Abstract
In the epithelial compartment of the uterus, estradiol-17β (E2) induces cell proliferation while progesterone (P4) inhibits this response and causes differentiation of the cells. In this study, we identified the mechanism whereby E2 and P4 reciprocally regulate the expression of minichromosome maintenance (MCM)-2, a protein that is an essential component of the hexameric MCM-2 to 7 complex required for DNA synthesis initiation. We show in the uterine epithelium that Kruppel-like transcription (KLF) factors, KLF 4 and 15, are inversely expressed; most importantly, they bind to the Mcm2 promoter under the regulation of E2 and P4E2, respectively. After P4E2 exposure and in contrast to E2 treated mice, the Mcm2 promoter displays increased histone 3 (H3) methylation and the recruitment of histone deacetylase 1 and 3 with the concomitant deacetylation of H3. This increased methylation and decreased acetylation is associated with an inhibition of RNA polymerase II binding, indicating an inactive Mcm2 promoter following P4E2 treatment. Using transient transfection assays in the Ishikawa endometrial cell line, we demonstrate that Mcm2 promoter activity is hormonally stimulated by E2 and that KLF15 inhibits this E2 enhanced transcription. KLF15 expression also blocks Ishikawa cell proliferation through inhibition of MCM2 protein level. Importantly, in vivo expression of KLF15 in an estrogenized uterus mimics P4’s action by inhibiting E2-induced uterine epithelial MCM-2 expression and DNA synthesis. KLF15 is therefore a downstream physiological mediator of progesterone’s cell cycle inhibitory action in the uterine epithelium.
Keywords: endometrium, implantation, menstrual cycle, endometriosis
Estradiol-17β (E2) and progesterone (P4) directs uterine preparation for pregnancy. These hormones synthesized cyclically in humans cause a sequential series of proliferative and differentiation events in the uterine stroma and epithelium that result in the epithelium being receptive to the blastocyst for attachment and subsequent implantation (1). Despite this close control of uterine cell proliferation, aberrant proliferative conditions of the human endometrium are common. For example, endometrial polyps and endometriosis are caused by inappropriate proliferation of the uterus, while unopposed estrogen stimulation is associated with menstrual irregularities and endometrial hyperplasia/adenocarcinoma (2). Endometrial cancer is the most common female genital tract malignancy and is responsible for 6% of cancer deaths of women in the United States and more worldwide (3). However, the molecular mechanisms underlying these pathologies are still obscure, as are the molecular mechanisms involved in normal hormonal regulation of cell proliferation in the endometrium, which is essential for successful pregnancy (4).
The mouse uterus provides a unique in vivo model to study the regulation of epithelial cell proliferation as the physiological actions of E2 and P4E2 can be recapitulated in ovariectomized animals by treatment with exogenous hormones (4). Physiologically in mice, E2, synthesized at proestrus, induces a wave of epithelial DNA synthesis followed by cell division. This E2-induced wave of epithelial DNA synthesis is completely inhibited by the P4 that is synthesized following copulation. Implantation is precipitated by a surge of estrogen on day four of pregnancy (4). Similar mechanisms of hormone action are found in humans where the E2 induced epithelial cell proliferation is also inhibited by P4 (5). There is doubt whether E2 is required for implantation in humans, as implantation can occur without ovaries providing P4 is supplied. However, given that the mouse uterus can synthesize E2 at implantation, the endometrium may be the source of this hormone in humans (6).
The effects of E2 and P4 on cell proliferation in the uterus are mediated by their cognate transcription factors estrogen receptor (ERα) and progesterone receptor (PR), respectively. These receptors act in cell autonomous and nonautonomous fashion together with cell type specific transcriptional cofactors to exercise their biological functions. Recent cell-specific gene targeting shows that E2 induced epithelial cell proliferation is regulated through stromal localized ER (7) that causes the synthesis of paracrine factors, including IGF-1, that act on the epithelial cells (8). PR is required in the uterine epithelium and stroma and causes inhibition of E2-induced epithelial cell proliferation (9, 10). Despite these cell-specific requirements for the receptor, each hormone induces a cascade of downstream effectors including transcription factors (TF) that propagate the hormonal signal (1).
In uterine epithelial cells, P4 exerts its inhibitory effects on the E2-induced cell cycle in G1 within 3 h of E2 treatment (11). In order to dissect the TFs and the pathways involved in the P4-inhibition of uterine epithelial DNA synthesis, we examined the transcriptome in this epithelium 3 h posttreatment of P4E2 or E2 alone. We found the coordinate down-regulation by P4E2 treatment of greater than 20 genes associated with DNA replication and chromatin assembly. These genes include Mcm family members, Pcna, Mki67, Fen1, Lig1, Cdk2, Tk1, and Mad2l1 (12). This coordinated response suggests action of TFs in the epithelial cells that mediate the cascade of events through the regulation of gene batteries involved in DNA replication. Several TFs were identified that were expressed differentially in response to E2 and P4E2 (12) including transcripts for the Kruppel-like transcription factors, Klf4 and Klf15, whose expression was increased by E2 and P4E2 treatment, respectively.
KLFs belong to the specificity family of proteins. They are zinc finger containing TFs named after the segmentation gene product Kruppel originally identified in Drosophila melanogaster, in which it plays an essential role in embryogenesis (13). In mammals they constitute a large family of factors containing at least seventeen members that bind to a CACCC or CGCCC DNA motif (14, 15). KLF’s have pleiotropic functions that both enhance and inhibit transcription (16), and through competition for binding to their target sequences, they can mutually antagonize each other (15, 17). They have a wide range of functions in regulating cell cycle, apoptosis, and differentiation (15). KLFs affect expression of proproliferative genes such as E, D and A type cyclins. They also regulate several antiproliferative genes, for example p53 and E cadherin (17). KLF9 expressed in the uterus has a role in reproduction; its loss results in a modest reduction in fertility in mice caused by an implantation failure secondary to the attenuation and delay of cell proliferation in the luminal and glandular epithelium in response to E2 (18–20). KLF9 acts as a PR coregulator and, depending on cell context, acts as a negative or positive regulator of ER signaling (18, 21). In this study, because of its regulation by P4E2 in the uterine epithelium at the important 3 h time point, we focused on KLF 15 and, as a contrast, analyzed KLF4 as an E2 regulated TF.
A major concept in cell cycle regulation is the assembly of the prereplication complex (RC) upon origins of replication at the end of mitosis and in early G1 by the association of CDC6 and CDT1 proteins to the origin recognition complex (ORC). The association of CDC6 and CDT1 to the ORC is a prerequisite for the loading of a hexameric complex containing six different minichromosome maintenance proteins (MCM 2-7) to chromatin, licensing the origin for a new replication round. At this stage, MCMs that are members of the AAA+ class of ATPases (22) acquire their helicase activity to unwind DNA. The molecular components and the basic interaction of pre-RC components are strikingly conserved from yeast to metazoa. However, the dynamics of the ancillary protein assembly vary in different species. After DNA synthesis is initiated, the MCM2-7 complex dissociates from the DNA and CDT1 is degraded, thus ensuring only one round of DNA replication per cell cycle. MCMs play a critical role in the initiation of DNA synthesis, and the removal of any one MCM blocks DNA synthesis in a wide range of organism from yeast to humans (23–27). Given their essential role in proliferation, they are used as a biomarker and prognostic indicator for different cancers (26).
In the uterine epithelium, E2 stimulates the expression of the MCMs and of the loading factor CDT1 (12, 28). Consequentially, chromatin association of the MCM2-7 complex is stimulated by E2 at the G1/S phase transition. P4E2 inhibits the transcript abundance of MCM 2 to 6, causes nuclear egress of the residual MCM proteins, and inhibits CDT1 synthesis. Thus the binding of the MCM2-7 complex to chromatin is inhibited and prereplication licensing is blocked (12). A similar action can be ascribed to P4E2 in human endometrial epithelium as a dramatic down-regulation of MCM2 transcripts; and loss of protein occurs in the secretory and therefore P4 dominated phase of the menstrual cycle (5). Despite the importance of this regulation in mice and humans, the molecular basis for the P4E2 regulation of DNA replication licensing is not understood.
In this study we tested the hypothesis that KLF15 mediates the actions of P4E2 in the uterine epithelium through inhibition of the transcription of MCM2, and thereby, of DNA replication licensing. We show that KLF15 binds to the MCM2 promoter in a P4E2 dependent fashion and that it negatively regulates RNA Pol II association. KLF15 expression suppresses E2 mediated MCM2 transcription. In vivo, KLF-15 expression in the E2 exposed uterus mimics P4 action by inhibiting MCM2 expression and epithelial cell DNA synthesis. These data establish KLF-15 as a downstream mediator of the antiproliferative action of progesterone on E2-induced epithelial cell proliferation.
Results
KLF4 and KLF15 Are Regulated by E2 and P4E2.
