Abstract
Humans respond to foreign antigen by generating plasma Abs and memory B cells (MBCs). The Ab response then declines, sometimes to below the limit of detection. In contrast, MBCs are generally thought to be long-lived. We tested and compared Plasmodium falciparum (Pf)-specific Ab and MBC responses in two populations of children: (i) previously exposed children who had documented Pf infections several years ago, but minimal exposure since then; and (ii) persistently exposed children living in a separate but nearby endemic area. We found that although Pf-specific plasma Abs were lower in previously exposed children compared with persistently exposed children, their cognate MBCs were maintained at similar frequencies. We conclude that serological analysis by itself would greatly underestimate the true memory of Pf-specific Ab responses in previously exposed children living in areas where Pf transmission has been reduced or eliminated.
Keywords: immunological, immunity, protection, longevity, maintenance
Despite optimism in the 1950s and 1960s that malaria could be eradicated, it remains a major public health problem in tropical countries (1, 2). Transmission intensity and disease incidence is declining in some areas of Africa (3–6), and elimination and even eradication of malaria is again part of the global health agenda (1). Naturally acquired immunity to malaria limits malaria morbidity and mortality in older children and adults (7–9). Immunity is acquired following exposure; therefore, as malaria transmission falls, so may immunity, rendering the immune individual or population susceptible again (10–12). Hence, the substantial public health gains from malaria control (13) may be threatened by the increased susceptibility of populations if malaria transmission were to be resurgent. However, it is not known to what extent naturally acquired immunity depends upon persistent exposure. Therefore, it is important that we gain a thorough understanding of the longevity of naturally acquired immunity to malaria in the absence of persisting exposure.
Circulating plasma Abs are associated with antimalarial immunity (14–16). However, these responses may be short-lived (particularly in young children) (17–19), though long-lived responses also develop, particularly in older individuals (20, 21).
Unlike plasma Abs (surrogates for plasma cells), which can decline to undetectable levels following antigen clearance, human memory B cells (MBCs) are generally long-lived (22–25). For example, antivaccinia IgG MBCs can persist for 50 y after vaccination with vaccinia (25). Hepatitis B virus (HBV)-specific MBCs persist after HBV vaccination (26–29), despite half of the vaccine-induced Ab being lost within a few years (30).
Rapid boosting of Ab responses to various Plasmodium falciparum (Pf) antigens has been reported after reexposure to malaria following prolonged periods of either sustained control or drought in both children and adults, suggesting that they can generate and retain Pf-specific MBC (31, 32). Our earlier attempts to enumerate Pf-specific MBCs in human peripheral blood mononuclear cells (PBMC) reported low frequencies of malaria-antigen specific MBCs (33). However, the more recent studies have reported higher frequencies of these cells because of progressive improvements of the enzyme-linked immunospot (ELISpot)-based assay for quantification of human MBCs (33–37). Wipasa et al., reported stable malaria-specific Ab and MBC levels in adults with a clear history of prior exposure to malaria but living in an area of extremely low transmission in Thailand (37). In a year-long prospective study of children and adults in Mali, Weiss et al., demonstrated that Pf-specific MBC and Ab titers increased after acute malaria and then, after 6 mo of decreased Pf exposure, contracted to a point slightly higher than preinfection levels (36). Unlike the earlier study in Thai adults, the Mali study could not determine the maintenance of Pf-specific MBC in the children and adults they tested because it was conducted in an area of high seasonal transmission, where transmission resumes after the 6-mo dry period following the transmission season.
The aim of our study was to determine whether Pf-specific IgG MBCs would reflect anti-Pf responses, even when circulating plasma Abs in children had decayed because of lack of ongoing exposure. We therefore compared circulating MBCs and Abs between previously exposed and persistently exposed children living in two geographically distinct villages of Kilifi but of similar socioeconomic status and access to health care.
Results
Characteristics of the Study Subjects.
IgG MBC and Ab responses to tetanus toxoid (TT) and the malaria antigens apical merozoite antigen (AMA) 1, merozoite surface protein (MSP) 1 42 kDa, as well as the total parasite antigens (Pf lysate) were determined in children from two cohorts: Ngerenya (previously exposed) and Junju (persistently exposed). The reduction in malaria transmission intensity from a parasite prevalence of 30% to 0% over 7 y in Ngerenya (4) is contrasted with the sustained transmission in Junju (Fig. S1). We selected 105 children from Ngerenya who have had at least one documented infection with Pf, but who subsequently remained free of malaria because of the dramatic reduction of transmission in their area (Table 1). The median time since the last documented malaria episode for these children was 82.6 [interquartile range (IQR), 74.9–89.3] mo to the time of sampling. For comparison, we selected 76 children from Junju, who similarly have had at least one documented Pf infection and who remain at risk of infection because of the ongoing malaria transmission. The median time since the last documented malaria episode for these children was 5.3 (IQR, 3.5–12.2) mo. Although afebrile at the time of sampling, 30% of the persistently exposed children had asymptomatic Pf parasitaemia by microscopy. None of the previously exposed children had detectable parasitaemia by blood smears or Pf-specific PCR.
Table 1.
