Abstract
Whether and how human populations exposed to the agricultural revolution are still affected by Darwinian selection remains controversial among social scientists, biologists, and the general public. Although methods of studying selection in natural populations are well established, our understanding of selection in humans has been limited by the availability of suitable datasets. Here, we present a study comparing the maximum strengths of natural and sexual selection in humans that includes the effects of sex and wealth on different episodes of selection. Our dataset was compiled from church records of preindustrial Finnish populations characterized by socially imposed monogamy, and it contains a complete distribution of survival, mating, and reproductive success for 5,923 individuals born 1760–1849. Individual differences in early survival and fertility (natural selection) were responsible for most variation in fitness, even among wealthier individuals. Variance in mating success explained most of the higher variance in reproductive success in males compared with females, but mating success also influenced reproductive success in females, allowing for sexual selection to operate in both sexes. The detected opportunity for selection is in line with measurements for other species but higher than most previous reports for human samples. This disparity results from biological, demographic, economic, and social differences across populations as well as from failures by most previous studies to account for variation in fitness introduced by nonreproductive individuals. Our results emphasize that the demographic, cultural, and technological changes of the last 10,000 y did not preclude the potential for natural and sexual selection in our species.
Keywords: Bateman gradient, Bateman principles, selection differential, human evolution, mating system
Disagreement about the strength of selection affecting humans has influenced how the theory of natural and sexual selection has been perceived since Darwin (1). In particular, humans exhibit a pronounced ecological flexibility through social and cultural adaptations (2) that is often invoked to cast doubt on the continued relevance of Darwinian selection in our species. Even among those applying evolutionary theory in humans, some scientists consider recent selective pressures weak and therefore only study human adaptations in the demographic, social, and physical environments of the Pleistocene, whereas others view selection as an effective force also operating in societies affected by the agricultural revolution (3).
Darwinian selection results from variation in fitness between individuals of the same population and the evolutionary response of traits to this selection depends on the genetic covariation between traits and fitness. Such evolutionary change is traditionally approximated by multiplying heritability (the proportion of phenotypic variance in the trait attributable to genetic differences among individuals) and phenotypic selection (the covariance between the trait and relative fitness) (4). It is therefore interesting to understand which genes or traits contribute to variation in fitness (5, 6). To assess the limit to phenotypic selection, it is also important to quantify per se the amount of variation in fitness, which determines the overall opportunity for total selection at the level of the organism (7–10). Because the methods of studying selection in natural populations are relatively well established, our understanding of selection in human populations is now limited mainly by the lack of suitable datasets (5, 11).
Recently, the establishment of large genomic and epidemiological datasets has enabled precise measurements of selection acting on specific loci and phenotypic traits in humans (5, 6). These studies demonstrate that selection has not stopped with the advent of agriculture and can act on certain traits even within contemporary modern populations. For example, Byars et al. (12) found some traits of medical significance in the United States to be currently influenced by selection (see also ref. 13). By using summary statistics for birth and death rates in a population, it is also possible to estimate opportunity for total selection (i.e., variance in relative fitness) and its two components—opportunity for selection because of survival to reproductive age and opportunity for selection because of fertility—at the scale of the entire organism (10). Cross-sectional anthropologic and demographic surveys have been used for partial measurements of selection in human populations (e.g., ref. 14). Survival to reproductive age appears to be the largest component of variation in fitness in most populations, especially before the demographic transition. A review of 53 different populations suggests that variation in fertility explains around one-third of the variation in opportunity for total selection both within and across populations (14). However, this fertility component encompasses multiple sources of variance in reproductive success with different biological meanings, such as competition for mates and fertility per se. In addition, the relationship between life-history traits and relative fitness can depend on wealth (15, 16), but analyses at a population level preclude controlling for social structure. Consequently, our understanding of life-history traits underpinning the opportunity for total selection remains poor, despite the long-lasting interest in the question.
Although the theory of sexual selection drives a considerable number of studies in evolutionary psychology and human behavioral ecology, the measurement of variation in fitness caused by variation in mating success has remained surprisingly unexplored. Sexual selection theory predicts that the sex experiencing stronger sexual selection should illustrate Bateman’s three 1948 principles: (i) a higher variance in reproductive success; (ii) a higher variance in mating success; and, crucially, (iii) a stronger association between mating and reproductive success, the so-called Bateman gradient (7, 17, 18). Because this latter property seems to result from primary sexual differentiation in the cost of reproduction, Bateman (17) argued that sexual selection is expected to be stronger in males than in females for many species, including mammals. Since then, several studies have stressed that differences in the strength of sexual selection between sexes can be influenced by factors other than the initial asymmetry in the cost of reproduction (19). Because human populations vary in important ways that should affect the relative strength of sexual selection between sexes (e.g., the adult sex ratio, population density, the level of paternal investment), Brown et al. (11) argued that humans are unlikely to conform to the single universal pattern proposed by Bateman. To our knowledge, only two attempts have been made to investigate in humans how variation in mating success affects variation in reproductive success in each sex (20, 21): one study focused on a colonizing population with a relatively high average fertility and the other on a population with a relatively high level of multiple mating.
