it has been known for a long time that a deletion of XRN1 is detrimental to other cellular functions. XRN1 mutants exhibit pleiotropic phenotypes, including slow growth, loss of viability upon nitrogen starvation, meiotic arrest, defective sporulation, defects in microtubule-related processes, telomere shortening, and chromosomal stability (10–15). It has yet to be shown that these phenotypes are directly related to a deficiency in exoribonuclease activity, however. More recently, a new class of noncoding RNAs, Xrn1-sensitive unstable transcripts named XUTs, has been described in Saccharomyces cerevisiae (16). Known regulatory RNAs belong to this class, and their stabilization in the absence of Xrn1 could explain the aberrant expression of some genes.
Few studies have investigated how the activity of exoribonucleases is modulated in connection to cell physiology (17, 18). Here, we focus on Dcs1, a factor that potentially controls the activity of the exoribonuclease Xrn1 (4). We have a particular interest in the fact that Dcs1 is also required for growth in glycerol medium because we show that Xrn1 is also necessary for growth on this carbon source. More precisely, we demonstrate that a cytoplasmic 5′-3′ exoribonuclease activity is required under these conditions, suggesting that a potential connection exists between the ability to grow on glycerol and the capacity to degrade RNA or to activate RNA degradation in the presence of Dcs1. We demonstrate that Dcs1 is a specific cofactor of Xrn1. We decided to examine the physiological consequences of this regulation by 2D protein gel analysis. We studied the impact of shifting cells from glucose to glycerol on the accumulation of specific proteins in the absence of Xrn1 or its activator Dcs1. A majority of the down-regulated proteins are essential for mitochondrial function such as respiration, a prerequisite for growth on nonfermentable carbon sources like glycerol. We thus show that 5′-3′ exoribonuclease activity is important for mitochondrial function and propose that one role of Dcs1 is the modulation of Xrn1 activity.
Results
5′-3′ Exoribonuclease Activity Is Required for Growth on Glycerol.
Dcs1 has been shown to stimulate Xrn1 activity (4), and a DCS1 deletion strain cannot grow on glycerol (19) (Fig. 1A). We asked whether these two observations were connected by spotting serial dilutions of the xrn1 mutant on glycerol plates. In fact, we observed that both mutants show a growth defect on nonfermentable carbon sources such as glycerol, ethanol, and lactate (Fig. S1). Complementation of this strain with a wild-type XRN1 gene restored growth on glycerol, whereas complementation with a catalytic mutant did not (Fig. 1B), showing that this defect is related to the enzyme’s exoribonuclease activity. We were also able to rescue this growth defect by expressing the nuclear 5′-3′ exoribonuclease Rat1 in the cytoplasm in the absence of its nuclear localization signal, Rat1ΔNLS (20) (Fig. 1B). Thus, a cytoplasmic 5′-3′ exoribonuclease activity is important for growth on glycerol. An interesting connection therefore exists between the ability to grow on this carbon source and the capacity to degrade RNA in the 5′-3′ direction or to activate this degradation through the presence of Dcs1.
Fig. 1.
Correlation between cytoplasmic 5′-3′ exoribonuclease activity and growth on glycerol. Serial dilutions of the indicated strains were spotted onto plates containing either glucose (Left) or glycerol (Right) as the sole carbon source. (A) Absence of Dcs1 impedes growth on glycerol. (B) Cytoplasmic 5′-3′ exoribonuclease activity is necessary for growth on glycerol. The xrn1Δ strain was transformed with different plasmids encoding wild-type (WT) XRN1, the XRN1 catalytic mutant (E176G substitution), or the mutated RAT1ΔNLS gene that restores cytoplasmic exoribonuclease activity by expressing Rat1 in the cytoplasm through removal of the nuclear localization signal (NLS). Growth on plates of xrn1Δ and dcs1Δ strains versus WT are shown in Fig. S1.
Xrn1 Activity Is Not Inhibited by Products or Substrates of Dcs1 in Vitro.
Dcs1 cleaves the m7GpppN cap of messenger RNAs that are digested by the exosome, and it was proposed that this catalytic activity is necessary for Xrn1-dependent RNA decay (4). Dcs1 produces m7GMP and a 5′ diphosphate-nucleotide upon cleavage of m7GpppN and is also able to convert the m7GDP that results from the cleavage of capped RNA by the Dcp1/Dcp2 complex to m7GMP (21, 22). The previous model suggested that one or more of these compounds, which accumulate or are missing in the absence of the activity of Dcs1, are effectors of Xrn1 activity (4). We therefore performed in vitro RNA degradation assays (RT-FeDEx; ref. 23) to measure Xrn1 activity in the presence of m7GDP, m7GMP, m7G, and m7GpppN. None of these potential effectors affected Xrn1 activity at concentrations above those expected in vivo (24), whereas a known inhibitor of Xrn1, 3′-phospho-adenosine-5′-phosphate (pAp), significantly reduced its activity (Fig. S2). We thus conclude that Xrn1 is not a target for the potential substrates or end-products of Dcs1 activity.