Characteristics of study participants
| Variable | Junju | Ngerenya |
| Total no. | 76 | 105 |
| Sex (%) | ||
| Female | 43 (56.58%) | 46 (43.81%) |
| Male | 33 (43.42%) | 59 (56.19%) |
| Age (y) | ||
| Mean (95% CI) | 6.95 (6.47–7.43) | 10.89 (10.46–11.31) |
| Range | 1–10 | 5–14 |
| Total no. of previous Plasmodium falciparum malaria episodes (no.)* | ||
| Mean (95% CI) | 6.22 (5.51–6.94) | 3.91 (3.24–4.59) |
| Range | 1–15 | 1–15 |
| Time since last episode (mo) | ||
| Median, IQR | 5.3 (3.5–12.2) | 82.6 (74.93–89.3) |
| Range | 0.67–40.6 | (3.27–128.63) |
| Plasmodium falciparum infection status and sampling (% positive) | ||
| By blood smears | 24 (31.57) | 0 |
| By PCR | ND | 0 (0) |
CI, confidence interval; ND, not determined.
*Data from weekly active surveillance and available from birth.
Pf-Specific MBCs but Not Their Cognate Plasma Abs Are Maintained at Similar Levels and Prevalence in the Presence or Absence of Persistent Exposure.
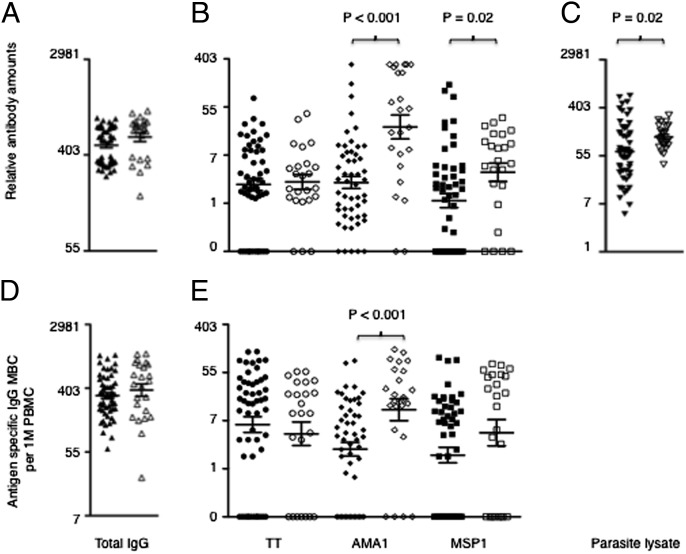
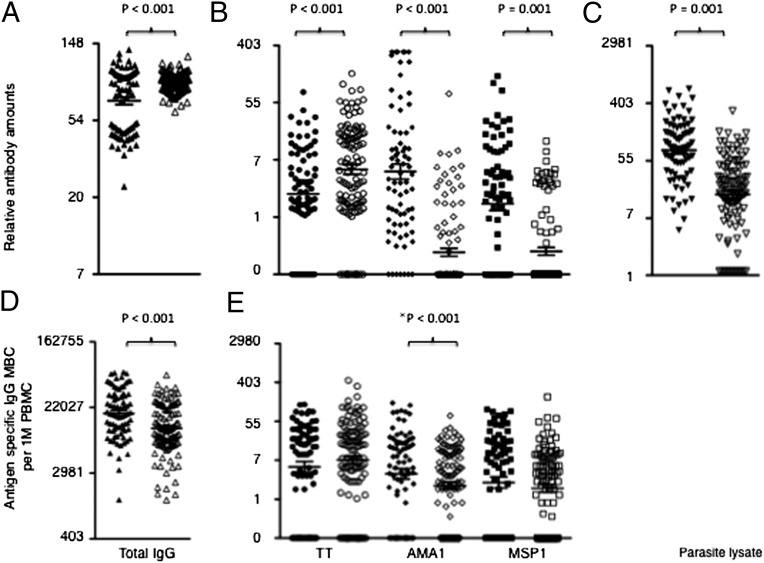
Because asymptomatic parasitaemia was associated with elevated anti-Pf plasma Abs and elevated MBC responses (Fig. 1), we adjusted for the presence of asymptomatic parasitaemia as a covariate in the comparisons and multivariable analyses that follow. Mean Ab levels to Pf antigens were higher among the persistently exposed than the previously exposed children, but Pf-specific MBC responses were similar (Fig. 2 B and E). Similarly, the prevalence of Ab responses to Pf antigens was higher among the persistently exposed than the previously exposed children, but prevalence of Pf-specific MBC responses was similar (Fig. S2).
Fig. 1.
Asymptomatic infections were associated with higher anti-Pf antigen-specific Ab and MBC responses. Levels of Abs and MBCs were determined from cross-sectional samples obtained at the end of a 4-mo dry period (during which there is minimal to nil Pf transmission in Junju) by antigen-specific ELISA and ELISpot, respectively. Of the 76 Junju children tested, 23 had Pf asymptomatic infections as determined by positive blood smears at the time of sampling. Frequencies of MBCs are expressed per million of cultured PBMCs. Shown are the comparisons of Ab levels and MBC frequencies between the uninfected (filled symbols) and infected (open symbols) persistently exposed children for: (A) levels of total serum IgG Abs; (B) levels of anti-TT, anti-AMA1, and anti-MSP1 Abs; (C) levels of anti-Pf lysate Abs; (D) frequencies of total IgG MBCs; and (E) frequencies of anti-TT, anti-AMA1, and anti-MSP1 MBCs. P values are only shown for the significant differences and were obtained using student t-test analyses of logarithmically transformed data. Bars indicate mean ± standard error.