Here, we present a study assessing the variance in relative lifetime reproductive success and its components in a human population with socially imposed monogamy and a low level of multiple mating (22) inhabiting an ecologically constraining environment and characterized by natural mortality and fertility rates typical of many preindustrial societies (23). Specifically, we studied the contribution to the opportunity for total selection of four different episodes of selection during the life cycle: survival to reproductive age, mate access, mating success, and fertility per mate. We take into account the effect of sex and wealth accumulation by contrasting two socio-economic classes in all analyses (landowners vs. landless). Data derived from church books maintained for tax purposes on every individual born in 18th- and 19th-century Finland (24) allowed us to compile a complete distribution of marital and reproductive success in four farming- and fishing-based populations (SI Materials and Methods). Our dataset avoids most of limitations of traditional demographic surveys while combining benefits characterizing alternative approaches: the dataset follows members of a large cohort individually from birth to death, as done in studies based on genealogic data (e.g., ref. 20), and accurately includes individuals who failed to survive to reproductive age, failed in the competition for spouses, and dispersed from their natal parish, similar to many ongoing longitudinal studies (e.g., ref. 21). Our sample is based on 5,923 individuals with complete information who were born between 1760 and 1849. It predates the demographic transition and the great famine of 1866–1868. Because extramarital relations were socially condemned (22) and extramarital births were rare, we used marriage data to infer matings.
Results
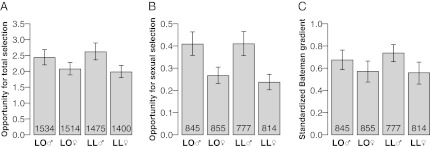
We first found evidence of an intense opportunity for total selection in the preindustrial Finnish population (Fig. 1A). The opportunity for total selection (I) sets the upper bound on the force of selection that can act on any phenotypic character (8) and is calculated as the variance in relative fitness, i.e., the variance in absolute fitness divided by the square of the mean fitness (10). To estimate I, we computed the variance in the number of offspring produced by all born individuals (including individuals not marrying or not reproducing) divided by the square of the mean, which is equivalent to computing the variance in the relative number of offspring. During the entire study period, the mean and variance in number of offspring were relatively stable (Fig. S1), so the data were pooled over birth cohorts. The number of offspring born over a lifetime ranged from 0 to 17 with an average (±SD) for all individuals born to, respectively, landless and landowning parents of 2.03 ± 3.28 and 2.18 ± 3.40 for males and 2.19 ± 3.09 and 2.26 ± 3.25 for females. For the entire sample, I equaled 2.27 [95% confidence interval (CI95%) = 2.16–2.38], indicating that within a single generation selection could potentially induce a more than twofold increase in the mean fitness value of the population. I was 24.2% higher in males than in females (P < 0.001) and 1.6% higher in landless than in landowners (P = 0.75) (Fig. 1A). The maximum change in mean phenotype resulting from one generation of directional selection—or the maximal selection differential for a trait—can be calculated as the SD of relative fitness, i.e., I1/2 (8). We found that the mean of a trait can increase by up to 1.51 SD (CI95% = 1.47–1.54) per generation if the covariance between the trait and the relative fitness and heritability are maximal (Fig. S2). Because differential selection measurements derive directly from I, the theoretically maximal evolutionary response to selection was also stronger in males than in females and of similar magnitude between landless and landowners.
Fig. 1.
Variance in reproductive success, mating success, and their relationship in a monogamous human population. The total opportunity for selection as measured by variance in relative reproductive success (A), the opportunity for sexual selection measured by variance in relative mating success (B), and the standardized Bateman gradient measured by the slope of the regression of relative reproductive success on relative mating success (C) are all higher for men than for women but are not different between landowners (LO) and landless (LL). Error bars are bootstrap CI95% values on the means. Sample sizes are indicated inside the bars.
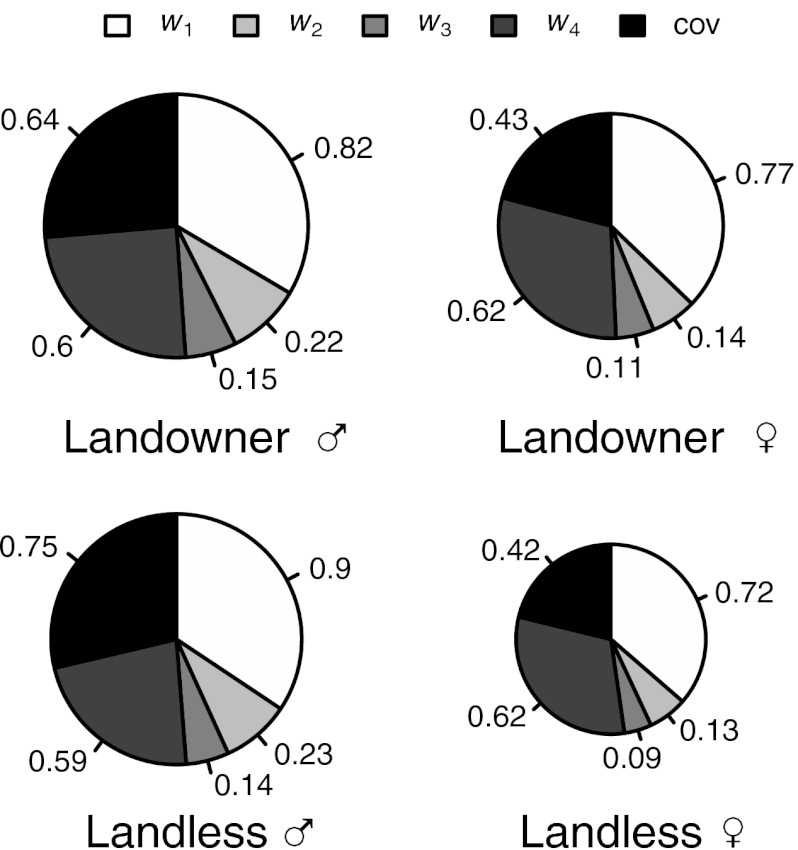
Second, we found that survival to adulthood and fertility were the two variance components responsible for most of the opportunity for selection (Fig. 2). The opportunity for total selection can be divided into additive components of variance in relative fitness, corresponding to multiplicative fitness components (8, 25). Here, we distinguished four episodes of selection: survival to reproductive age (w1), mate access (w2), mating success (w3), and fertility per mate (w4). The probability of surviving to reproductive age (w1) was lower in males than in females (0.54 and 0.57, respectively, logistic regression, 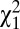 = 6.8, P < 0.001) and similar for both social classes (
= 6.8, P < 0.001) and similar for both social classes (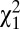 = 0.097, P = 0.75). Among individuals who survived to reproductive age (age 15 y), the probability of marrying at least once (w2) was also lower in men than in women (0.82 and 0.88, respectively,