Catalytic Activity of Dcs1 Is Not Necessary for Xrn1 Activity in Vivo.
The correlation between growth on glycerol and Xrn1 activation by Dcs1 is inconsistent with a previous observation that a catalytic mutant of Dcs1, Dcs1H-N (SI Materials and Methods), could grow on glycerol but was unable to activate Xrn1 (4).
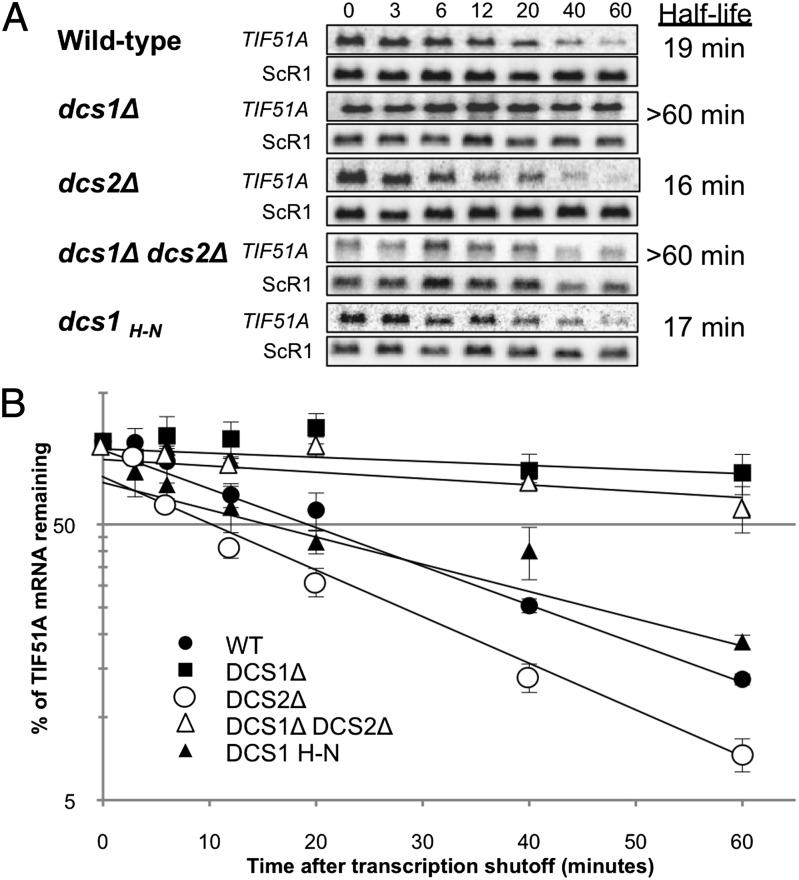
We therefore revisited the original observation by measuring mRNA half-life to determine the impact of Dcs1H-N on Xrn1-dependent mRNA decay in vivo. Northern blots were performed to detect TIF51A mRNA, a known substrate of Xrn1 (4), at times after addition of thiolutin to stop transcription (Fig. 2A). Quantifications are shown in Fig. 2B and showed that the absence of the catalytic activity of Dcs1 has no real impact on TIF51A mRNA stability in vivo. Only the DCS1 deletion strain and double deletion strain, in which a catalytically inactive paralog of Dcs1, Dcs2 is also deleted, showed increased stabilization of TIF51A mRNA compared with the WT, dcs2Δ, or DCS1H-N strains. A clear correlation therefore exists between the ability to grow on glycerol and the capacity of Dcs1 to activate 5′-3′ RNA degradation by Xrn1. A possible explanation for the discrepancy with the previous observation is given in the discussion. We also verified that TIF51A mRNA has a similar half-life (>60 min) in a dsc1 mutant as an xrn1 mutant when cells are shifted from glucose to glycerol media (Fig. S3A), and that the protein Xrn1 is similarly expressed in the wild-type, dcs1Δ, dcs2Δ, or dcs1Δ/dcs2Δ strains (Fig. S3B). Therefore, Xrn1 is essentially inactive for TIF51A mRNA degradation in the absence of Dcs1 in vivo, and Dcs1 is not a regulator of XRN1 gene expression.
Fig. 2.
The catalytic activity of Dcs1 is not required for activation of TIF51A mRNA degradation. DCS1H-N denotes the catalytically inactive DCS1 mutant harboring a H268N substitution in its HIT motif (4). Degradation of TIF51A mRNA was monitored after transcriptional shutoff with thiolutin (15 μg/mL), and RNA was isolated at the indicated time points. (A) Northern blot showing degradation in dcs1Δ and/or dcs2Δ mutant backgrounds. The ScR1 RNA served as a loading control. (B) Half-life measurements of TIF51A transcripts were carried out by quantification of the mRNA remaining at each time point after correcting for loading differences using ScR1 RNA. The average value and SDs were obtained from at least three independent experiments.