Fig. 2.
Anti-Pf MBCs, but not the relatively short-lived serum Abs, are maintained at similar frequencies in previously compared to persistently exposed children. Levels of MBCs and Abs were determined from cross-sectional samples obtained at the end of a 4-mo dry period by ELISA and ELISpot, respectively. MBC frequencies are expressed per million cultured PBMCs. Shown are the comparisons of B-cell memory responses between persistently (filled symbols) and previously exposed children (open symbols) for: (A) levels of total serum IgG Abs; (B) levels of anti-TT, anti-AMA1, and anti-MSP1 Abs; (C) levels of anti-Pf lysate Abs; (D) frequencies of total IgG MBCs; and (E) frequencies of anti-TT, anti-AMA1, and anti-MSP1 MBCs. P values are only shown for the significant differences, and were obtained using student t-test analyses of logarithmically transformed data. Bars indicate mean ± standard error. An asterisk indicates where the P value increased above 0.05 after controlling for asymptomatic infections among the persistently exposed children.
Unexpectedly, TT-specific Ab levels were higher among the previously exposed than the persistently exposed children, but TT-specific MBC frequencies were similar (Fig. 2B). Similarly, total plasma IgG Ab levels were higher among previously exposed than persistently exposed children (Fig. 2A), but the converse was true for total IgG MBC frequencies (Fig. 2D).
We found positive correlations between levels of AMA1- and MSP1-specific Abs (r = 0.7, P < 0.005), but not between AMA1- and TT-specific Abs (r = 0.15, P = 0.14), or between MSP1- and TT-specific Abs (r = 0.06, P = 0.57).
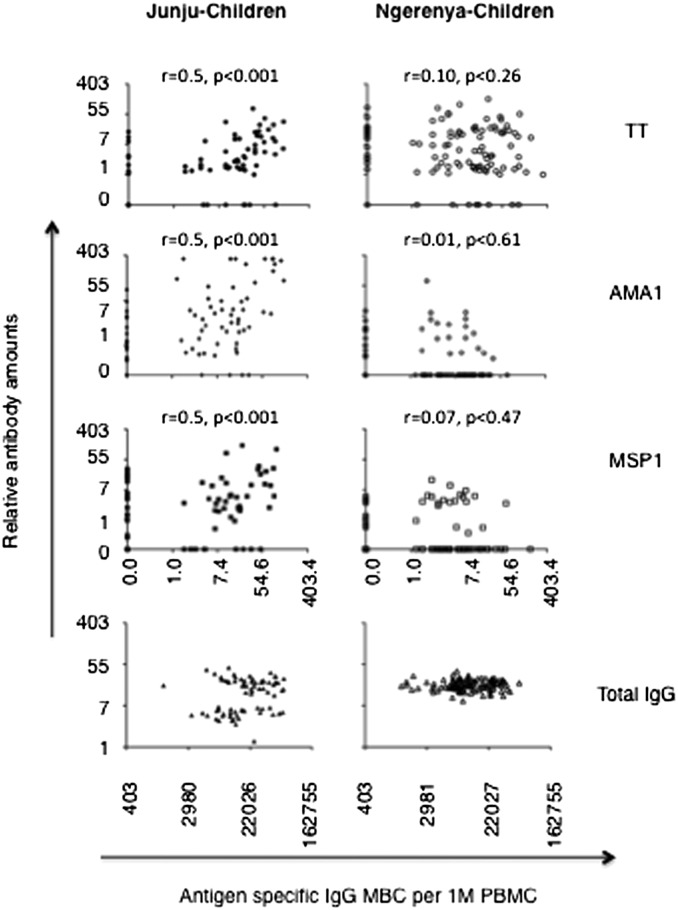
Pf-specific MBC levels were positively correlated with the levels of their respective cognate plasma Abs among the persistently exposed but not previously exposed children (Fig. 3). Unexpectedly, anti-TT MBCs and their respective Abs were correlated among the persistently exposed, but not the previously exposed children.
Fig. 3.
Frequencies of antigen-specific MBCs correlate with levels of cognate Abs in the presence but not in the absence of persistent Pf transmission. Correlation plots for Abs versus MBCs among persistently and previously exposed children. Spearman correlation coefficients and their respective P values were obtained using logarithmically transformed data.
Frequencies of AMA1- and MSP1-Specific MBCs and Ab Levels and Prospective Risk of Malaria Episode.
We determined prospectively whether Pf-specific B-cell responses measured after a 4-mo dry period with minimal Pf-transmission, just prior to the main Pf transmission season in Junju (of approximately 9 mo), were associated with the subsequent risk of malaria. A malaria episode was defined as an axillary temperature of 37.5 °C associated with a Pf asexual parasitaemia of at least 2,500 parasites per milliliter (38, 39). Among children without asymptomatic parasitaemia at baseline, high AMA1-specific MBC frequencies were associated with a lower hazard for subsequent malaria, but there was a nonsignificant higher hazard for subsequent malaria among children with asymptomatic parasitaemia at baseline (Table 2 and Fig. S3). In stark contrast, high MSP1-specific MBC frequencies were associated with a higher hazard of subsequent malaria among children without asymptomatic parasitaemia, but a lower hazard of malaria among children with asymptomatic parasitaemia at baseline. Hence, these results suggest that asymptomatic parasitaemia have a strong interaction with malaria-specific MBCs, and that the predictive effect of these interactions was in opposing directions for the two antigens tested. The multivariable model with interaction variables for AMA1- and MSP1-specific MBC with asymptomatic parasitaemia was strongly significant when compared with the respective model without interactions with a likelihood ratio χ2 improvement test of 17.7, P = 0.007.