= 0.097, P = 0.75). Among individuals who survived to reproductive age (age 15 y), the probability of marrying at least once (w2) was also lower in men than in women (0.82 and 0.88, respectively, 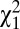 = 27.8, P < 0.001) but similar for both social classes (
= 27.8, P < 0.001) but similar for both social classes (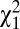 = 0.014, P = 0.68). Because w1 and w2 are binary variables with values 0 or 1, the amount of variance in relative fitness induced by these two episodes of selection is directly linked to the mean. Specifically, the variance in relative fitness is equal to (1 − p)/p, where p is the probability of surviving to reproductive age (w1) or of marrying (w2). Therefore, opportunities for selection induced by survival to reproductive age (w1) and by mate access (w2) were higher in males than in females. Overall, the opportunity for selection because of survival (w1) accounted for 35.3% and mate access (w2) accounted for 7.8% of I (Table S1). In addition to the ability to marry (w2), the variance in relative fitness could also be driven by the total number of sequential marriages (w3). In preindustrial Finland, monogamy was socially imposed and divorce was forbidden, so men and women could remarry only if widowed (22), resulting in a nonextensive serially monogamous mating system in which 7.7% of all born individuals obtained several partners through remarriages (26). The probability of remarrying was higher in males than in females (19% and 14%, respectively, among married individuals, logistic regression,
= 0.014, P = 0.68). Because w1 and w2 are binary variables with values 0 or 1, the amount of variance in relative fitness induced by these two episodes of selection is directly linked to the mean. Specifically, the variance in relative fitness is equal to (1 − p)/p, where p is the probability of surviving to reproductive age (w1) or of marrying (w2). Therefore, opportunities for selection induced by survival to reproductive age (w1) and by mate access (w2) were higher in males than in females. Overall, the opportunity for selection because of survival (w1) accounted for 35.3% and mate access (w2) accounted for 7.8% of I (Table S1). In addition to the ability to marry (w2), the variance in relative fitness could also be driven by the total number of sequential marriages (w3). In preindustrial Finland, monogamy was socially imposed and divorce was forbidden, so men and women could remarry only if widowed (22), resulting in a nonextensive serially monogamous mating system in which 7.7% of all born individuals obtained several partners through remarriages (26). The probability of remarrying was higher in males than in females (19% and 14%, respectively, among married individuals, logistic regression, 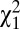 = 13.6, P < 0.001) and similar for both social classes (
= 13.6, P < 0.001) and similar for both social classes (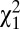 = 0.37, P = 0.54). This finding was also true when restricting our sample to only individuals who had the possibility to remarry (i.e., whose first spouse died before them). For this episode, the variation in relative fitness was again greater in males than in females (P < 0.001) but not significantly different between the social classes (P = 0.10). This fitness component contributed 5.6% of I. Finally, among married individuals, the number of offspring produced over lifetime to landless and landowning individuals was, respectively, 4.73 ± 3.50 and 4.84 ± 3.58 for men and 4.27 ± 3.11 and 4.55 ± 3.30 for women. The opportunity for selection because of variation in average number of offspring per mate (w4) did not differ significantly between the four categories of individuals by wealth and sex (P = 0.81) and accounted for 26.8% of I. Thus, the greater I measured in men results from their greater variance in early survival, in ever marrying, and in number of marriages.
= 0.37, P = 0.54). This finding was also true when restricting our sample to only individuals who had the possibility to remarry (i.e., whose first spouse died before them). For this episode, the variation in relative fitness was again greater in males than in females (P < 0.001) but not significantly different between the social classes (P = 0.10). This fitness component contributed 5.6% of I. Finally, among married individuals, the number of offspring produced over lifetime to landless and landowning individuals was, respectively, 4.73 ± 3.50 and 4.84 ± 3.58 for men and 4.27 ± 3.11 and 4.55 ± 3.30 for women. The opportunity for selection because of variation in average number of offspring per mate (w4) did not differ significantly between the four categories of individuals by wealth and sex (P = 0.81) and accounted for 26.8% of I. Thus, the greater I measured in men results from their greater variance in early survival, in ever marrying, and in number of marriages.
Fig. 2.
Partitioning of total opportunity for selection. Variance in relative reproductive success is divided into four fitness components or episodes of selection: survival to reproductive age (w1), mate access (w2), mating success (w3), and fertility per mate (w4). All covariance terms have been summed (cov) (see Table S1 for details). Survival and fertility account for most of the total opportunity. Diameters of pie charts are proportional to the total opportunity for selection.