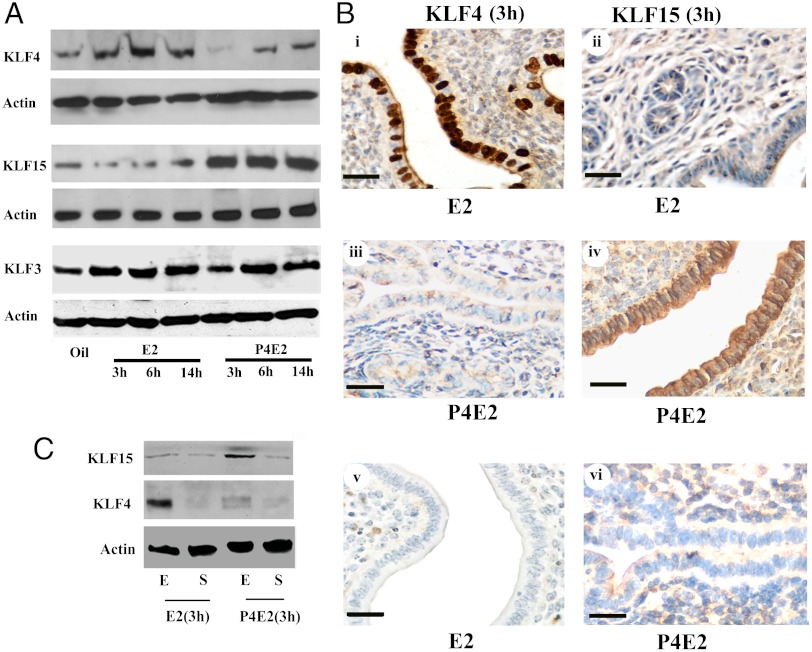
To identify the molecular basis of uterine epithelial DNA synthesis, it is necessary to identify the steroid hormone regulated cell type specific TFs that are responsible for this process. Because KLFs have diverse functions in cell cycle regulation, and KLF4 and KLF15 transcripts are differentially regulated in the uterine epithelium 3 h after E2 and P4E2 treatment, we chose to study these two TFs. First, to ensure whether the differential expression in transcript abundance leads to changes in relative protein expression, we performed Western analysis of epithelial lysates isolated at different times after physiologically relevant hormonal treatment of ovariectomized mice. E2 treatment resulted in an up-regulation of KLF4 protein within 3 h that persisted through 14 h posttreatment compared to vehicle treated controls (Fig. 1A). P4 pretreatment inhibited this E2 up-regulation of KLF4 and resulted in a significant decline over the first 3 h of treatment, followed by an increase that was still significantly lower than the E2 treated group through the 14 h observation period (Fig. 1A). KLF 15 protein concentration was reduced by E2 treatment at the 3 h mark compared to the oil treated control. Although there is some recovery in concentration, this suppression persisted until 14 h posttreatment. In contrast, P4E2 treatment significantly elevated KLF15 protein concentration over the control and E2 treated groups through the 14 h time course (Fig. 1A). KLF3, whose mRNA was not shown to be differently regulated by P4E2 but is expressed in the uterine epithelium, responded to E2 treatment modestly in protein concentration regardless of P4 pretreatment (Fig. 1A).
Fig. 1.
Hormonal regulation of uterine epithelial KLF4 and KLF15 expression. (A) Regulation of KLF4 and KLF15 protein expression by E2 and P4E2, respectively. Equivalent amount of protein lysate isolated from uterine epithelial cells of mice treated with hormones as indicated were separated by SDS PAGE and subjected to Western blotting with specific antibodies as shown. Actin was used as a protein loading control. (B) Cross section of the midportion of the uterus immunostained using antibodies against KLF4 (i, iii) or KLF15 (ii, iv) 3 h after corresponding hormone treatment or with control IgG (v, vi). Brown staining indicates positive reactivity. The data described here are representative of the analysis of at least 5 different mice for each group. Scale bar: 50 um. (C) Regulation of KLF4, KLF15 protein expression 3 h after E2 and P4E2 treatment in uterine epithelial and stromal compartments. Equivalent amount of protein lysate isolated from uterine epithelial or stromal cells of mice treated with hormones as indicated were separated by SDS PAGE and subjected to Western blotting with specific antibodies as shown. Actin was used as a protein loading control.
Cell type specificity of expression at the important 3 h time point posthormonal treatment was investigated by immunohistochemistry (IHC) in transverse uterine sections and by Western blotting of lysates of separated epithelial and stromal cells using anti- KLF4 and KLF15 antibodies. KLF4 as assessed by IHC is highly expressed in the uterine epithelium of E2 treated mice with minimal expression in the stroma (Fig. 1B, i). In the P4E2 mice, staining was dramatically reduced (Fig. 1B, iii), almost to the control level of staining seen with the IgG control (Fig. 1B, v). In contrast, KLF15 immunostaining shows a dramatic increase in expression in the uterine epithelium of P4E2 treated mice with only background levels detected in the E2 treated epithelium (Fig. 1B, ii and vi). There is some immunostaining in the stroma of the P4E2 group, but only slightly more than the background obtained with a control antibody (Fig. 1B, iv and vi). These data are confirmed by Western blotting of separated epithelial and stromal uterine cell types prepared as described in the Materials and Methods. KLF4 is expressed at high levels only in the epithelium isolated from E2 treated mice (Fig. 1C). In contrast, KLF15 is expressed predominantly in the P4E2 treated uterine epithelium with a low level in the stroma and only minimal expression in the two tissues isolated from the estrogenized uterus (Fig. 1C). It should also be noted that the stromal preparations are contaminated by glandular epithelium (29) and are not as pure as the > 95% purity of the epithelial fraction (30). Thus, the expression of KLF15 in stromal cells is likely even lower than the low level detected on the Western. From these data we can conclude that there is a preferential expression of KLF4 and KLF15 in the uterine epithelium following E2 and P4E2 treatment, respectively. Thus the relative balance of the concentration of these TFs in the uterine epithelium depends on hormonal treatment.
KLF4 and KLF15 Bind to the Mcm2 Promoter and Predict RNA Pol II Association.
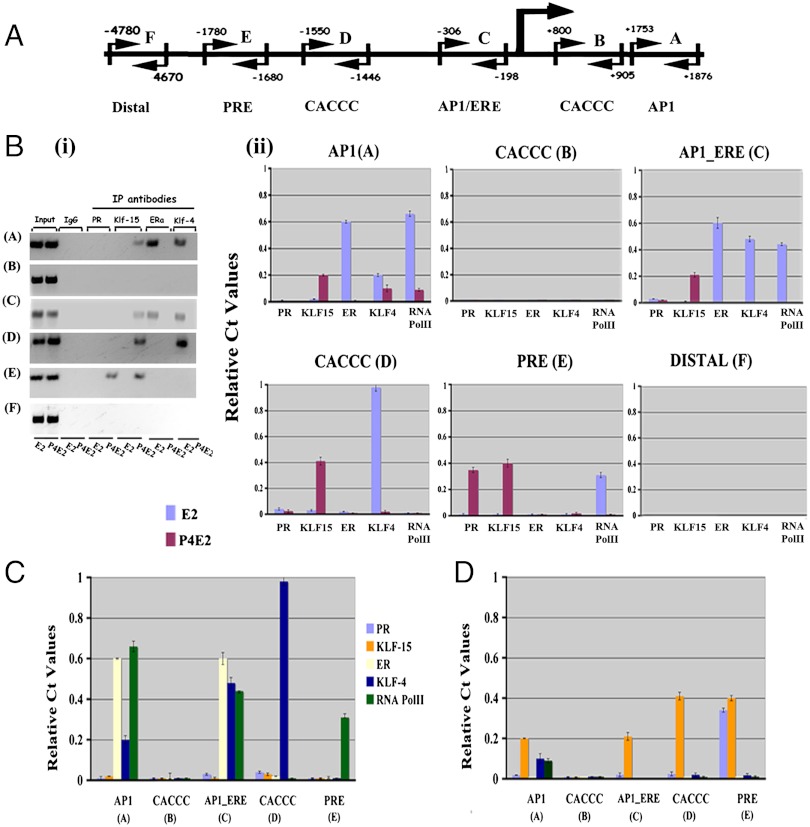
The up-regulation of KLF15 by P4E2, coincident with the down-regulation of Mcm2 transcript abundance, led to the hypothesis that KLF15 could negatively regulate Mcm2 transcription. To test this hypothesis we first determined whether this transcription factor binds to the putative Mcm2 promoter. As a control, we also determined the binding of the E2 induced KLF4 to the same promoter region. In order to do this analysis, a region containing 5,000 bp upstream and 2,000 bp downstream, encompassing the first two introns from the MCM2 transcriptional start site (TSS), was identified using the UCSF genome browser and verified with the Ensemble browser. Within this sequence, putative TF binding sites for ERα and PR, AP1 sites (since these can also be sites of ER binding through c-jun and c-fos), and the KLF binding sequence CACCC (16) were mapped (Fig. 2A). Three sites were in the upstream region while sites B (CACCC) and A (AP1) were in the first and second intron, respectively. PCR Primers were made to amplify these regions as indicated in Fig. 2A, as well as an irrelevant DNA sequence 4670 bp distal to the putative transcription start sites as shown (Fig. 2A). Chromatin immunoprecipitation (ChIP) was performed on uterine chromatin using antibodies to PR, KLF15, ERα, KLF4, and RNA polymerase II followed by PCR (to test whether the band size corresponds with the expected one; Fig. 2B, i) as well as Quantitative real time-PCR (Q-PCR; Fig. 2B, ii) 3 h after E2 or P4E2 treatment. There was minimal binding detected at this cycle number with an irrelevant IgG or with any of the antibodies used to the distal region (F) or to the downstream CACCC (B) sequence (Fig. 2B). This lack of significant binding with the nonspecific IgG was found in all experiments and was always run as a specificity control. In contrast, hormone specific binding of individual transcription factors was readily detected at this low cycle number. KLF15 bound to the AP1 (A), AP1_ERE (C), CACCC (D), and PRE (E) in chromatin extracted from P4E2 treated mice (Fig. 2B). This binding of KLF15 was significantly diminished in chromatin from the E2 treated group (Fig. 2B). In contrast, KLF4 was bound to the AP1 (A), AP1_ERE (C), and CACCC (D) sites in chromatin from E2 treated uteri, but was significantly diminished in chromatin from the P4E2 group (Fig. 2B). This ChIP analysis also revealed that PR bound to the PRE (E) and ERα to the AP1 (A) and AP1_ERE (C) sites again in a hormone regulated fashion (Fig. 2B). In addition to these TFs, the binding of RNA Pol II was also determined. RNA Pol II binding was highly up-regulated by E2 treatment at the AP1 (A), AP1_ERE (C), and PRE (E) sites. RNA Pol II association is at least sixfold higher in the E2 than P4E2 treated uterus at site A (AP1); on sites C (AP1_ERE ) and E (PRE ), its association is dramatically higher as it is essentially undetectable in P4E2 treated samples (Fig. 2B). Thus RNA Pol II is significantly recruited to most of these binding sites in an estrogen dependent manner and this was significantly inhibited, in many cases to levels at the limit of detection, by P4E2 pretreatment (Fig. 2B, i and ii).