Direct and Specific Activation of Xrn1 by Dcs1.
To determine whether the activation of Xrn1 by Dcs1 was direct, we assayed Xrn1 in the presence of Dcs1 in vitro. RNA degradation assays were performed by using either RT-FeDEx assays (23) (Fig. 3) or a 5′-labeled 30-nt substrate (Fig. S4). Both approaches showed that the activity of Xrn1 is increased by the presence of Dcs1. Furthermore, both wild-type Dcs1 and the catalytic mutant, Dcs1H-N, were able to enhance Xrn1 activity in vitro. Control experiments showed that neither form of Dcs could degrade RNA alone, excluding the possibility that these preparations contained a contaminating RNase activity (Fig. 3). Moreover, all Dcs factors tested, such as DcpS, the Dcs1 human ortholog, or Dcs2, activated Xrn1 in vitro (Table 1). The activation ability of Dcs factors is apparently specific to Xrn1, because Dcs factors were unable to stimulate exoribonuclease activity of other 5′-3′ RNases, such as Rat1, RNase J1, or the 3′-5′ exoribonuclease RNase PH (Table 1).
Fig. 3.
Direct and specific activation of Xrn1 by Dcs1 or Dcs1H-N in vitro. In vitro assays of 5′ to 3′-exoribonuclease activity by RT-FeDEX (23). The fluorophore (FAM) linked to RNA is hybridized to a DNA oligonucleotide containing a quencher (TAMRA). The generation of FAM fluorescence signal was monitored in real time in the presence of 50 nM Xrn1 exoribonuclease. RT-FeDEx analysis shows the increased activity of Xrn1 in the presence of both wild-type 100 nM purified Dcs1 protein or catalytic mutant 100 nM purified Dcs1 protein (H268N), whereas no contaminating exoribonuclease activity of Dcs1 (wild-type or mutant) can be detected.
Table 1.
Dcs family proteins and activation of exoribonucleases in vitro
| Protein added in RNase assays | Xrn1 | Rat1/Rai1 | RNase J1 | RNase PH |
| Dcs1-his tag (E. coli) | + | — | — | — |
| DcpS-flag (human) | + | — | — | — |
| Dcs1-his tag catalytic mutant (E. coli) | + | — | — | N/D |
| Dcs2-his tag (E. coli) | + | N/D | — | N/D |
+, activation; —, Dcs-independent exoribonuclease activity; N/D, not done.
Because Rat1 is active independently of Dcs1 in vitro (Table 1), we asked whether Rat1ΔNLS could compensate for the absence of cytoplasmic Xrn1 for the degradation of TIF51A mRNA in the absence of Dcs1 in vivo. In Table 2, we show that Rat1ΔNLS can destabilize the TIF51A mRNA (twofold) in the absence of Dcs1, albeit not as efficiently as Xrn1 in the presence of Dcs1 (fourfold).
Table 2.
Cytoplasmic mRNA degradation by Rat1ΔNLS does not require the presence of Dcs1
| Strains | Plasmids | TIF51A mRNA half-life, min |
| Wild-type | + Xrn1 | 17 ± 0.5 |
| + Rat1ΔNLS | 15 ± 1 | |
| dcs1Δ | + Xrn1 | >60 |
| + Rat1ΔNLS | 27 ± 1.7 |
Specific Interaction Between Dcs1 and Xrn1.
The experiments described above suggest a specific interaction between Dcs1 and Xrn1. We therefore performed Far Western blot experiments to demonstrate this interaction in vitro (Fig. S5A). Different quantities of Xrn1 were spotted on membranes, incubated in the presence of Dcs1 protein, and then revealed with anti-Dcs1 antibody. A clear positive signal was seen with Xrn1 and the Dcs1-positive control, whereas no signal was seen with either BSA or RNase J1. To determine at what level Dcs1 affected Xrn1 activity, we also performed RNA gel-shift assays in the absence of Mg2+ to inhibit the exoribonuclease activity of Xrn1 (Fig. 4). These experiments revealed that the presence of Dcs1 improves the apparent affinity of Xrn1 for its RNA substrate, whereas it has no effect on the binding of the Rat1/Rai1 complex to the same RNA. As expected based on its primary function, Dcs1 did not bind the RNA substrate of Xrn1, a 5′ monophosphorylated RNA. We were unable to visualize the formation of a stable RNA/Xrn1/Dcs1 complex (Fig. 4), but on the hypothesis that the complex between Dcs1 and Xrn1 is transitory, we performed formaldehyde cross-link experiments to try to trap the complex. Under denaturing gel conditions, a supershift in the mobility of Dcs1 was visible specifically in the presence of Xrn1 (Fig. S5B). These experiments are consistent with Dcs1 forming a transitory complex with Xrn1.