Third, we detected an opportunity for sexual selection (Fig. 1B) even if natural selection exceeded selection triggered by competition for mates. The opportunity for sexual selection (IS) sets the upper bound on the force of sexual selection that can act on any phenotypic character and can be measured as the variance in relative mating success, i.e., the variance in absolute number of mates divided by the square of the mean number of mates (27). In our sample, it can be computed directly, either as the variance in relative number of marriages for the subsample of individuals who survived to age 15 y (to exclude variation in mating success because of death before reproductive age) or by investigating the proportion of variation in fitness captured by the combination of episodes w2 and w3 (w2w3). Both methods led to similar estimates with IS equaled 0.33 (CI95% = 0.30–0.35), representing 14.5% of I. This variance in relative mating success was 62.0% higher in males than in females (P < 0.001) and was not significantly influenced by social class (P = 0.52).
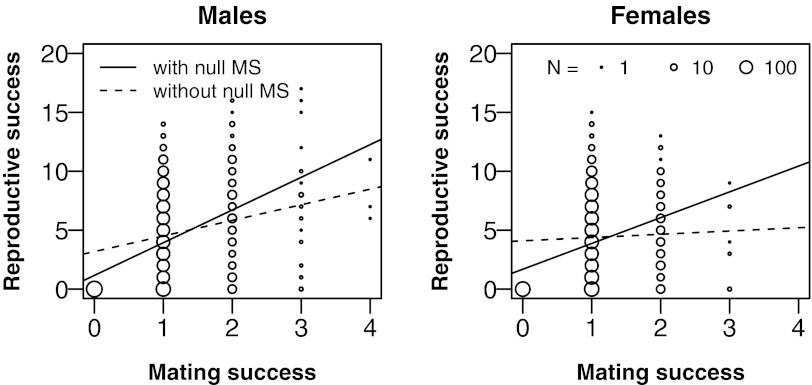
Fourth, we studied the relationship between mating and reproductive success and found that variation in mating success contributed more to reproductive success in males than in females (Fig. 3). We quantified the influence of mating success on reproductive fitness with the Bateman gradient, measured here in its standardized version as the slope of the least-squares regression of the relative reproductive success on the relative mating success (βSS) (9, 18). To remove variation attributable to early death, we only included individuals surviving to reproductive age. The overall βSS equaled 0.65 (CI95% = 0.61–0.70), indicating that improved mating success increased reproductive success. The gradient value was 23.9% higher for males than for females (P = 0.002), suggesting that mating success improved reproductive success more in men than in women. There was no significant difference in gradient values between the social classes (P = 0.39). For all categories of individuals, mating success influenced reproductive success as indicated by nonzero βSS (Fig. 1C). To study the influence of mating with several partners, we also analyzed the relationship between mating and reproductive success in a subsample of individuals who married at least once (Fig. 3). The results show that this derivation of the standardized Bateman gradient (βSS′) is positive for males (βSS′ = 0.34, CI95% = 0.22–0.46), potentially zero for females (βSS′ = 0.07, CI95% = −0.04–0.20), and does not significantly differ between social classes (P = 0.66; Fig. S3). The fact that only men benefited from mating successively with different partners followed from the age difference between remarried women and the wives of remarried men: whereas 42.1% of women remarrying were already older at the time of their second marriage than the median age of female reproductive cessation at 41 y (28), only 19.9% of men married a second spouse older than 41 y (26).
Fig. 3.
Relationship between number of marriages and number of children in males (Left) and females (Right). Lines represent regression lines including all individuals surviving to reproductive age (continuous line) or only those married at least once (dashed line). The diameters of circles are proportional to the logarithm of the number of individuals.
Finally, using our estimates of the opportunity for sexual selection and the Bateman gradients, we computed the maximal selection differential caused by sexual selection (S′max) (9) and found that sexual selection could have exerted important evolutionary changes in this population (Fig. S2). The magnitude of S′max is given by the product of IS1/2 and βSS (9). Our data show that sexual selection alone could have triggered a shift in the average value of a trait corresponding to up to 0.37 SD (CI95% = 0.34–0.40) after a single generation of directional selection. It therefore represents 24.8% of the maximal magnitude of the shift engendered by total selection. S′max was 57.8% higher in males than in females (P < 0.001) and of similar magnitude in both social classes (P = 0.65).
Discussion
We investigated the variance in relative lifetime reproductive success (i.e., the opportunity for total selection) and its components in a historical monogamous human population. We showed that the intensity of Darwinian selection in this population was in line with empirical measurements of the opportunity for selection reported for other species (e.g., refs. 9 and 29–33). The important demographic, cultural, and technological changes that have occurred during the Holocene (the last 10,000 y) have not prevented the opportunity for selection and the potential for evolution in our species. This result contrasts with the traditional view of evolutionary psychologists and social scientists but is consistent with recent findings from a number of disciplines (34). We also demonstrate that, even when the marriage system is strictly monogamous with limited serial monogamy, all requirements for sexual selection to operate can be met (9). Importantly, our findings are likely to be conservative with respect to unmeasured but usually rare extrapair paternity events (35) because they increase the opportunity for sexual selection in monogamous populations when mated males are responsible for these paternities (36). Consequently, although the practice of nonextensive serial monogamy and the advent of agriculture may have constrained the opportunity for selection, our study shows that there is still room for substantial selection in such populations.