Fig. 2.
Binding of transcription factors and RNA Pol II on the putative Mcm2 promoter region 3h after E2 or P4E2 treatment. (A) Map of putative PR (PRE), ERα (AP1/ERE, AP1), and KLF15/KLF4 (CACCC) binding sites on the Mcm2 promoter. Large arrow shows the transcription start site, small arrows position of PCR primer pairs. (B) ChIP assay was performed with uterine chromatin isolated 3 h after E2 (blue) and P4E2 (magenta) treatment using anti-PR, -KLF15, -ERα, -KLF4, and RNA Pol II antibodies followed by PCR (i) and real time PCR (ii). The precipitated DNA was amplified using primers as described in Materials and Methods. All data are normalized against IgG control. Results are expressed as relative Ct values compared to input. (C) and (D) Relationship between RNA Pol II binding (green) with ERα (yellow), PR (light blue), KLF4 (dark blue), or KLF15 (orange) recruitment 3 h after E2 (C) and P4E2 (D) treatment.
ChIP demands a significant input of material together with rapid cross-linking to obtain reliable data from in vivo sources and with multiple antibodies. Therefore, given the predominant expression of KLF4 and 15 in the uterine epithelium of E2 and P4E2 treated mice respectively, we used chromatin from whole uteri to perform the experiments. However, to ensure the data is representative we also performed ChIP on isolated epithelium 3 hrs post-E2 and P4E2 treatment. These data show the same pattern of binding of KLF15, KLF4, and RNA Pol II that was determined in whole uterine homogenates (Fig. S1). This result indicates that the data from whole uteri are representative because of the preferential expression of both KLF4 and KLF15 in the uterine epithelium.
Because binding of these TFs is hormone responsive, in Fig. 2 C and D we display the Q-PCR data from the ChIP analysis of the Mcm2 promoter in uteri according to their E2 (Fig. 2C) and P4E2 (Fig. 2D) treatment, respectively. These data show that following E2 treatment of KLF4, ERα and RNA Pol II are bound to the putative MCM2 promoter with minimal binding of KLF15 (Fig. 2C) at each position. In contrast, P4E2 treatment ablates binding of KLF4, ERα, and RNA Pol II while PR and particularly KLF 15 binding is highly enriched (Fig. 2D). Together, these data show that P4E2 treated mice have higher amounts of KLF-15 binding on each of three positions and that this binding corresponds with lower RNA Pol II association.
The Temporal Regulation of KLF15 Binding Controls RNA Pol II Association.
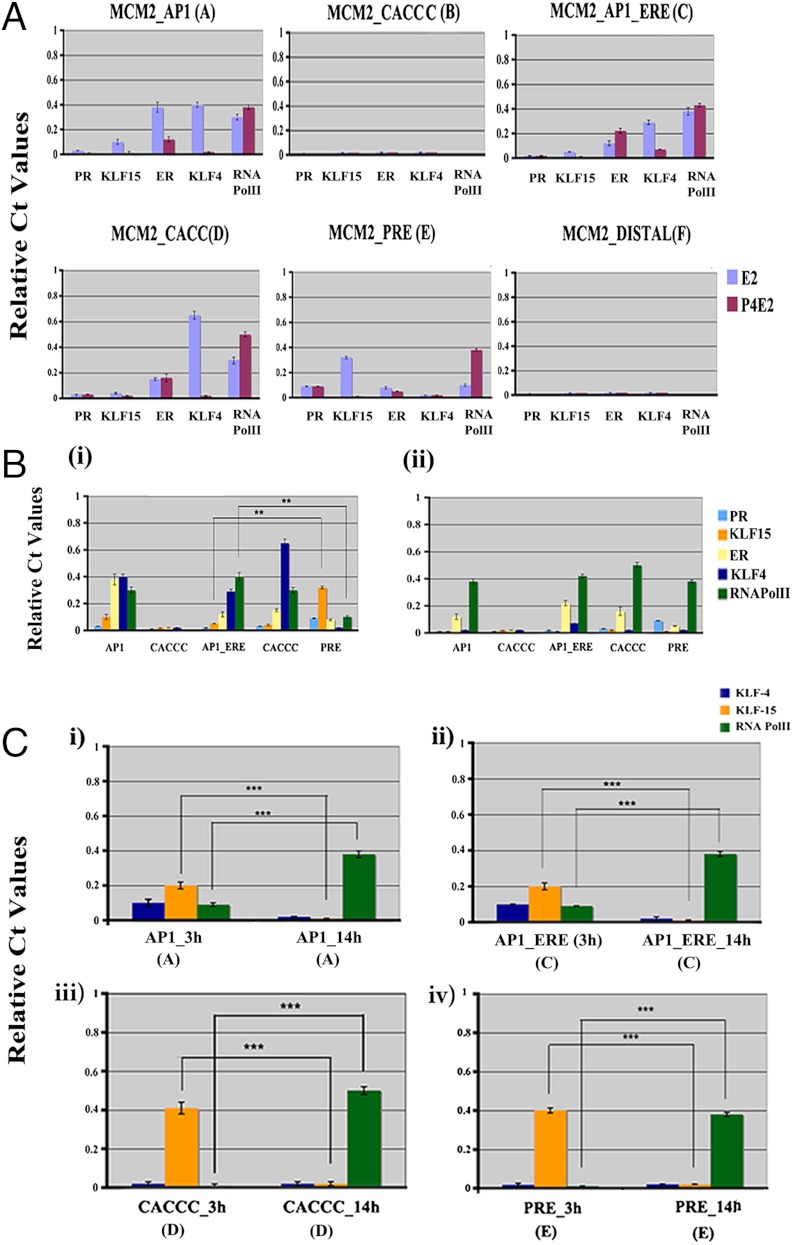
Physiologically, by 14 h the effects of P4 on cell proliferation are attenuating in preparation for the next wave of E2 stimulation, and there is a rebound in the expression of many of the genes that are inhibited by P4 early in the process (28, 31, 32). Thus, to test the hypothesis that KLF-15 inhibits Mcm2 transcription by blocking RNA polymerase association with the chromatin, we determined the changing pattern of recruitment of KLF-4, KLF-15, and RNA Pol II at 14 h after treatment using ChIP and Q-PCR of uterine chromatin. Notably, at 14 h, RNA Pol II is recruited to the AP1 (A), AP1_ERE (C), CACCC (D), and PRE (E) sites (Fig. 3A) in the P4E2 treated uterine samples to an even greater extent than in the samples treated with E2 alone. This result is shown in more detail in Fig. 3B, where the two hormone treatments are separated graphically. Specifically, binding of KLF-15 vs. RNA Pol II at the site AP1_ERE (C) vs. PRE (E) shows a significantly higher binding of KLF-15 at the PRE (E) that corresponds with reduced RNA Pol II association (Fig. 3B, i). Furthermore, KLF15 binding that dominated at 3 h after P4E2 treatment is now lost, and RNA Pol II is associated at each of the positions (Fig. 3 A and B, ii). We hypothesized that there is an inverse relationship between KLF-15 binding with RNA Pol II recruitment; therefore, we compared their binding at 3 h versus at the 14 h time point after P4E2 treatment at four individual motifs (Fig. 3C, i–iv). In each position, the significantly higher association of KLF-15 at 3 h after P4E2 treatment (p < 0.001) is correlated with a corresponding lower RNA Pol II association (p < 0.001). Our data strongly suggest that KLF15 negatively regulates RNA Pol II binding.
Fig. 3.
Temporal binding patterns of KLF4 and KLF15 compared to RNA Pol II on the Mcm2 promoter. (A) Analysis of recruitment of ERα, PR, KLF-4, KLF-15, and RNA Pol II by ChIP on the uterine Mcm2 promoter 14 h after hormone treatment. The results presented are compared to input and normalized against IgG control. (B) Correlation between KLF15 recruitment and RNA Pol II recruitment 14 h after E2 (i) and P4E2 (ii) treated samples. Shown are representative bar diagrams that established a reciprocal relationship between KLF15 and RNA Pol II association. (Differences between binding ***p < 0.001, n = 5). (C) Identification of a direct link between KLF15 and RNA Pol II association by comparing 3 h vs. 14 h in the P4E2 treated samples at four discrete positions on the Mcm2 promoter. In each case a reduction in KLF15 binding at 14 h is correlated with an increase in RNA Pol II binding. Differences in binding at each position ***p < 0.001, n = 5. Bar identification shown in figure is as described in Fig. 2.
E2 and P4E2 Alter Histone Acetylation and Methylation of the MCM2 Promoter.
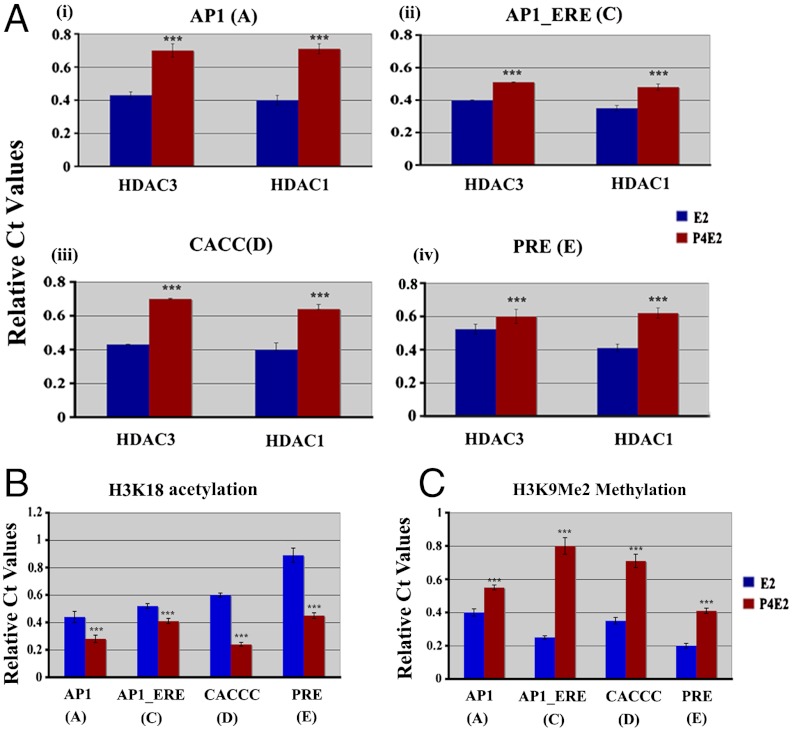
The activity of promoters is partially defined by the state of histone acetylation and methylation. Hyper-acetylated histones are linked to transcriptionally active domains, whereas hypo-acetylated histones are generally associated with transcriptionally silent domains (33–35). Therefore we examined these epigenetic signatures in response to hormone exposure utilizing ChIP on the uterine Mcm2 chromatin 3 h after hormone treatment. Assays were performed with antibodies against the histone modifying enzymes, HDAC1 or HDAC3, to determine their association with these positions (A, C, D, and E). In P4E2 treated mice, uterine binding of HDAC3 was higher at all four sites compared to E2 treated samples. Similarly, HDAC1 association was significantly higher in P4E2 treated samples at the AP1 (A), AP1_ERE (C), CACCC (D), and PRE (E) sites compared to chromatin from E2 treated mice. Consistent with this binding of the HDACs to the Mcm2 promoter region, ChIP using antibodies specific for the acetylated lysine 18 of Histone H3 (H3K18) indicated that the acetylation state of the Mcm2 promoter was lower at all four sites in the P4E2 treated versus the E2 treated group (Fig. 4B).