Fig. 4.
Dcs1 improves apparent affinity of Xrn1 for RNA in vitro. (A and B) Electrophoretic mobility shift assay of FAM-linked RNA by Xrn1. FAM-linked RNA (1.5 μM) was incubated with increased amounts of purified Xrn1protein (in a buffer without Mg2+, in the absence (A) or in the presence of 1μM of purified Dcs1 (B), and electrophoretically separated on a 4% nondenaturing polyacrylamide gel. (C) Comparison of the quantification of Xrn1-associated RNA as a percentage of the RNA input, in the absence or presence of purified Dcs1 (A and B). (D and E) Electrophoretic mobility shift assay of FAM-linked RNA by Xrn1 or Rat1. FAM-linked RNA (1.5 μM) was incubated with increased amounts of purified Dcs1 protein in a buffer without Mg2+, in the presence of 0.5 μM Xrn1 (D) or 1.3 μM of Rat1/Rai1 (B) exoribonucleases, and electrophoretically separated on a 4% nondenaturing polyacrylamide gel. (F) Comparison of the quantification of Xrn1-associated RNA (D) with the quantification of Rat1/Rai1-associated RNA (E) in the presence of purified Dcs1, as a percentage of the RNA input.
Importance of Xrn1 Activation by Dcs1 for Mitochondrial Function.
The observations that Dcs1 is a cofactor of Xrn1 and that Xrn1 exoribonuclease activity is important for growth on glycerol suggests that activation of Xrn1 by Dcs1 is physiologically important. DCS1, and even more so DCS2, gene expression is induced when the carbon source is limited (24). To examine this phenomenon using 2D protein gel analysis, we decided to measure the impact of an XRN1 deletion and a DCS double deletion on the accumulation of specific proteins when cells are shifted from glucose to glycerol medium for 3 h. We used the dcs1Δdcs2Δ strain because Dcs2 was also found to be a potential activator of Xrn1 in vitro (Table 1). We analyzed proteins extracted from wild-type and both xrn1Δ and dcs1Δ/dcs2Δ cells. Protein spots (230) were quantified that were common to three independent experiments. All spots showing at least a twofold decrease in mutant versus wild-type strain were identified by mass spectrometry (16 spots in the xrn1 mutant and 11 in the dcs double deletion mutant). These proteins could be classified into three categories, those down-regulated in both mutants and those down-regulated in xrn1 or in dcs mutants only (Fig. 5). We found that many of the proteins in the first category are involved in mitochondrial function (Fig. 5A) and are essential for respiration. Because this 2D protein gel approach is limited to a portion of the proteome, under specific growth conditions, it is not possible to say whether these are the primary targets of a defective 5′-3′ RNA degradation pathway. However, this degradation defect directly or indirectly produces a down-regulation of mitochondrial factors that is sufficient to explain the absence of growth on a nonfermentable carbon source such as glycerol.
Fig. 5.
Different classes of down-regulated proteins in glycerol in the context of a defective 5′-3′ exoribonuclease activity. (A) Down-regulated (>twofold) proteins analyzed by 2D gel electrophoresis in the wild-type, xrn1Δ, and dcs1Δ dcs2Δ strains. Total proteins were isolated from cultures grown in rich media containing glucose and shifted for 3 h into media containing glycerol as the carbon source. The average value and SDs for each time point were obtained from at least three independent experiments. Genes involved in mitochondrial function are underlined, and essential genes for respiration are marked with an asterisk (http://www.yeastgenome.org). (B) Western blot of Por1 protein in wild-type, dcs1Δ dcs2Δ and xrn1Δ strains. Prp4 was used as a loading control. (C) POR1 mRNA expression in wild-type, dcs1Δdcs2Δ, and xrn1Δ strains. POR1 mRNA levels were analyzed by Northern blot analysis. The ScR1 RNA served as a loading control. (B and C) Total proteins or total RNA were isolated from cultures grown in media containing either glucose (Left) or shifted for 3 h into medium containing glycerol (Right) as the sole carbon source.
The mitochondrial porin, Por1 (25), which is essential for mitochondrial function, is one of the most affected proteins in both mutant strains. We confirmed the results of the 2D protein-gel analysis by performing Western and Northern blot analysis for POR1 (Fig. 5 B and C). The expression of POR1 clearly depends on both XRN1 and DCS1 (see also Fig. S5 that shows the specific role of DCS1 in POR1 mRNA expression). In conclusion, the activation of Xrn1 by its cofactor Dcs1 is critical for the expression of genes necessary for mitochondrial function and respiration.