Table 2.
Interactions between anti-Pf Ab and MBC levels and parasitaemia at baseline influences the prospective risk of clinical malaria
| All children |
Parasite-negative at baseline |
Parasite-positive at baseline |
||||
| Covariate | Hazard ratio | P value | Hazard ratio | P value | Hazard ratio | P value |
| AMA1 Ab | 0.99 (0.82–1.19) | 0.91 | 0.95 (0.74–1.21) | 0.67 | 0.96 (0.57–1.62) | 0.88 |
| AMA1 MBC | 0.84 (0.63–1.11) | 0.22 | 0.65 (0.47–0.91) | 0.01 | 1.62 (0.69–3.85) | 0.27 |
| MSP1 Ab | 1.07 (0.81–1.35) | 0.72 | 1.13 (0.82–1.56) | 0.45 | 0.76 (0.38–1.50) | 0.43 |
| MSP1 MBC | 1.11 (0.88–1.41) | 0.38 | 1.41 (1.04–1.91) | 0.03 | 0.62 (0.40–0.98) | 0.04 |
| Pf-lysate Ab | 0.85 (0.62–1.17) | 0.32 | 0.78 (0.55–1.11) | 0.17 | 0.54 (0.68–4.29) | 0.56 |
| Previous episodes | 1.17 (1.03–1.33) | 0.02 | 1.15 (0.98–1.36) | 0.09 | 1.35 (1.01–1.79) | 0.04 |
| Age (y) | 0.99 (0.98–1.01) | 0.71 | 1.01 (0.99–1.02) | 0.50 | 1.01 (0.97–1.05) | 0.77 |
| Parasitaemia | 0.59 (0.29–1.23) | 0.16 | ||||
Three multivariable Cox regression models are presented, where the parasite-negative and parasite-positive models are synonymous with the model with interactions. In the absence of interactive variables between B-cell responses and parasitaemia at baseline, the hazard ratios were not different from unity. However, inclusion of interactive variables between asymptomatic infections and B-cell responses improved the multivariable model with a log-likelihood ratio (χ2 test) of 17.7, P = 0.007.
Discussion
Our study demonstrates that although Pf-specific Abs declined to undetectable levels in the absence of persistent Pf exposure, their cognate MBCs were long-lived and sustained at similar frequencies to those observed in persistently exposed children. This finding was demonstrable because of the well-defined local epidemiology in Kilifi, where Pf transmission has become negligible in one formerly endemic study area (Ngerenya) but not in another (Junju).
Whether Pf infections can induce long-lived B-cell memory responses to malaria antigens in children has long been a matter of debate (7, 40, 41). A recent study by Wipasa et al., demonstrated that Thai adults exposed to infrequent Pf transmission had generated long-lived Plasmodium-specific MBCs (37). Weiss et al. showed that both children and adults exposed to high Pf transmission in Mali generated Pf-specific B-cell memory responses that increased in magnitude after acute malaria, and thereafter contracted to a point slightly higher than preinfection levels by the start of the subsequent transmission season, which followed 6 mo of decreased Pf exposure (36). In Thailand, malaria transmission has been very low for many years, and hence only adults could be studied. In Mali, transmission is very seasonal but ongoing, hence the longest period of nonexposure was the 6-mo dry season. Similar to the Thai study, our study analyzed B-cell memory responses long after the rapid-decay phase of B-cell responses that immediately follows acute responses and, hence, did not observe a similar kinetic of contracting responses as those observed in the Mali study. Our study could therefore identify stable Pf-specific MBC in previously exposed children, but who had subsequently lived free of malaria for years. As malaria transmission reduces dramatically in several parts of Africa (3–6), and we do not know if rapidly waning immunity poses a substantial threat should malaria transmission recover, it is therefore reassuring that we were able to demonstrate long-lived Pf-specific IgG MBCs in previously exposed children who are now living in areas where Pf transmission has reduced to almost nil.
Although the frequencies of total IgG MBCs were higher in the persistently exposed than the previously exposed children, we found higher concentrations of both total circulating and TT-specific Abs in the absence of continuing Pf transmission. The expanded population of total IgG MBCs in the persistently exposed children may be attributable to continuous activation of MBCs by Pf antigens or their associated polyclonal stimulants (21, 32, 33). Nogaro et al. and Weiss et al. (34, 36) reported possible polyclonal activation of TT and Diptheria-specific B-cell responses following clinical malaria infections. However, TT-specific B-cell responses were not increased in either the presence of asymptomatic parasitaemia or among the persistently exposed children, suggesting that polyclonal activation of other MBC specificities by parasite products or associated cytokine milieu, as seen by Nogaro at al. (34) and Weiss et al. (35) in clinical malaria, is less likely in asymptomatic infection. The reduced total IgG and TT-specific antibodies may be a consequence of continuous activation of MBC by successive Pf infections, generating new plasmablasts that displace older plasma cells from the bone marrow (42), and so in turn accelerating the decay rate for Abs to antigens like TT. The presence of asymptomatic parasitaemia among the persistently exposed children was associated with elevated numbers of AMA1-, but not MSP1-specific MBC. This finding probably reflects inherent differences in immunogenicity between different antigens (42), with the increase in MBC being driven by the asymptomatic parasitaemia.