Most of the variation in relative fitness resulted from two episodes of selection that correspond to natural selection: survival to adulthood and fertility. Nonetheless, sexual selection was also potentially effective; this was true even among women who, in sharp contrast with men, did not benefit from multiple pairings. The variance in reproductive success was largely determined by the fitness difference between mating and nonmating individuals in both sexes. In our historical population, the maximal selection differential that sexual selection could induce was around one-quarter of that induced by total selection, which is in line with results reported by Moorad et al. (20) for a historical frontier population in Utah for a period when polygyny had vanished (early 1890s). Because historical Finland was characterized by nonextensive serial monogamy and high infant and child mortality, our estimates are conservative concerning the relative role of sexual vs. natural selection. Thus, even though socially imposed monogamy can reduce the relative role of sexual selection (20), by demonstrating that all requirements for sexual selection to operate can be met with the opportunity for total selection, the opportunity for sexual selection, and the Bateman gradient all being positive, our findings extend those recently published for the Utah frontier population (20) and suggest that sexual selection can still have a substantial role for the evolution of monogamous human populations with low levels of multiple matings.
We observed considerable differences between the sexes in the maximum strength of both natural and sexual selection. Concerning natural selection, a higher early-mortality rate contributed to the higher opportunity for selection in males. Because sex differences in early mortality may be common, isolating the variance in relative fitness induced by this episode of selection allowed us to exclude from sexual selection estimates variation not caused by the competition for mates. Nevertheless, our results show that sexual selection was the main source of the difference in opportunity for total selection between the sexes, in accordance with Bateman’s 1948 principles (7, 17). At a proximal level, we cannot exclude that sex differences in the opportunities for sexual selection could result from sex differences in survival in adulthood allowing more remarriage opportunities for males than for females (26, 37). Nonetheless, differences in the opportunities for sexual selection are also likely to result from lactational amenorrhea and female menopause (28), which led males to experience a longer reproductive life than females did despite a slightly female-biased adult sex ratio in this population (51.1% of females at age 15 y; see also ref. 38). Indeed, the longer reproductive lifetime of males can explain the stronger variance in male mating and reproductive success because (i) the greater number of males than females available for reproduction increased intrasexual competition for mates among males; (ii) it created a greater remarriage probability among men than among women; and (iii) multiple marriages increased male fitness because men could have additional children with their younger new spouse, whereas women who were remarrying were usually too old to produce more offspring. Our results therefore stress the importance of using methods that allow the isolation of sex differences caused by natural selection from sexual selection estimates and using data covering the full lifespan of individuals to capture reproductive events at old ages in men, to thus provide an unbiased estimate of the opportunity for sexual selection.
In sharp contrast to the sex differences, social class did not influence our measures of selection. Our results were similar for the two social classes representing one group of individuals with parents whose subsistence did not rely on wealth inheritance practices (landless) and another group of individuals from parents whose subsistence relied on transmitted wealth (landowners). We used parental rather than offspring social class to be able to attribute a social class to each individual, including those who died young. It is therefore possible that changes in social class between generations reduced the social class differences in opportunity for selection in our analysis. However, parents influenced the marriages of their children, and the inheritance of social class was moderately high in the studied populations (39), suggesting that the undetected social differences, if real, cannot be large. Many theoretical studies assume that resource accumulation is crucial for selection affecting humans (37), and phenotypic selection has been shown to be related to male wealth (15). Compared with other historical populations in Europe, variation in socio-economic status within the studied rural population was low (16). Our results therefore suggest that the degree of wealth accumulation needed to shape selection may be substantial and achieved only under conditions of strong social stratification. Further studies are needed to determine the generality of this finding in different socio-ecological environments.
Compared with results reported for other human populations, our estimate for the opportunity for total selection appears high (∼2.3, sexes pooled). For example, similar values in a review of 18 populations ranged from 0.10 to 2.15 (11) and equaled 0.65 for Utah in the early 1890s (20) and 0.55 for contemporary United States (21). The main reason for the relatively high opportunity for total selection in Finland comes from a large (∼60%) nulliparous fraction of each birth cohort. Previous studies measuring the opportunity for selection in humans report lower values for the total opportunity for selection for two main reasons. First, most previous studies relied on data only from adults and/or married individuals and/or parents instead of including all born individuals and therefore excluded nulliparous individuals from their samples (e.g., in ref. 11). Such a practice can lead to an underestimation of the variation in relative fitness. Indeed, remeasuring the opportunity for total selection in our dataset but considering only parents led to a drastic estimate reduction (from 2.52 to 0.31 for males and from 2.03 to 0.32 for females). Moreover, because the proportion of nulliparous individuals can differ between the sexes, such methodological artifacts could also explain the absence of differences in the opportunity for total selection between the sexes reported for several populations (11). For example, Brown et al. (11) reported for Finns no evidence of higher variance in reproductive success of males compared with females when using a sample of parents only (from study in ref. 40). However, as shown above, we too find no differences between the sexes if we exclude the nulliparous individuals from the sample, although such differences are present for the entire cohort.