Discussion
In this paper, we show that a cytoplasmic 5′-3′ exoribonucleolytic activity is necessary for growth on glycerol and other nonfermentable carbon sources in yeast. This activity is usually provided by Xrn1, the major cytoplasmic exoribonuclease in S. cerevisiae, but we were able to demonstrate that Rat1 lacking a nuclear localization signal can compensate for the growth defect in an XRN1 deletion strain. This observation reveals that degradation must be optimal to ensure respiration on glycerol. It is remarkable that the cell's 3′-5′ exoribonuclease activities are not able to compensate for the absence of Xrn1 when cells are grown on glycerol.
The absence of Dcs1 or Xrn1 led to a strong defect in POR1 expression (Fig. 5 A–C and Fig. S6). Although defects in the expression of other mRNAs may also play a role, the defect in the expression of the POR1 gene alone, which is essential gene for respiration, is sufficient to explain the observation that 5′-3′ cytoplasmic exoribonuclease activity is required for growth on glycerol (Fig. 1). We also considered the possibility that the absence of Xrn1 or Dcs1 could have caused a loss of mitochondrial DNA over several generations. However, replacing the deleted XRN1 locus by a wild-type copy of XRN1 allowed the new strain to grow on glycerol again, ruling out a permanent loss of mitochondrial DNA in xnr1Δ strains (Fig. S7A). Similar observations have been made for a DCS1 deletion strain (4, 19).
Our results showing that the catalytic activity of Dcs1 is not important to stimulate 5′-3′ mRNA degradation are contradictory with a prior study (4). To measure mRNA half-life in this study, we used thiolutin to block RNA synthesis at 30 °C, whereas in the previous study, transcription was stopped by shifting temperature from 25 °C to 37 °C in a strain harboring a thermosensitive RNA polymerase mutant rbp1-1 (4). To determine whether the Dcs1H-N catalytic mutant was temperature sensitive, we therefore performed mRNA half-life measurements at 30 °C and 37 °C in the presence of thiolutin and observed that the Dcs1H-N catalytic mutant loses its ability to activate TIF51A mRNA degradation at 37 °C, and not at 30 °C (Fig. S8A). The Dcs1H-N mutant also shows a growth defect on glycerol at 37 °C, and not at 30 °C, in agreement with previous observations (Fig. S8B). The apparent contradiction with the previous study is therefore likely explained by a thermosensitivity of the catalytic mutant.
In vitro experiments with purified proteins showed that Dcs1 acts directly on Xrn1 (Fig. 2). A similar observation was made for Dcs1H-N, Dcs2 (Table 1). Although Dcs2 also stimulated Xrn1 activity in vitro, it is not implicated in the 5′-3′ RNA degradation pathway, and it is not required for respiration in vivo (Fig. 1). DCS2 is poorly expressed in glucose media but is induced under conditions of carbon source limitation such as glycerol (24), which could explain the lack of phenotype in a DCS2 deletion strain in the presence of glucose. We asked whether Dcs2 could play a role in the kinetics of TIF51A mRNA degradation, when cells were shifted from glucose to glycerol. However, only the dcs1Δ mutation affects the degradation rate of the TIF51A mRNA, even in glycerol growth conditions (Fig. S3A). A particular cellular localization of Dcs2 could also explain why Dcs2 does not impact on TIF51A mRNA decay like Dcs1; Dcs2 has been reported to be localized in processing bodies under stress conditions (24).
RNA gel shift experiments, cross-link experiments using formaldehyde, and Far Western blot analysis of the interaction between Dcs1 and Xrn1 showed that Dcs1 interacts with Xrn1 and that this interaction probably activates RNA degradation by increasing the affinity of Xrn1 for its substrate, perhaps through a conformational change after a transitory interaction. The role of Dcs1 is specific to Xrn1 because no such activation was observed for the other RNases tested in vitro, such as Rat1 (Table 1). We were able to demonstrate that, in contrast to Xrn1, a cytoplasmic Rat1ΔNLS still shows detectable RNA decay activity in the absence of Dcs1 in vivo (Table 2).
Our data show that one important role of Dcs1 is to maintain Xrn1 activity and, because 5′-3′ exoribonuclease activity is critical for growth on glycerol, this interaction clearly has physiological importance. The 2D protein gel analysis revealed, however, that the absence of Xrn1 and Dcs1 has distinct effects on the expression of some genes. Dps1 and Cys3 for example, which have both been shown to be involved in respiration (26, 27), are affected differently by Dcs1 and Xrn1 (Fig. 5A). Thus, Xrn1 and Dcs1 appear to be involved in other pathways related to the mitochondrial function independently of their functions as an exoribonuclease and an exoribonuclease cofactor. The activation of Xrn1 by Dcs1 is therefore necessary, but not sufficient, to maintain an optimal cellular respiration.