Although MBCs have a multiplicity of functions, their most direct contribution to protection of the host from disease is via production of protective Abs, whereby they either contribute to the maintenance, or rapid deployment of protective Ab levels upon reinfection. In the latter scenario, the rate of proliferation and differentiation of MBCs into antibody-secreting cells (ASCs) must overtake the pathogen’s replication rate and development of associated pathogenesis. Because it takes ∼5–6 d for MBC to differentiate into ASCs, it has been argued that MBCs specific to the red-blood stage of Pf, which causes disease, may not deploy Abs fast enough to limit pathogenesis (36). However, the expression of some merozoite-stage antigens begins earlier, during liver-stage development (43), raising the possibility that proliferation and differentiation of MBC into ASC could be initiated much earlier in the infection. In multivariable prospective analyses we found evidence for interactions between Pf-specific MBCs and asymptomatic parasitaemia that influenced the associated risk. High frequencies of anti-MSP1 MBCs were associated with increased risk for subsequent malaria episodes among the children who did not have asymptomatic parasitaemia. In contrast, high frequencies of anti-AMA1 MBCs were associated with higher risk for subsequent malaria attack among the children with asymptomatic parasitaemia. We have also previously identified interactions between Pf-specific Abs and asymptomatic parasitaemia in determining risk of clinical malaria (44, 45). The reasons for these interactions are complex, and may reflect confounding by exposure (46), premunition (47), or persistent exposure to antigen (17). Nonetheless, the differential mortality in previously exposed versus unexposed individuals during malaria epidemics in Madagascar (48) suggests that immunological memory may be critical when facing resurgent malaria.
In summary, we conclude that although persistent exposure to Pf antigen is required for the maintenance of circulating Pf-specific Abs in the majority of children in endemic areas, Pf-specific MBCs are maintained independently of sustained exposure to the parasite. There are three major implications. (i) Studies investigating humoral responses to Pf antigens in areas of declining Pf transmission by serology alone would greatly underrepresent past Pf-specific Ab responses. The solution is to combine serology with cellular assays that enable enumeration of Pf-specific MBC. (ii) There is now a need for studies investigating the protective effect of Pf-specific MBCs in areas experiencing recurring epidemics. (iii) Children can generate and maintain long-lived Pf-specific MBC in the absence of continuing Pf exposure should encourage efforts to develop vaccines that induce long-lasting B-cell memory for use in protecting people living in previously endemic areas.
Ultimately, if elimination of Pf malaria is going to be achieved, many former endemic areas will go through transitional periods where reintroductions occur from time to time, especially in areas adjacent to pockets of residual transmission. In such areas, vaccines that induce long-lasting immunity will provide the best long-term protection. In the short term, we can be reassured that Pf-specific immunological memory is not as short-lived as the rapidly lost Ab responses suggest.
Materials and Methods
Ethics.
This study was approved by the Kenyan Medical Research Institute National Ethics Committee. Informed consent was requested from the parents/guardians of the children, as required.
Study Site.
The study was done at the Kenya Medical Research Institute, Centre for Geographic Medicine Research Coast situated at Kilifi District Hospital, Kenya. The hospital serves ∼240,000 people living in Kilifi District. The children investigated were residents in two villages, located within 20 km of each other, with Junju lying on the southern side and Ngerenya on the northern side of an Indian Ocean creek. These study sites are inhabited by predominantly the Mijikenda people, who share similar beliefs and customs and are described in detail elsewhere (38, 39).
Study Population.
Although there has been a gradual decline of Pf transmission in Kilifi District (4, 5), Junju remains stably endemic with two high-transmission seasons (May to August and October to December) and a parasite prevalence of 30% (49, 50). In contrast, Pf transmission has dramatically reduced in Ngerenya, which was endemic with a parasite prevalence of 40% in 1998, and a transmission intensity of 10 infective bites per person per year (51) (Fig. 1). Pf prevalence had declined to between nil and minimal by 2005 and has remained at this level ever since. Children are recruited into the cohorts at birth and actively followed weekly (49) for detection of malaria episodes until the age of 13 y. We maintain extensive and detailed records of the numbers and dates of malaria experiences for each child, either from birth or the time of recruitment.
PBMC and Plasma.
Five milliliters of venous blood samples and blood smears were collected in a preseason cross-sectional survey in May 2010, a time preceded by 4 mo of minimal Pf transmission in Junju. PBMC and plasma for ELISpot and ELISA were harvested and stored in liquid nitrogen and −80o, respectively.
Antigens.
Pf-specific IgG MBC and serum Ab responses were quantified against recombinant Pf AMA1-FVO/3D7 and MSP1 42 kDa, to which circulating IgG Abs have been associated with clinical protection in our study population (44, 52–54). Recombinant Pf antigens were provided by L. H. Miller (National Institutes of Health, Rockville, MD), while the Pf-lysate was home-made. TT was obtained from The National Institute for Biological Standards and Control (United Kingdom).