Second, real differences in the proportion of nulliparous individual can also occur naturally across human populations. For example, although the absolute variance in reproductive success is very similar in Utah and Finland (10.4 vs. 10.6), the large discrepancy in nulliparity between the populations (∼19% vs. 60%) induced an important difference in the average fertility of all born individuals (3.6 vs. ∼2.16). Consequently, Moorad et al. (20) reported a much lower opportunity for total selection than we do, despite considering the nulliparous class of individuals in their analyses. The difference in average fertility between these historical populations appears to have mainly originated from differences in child mortality rate as well as differences in fertility rates between mothers. Although more than 80% of all born Utahns survived to 16 y old in the 1890s (41), Finland was characterized by ∼60% of individuals surviving to 15 y old and therefore presented an early-mortality rate much closer to values reported for contemporary hunter-gatherer populations (42). Consequently, when measuring the opportunity for total selection in our dataset only on individuals surviving to the reproductive age, we obtained an estimate of 0.82 (sexes pooled) more similar to the one reported for Utah. By lowering the competition for resources and so prereproductive mortality, colonizing human populations might undergo a reduction in selection. This finding should be considered, together with founder effects, to understand the evolution of genetic diversity in such pioneer populations. Concerning differences in fertility rates, Utahn mothers had ∼5.0 children in their lifetime, compared with 4.4 for Finns. In addition to the demographic and environmental characteristics of pioneer populations, economic returns to land are usually low relative to labor in frontier environments (43) and could have contributed to the socially supported desire for high family size of these Mormons. All in all, comparing Bateman principles across populations as suggested by Brown et al. (11), or more generally the contribution of the different episodes of selection on the opportunity for selection as introduced here (8, 25), can shed light on the causes and consequences of human diversity. For such efforts, ensuring that the estimates from different populations accurately reflect differences in phenotypic selection rather than methodological discrepancies is crucial (see also ref. 44).
We recall that measuring the opportunity for selection does not necessarily equal measurements of actual selection acting on traits other than fitness. Variation in fitness is caused by several traits that are not all independent (e.g., tradeoffs, pleiotropy), and some variation in reproductive or mating success is unrelated to the phenotype and genotype of an individual. This fact has triggered criticisms of the relevance of global measurements of selection at the phenotypic level, such as the opportunity for selection (e.g., refs. 45, 46). Despite these criticisms and the inherent limitations shared by any phenotypic approach to selection, there are at least five reasons why much can be learned by global measurements of selection. First, measurements of opportunity for selection are necessary for identifying whether natural and sexual selection can operate at all (47). For example, we showed that sexual selection can act in both sexes despite socially constrained monogamy. Second, from the opportunity for selection we can derive the maximum evolutionary rate for any trait, given the proportion of variation in fitness transmitted to the next generation that this trait can explain (8, 9). For example, our analysis shows that a hypothetical trait explaining 5% of transmitted variation in fitness may have evolved up to 0.08 SD per generation of directional selection. Such estimates provide guidance for discussing the possible scope and limits of phenotypic evolution, which is especially relevant for historical populations among which most phenotypic characteristics are no longer accessible. Third, global measurements of selection allow us to decompose the variance in fitness into episodes of selection, indicating where selection has the greatest potential to act (8, 47). Our results suggest that traits influencing survival and fertility were likely to be under stronger selection than those influencing mate choice in agrarian Finland. Fourth, measurement of the opportunity for selection allows comparison across classes of individuals. We showed that possession of wealth does not necessarily constrain selection and that competition for mates is likely to be a stronger selective pressure for males than for females. Finally, we also demonstrated the benefit of comparing measurements performed in different populations by illustrating that differences in environmental and social constraints might explain differences in opportunity for selection between preindustrial Finland and Utah. We therefore subscribe to the view of Krakauer et al. (47), who see approaches relying on opportunity for selection as a complementary approach to that focusing on phenotypic measurements of selection at the level of specific traits.
To conclude, monogamy may have limited the potential for selection to operate in agricultural human populations (20). Nonetheless, such a change did not introduce insurmountable constraint on the variation in fitness, implying that there is still room for relatively strong selection to operate in preindustrial Finland. Whether this finding is general among all populations with enforced monogamy remains an open question because other factors can also influence sex roles (11). This question calls for replications of measurements of the different components of the opportunity for selection using either samples from other populations that are not biased toward individuals with offspring or methods allowing correction for potential bias (see ref. 20). Our results stress that it is misleading to assume that all human populations have remained unaffected by selection for the last few millennia. In addition to genetic studies and phenotypic studies focusing on particular traits, global measurements of selection advance our understanding of human evolution, and studies focusing on evolutionary changes in both historical and current populations should be encouraged.
Materials and Methods
Demographic Data.
We used demographic data (n = 5,923) collected by the Lutheran Church from four geographically separate populations in Finland, one inland parish (Ikaalinen) and three coastal parishes (Hiittinen, Kustavi, and Rymättylä), for individuals born between 1760 and 1849. For more details on all methods, see SI Materials and Methods.
Variables.
We used marriage number as a proxy to measure mating success because the number of mating partners before marriage was low (22) and because it is unlikely that extrapair paternities would have exceeded the current worldwide estimate of 3% for populations with high paternity certainty (35). We measured reproductive success as the lifetime number of children of all born individuals. This measurement includes survival and fertility components and therefore corresponds to an estimate of the lifetime reproductive success. We grouped individuals into two social classes depending on their father’s occupation (landowners vs. landless). Although parental and offspring social class could differ, inheritance of social class was moderately high (39). This classification enabled us to attribute a social class to all individuals, including those who died before adulthood, and to compare opportunities for selection between social classes throughout successive episodes of selections occurring during the life cycle.
Statistics.