The ability of Rat1ΔNLS to compensate for Xrn1 shows that 5′-3′ exoribonuclease activity is important for mitochondrial function. Because Rat1ΔNLS 5′-3′ exoribonuclease activity is less affected by the absence of Dcs1 in vivo, we wondered whether a DCS1 deletion strain could grow on glycerol with Rat1ΔNLS replacing Xrn1. The answer was no (Fig. S7B), and one reason is probably, as mentioned above, that Dcs1 affects the expression of other genes important for respiration independently of the 5′-3′ RNA degradation pathway (such as Dps1; Fig. 5A).
One could imagine that the accumulation of a particular class of RNAs is potentially inhibitory for the expression of key genes of the respiration pathway. If these inhibitory RNAs exist, our data suggest that they are likely to belong to a class of specific targets of the 5′-3′ degradation pathway. Recently, a novel class of RNAs mainly degraded by Xrn1 was discovered that includes potential regulatory antisense noncoding RNAs (ncRNAs) (16). Accumulation of these ncRNAs, called XUTs, in the absence of 5′-3′ exoribonuclease activity could explain the down-regulation of the expression of specific genes. This model also relies on previously published work in which it was demonstrated that the expression of the PHO84 and TY1 genes can be down-regulated by the accumulation of antisense transcripts that are substrates of the exoribonucleases Rrp6 and Xrn1, respectively (28–30). We therefore checked to see whether any of the genes corresponding to the down-regulated proteins in our 2D gel analysis also contained potential XUTs (16), but did not find any.
The xrn1 mutant has been analyzed by microarrays and, more recently, in a high-throughput sequencing approach (16, 31), but we do not observe a major correlation with our 2D gel analysis. These earlier studies were conducted on total RNA, polyadenylated RNAs, or total RNA depleted of ribosomal RNAs. RNAs isolated from xrn1 mutants are known to be enriched for decapped mRNAs, making it impossible to accurately quantify functional messenger RNAs, possibly explaining why we do not observe a significant correlation between mRNAs and protein levels. Moreover, no transcriptome data are available on xrn1 mutant cells grown in glycerol medium.
The interaction between Dcs1 and Xrn1 activates Xrn1. Dcs1 is a conserved pyrophosphatase of the histidine triad (HIT) family that catalyses the cleavage of m7GpppN resulting from the 3′-5′ mRNA degradation process (32). Cytoplasmic Xrn1 and nuclear Rat1 belong to a large family of conserved 5′-3′ exoribonucleases (33). Interestingly, Rat1 also forms a complex with a pyrophosphatase, Rai1, that was recently shown to cleave unmethylated capped RNA (34). Rai1 helps the exoribonuclease activity by stabilizing Rat1, and the catalytic activity of Rai1 is also dispensable for the activation of Rat1. The complex formation between a pyrophosphatase and a 5′-3′ exoribonuclease might have a particular importance in evolution.
Materials and Methods
mRNA decay measurements (Northern blot analysis) were done by following standard procedures with details given in SI Materials and Methods. Transcription was blocked by adding thiolutin (15 μg/mL) to the cultures (generous gift from Pfizer). Scans were performed with a Typhoon (Amersham Bioscience), and quantifications by using ImageQuant software. All half-life values reported are an average of at least three experiments.
RNA Degradation by Fluorescence Analysis.
The fluorescence assay was performed by RT-FeDeX assays as described (23). See SI Materials and Methods for reaction buffers.
Measuring Protein–RNA Affinity by EMSA.
We used the FAM-modified RNA (23) at a constant concentration (1 μM) and varied the protein concentration. The protein and RNA were incubated together on ice in the Xrn1/Rat1-Rai1 buffer without Mg2+ (30 mM Tris⋅HCl at pH 8, 50 mM NH4Cl, 0.5 mM DTT, and 20 mg/mL Acetylated Bovine Serum). The samples were separated on 4% (wt/vol) nondenaturing polyacrylamide (19:1) gels in Tris borate/EDTA buffer at room temperature. Gel images were scanned with a Typhoon (Amersham Bioscience).
Western Blot Analysis.
Crude extracts were prepared from cultures by vortexing with glass beads. Samples were resolved by SDS/PAGE and transferred onto nitrocellulose membranes. Membranes were probed with either a 1:5,000 dilution of polyclonal anti-Prp4 antibodies with a 1:2,000 dilution of monoclonal anti-Porin antibodies (Invitrogen). The blotted membranes were developed with a 1:20,000 dilution of peroxidase-conjugated anti-rabbit and anti-mouse IgG (Sigma), respectively, and an enhanced chemiluminescence substrate (ECL; Amersham Bioscience).
2D Gel Electrophoresis.
Three independent cultures were grown to exponential phase in YPD and shifted to glycerol for 3 h at 30 °C. Soluble proteins were prepared according to ref. 35. First-dimension electrophoresis was carried out on a nonlinear immobilized pH 3–11 gradient IPG strip (Bio-Rad) and the second dimension by 12% SDS/PAGE. The six samples were analyzed in parallel. Densitometric quantification of the six blue-colloidal-stained 2D gels was performed by using the PDQuest 2D Analysis software. Protein spots were excised from gels for trypsin in-gel digestion and MALDI-TOF analysis.