Determination of Parasitaemia.
Thick and thin blood smears were stained with Giemsa and Pf-infected red cells counted against 500 leukocytes and 1,000 red blood cells, respectively, by expert microscopists. To further confirm that previously exposed were uninfected, a Pf-specific PCR was performed, as previously described (55).
ELISA.
Plasma samples were tested for human IgG Abs specific for Pf and TT antigens using a standard ELISA protocol. For AMA1, ELISA plates were coated with a 1:1 mixture of FVO and 3D7 alleles. Plates were coated overnight at 4 °C, with recombinant proteins and TT at 1 μg/mL, and Pf-lysate and the accompanying red blood cell control lysate at 1 in 500 dilution in bicarbonate buffer (100 μL/well). One-hundred microliters per well of 1 in 1,000 dilution of test plasma in 0.3% (vol/vol) PBST + EDTA was added after plates had been washed three times with 0.05% (vol/vol) Tween in phosphate buffered saline (PBST), and thereafter blocked with 10% (vol/vol) fetal calf serum (FCS)/PBS (200 μL/well). Plates with test plasma were then incubated for 1.5 h at room temperature in a humidified chamber. Plates were then washed five times before the addition of alkaline phosphatase (AP)-labeled goat anti-human IgG Abs (Sigma) conjugate at 1:2,000 dilution 0.05% PBST at 100 μL/well. After 1-h incubation with the conjugate, the plates were washed five times and the human IgG complexed with the AP-labeled conjugate revealed with and P-nitrophenyl phosphate (Sigma). The substrate reaction was stopped with 50 μL/well of 3 M NaOH, after which the plates were left for 5 min in the dark before being read at 405/570 nm. Purified hyperimmune IgG was used as a standard for the Pf-specific ELISAs. Anti-TT IgG Abs were quantified against hyperimmune plasma from an adult, who received booster immunizations with the TT vaccine prior to this study. Ab concentrations were expressed in arbitrary units determined against the respective standard curves on each plate.
ELISpot.
Pf-specific MBCs were quantified by a recently optimized ELISpot assay (35) modeled on the standard assay as previously developed by Crotty et al. (25), where MBCs but not naive B cells differentiate into ASCs in response to polyclonal stimulation in 5- to 6-d PBMC cultures. Briefly, PBMCs were cultured for 5 d at 1 × 106 cells/mL of RPMI complete media with 2.5 μg/mL CpG oligodeoxynucleotide-2006 (Eurofins MWG/Operon), 1/10,000 dilution SAC, 1/100,000 dilution pokeweed mitogen (Sigma-Aldrich), and 50 ng/mL of IL10 (R&D Systems) in 24-well plates. The 96-well ELISpot plates (Millipore Multiscreen-HA) were precoated by incubating plates overnight at 4 °C with either: 10 μg/mL polyclonal goat Abs specific for human IgG (Caltag) to detect all IgG-secreting cells, or 1% BSA as a nonspecific protein control or 5 μg/mL of TT, MSP1, or AMA1 in PBS. Plates were blocked by incubation with a solution of 10% FCS in RPMI for 2 h at 37 °C. PBMCs from 5-d cultures were serially diluted by a factor of one-half in duplicates or triplicates to final concentrations of between 0.125 × 104 and 1 × 104 PBMC per well to detect total IgG+ ASCs, and between 0.062 × 105 and 2 × 105 PBMC per well to detect antigen-specific ASCs. ELISpot plates were kept at 37 °C in a 5% CO2 incubator for 5 h and then washed four times with PBS and four times with PBS-0.05% Tween 20. AP-conjugated goat anti-human IgG Fc Ab (Jackson ImmunoResearch Laboratories) diluted 1:1,000 in PBS-0.05% Tween 20 with 1% FCS was added to wells and incubated overnight at 4 °C. Plates were washed four times with PBS-0.05% Tween 20, four times with PBS, and three times with distilled water before adding BCIP/NBT 100 μL/well (Bio-Rad). The plates were dried in the dark and spots scanned with the ImmunoSpot series 4 analyzer (Cellular Technologies) and results analyzed using Immunospot v5 software (Cellular Technologies). We determined the threshold criteria for positivity based on the upper range of the frequency obtained for 1% BSA coating, which was three spots per 1 × 106 PBMC (Fig. S4).
Statistical Analyses.
Log-transformed Ab and MBC data were analyzed using Stata (v11, Stata Corp) and GraphPad Prism for Macintosh (GraphPad Software, v5.01). Correlations between different continuous measures was determined by using the Pearson correlation coefficient. Two-sample t test was used to compare continuous variables between groups. Associations between levels of B-cell responses, age, asymptomatic parasitaemia, gender, and total numbers of previous malaria episodes with the risk to the first or only episode of Pf malaria were determined by Cox-regression analyses. Poisson regression models were fitted to determine the number of multiple malaria episodes associated with B-cell responses, age, asymptomatic parasitaemia, gender, and total numbers of previous malaria episodes. Different models were compared by likelihood ratio χ2 test. For all tests, two-tailed P values were considered significant if P < 0.05.