Fig. 4.
Acetylation and methylation signature on Mcm2 chromatin 3 h after hormone treatment. Uterine tissues were collected from ovariectomized mice after 3 h of E2 or P4E2 treatment and subjected to ChIP using antibodies against (A) HDAC1 or HDAC3, as described in the Materials and Methods. The results presented are compared to input and normalized against IgG control. Each site is independently shown. Differential binding as indicated ***P < 0.001, n = 5. (B) H3K18Ac levels at different sites were determined by Chip-QPCR 3 h after E2 or P4E2 treated uterine chromatin. (C) Levels of H3K9Me2 on different sites were determined by Chip-QPCR 3 h after E2 or P4E2 treated uterine chromatin. Statistical analysis (B and C) represents the difference between E2 treated samples with P4E2 treated one at four different sites, ***P < 0.001, n = 5.
Inactive promoters also harbor higher levels of methylation of lysine 9 on histone 3 (H3K9Me2) (36, 37). Therefore, we performed ChIP on the Mcm2 promoter with an antibody against H3K9Me2 in P4E2 and E2 treated samples 3 h after treatment. Our results detected this modification at the AP1 (A), AP1_ERE (C), CACCC (D), and PRE (E) positions in the Mcm2 promoter. Consistent with an inactive promoter in the P4E2 treated samples, there was a greater amount of H3K9Me2 at all four positions (Fig. 4C).
Together, these data confirmed that the uterine Mcm2 promoter is in a more active configuration in E2 than in P4E2 treated mice.
The Mcm2 Promoter Region Is Regulated by E2 and Inhibited by KLF15.
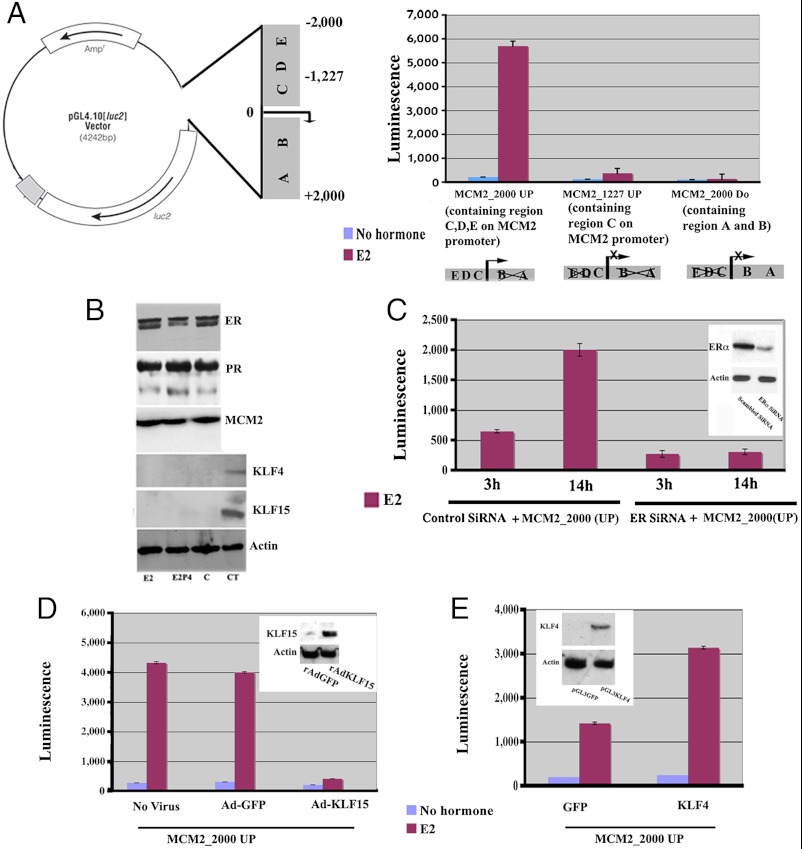
Our results from the ChIP analysis define the temporal and hormone dependent recruitment of TFs to the putative Mcm2 promoter region. These in vivo studies, particularly those pertaining to RNA Pol II recruitment and histone acetylation and methylation, also suggest that female sex steroid hormones regulate Mcm2 promoter activity. To test this hypothesis directly, we performed luciferase (LUC) assays using constructs containing the putative promoter regions separately fused to a promoterless LUC expression vector (pGL4-Luc basic) (Fig. 5A). These constructs were transfected into the endometrial adenocarcinoma Ishikawa cell line, which is one of the few uterine cell lines that expresses functional ERα and PR (38). This cancer cell line is modestly responsive to E2 but has lost its inhibitory response to P4 despite expression of receptors for both hormones. Before performing the luciferase assays, we determined the expression of ERα, PR, KLF4, KLF15, and MCM2 in our clone of Ishikawa cells by Western blotting with appropriate antibodies. This analysis demonstrated that ERα, PR, and MCM2 proteins are abundantly expressed in Ishikawa cells although no hormonal regulation of these proteins was obtained in this cell line (Fig. 5B). In contrast, we could not detect KLF4 or KLF15 in Ishikawa cell lysates even though these proteins were readily detected in the control (CT) uterine lysates (Fig. 5B). Having established that Ishikawa cells express ERα, we next analyzed promoter activity of the various genomic DNA fragments by transient transfection methods. Serum deprived Ishikawa cells were separately transfected with one of the three constructs containing MCM2_2000-Luc (2000 bp upstream of the TSS UP), MCM2_1227-Luc (1227 bp upstream from the TSS UP), and MCM2_2000-Luc (2000 bp downstream from the TSS Do) region together with a Renilla luciferase plasmid followed by hormonal treatment; the normalized firefly activity was determined. Fourteen hours after hormone treatment, only the MCM2_2000 (up) sequence exhibited significant promoter activity, and this activity was dependent on E2 with very little luciferase activity being detected in the untreated control (Fig. 5A). Neither the downstream nor the immediate 1227 base pair up-stream regions exhibited significant transcriptional activity (Fig. 5A).
Fig. 5.
Transcriptional activity of the Mcm2 promoter in Ishikawa cells. (A) Shown is the representative diagram of PGL4 (luc2) vector containing the putative Mcm2 promoter region containing the specific sites for binding of transcription factors identified in Fig. 2. Ishikawa cells were transiently cotransfected with a control renilla plasmid and the luciferase derivatives containing three separate genomic fragments 2000 bp upstream region of TSS (MCM2_2000 UP), downstream region containing 2000 bp downstream in relation to TSS (MCM2_2000 DO), and region containing 1227 upstream in relation to TSS (MCM2_1227 UP) for 36 h followed by vehicle alone (blue bar) or E2 (red bar) treatment and assessed for luciferase activity 14 h after hormone treatment. Results in all panels are presented as normalized by firefly luciferase activity compared to Renilla control vector. (B) Serum deprived Ishikawa cells were treated with E2 or P4E2 and whole cell lysates were collected and analyzed by western blotting for expression of ERα, PR, MCM2, KLF4 and KLF15. The vehicle treated cells (C) along with uterine epithelial tissue lysates (CT) are used as control for KLF4 and 15, respectively. (C) Effects of silencing of ERα expression on Mcm2 transactivation. Ishikawa cells were cotransfected with ERα SiRNA along with the MCM2_2000(UP) construct followed by E2 treatment for 3 h or 14 h when the cells were harvested as described in the Materials and Methods. Insert is the Western blot analysis of ERα expression compared to actin control after transfection of specific SiRNA or scrambled SiRNA in Ishikawa at the end of the experiment. (D) Role of KLF15 in MCM2_2000 transactivation. Ishikawa cells were transfected with MCM2_2000 UP followed by infection with AdKLF15 or Ad-GFP. Cells were incubated for 36 h, then treated with E2 or vehicle for 14 h. Luciferase assays were performed on the isolated cells extracts. Insert is the Western blot analysis of KLF15 expression after infection with AdGFP (control) or AdKLF15 (experiment) compared to actin control in Ishikawa followed by E2 treatment for 14 h. (E) Role of KLF4 in MCM2_2000 transactivation. Ishikawa cells were cotransfected with MCM2_2000 UP along with KLF4 or vector containing GFP. Cells were incubated for 36 h then treated with E2 or vehicle for 14 h. Luciferase assays were performed on the cells extracts. Insert is the Western blot analysis of KLF4 expression compared to actin control after transfection of specific pGL3KLF4 (experiment) or pGL3GFP (control) in Ishikawa followed by E2 treatment for 14 h.