Plasmids, Yeast Strains, and Growth Condition.
All details can be found in SI Materials and Methods and Tables S1 and S2.
Purification of Enzymes.
Details can be found in SI Materials and Methods.
Extraction of RNA.
Preparation of total RNA was performed according standard procedures (36). Additional details are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Arlen W. Johnson for plasmids pAJ228, pAJ152, and pRDK307; Roy Parker for strains yRP840 and yRP844; and Josette Banroques for providing polyclonal anti-Prp4 antibodies. This work was supported by funds from the Centre National de la Recherche Scientifique, Université Pierre et Marie Curie, Université Paris VII-Denis Diderot, Association pour la Recherche sur le Cancer Grant A07/2/4831, National Institutes of Health Grant GM67005, and a grant from the Agence Nationale pour la Recherche (ANR REGULncRNA). F.S. was a recipient of a fellowship from the Ministère pour la Recherche et la Technologie and from la Fondation pour la Recherche Médicale.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120090109/-/DCSupplemental.
References
- 1.Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- 2.Parker R, Song H. The enzymes and control of eukaryotic mRNA turnover. Nat Struct Mol Biol. 2004;11:121–127. doi: 10.1038/nsmb724. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Kiledjian M. Regulation of mRNA decapping. Wiley Interdiscip Rev RNA. 2010;1:253–265. doi: 10.1002/wrna.15. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Kiledjian M. Scavenger decapping activity facilitates 5′ to 3′ mRNA decay. Mol Cell Biol. 2005;25:9764–9772. doi: 10.1128/MCB.25.22.9764-9772.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu CL, Stevens A. Yeast cells lacking 5′—>3′ exoribonuclease 1 contain mRNA species that are poly(A) deficient and partially lack the 5′ cap structure. Mol Cell Biol. 1993;13:4826–4835. doi: 10.1128/mcb.13.8.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muhlrad D, Decker CJ, Parker R. Turnover mechanisms of the stable yeast PGK1 mRNA. Mol Cell Biol. 1995;15:2145–2156. doi: 10.1128/mcb.15.4.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isken O, Maquat LE. Quality control of eukaryotic mRNA: Safeguarding cells from abnormal mRNA function. Genes Dev. 2007;21:1833–1856. doi: 10.1101/gad.1566807. [DOI] [PubMed] [Google Scholar]
- 8.Stevens A, Hsu CL, Isham KR, Larimer FW. Fragments of the internal transcribed spacer 1 of pre-rRNA accumulate in Saccharomyces cerevisiae lacking 5′----3′ exoribonuclease 1. J Bacteriol. 1991;173:7024–7028. doi: 10.1128/jb.173.21.7024-7028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chernyakov I, Whipple JM, Kotelawala L, Grayhack EJ, Phizicky EM. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev. 2008;22:1369–1380. doi: 10.1101/gad.1654308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heyer WD, Johnson AW, Reinhart U, Kolodner RD. Regulation and intracellular localization of Saccharomyces cerevisiae strand exchange protein 1 (Sep1/Xrn1/Kem1), a multifunctional exonuclease. Mol Cell Biol. 1995;15:2728–2736. doi: 10.1128/mcb.15.5.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solinger JA, Pascolini D, Heyer WD. Active-site mutations in the Xrn1p exoribonuclease of Saccharomyces cerevisiae reveal a specific role in meiosis. Mol Cell Biol. 1999;19:5930–5942. doi: 10.1128/mcb.19.9.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Interthal H, et al. A role of Sep1 (= Kem1, Xrn1) as a microtubule-associated protein in Saccharomyces cerevisiae. EMBO J. 1995;14:1057–1066. doi: 10.1002/j.1460-2075.1995.tb07088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tishkoff DX, Rockmill B, Roeder GS, Kolodner RD. The sep1 mutant of Saccharomyces cerevisiae arrests in pachytene and is deficient in meiotic recombination. Genetics. 1995;139:495–509. doi: 10.1093/genetics/139.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Lee A, Gilbert W. Gene disruption of a G4-DNA-dependent nuclease in yeast leads to cellular senescence and telomere shortening. Proc Natl Acad Sci USA. 1995;92:6002–6006. doi: 10.1073/pnas.92.13.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kipling D, Tambini C, Kearsey SE. rar mutations which increase artificial chromosome stability in Saccharomyces cerevisiae identify transcription and recombination proteins. Nucleic Acids Res. 1991;19:1385–1391. doi: 10.1093/nar/19.7.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dijk EL, et al. XUTs are a class of Xrn1-sensitive antisense regulatory non-coding RNA in yeast. Nature. 2011;475:114–117. doi: 10.1038/nature10118. [DOI] [PubMed] [Google Scholar]