Supplementary Material
Acknowledgments
We thank L. H. Miller, P. Crompton, S. Pierce, and colleagues at the National Institutes of Health for provision of recombinant Plasmodium falciparum antigens and helpful discussions; W. Nahrendorf and G. Macharia for participating in some of the laboratory assays; L. Murungi for advice on Ab quantification; M. Mackinnon for help with Pf-specific PCR; S. Roetynk, E. Nduati, D. Muema, F. Osier, S. Kinyanjui, and P. Bull for helpful advice and criticism; and the families that participated. This work was funded by Wellcome Trust Grant B9RTIR0. F.M.N. is a postdoctoral fellow under the Malaria Vectored Vaccines Consortium (MVVC); the MVVC is a 4-y project funded by the European and Developing Countries Clinical Trials Partnership.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200472109/-/DCSupplemental.
References
- 1.Mendis K, et al. From malaria control to eradication: The WHO perspective. Trop Med Int Health. 2009;14:802–809. doi: 10.1111/j.1365-3156.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- 2.Nájera JA. Malaria control: Achievements, problems and strategies. Parassitologia. 2001;43:1–89. [PubMed] [Google Scholar]
- 3.Ceesay SJ, et al. Changes in malaria indices between 1999 and 2007 in The Gambia: A retrospective analysis. Lancet. 2008;372:1545–1554. doi: 10.1016/S0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Meara WP, et al. The impact of primary health care on malaria morbidity—Defining access by disease burden. Trop Med Int Health. 2009;14:29–35. doi: 10.1111/j.1365-3156.2008.02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okiro EA, et al. The decline in paediatric malaria admissions on the coast of Kenya. Malar J. 2007;6:151. doi: 10.1186/1475-2875-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceesay SJ, et al. Continued decline of malaria in The Gambia with implications for elimination. PLoS ONE. 2010;5:e12242. doi: 10.1371/journal.pone.0012242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: More questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 8.Marsh K. Malaria—A neglected disease? Parasitology. 1992;104(Suppl):S53–S69. doi: 10.1017/s0031182000075247. [DOI] [PubMed] [Google Scholar]
- 9.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 10.Ghani AC, et al. Loss of population levels of immunity to malaria as a result of exposure-reducing interventions: Consequences for interpretation of disease trends. PLoS ONE. 2009;4:e4383. doi: 10.1371/journal.pone.0004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bejon P, Ogada E, Peshu N, Marsh K. Interactions between age and ITN use determine the risk of febrile malaria in children. PLoS ONE. 2009;4:e8321. doi: 10.1371/journal.pone.0008321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aponte JJ, et al. Age interaction in the development of naturally acquired immunity to Plasmodium falciparum and its clinical presentation. PLoS Med. 2007;4:e242. doi: 10.1371/journal.pmed.0040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleinschmidt I, et al. Marked increase in child survival after four years of intensive malaria control. Am J Trop Med Hyg. 2009;80:882–888. [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen S, McGREGOR IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 15.Fowkes FJ, Richards JS, Simpson JA, Beeson JG. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 2010;7:e1000218. doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabchareon A, et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 17.Akpogheneta OJ, et al. Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun. 2008;76:1748–1755. doi: 10.1128/IAI.01333-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavanagh DR, et al. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J Immunol. 1998;161:347–359. [PubMed] [Google Scholar]
- 19.Kinyanjui SM, Bull P, Newbold CI, Marsh K. Kinetics of antibody responses to Plasmodium falciparum-infected erythrocyte variant surface antigens. J Infect Dis. 2003;187:667–674. doi: 10.1086/373994. [DOI] [PubMed] [Google Scholar]
- 20.Drakeley CJ, et al. Altitude-dependent and -independent variations in Plasmodium falciparum prevalence in northeastern Tanzania. J Infect Dis. 2005;191:1589–1598. doi: 10.1086/429669. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RR, et al. Selective recognition of malaria antigens by human serum antibodies is not genetically determined but demonstrates some features of clonal imprinting. Int Immunol. 1996;8:905–915. doi: 10.1093/intimm/8.6.905. [DOI] [PubMed] [Google Scholar]
- 22.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 23.Böttiger M, Gustavsson O, Svensson A. Immunity to tetanus, diphtheria and poliomyelitis in the adult population of Sweden in 1991. Int J Epidemiol. 1998;27:916–925. doi: 10.1093/ije/27.5.916. [DOI] [PubMed] [Google Scholar]
- 24.Crotty S, Ahmed R. Immunological memory in humans. Semin Immunol. 2004;16:197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Crotty S, et al. Cutting edge: Long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 26.Banatvala J, Van Damme P, Oehen S. Lifelong protection against hepatitis B: The role of vaccine immunogenicity in immune memory. Vaccine. 2000;19:877–885. doi: 10.1016/s0264-410x(00)00224-3. [DOI] [PubMed] [Google Scholar]
- 27.Bauer T, Jilg W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine. 2006;24:572–577. doi: 10.1016/j.vaccine.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 28.Wainwright RB, Bulkow LR, Parkinson AJ, Zanis C, McMahon BJ. Protection provided by hepatitis B vaccine in a Yupik Eskimo population—Results of a 10-year study. J Infect Dis. 1997;175:674–677. doi: 10.1093/infdis/175.3.674. [DOI] [PubMed] [Google Scholar]