To divide the opportunity for total selection into different episodes of selection, we used methods described in refs. 8 and 25. Opportunity for selection, Bateman gradients, and maximum selection differential were computed following ref. 9. P values with no statistics were computed by using permutation tests. CI95% values were obtained from bootstraps. Each permutation test and bootstrap involved 10,000 data shufflings. Analyses were performed with R 2.10.
Supplementary Material
Acknowledgments
We thank K. Pokkinen, A. Siitonen, L. Iso-Iivari, and V. P. Toropainen for data collection as well as M. Borgerhoff-Mulder, P. David, A. Jones, J. Moorad, I. Rickard, and anonymous referees for comments on the manuscript. We also thank Wissenschaftskolleg zu Berlin, the Kone Foundation, the Royal Society, and the European Research Council for funding.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118174109/-/DCSupplemental.
References
- 1.Cronin H. The Ant and the Peacock: Altruism and Sexual Selection from Darwin to Today. Cambridge, UK: Cambridge Univ Press; 1993. [Google Scholar]
- 2.Runciman W. The Theory of Cultural and Social Selection. Cambridge, UK: Cambridge Univ Press; 2009. [Google Scholar]
- 3.Smith EA, Borgerhoff Mulder M, Hill K. Controversies in the evolutionary social sciences: A guide for the perplexed. Trends Ecol Evol. 2001;16(3):128–135. doi: 10.1016/s0169-5347(00)02077-2. [DOI] [PubMed] [Google Scholar]
- 4.Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- 5.Stearns SC, Byars SG, Govindaraju DR, Ewbank D. Measuring selection in contemporary human populations. Nat Rev Genet. 2010;11:611–622. doi: 10.1038/nrg2831. [DOI] [PubMed] [Google Scholar]
- 6.Kelley JL, Swanson WJ. Positive selection in the human genome: From genome scans to biological significance. Annu Rev Genomics Hum Genet. 2008;9(1):143–160. doi: 10.1146/annurev.genom.9.081307.164411. [DOI] [PubMed] [Google Scholar]
- 7.Shuster SM, Wade MJ. Mating Systems and Strategies. Princeton, NJ: Princeton Univ Press; 2003. [Google Scholar]
- 8.Arnold SJ, Wade MJ. On the measurement of natural and sexual selection: Theory. Evolution. 1984;38:709–719. doi: 10.1111/j.1558-5646.1984.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones AG. On the opportunity for sexual selection, the Bateman gradient and the maximum intensity of sexual selection. Evolution. 2009;63:1673–1684. doi: 10.1111/j.1558-5646.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 10.Crow JF. Some possibilities for measuring selection intensities in man. Hum Biol. 1958;30(1):1–13. [PubMed] [Google Scholar]
- 11.Brown GR, Laland KN, Borgerhoff Mulder M. Bateman’s principles and human sex roles. Trends Ecol Evol. 2009;24(6):297–304. doi: 10.1016/j.tree.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byars SG, Ewbank D, Govindaraju DR, Stearns SC. Colloquium papers: Natural selection in a contemporary human population. Proc Natl Acad Sci USA. 2010;107(Suppl 1):1787–1792. doi: 10.1073/pnas.0906199106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milot E, et al. Evidence for evolution in response to natural selection in a contemporary human population. Proc Natl Acad Sci USA. 2011;108:17040–17045. doi: 10.1073/pnas.1104210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spuhler JN. The maximum opportunity for natural selection in some human populations. In: Zubrow EBW, editor. Demographic Anthropology: Quantitative Approaches. Albuquerque: Univ of New Mexico Press; 1976. pp. 185–226. [Google Scholar]
- 15.Nettle D, Pollet TV. Natural selection on male wealth in humans. Am Nat. 2008;172:658–666. doi: 10.1086/591690. [DOI] [PubMed] [Google Scholar]
- 16.Korpelainen H. Fitness, reproduction and longevity among European aristocratic and rural Finnish families in the 1700s and 1800s. Proc Biol Sci. 2000;267:1765–1770. doi: 10.1098/rspb.2000.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bateman AJ. Intra-sexual selection in Drosophila. Heredity (Edinb) 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- 18.Arnold SJ, Duvall D. Animal mating systems: A synthesis based on selection theory. Am Nat. 1994;143:317–348. [Google Scholar]
- 19.Kokko H, Jennions MD. Parental investment, sexual selection and sex ratios. J Evol Biol. 2008;21:919–948. doi: 10.1111/j.1420-9101.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 20.Moorad JA, Promislow DEL, Smith KR, Wade MJ. Mating system change reduces the strength of sexual selection in an American frontier population of the 19th century. Evol Hum Behav. 2011;32(2):147–155. doi: 10.1016/j.evolhumbehav.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jokela M, Rotkirch A, Rickard IJ, Pettay JE, Lummaa V. Serial monogamy increases reproductive success in men but not in women. Behav Ecol. 2010;21:906–912. [Google Scholar]
- 22.Saarimäki P. The Norms, Practices and Conflicts of Sex and Marriage - Premarital and Marital Sexual Activity in Rural Central Finland in the Late Nineteenth Century. Jyväskylä, Finland: Jyväskylä University; 2010. [Google Scholar]