- 17.Dichtl B, Stevens A, Tollervey D. Lithium toxicity in yeast is due to the inhibition of RNA processing enzymes. EMBO J. 1997;16:7184–7195. doi: 10.1093/emboj/16.23.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todeschini AL, Condon C, Bénard L. Sodium-induced GCN4 expression controls the accumulation of the 5′ to 3′ RNA degradation inhibitor, 3′-phosphoadenosine 5′-phosphate. J Biol Chem. 2006;281:3276–3282. doi: 10.1074/jbc.M511688200. [DOI] [PubMed] [Google Scholar]
- 19.Malys N, Carroll K, Miyan J, Tollervey D, McCarthy JE. The ‘scavenger’ m7GpppX pyrophosphatase activity of Dcs1 modulates nutrient-induced responses in yeast. Nucleic Acids Res. 2004;32:3590–3600. doi: 10.1093/nar/gkh687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson AW. Rat1p and Xrn1p are functionally interchangeable exoribonucleases that are restricted to and required in the nucleus and cytoplasm, respectively. Mol Cell Biol. 1997;17:6122–6130. doi: 10.1128/mcb.17.10.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Rodgers ND, Jiao X, Kiledjian M. The scavenger mRNA decapping enzyme DcpS is a member of the HIT family of pyrophosphatases. EMBO J. 2002;21:4699–4708. doi: 10.1093/emboj/cdf448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu SW, Jiao X, Welch S, Kiledjian M. Analysis of mRNA decapping. Methods Enzymol. 2008;448:3–21. doi: 10.1016/S0076-6879(08)02601-3. [DOI] [PubMed] [Google Scholar]
- 23.Sinturel F, et al. Real-time fluorescence detection of exoribonucleases. RNA. 2009;15:2057–2062. doi: 10.1261/rna.1670909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malys N, McCarthy JE. Dcs2, a novel stress-induced modulator of m7GpppX pyrophosphatase activity that locates to P bodies. J Mol Biol. 2006;363:370–382. doi: 10.1016/j.jmb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Mihara K, Sato R. Molecular cloning and sequencing of cDNA for yeast porin, an outer mitochondrial membrane protein: A search for targeting signal in the primary structure. EMBO J. 1985;4:769–774. doi: 10.1002/j.1460-2075.1985.tb03695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merz S, Westermann B. Genome-wide deletion mutant analysis reveals genes required for respiratory growth, mitochondrial genome maintenance and mitochondrial protein synthesis in Saccharomyces cerevisiae. Genome Biol. 2009;10:R95. doi: 10.1186/gb-2009-10-9-r95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimmer KS, et al. Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:847–853. doi: 10.1091/mbc.01-12-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camblong J, Iglesias N, Fickentscher C, Dieppois G, Stutz F. Antisense RNA stabilization induces transcriptional gene silencing via histone deacetylation in S. cerevisiae. Cell. 2007;131:706–717. doi: 10.1016/j.cell.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Berretta J, Pinskaya M, Morillon A. A cryptic unstable transcript mediates transcriptional trans-silencing of the Ty1 retrotransposon in S. cerevisiae. Genes Dev. 2008;22:615–626. doi: 10.1101/gad.458008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camblong J, et al. Trans-acting antisense RNAs mediate transcriptional gene cosuppression in S. cerevisiae. Genes Dev. 2009;23:1534–1545. doi: 10.1101/gad.522509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He F, et al. Genome-wide analysis of mRNAs regulated by the nonsense-mediated and 5′ to 3′ mRNA decay pathways in yeast. Mol Cell. 2003;12:1439–1452. doi: 10.1016/s1097-2765(03)00446-5. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Kiledjian M. Functional link between the mammalian exosome and mRNA decapping. Cell. 2001;107:751–762. doi: 10.1016/s0092-8674(01)00592-x. [DOI] [PubMed] [Google Scholar]
- 33.Xiang S, et al. Structure and function of the 5′—>3′ exoribonuclease Rat1 and its activating partner Rai1. Nature. 2009;458:784–788. doi: 10.1038/nature07731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiao X, et al. Identification of a quality-control mechanism for mRNA 5′-end capping. Nature. 2010;467:608–611. doi: 10.1038/nature09338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gobeil LA, Plante P, Rohani M, Ouellette M, Provost P. Involvement of Dcr1 in post-transcriptional regulation of gene expression in Schizosaccharomyces pombe. Front Biosci. 2008;13:2203–2215. doi: 10.2741/2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benard L, Carroll K, Valle RC, Wickner RB. Ski6p is a homolog of RNA-processing enzymes that affects translation of non-poly(A) mRNAs and 60S ribosomal subunit biogenesis. Mol Cell Biol. 1998;18:2688–2696. doi: 10.1128/mcb.18.5.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.