As E2 causes a stimulation of Mcm2 promoter activity, we determined whether this was ERα dependent by knocking down this receptor expression using a SiRNA. This SiRNA reduced ERα protein by approximately 80%, and significantly reduced Mcm2 transcriptional activity (Fig. 5C). This result indicates that the ERα drives the transcriptional response of the Mcm2 promoter and that the MCM2 upstream region acts as an E2 responsive element with the regulatory region between 2000 and 1227 bp upstream from the TSS.
Our ChIP data obtained above suggests negative regulation of Mcm2 transcription by KLF 15. To test this hypothesis directly, serum deprived Ishikawa cells were infected with adenovirus containing KLF-15 or control GFP followed by transfection with the MCM2_2000 UP (LUC) construct. The resultant cells that express KLF15 (Fig. 5D) were treated with E2 and the level of luciferase activity was directly determined in cell extracts. The expression of GFP from the adenovirus had no effect on luciferase activity (Fig. 5D). However, expression of KLF15 almost completely inhibited Mcm2 transcription in the E2 treated cells (Fig. 5D). As a control for specificity of the KLF, KLF4 was also overexpressed (Fig. 5E) in a parallel experiment. In contrast to KLF15, KLF4 increased MCM2-2000 luciferase expression (Fig. 5E). Thus the effect of KLF15 on Mcm2 transcription is not simply due to a KLF indiscriminately inhibiting its transcription.
Together, these data show that the Mcm2 promoter is transcriptionally regulated by E2 and that this transcription can be significantly inhibited by KLF15.
Ishikawa Cell Proliferation Is Negatively Regulated by KLF15 and Requires MCM2.
The rapid down-regulation of MCM proteins by P4E2 (28) led to the hypothesis that inhibition of the MCM mediated pathway plays a major role in the P4 negative regulation of uterine epithelial cell proliferation. This hypothesis was based on observations in lower organisms that reduction in any of the MCM’s level resulted in an inhibition of DNA synthesis (39). In this study, we showed that KLF15 is a negative regulator of Mcm2 transcription. Therefore we hypothesized that KLF15 is the mediator of P4 inhibition of DNA synthesis in uterine epithelial cells by suppression of Mcm2 transcription.
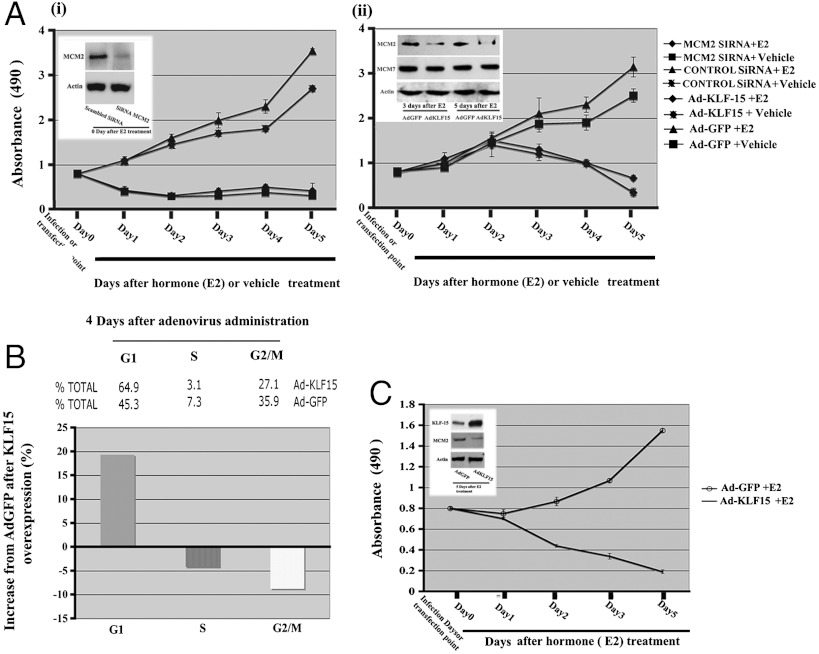
To test this hypothesis, we first inhibited Mcm2 expression using a SiRNA and determined the effects on cell proliferation in Ishikawa cells. In these experiments, E2 modestly stimulates Ishikawa cell proliferation, as determined using a MTT assay (Fig. 6A, i) as previously reported (38). This proliferative response was unaffected by transfection with a scrambled SiRNA. However, transfection with a specific SiRNA against Mcm2 mRNA,which reduced MCM2 protein levels by approximately 80%, resulted in a complete suppression of cell proliferation in both the control and E2 treated groups. This effect was seen within 24 h of the nonhormone treated state.
Fig. 6.
Effects of MCM2 knock down and KLF15 expression on Ishikawa cell proliferation. (A) Ishikawa cells were cultured in hormone free media for 24 h after MCM2 or scrambled SiRNA transfection (i) or infection with Ad-KLF15 or Ad-GFP (ii) followed by E2 or vehicle (ethanol) treatment. Cell proliferation was assayed in triplicate wells daily for 5 d after hormone treatment as described in the Materials and Methods, using the MTT assay (absorbance at 490 nm). Inserts (i, ii) indicate the Western blot analysis of equivalent amounts of protein isolated from these cell and detected with antibodies against MCM2, MCM7 (only ii) or actin as control after silencing of MCM2 SiRNA (i) or after KLF-15 overexpression (ii) for the indicated times. (B) Role of KLF15 overexpression on cell cycle parameters. Four days after postinfection, Ishikawa cells were collected and analyzed by flow cytometry to determine distribution of cells in each phase of the cell cycle. (C) Role of KLF15 overexpression on T47D cell proliferation. Human breast cancer T47D cells in culture were infected with rAdKLF-15 or rAd-GFP. Twenty-four hours after infection, cells were treated with E2 or vehicle and cell proliferation was assessed by the MTT method from 0 d to 5 d after treatment. Insert shows the Western blot analysis using antibodies against KLF15 and MCM2 compared to the control actin 5 d after treatment, indicating the reciprocal expression of KLF15 and MCM2. Values in (A) and (C) are presented as mean ± SE of five independent experiments (A and C).
Next we tested the effects of KLF15 overexpression in Ishikawa cells. These cells were infected with a KLF15 expressing adenovirus (rAdKLF15) or control GFP virus (rAdGFP) 24 h before hormone addition (Fig. 6A, ii). In the control virus treatment group, there was no effect on cell proliferation. However, infection of the cells with KLF15 expressing adenovirus reduced cell proliferation in both vehicle and E2 treated groups. But in contrast to the response to the MCM2 SiRNA, this reduction in proliferation occurred after approximately 2 d of expression. To determine whether this KLF15 overexpression affected MCM2 protein levels, we measured its expression. Within 4 d of infection (3 d after E2 treatment), KLF15 overexpression reduced MCM2 expression in these cells, an effect that was enhanced by 5 d of treatment (Fig. 6A, ii, insert). To determine specificity of the effect of KLF15, we also examined MCM7 expression because, in vivo, its expression is unresponsive to hormone treatment. KLF15 overexpression did not inhibit expression of MCM7 in Ishikawa cells, indicating specificity of the response (Fig. 6A, ii). We determined the effects of KLF15 on cell cycle progression by flow cytometry. This flow cytometric analysis indicated that, following KLF15 expression, cells accumulated in G1, showing that KLF15 inhibited cell cycle progression in the G1 phase of the cell cycle (Fig. 6B).
The inhibition of cell proliferation in Ishikawa cells by KLF15 was profound. Therefore, we sought to determine whether this was the case in other hormone responsive cell lines. We used the breast cancer cell line T47D to perform these experiments. Infection with an adenovirus expressing KLF15 inhibited T47D cell proliferation with a time-course similar to that observed in Ishikawa cells, whereas the control GFP virus had no effect (Fig. 6C).
Therefore, we can conclude that KLF15 is a negative regulator of cell proliferation in hormone responsive cell lines, at least in part through its inhibition of Mcm2 transcription.
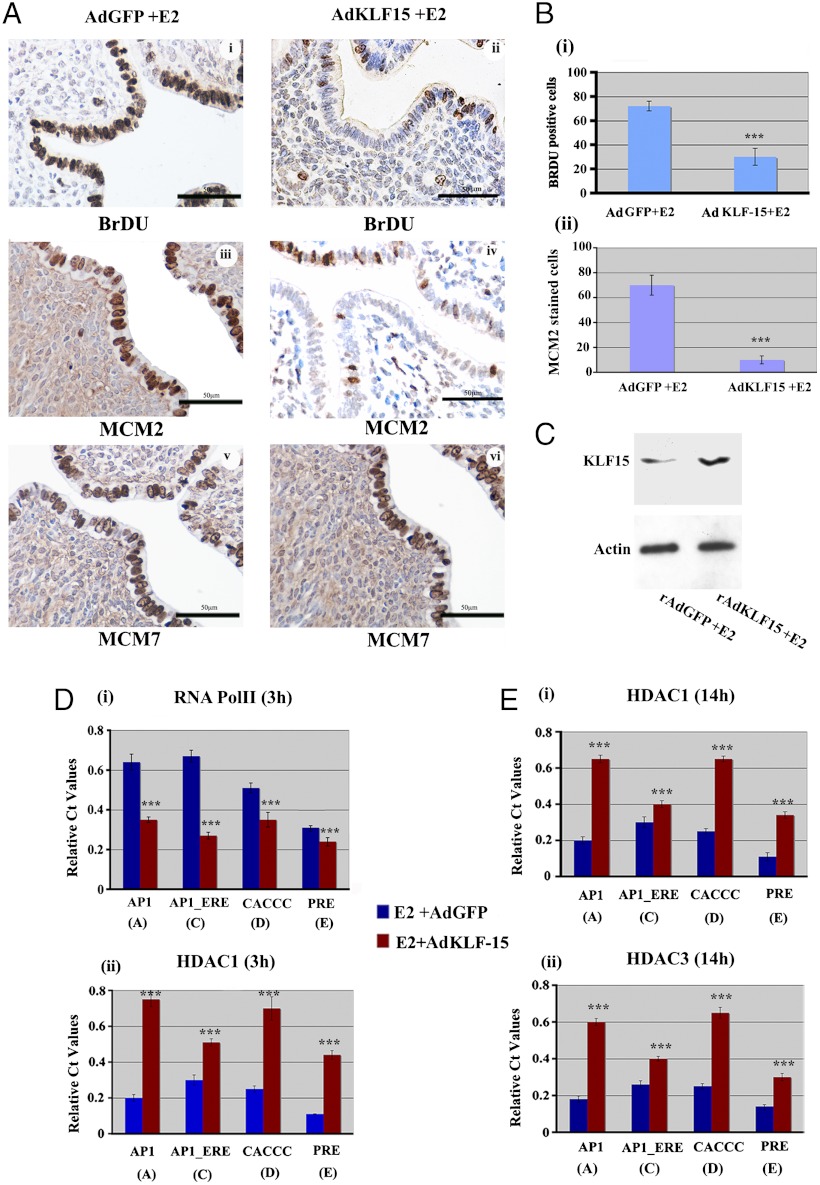
KLF15 Inhibits E2 Induced Epithelial Cell Proliferation in Vivo and Deacetylates the Mcm2 Chromatin.