- 29.West DJ, Calandra GB. Vaccine induced immunologic memory for hepatitis B surface antigen: Implications for policy on booster vaccination. Vaccine. 1996;14:1019–1027. doi: 10.1016/0264-410x(96)00062-x. [DOI] [PubMed] [Google Scholar]
- 30.Jilg W, Schmidt M, Deinhardt F. Decline of anti-HBs after hepatitis B vaccination and timing of revaccination. Lancet. 1990;335:173–174. doi: 10.1016/0140-6736(90)90050-f. [DOI] [PubMed] [Google Scholar]
- 31.Vande Waa JA, Jensen JB, Akood MA, Bayoumi R. Longitudinal study on the in vitro immune response to Plasmodium falciparum in Sudan. Infect Immun. 1984;45:505–510. doi: 10.1128/iai.45.2.505-510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Migot F, et al. Human immune responses to the Plasmodium falciparum ring-infected erythrocyte surface antigen (Pf155/RESA) after a decrease in malaria transmission in Madagascar. Am J Trop Med Hyg. 1993;48:432–439. doi: 10.4269/ajtmh.1993.48.432. [DOI] [PubMed] [Google Scholar]
- 33.Crompton PD, et al. The TLR9 ligand CpG promotes the acquisition of Plasmodium falciparum-specific memory B cells in malaria-naive individuals. J Immunol. 2009;182:3318–3326. doi: 10.4049/jimmunol.0803596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nogaro SI, et al. The breadth, but not the magnitude, of circulating memory B cell responses to P. falciparum increases with age/exposure in an area of low transmission. PLoS ONE. 2011;6:e25582. doi: 10.1371/journal.pone.0025582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss GE, et al. High efficiency human memory B cell assay and its application to studying Plasmodium falciparum-specific memory B cells in natural infections. J Immunol Methods. 2012;375:68–74. doi: 10.1016/j.jim.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss GE, et al. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog. 2010;6:e1000912. doi: 10.1371/journal.ppat.1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wipasa J, et al. Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 2010;6:e1000770. doi: 10.1371/journal.ppat.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mwangi TW, Mohammed M, Dayo H, Snow RW, Marsh K. Clinical algorithms for malaria diagnosis lack utility among people of different age groups. Trop Med Int Health. 2005;10:530–536. doi: 10.1111/j.1365-3156.2005.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis. 2005;191:1932–1939. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierce SK. Understanding B cell activation: From single molecule tracking, through Tolls, to stalking memory in malaria. Immunol Res. 2009;43:85–97. doi: 10.1007/s12026-008-8052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Struik SS, Riley EM. Does malaria suffer from lack of memory? Immunol Rev. 2004;201:268–290. doi: 10.1111/j.0105-2896.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 42.Radbruch A, et al. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 43.Combe A, et al. Clonal conditional mutagenesis in malaria parasites. Cell Host Microbe. 2009;5:386–396. doi: 10.1016/j.chom.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Osier FH, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 2008;76:2240–2248. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackintosh CL, et al. Failure to respond to the surface of Plasmodium falciparum infected erythrocytes predicts susceptibility to clinical malaria amongst African children. Int J Parasitol. 2008;38:1445–1454. doi: 10.1016/j.ijpara.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bejon P, et al. Immunity to febrile malaria in children: An analysis that distinguishes immunity from lack of exposure. Infect Immun. 2009;77:1917–1923. doi: 10.1128/IAI.01358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith T, Felger I, Tanner M, Beck HP. Premunition in Plasmodium falciparum infection: Insights from the epidemiology of multiple infections. Trans R Soc Trop Med Hyg. 1999;93(Suppl 1):59–64. doi: 10.1016/s0035-9203(99)90329-2. [DOI] [PubMed] [Google Scholar]
- 48.Migot F, et al. Anti-malaria antibody-producing B cell frequencies in adults after a Plasmodium falciparum outbreak in Madagascar. Clin Exp Immunol. 1995;102:529–534. doi: 10.1111/j.1365-2249.1995.tb03848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bejon P, et al. The induction and persistence of T cell IFN-gamma responses after vaccination or natural exposure is suppressed by Plasmodium falciparum. J Immunol. 2007;179:4193–4201. doi: 10.4049/jimmunol.179.6.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mbogo CM, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg. 2003;68:734–742. [PubMed] [Google Scholar]
- 51.Mbogo CN, et al. Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at nine sites on the Kenyan coast. Am J Trop Med Hyg. 1995;52:201–206. doi: 10.4269/ajtmh.1995.52.201. [DOI] [PubMed] [Google Scholar]
- 52.Polley SD, et al. High levels of serum antibodies to merozoite surface protein 2 of Plasmodium falciparum are associated with reduced risk of clinical malaria in coastal Kenya. Vaccine. 2006;24:4233–4246. doi: 10.1016/j.vaccine.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 53.Polley SD, et al. Human antibodies to recombinant protein constructs of Plasmodium falciparum Apical Membrane Antigen 1 (AMA1) and their associations with protection from malaria. Vaccine. 2004;23:718–728. doi: 10.1016/j.vaccine.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 54.Polley SD, et al. Plasmodium falciparum merozoite surface protein 3 is a target of allele-specific immunity and alleles are maintained by natural selection. J Infect Dis. 2007;195:279–287. doi: 10.1086/509806. [DOI] [PubMed] [Google Scholar]
- 55.Rougemont M, et al. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol. 2004;42:5636–5643. doi: 10.1128/JCM.42.12.5636-5643.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.