- 23.Rickard IJ, et al. Food availability at birth limited reproductive success in historical humans. Ecology. 2010;91:3515–3525. doi: 10.1890/10-0019.1. [DOI] [PubMed] [Google Scholar]
- 24.Lahdenperä M, Lummaa V, Helle S, Tremblay M, Russell AF. Fitness benefits of prolonged post-reproductive lifespan in women. Nature. 2004;428(6979):178–181. doi: 10.1038/nature02367. [DOI] [PubMed] [Google Scholar]
- 25.Arnold SJ, Wade MJ. On the measurement of natural and sexual selection: Applications. Evolution. 1984;38:720–734. doi: 10.1111/j.1558-5646.1984.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 26.Lahdenperä M, Lummaa V, Russell AF. Selection on male longevity in a monogamous human population: late-life survival brings no additional grandchildren. J Evol Biol. 2011;24(5):1053–1063. doi: 10.1111/j.1420-9101.2011.02237.x. [DOI] [PubMed] [Google Scholar]
- 27.Wade MJ, Arnold SJ. The intensity of sexual selection in relation to male sexual behaviour, female choice, and sperm precedence. Anim Behav. 1980;28:446–461. [Google Scholar]
- 28.Broekmans FJ, Knauff EAH, te Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: Current knowledge and future trends. Trends Endocrinol Metab. 2007;18(2):58–65. doi: 10.1016/j.tem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Vanpé C, et al. Mating system, sexual dimorphism, and the opportunity for sexual selection in a territorial ungulate. Behav Ecol. 2008;19:309–316. [Google Scholar]
- 30.Tatarenkov A, Healey CIM, Grether GF, Avise JC. Pronounced reproductive skew in a natural population of green swordtails, Xiphophorus helleri. Mol Ecol. 2008;17:4522–4534. doi: 10.1111/j.1365-294X.2008.03936.x. [DOI] [PubMed] [Google Scholar]
- 31.Coltman DW, et al. Density-dependent variation in lifetime breeding success and natural and sexual selection in Soay rams. Am Nat. 1999;154(6):730–746. doi: 10.1086/303274. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Muñoz R, Bretman A, Slate J, Walling CA, Tregenza T. Natural and sexual selection in a wild insect population. Science. 2010;328:1269–1272. doi: 10.1126/science.1188102. [DOI] [PubMed] [Google Scholar]
- 33.Rossiter SJ, Ransome RD, Faulkes CG, Dawson DA, Jones G. Long-term paternity skew and the opportunity for selection in a mammal with reversed sexual size dimorphism. Mol Ecol. 2006;15:3035–3043. doi: 10.1111/j.1365-294X.2006.02987.x. [DOI] [PubMed] [Google Scholar]
- 34.Bolhuis JJ, Brown GR, Richardson RC, Laland KN. Darwin in mind: New opportunities for evolutionary psychology. PLoS Biol. 2011;9:e1001109. doi: 10.1371/journal.pbio.1001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson KG. How well does paternity confidence match actual paternity? Curr Anthropol. 2006;47:513–520. [Google Scholar]
- 36.Webster MS, Pruett-Jones S, Westneat DF, Arnold SJ. Measuring the effects of pairing success, extra-pair copulations and mate quality on the opportunity for sexual selection. Evolution. 1995;49:1147–1157. doi: 10.1111/j.1558-5646.1995.tb04441.x. [DOI] [PubMed] [Google Scholar]
- 37.Käär P, Jokela J, Merilä J, Helle T, Kojola I. Sexual conflict and remarriage in preindustrial human populations: Causes and fitness consequences. Evol Hum Behav. 1998;19(3):139–151. [Google Scholar]
- 38.Ranta E, Lummaa V, Kaitala V, Merilä J. Spatial dynamics of adaptive sex ratios. Ecol Lett. 2000;3(1):30–34. [Google Scholar]
- 39.Pettay JE, Helle S, Jokela J, Lummaa V. Natural selection on female life-history traits in relation to socio-economic class in pre-industrial human populations. PLoS ONE. 2007;2:e606. doi: 10.1371/journal.pone.0000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettay JE, Kruuk LEB, Jokela J, Lummaa V. Heritability and genetic constraints of life-history trait evolution in preindustrial humans. Proc Natl Acad Sci USA. 2005;102:2838–2843. doi: 10.1073/pnas.0406709102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jorde LB. Consanguinity and prereproductive mortality in the Utah Mormon population. Hum Hered. 2001;52(2):61–65. doi: 10.1159/000053356. [DOI] [PubMed] [Google Scholar]
- 42.Gurven M, Kaplan H. Longevity among hunter-gatherers: A cross-cultural examination. Popul Dev Rev. 2007;33:321–365. [Google Scholar]
- 43.Carr DL, Pan WKY, Bilsborrow RE. Declining fertility on the frontier: The Ecuadorian Amazon. Popul Environ. 2006;28(1):17–39. doi: 10.1007/s11111-007-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klug H, Lindström K, Kokko H. Who to include in measures of sexual selection is no trivial matter. Ecol Lett. 2010;13:1094–1102. doi: 10.1111/j.1461-0248.2010.01495.x. [DOI] [PubMed] [Google Scholar]
- 45.Grafen A. Sexual Selection: Testing the Alternatives. Chichester, UK: Wiley; 1987. pp. 221–233. [Google Scholar]
- 46.Klug H, Heuschele J, Jennions MD, Kokko H. The mismeasurement of sexual selection. J Evol Biol. 2010;23:447–462. doi: 10.1111/j.1420-9101.2009.01921.x. [DOI] [PubMed] [Google Scholar]
- 47.Krakauer AH, Webster MS, Duval EH, Jones AG, Shuster SM. The opportunity for sexual selection: Not mismeasured, just misunderstood. J Evol Biol. 2011;24:2064–2071. doi: 10.1111/j.1420-9101.2011.02317.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.