The hypothesis tested in this paper is that KLF 15 inhibits transcription of genes such as Mcm2, and thereby inhibits E2 induced epithelial DNA synthesis, and therefore cell proliferation. This is clearly the case in tissue culture, so we tested whether KLF15 could block DNA synthesis in the mouse uterine epithelium in vivo. In order to test this hypothesis, we infected ovariectomized mice with the adenovirus expressing KLF15 or GFP as a control through intravenous and uterine intraluminal routes and subsequently treated the mice with E2. Mice were killed 15 h after E2 injection at the peak of DNA synthesis (40) and 2 h after an ip injection of BrDU. Following histological preparation of the uterus, the level of DNA synthesis was measured by BrDU incorporation per cell (Fig. 7A). Compared with the GFP control virus, the overexpression of KLF-15 (Fig. 7C) significantly inhibited E2 induced uterine DNA synthesis by approximately 65% in response to E2 (Fig. 7 A, i and ii; and B, i). To determine whether this inhibition of DNA synthesis by KLF15 was coincident with an inhibition of Mcm2 expression, we immunostained uterine transverse sections for MCM2. KLF15 expression compared to the control GFP expressing virus resulted an approximately 80% reduction in the number of MCM2 expressing cells in presence of E2, similar in extent to that observed for BrDU incorporation (Fig. 7 A, iii and iv; B).
Fig. 7.
KLF15 expression in the mouse uterus suppresses MCM2 expression and inhibits E2-induced epithelial DNA synthesis. (A) Ovariectomized CD1 mice were exposed to rAdGFP (control) (i, iii, v) or rAdKLF15 (ii, iv, vi) as described in the Materials and Methods, followed by injection of E2 for 14 h. Two hours before killing, mice were given BrDU ip. (I and ii). Transverse sections of formalin fixed uteri were immunostained for (i, ii) BrDU incorporation, (iii and iv) MCM2 and (v and vi) MCM7 followed by hematoxylin staining as described. Brown staining indicates positive reactivity. The data described here are representative of the analysis of at least five different mice for each group. Scale bar: 50 um. (B) Differential BrDU incorporation (i) or MCM2 staining (ii). Percentage of positive epithelial cells staining for nuclear BrDU (i) or MCM2 (ii) in Ad-KLF-15 or Ad-GFP infected mice 14 h after E2 administration. ***P < 0.001, n = 5. (C) KLF15 protein expression after rAdKLF15 or rAdGFP treated uterus. Equivalent amount of protein lysate isolated from uterine epithelial cells of mice treated with rAdKLF15 (experiment) or rAdGFP (control) followed by E2 for 14 h were separated by SDS PAGE and subjected to Western blotting with specific antibodies as shown. Actin was used as a protein loading control. (D) Analysis of RNA Pol II and HDAC1 association on Mcm2 promoter after KLF15 overexpression. Uterine tissues were collected after administration of rAdKLF15 (experiment: red bars) or rAd-GFP (control: blue bars) as described in Materials and Methods followed by E2 treatment for 3 h. ChIP analysis on isolated chromatin was performed with antibody against RNA Pol II (i) and HDAC1 (ii) on the indicated regions of Mcm2 gene promoter. Data was analyzed by Student t test. Difference in binding in each position ***P < 0.001, n = 5. (E) Analysis of acetylation signature on Mcm2 promoter after AdKLF-15 overexpression. ChIP analysis was performed and data expressed as defined in Fig. 2 using chromatin isolated from uterine tissues after rAd-KLF-15 (red bars) or rAd-GFP (blue bars) infection followed by E2 treatment for 14 h with antibody against HDAC1 (i) or HDAC3 (ii). (Difference between binding ***P < 0.001, n = 5).
Our previous results indicated that neither the expression of Mcm7 transcript nor the protein is significantly changed in uterine luminal epithelium in E2 vs. P4E2 treated samples (28). Thus we determined the effect of KLF15 on MCM7 expression. In contrast to the effect on MCM2 expression, there was no significant difference in MCM7 levels between the GFP and KLF15 treated groups in presence of E2 (Fig. 7A, v and vi). These data, together with the normal structure of the cells in the epithelium and stroma, showed that the effects of KLF15 overexpression are specific and not the result of toxicity.
Because we demonstrated an inverse relationship between KLF15 association with RNA Pol II binding on Mcm2 uterine chromatin during E2 and P4E2 treatments, we determined the status of RNA Pol II association after KLF15 overexpression in the uterus 3 h after E2 treatment. ChIP analysis was performed using RNA Pol II specific antibodies with uterine tissues collected from rAdKLF-15 or rAdGFP treated mice. Our results showed that KLF15 administration inhibited RNA Pol II association at the AP1 (A), AP1_ERE (C), CACCC (D), and PRE (E) sites on the Mcm2 chromatin even in the presence of E2 (Fig. 7D, i). Because E2 inhibits HDAC association and increases histone acetylation, next we determined the effect of KLF15 expression on HDAC association, on this Mcm2 promoter. KLF15 facilitated HDAC1 recruitment on chromatin in the estrogenized uterus at 3 h (Fig. 7D, ii) and 14 h (Fig. 7E, i) after E2 administration when compared to rAd-GFP treated mice. Increased HDAC-3 binding was also found 14 h after hormone treatment in rAd-KLF-15 mice (Fig. 7E, ii). Overall, KLF15 facilitates the recruitment of the HDACs onto the Mcm2 promoter even in the presence of E2 administration, consistent with its negative regulation of Mcm2 transcription.
In summary, we conclude that KLF15 is a negative regulator of E2 induced cell proliferation and, physiologically, is a downstream mediator of P4 action.
Discussion
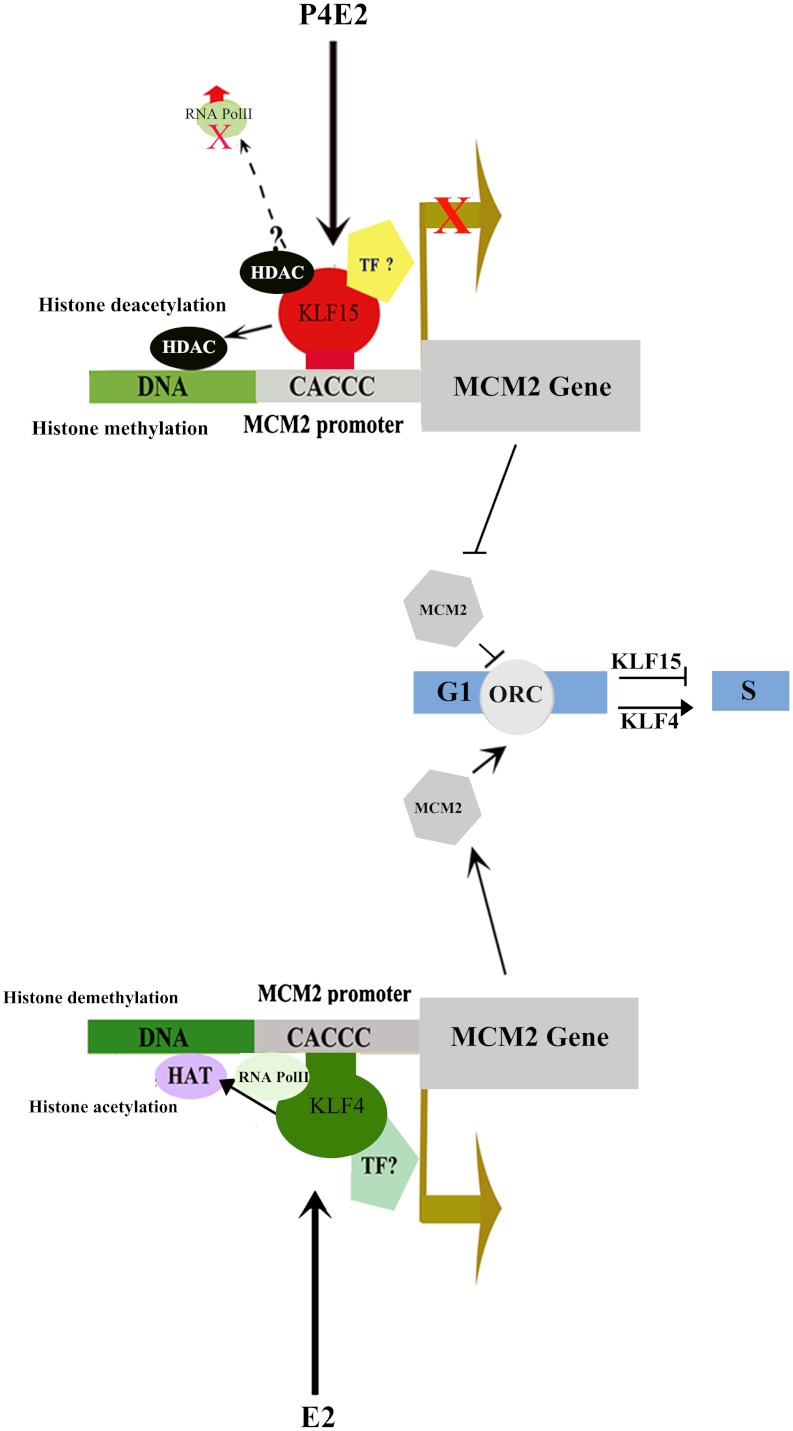
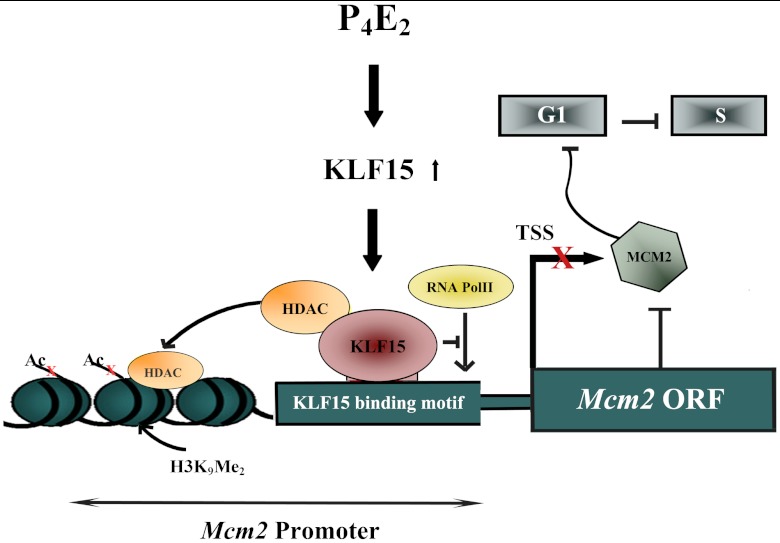
E2 induces and P4 inhibits uterine epithelial cell proliferation. In this study, as summarized in Fig. 8, our results show that two members of the Kruppel-like family of transcription factors, KLF4 and KLF15, mediates these hormones effects within the uterine epithelium. This is achieved by modulating the expression of Mcm2, whose product is required for DNA replication. E2 and P4E2 induce KLF4 and KLF15 recriprocally in uterine epithelial cells. KLF4 stimulates and KLF15 inhibits MCM2 transcription through modulating histone acetylation and methylation, and thereby RNA Pol II binding. The increased MCM2 as a result of KLF4 action in the estrogenized uterine epithelium allows for prereplication complex formation and thereby DNA synthesis initiation. In contrast, KLF15 inhibits Mcm2 expression with a reduction in Mcm2 mRNA and MCM2 protein, and thus DNA synthesis is inhibited. KLF15 expression alone is sufficient to block E2-induced epithelial DNA synthesis both in vivo and in vitro, indicating that this TF is a key mediator of P4E2 physiological action.
Fig. 8.
Proposed model for KLF4 and KLF15 mediation of E2 and P4E2 action in the uterine epithelium. In E2 exposed uterine epithelial cells, KLF4 binds to the MCM2 promoter and promotes histone demethylation and acetylation with the concomitant binding of RNA Pol II and transcription of the Mcm2 gene. This increased mRNA level leads to MCM protein accumulation and the assembly of the prereplication complex with the resultant DNA synthesis. In contrast, P4E2 treatment stimulates KLF15 and inhibits KLF4 epithelial cell expression. KLF15 in turn binds to the Mcm2 promoter and recruits HDACs that deacetylates the histones on the promoter. This deacetylation together with increased DNA methylation results in loss of RNA Pol II binding and suppression of Mcm2 transcription. Consequently, MCM2 levels are reduced in the cell, the binding of the hexameric MCM complex to the origins of DNA replication is blocked, and DNA synthesis is inhibited.
In their regulation of physiological events in the uterus, these sex steroid hormones act both cell autonomously and nonautonomously through their TF receptors (1, 10). In either case they induce the expression of downstream TFs in the target cell that propagate the initial signal. For example, P4 regulates epithelial synthesis of Indian Hedgehog (IHH). This IHH acts via the smoothened receptor expressed on stromal cells to induce the TF Coup-TF11, which in turn mediates downstream signaling, including the expression of bone morphogenic protein 2 that is required for stromal cell proliferation during the preimplantation period (41, 42). Meanwhile, over the same physiological period, P4 induces the helix-loop-helix TF, HAND2, in stromal cells to negatively regulate the stromal expression of FGFs that can induce epithelial proliferation (9). Similarly in the epithelium, E2-induced cell proliferation is regulated by stromal synthesized IGF-1, which binds to the IGF-1 receptor (IGF-1R), resulting in the engagement of the canonical cell cycle machinery that causes phosphorylation of the Rb family of transcriptional repressors (8, 31, 43). In this study we identified two additional transcription factors, KLF4 and KLF15, whose expression in the epithelium is reciprocally controlled by E2 and P4E2 and that, in turn, control DNA synthesis through modulating MCM2 expression.
The regulation of DNA synthesis through the loading of MCMs onto the origins of replication is a central control point of hormone activity as their function is essential for DNA synthesis in several organisms including yeast, Xenopus laevis and mammalian cells in culture (44). P4E2 negatively regulates the transcript abundance of the Mcm2-6 genes (28). Similarly in human endometrial epithelium, MCM2 transcripts are down-regulated in the P4 dominated secretory phase (5). In this study we demonstrated, using ChIP, that in vivo binding of KLF15 to the Mcm2 promoter in uterine tissue was dependent on P4E2 treatment. In contrast, in the E2 treated mice, KLF4 bound to the same sites in the Mcm2 promoter while KLF15 was relatively absent. Thus KLF15 and 4 have reciprocal binding to the Mcm2 promoter in P4E2 and an E2 dependent manner. We also showed that the MCM2 promoter is E2 inducible in tissue culture and, consistent with our hypothesis, KLF15 inhibited the E2-induced Mcm2 promoter transcriptional activity to basal, nonhormone stimulated levels. This effect was specific to KLF15, as overexpression of KLF4 did not inhibit Mcm2 promoter directed transcription, but instead stimulated it.
Covalent modification of histone tails has a fundamental role in chromatin structure and function. Histone acetylation is a dynamic process that is regulated by two classes of enzymes, histone acetyltransferase and HDACs. HDACs remove acetyl groups, increasing the positive charge of histone 3 and 4 tails, thus encouraging the high affinity binding between the histones and DNA backbone. The increased DNA binding condenses DNA structure and is linked with silencing of gene activity (33, 45). Consistent with P4 as a negative regulator of Mcm2 transcription, we showed that the in vivo binding of KLF15 to the Mcm2 promoter in the uterus was associated with the recruitment of HDACs and reduced histone H3 acetylation at K18. Histone 3 bimethylation (H3K9Me2) is a marker of inactive promoters and P4E2 increased H3K9Me2 methylation of the Mcm2 promoter compared to that observed in E2 treated uteri. Furthermore KLF15, overexpression in the estrogenized uterus increased the association of HDAC1 and 3 on the Mcm2 promoter. As decreased histone acetylation and increased methylation are associated with inactive chromatin, these data strongly suggest that KLF15 is a negative regulator of Mcm2 transcription in vivo as suggested by the luciferase assays. This conclusion was supported by the observation at the 3 and 14 h posttreatment time points that KLF15 is negatively correlated with RNA Pol II association on the Mcm2 promoter. In large scale genomic surveys, RNA Pol II binding to promoters predicted their transcriptional activity (46). This observation is consistent with our data on the activity of the Mcm2 promoter in response to E2 and P4E2. Overall, these cell culture and in vivo studies in the uterus define the Mcm2 promoter as a hormonally responsive one whose activity can be negatively regulated by KLF15.
To test the role of KLF15 as a downstream mediator of P4 in its inhibitory action on DNA synthesis, we expressed it in Ishikawa cells. KLF15 inhibited cell proliferation coincident with the down-regulation of MCM2 expression. This caused arrest of the cells in the G1 phase of the cell cycle. In contrast to this result, inhibition of KLF9 expression resulted in a reduced cell proliferation response to E2 in Ishikawa cells, while its expression elevated proliferation in the absence of this hormone (21). Nevertheless, this effect on proliferation of KLF9 was significantly less than that of the complete inhibition observed with KLF15 in this study. To confirm that this negative regulation of cell proliferation has physiological relevance, we expressed KLF15 in the uterus by adenovirus mediated gene transfer coincident with E2 treatment. This treatment resulted in inhibition of the E2-induced epithelial DNA synthesis (approximately 65%) that was accompanied by down-regulation of MCM2 but not MCM7 expression. These data indicate a specific effect of KLF15 and show that KLF15 alone is sufficient to inhibit E2 induced DNA synthesis in vivo in the absence of P4. These effects phenocopied the physiological action of P4 on E2 induced DNA synthesis. These results strongly argue that KLF15 is a downstream mediator of P4 antiproliferative effects on E2 induced DNA synthesis in the uterine epithelium.
Expression of MCMs is a marker for cycling cells, as their expression is lost in cells in G0. Recently this has been exploited as a biomarker in cancer. Indeed, several studies have identified MCMs, including MCM2, as powerful predictors of survival in many cancers including hormonally regulated ones such as breast and prostate (26). In other diseases of the endometrium that display abnormal proliferation and P4 resistance such as in endometriosis, the regulation of MCMs by P4 is also lost (47). P4 represents a rare example in vivo of a well-characterized physiological negative regulator of cell division. Our results establish that KLF15 inhibits endometrial and breast epithelial DNA synthesis. KLF15 therefore represents an important factor to be considered therapeutically for the inhibition of E2 proliferation action in the uterus and breast.
Materials and Methods
In brief, CD1 mice were ovariectomized and treated with hormonal regimens (31). At different times after the last E2 injection they were killed and their uteri fixed for IHC, or the uterine epithelium prepared and processed for Western blotting or ChIP as described (48). Ishikawa and T47D cells were cultured and transfected with Mcm2 promoter luciferase constructs and luminescence measure. Mice or cells were infected with Adenovirus expressing KLF15 or cells with a cDNA expressing KLF4 (48). All data were repeated at least three times and statistical analysis was performed using Prism 5. For details on all the above, see SI Materials and Methods.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dr. Mukesh K Jain and Dr. S. Halder (Case Western Reserve University, Cleveland OH) for the generous gift of the adenovirus expressing KLF15 and construct containing KLF15. This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement [U54 HD058155] as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and National Institutes of Health R01 HD50614 (to J.W.P.). J.W.P. is the Louis Goldstein Swan Chair in Women’s Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 7957 (volume 109, number 21).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118515109/-/DCSupplemental.
References
- 1.Rubel CA, Jeong JW, Tsai SY, Lydon JP, Demayo FJ. Epithelial-stromal interaction and progesterone receptors in the mouse uterus. Semin Reprod Med. 2010;28:27–35. doi: 10.1055/s-0029-1242990. [DOI] [PubMed] [Google Scholar]
- 2.Korhonen MO, Symons JP, Hyde BM, Rowan JP, Wilborn WH. Histologic classification and pathologic findings for endometrial biopsy specimens obtained from 2,964 perimenopausal and postmenopausal women undergoing screening for continuous hormones as replacement therapy (CHART 2 Study) Am J Obstet Gynecol. 1997;176:377–380. doi: 10.1016/s0002-9378(97)70502-7. [DOI] [PubMed] [Google Scholar]
- 3.Pike MC, et al. Estrogen-progestin replacement therapy and endometrial cancer. J Natl Cancer Inst. 1997;89:1110–1116. doi: 10.1093/jnci/89.15.1110. [DOI] [PubMed] [Google Scholar]
- 4.Tong W, et al. Female sex steroid hormone regulation of cell proliferation in the endometrium. In: Aplin JDFS, Glasser SR, Gludice LC, editors. The Endometrium: Molecular, Cellular and Clinical Persectives. 2nd Ed. London: Taylor and Francis; 2008. pp. 99–122. [Google Scholar]
- 5.Niklaus AL, et al. Assessment of the proliferative status of epithelial cell types in the endometrium of young and menopausal transition women. Hum Reprod. 2007;22:1778–1788. doi: 10.1093/humrep/dem032. [DOI] [PubMed] [Google Scholar]
- 6.Das A, et al. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci USA. 2009;106:12542–12547. doi: 10.1073/pnas.0901647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci USA. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu L, Pollard JW. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci USA. 2007;104:15847–15851. doi: 10.1073/pnas.0705749104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco HL, et al. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2011;26:1218–1227. doi: 10.1096/fj.11-193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin L, Finn CA, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: An autoradiographic study. J Endocrinol. 1973;56:133–144. doi: 10.1677/joe.0.0560133. [DOI] [PubMed] [Google Scholar]
- 12.Pan H, Zhu L, Deng Y, Pollard JW. Microarray analysis of uterine epithelial gene expression during the implantation window in the mouse. Endocrinology. 2006;147:4904–4916. doi: 10.1210/en.2006-0140. [DOI] [PubMed] [Google Scholar]
- 13.Wieschaus E, Nusslein-Volhard C, Kluding H. Kruppel, a gene whose activity is required early in the zygotic genome for normal embryonic segmentation. Dev Biol. 1984;104:172–186. doi: 10.1016/0012-1606(84)90046-0. [DOI] [PubMed] [Google Scholar]
- 14.Dang DT, Pevsner J, Yang VW. The biology of the mammalian Kruppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32:1103–1121. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Kruppel-like transcription factors: a functional family. Int J Biochem Cell Biol. 2008;40:1996–2001. doi: 10.1016/j.biocel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Lomberk G, Urrutia R. The family feud: Turning off Sp1 by Sp1-like KLF proteins. Biochem J. 2005;392:1–11. doi: 10.1042/BJ20051234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmen RC, et al. The emerging role of Kruppel-like factors in endocrine-responsive cancers of female reproductive tissues. J Endocrinol. 2010;204:223–231. doi: 10.1677/JOE-09-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velarde MC, Geng Y, Eason RR, Simmen FA, Simmen RC. Null mutation of Kruppel-like factor9/basic transcription element binding protein-1 alters peri-implantation uterine development in mice. Biol Reprod. 2005;73:472–481. doi: 10.1095/biolreprod.105.041855. [DOI] [PubMed] [Google Scholar]
- 19.Simmen RC, et al. Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Kruppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem. 2004;279:29286–29294. doi: 10.1074/jbc.M403139200. [DOI] [PubMed] [Google Scholar]
- 20.Pabona JM, Velarde MC, Zeng Z, Simmen FA, Simmen RC. Nuclear receptor co-regulator Kruppel-like factor 9 and prohibitin 2 expression in estrogen-induced epithelial cell proliferation in the mouse uterus. J Endocrinol. 2009;200:63–73. doi: 10.1677/JOE-08-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velarde MC, Zeng Z, McQuown JR, Simmen FA, Simmen RC. Kruppel-like factor 9 is a negative regulator of ligand-dependent estrogen receptor alpha signaling in Ishikawa endometrial adenocarcinoma cells. Mol Endocrinol. 2007;21:2988–3001. doi: 10.1210/me.2007-0242. [DOI] [PubMed] [Google Scholar]
- 22.Remus D, et al. Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing. Cell. 2009;139:719–730. doi: 10.1016/j.cell.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eward KL, et al. DNA replication licensing in somatic and germ cells. J Cell Sci. 2004;117:5875–5886. doi: 10.1242/jcs.01503. [DOI] [PubMed] [Google Scholar]
- 24.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 25.Coleman TR, Carpenter PB, Dunphy WG. The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez MA, Tachibana KE, Laskey RA, Coleman N. Control of DNA replication and its potential clinical exploitation. Nat Rev Cancer. 2005;5:135–141. doi: 10.1038/nrc1548. [DOI] [PubMed] [Google Scholar]
- 27.Tye BK. MCM proteins in DNA replication. Annu Rev Biochem. 1999;68:649–686. doi: 10.1146/annurev.biochem.68.1.649. [DOI] [PubMed] [Google Scholar]
- 28.Pan H, Deng Y, Pollard JW. Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci USA. 2006;103:14021–14026. doi: 10.1073/pnas.0601271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mairesse N, Galand P. Estrogen-induced proteins in luminal epithelium, endometrial stroma and myometrium of the rat uterus. Mol Cell Endocrinol. 1982;28:671–679. doi: 10.1016/0303-7207(82)90154-x. [DOI] [PubMed] [Google Scholar]
- 30.Tong W, Pollard JW. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol. 1999;19:2251–2264. doi: 10.1128/mcb.19.3.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen B, Pan H, Zhu L, Deng Y, Pollard JW. Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase → AKT → GSK → 3beta → cyclinD1 → pRB pathway to block uterine epithelial cell proliferation. Mol Endocrinol. 2005;19:1978–1990. doi: 10.1210/me.2004-0274. [DOI] [PubMed] [Google Scholar]
- 32.Martin L, Das RM, Finn CA. The inhibition by progesterone of uterine epithelial proliferation in the mouse. J Endocrinol. 1973;57:549–554. doi: 10.1677/joe.0.0570549. [DOI] [PubMed] [Google Scholar]
- 33.Barth TK, Imhof A. Fast signals and slow marks: the dynamics of histone modifications. Trends Biochem Sci. 2010;35:618–626. doi: 10.1016/j.tibs.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Kurdistani SK, Tavazoie S, Grunstein M. Mapping global histone acetylation patterns to gene expression. Cell. 2004;117:721–733. doi: 10.1016/j.cell.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 35.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 36.Jacob Y, et al. ATXR5 and ATXR6 are H3K27 monomethyltransferases required for chromatin structure and gene silencing. Nat Struct Mol Biol. 2009;16:763–768. doi: 10.1038/nsmb.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447(7143):407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 38.Croxtall JD, Elder MG, White JO. Hormonal control of proliferation in the Ishikawa endometrial adenocarcinoma cell line. J Steroid Biochem. 1990;35:665–669. doi: 10.1016/0022-4731(90)90306-d. [DOI] [PubMed] [Google Scholar]
- 39.Forsburg SL. Eukaryotic MCM proteins: Beyond replication initiation. Microbiol Mol Biol Rev. 2004;68:109–131. doi: 10.1128/MMBR.68.1.109-131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin L, Pollard JW, Fagg B. Oestriol, oestradiol-17beta and the proliferation and death of uterine cells. J Endocrinol. 1976;69:103–115. doi: 10.1677/joe.0.0690103. [DOI] [PubMed] [Google Scholar]
- 41.Franco HL, et al. Ablation of Indian hedgehog in the murine uterus results in decreased cell cycle progression, aberrant epidermal growth factor signaling, and increased estrogen signaling. Biol Reprod. 2010;82:783–790. doi: 10.1095/biolreprod.109.080259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee K, et al. Molecular mechanisms involved in progesterone receptor regulation of uterine function. J Steroid Biochem Mol Bio. 2006;102:41–50. doi: 10.1016/j.jsbmb.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richards RG, Walker MP, Sebastian J, DiAugustine RP. Insulin-like growth factor-1 (IGF-1) receptor-insulin receptor substrate complexes in the uterus. Altered signaling response to estradiol in the IGF-1(m/m) mouse. J Biol Chem. 1998;273:11962–11969. doi: 10.1074/jbc.273.19.11962. [DOI] [PubMed] [Google Scholar]
- 44.Remus D, Diffley JF. Eukaryotic DNA replication control: Lock and load, then fire. Curr Opin Cell Biol. 2009;21:771–777. doi: 10.1016/j.ceb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Eberharter A, Becker PB. Histone acetylation: A switch between repressive and permissive chromatin. EMBO Rep. 2002;3:224–229. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasowski M, et al. Variation in transcription factor binding among humans. Science. 2010;328:232–235. doi: 10.1126/science.1183621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burney RO, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 48.Ray S, Hou X, Zhou HE, Wang H, Das SK. Bip is a molecular link between the phase I and phase II estrogenic responses in uterus. Mol Endocrinol. 2006;20:1825–1837. doi: 10.1210/me.2006-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]