Abstract
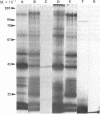
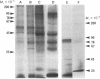
Lymphocytes were assessed for the presence of surface actin and myosin by lactoperoxidase-catalyzed iodination and indirect immunofluorescence using antisera against purified pig skeletal muscle actin and pig smooth muscle myosin. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis of 125I-labeled pig, mouse, and human B lymphocytes revealed an intense radioactive band of 43,000 molecular weight, whereas pig and mouse T lymphocytes gave a much less intense band. This band comigrated with actin, was nonglycosylated as judged by lack of binding to lentil lectin-Sepharose, was bound specifically by myosin fibers, and could be distinguished from a polypeptide of similar mobility derived from the major histocompatibility antigens. These results suggest that actin is present on the surface of B lymphocytes and, to a lesser extent, on T lymphocytes. Pig, mouse, and human Ig-bearing cells were stained by antiactin and antimyosin antisera, as judged by indirect immunofluorescence, whereas non-Ig-bearing cells were not stained. Antibody binding, however, was depleted by adsorbing the antisera with Ig-Sepharose. It was concluded that the immunofluorescence results are misleading and reflect the presence of antibodies that crossreact with Ig.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce-Gomez B., Jones E. A., Barnstable C. J., Solomon E., Bodmer W. F. The genetic control of HLA-A and B antigens in somatic cell hybrids: requirement for beta2 microglobulin. Tissue Antigens. 1978 Feb;11(2):96–112. doi: 10.1111/j.1399-0039.1978.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Barber B. H., Crumpton M. J. Actin associated with purified lymphocyte plasma membrane. FEBS Lett. 1976 Jul 15;66(2):215–220. doi: 10.1016/0014-5793(76)80507-8. [DOI] [PubMed] [Google Scholar]
- Bottomley R. C., Trayer I. P. Affinity chromatography of immobilized actin and myosin. Biochem J. 1975 Aug;149(2):365–379. doi: 10.1042/bj1490365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Fagraeus A., Lidman K., Biberfeld G. Reaction of human smooth muscle antibodies with human blood lymphocytes and lymphoid cell lines. Nature. 1974 Nov 15;252(5480):246–247. doi: 10.1038/252246a0. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Chaponnier C., Zumbe A., Vassalli P. Actin and tubulin co-cap with surface immunoglobulins in mouse B lymphocytes. Nature. 1977 Oct 20;269(5630):697–698. doi: 10.1038/269697a0. [DOI] [PubMed] [Google Scholar]
- Gröschel-Stewart U. Comparative studies of human smooth and striated muscle myosins. Biochim Biophys Acta. 1971 Feb 16;229(2):322–334. doi: 10.1016/0005-2795(71)90191-7. [DOI] [PubMed] [Google Scholar]
- Handwerger B. S., Schwartz R. H. Separation of murine lymphoid cells using nylon wool columns. Recovery of the B cell-enriched population. Transplantation. 1974 Dec;18(6):544–548. doi: 10.1097/00007890-197412000-00013. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Crumpton M. J. Isolation of glycoproteins from pig lymphocyte plasma membrane using Lens culinaris phytohemagglutinin. Biochem Biophys Res Commun. 1972 May 26;47(4):923–930. doi: 10.1016/0006-291x(72)90581-5. [DOI] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G. L., Smith M. J. An association between actin and the major histocompatibility antigen H-2. Nature. 1978 May 25;273(5660):274–278. doi: 10.1038/273274a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loken M. R., Herzenber L. A. Analysis of cell populations with a fluorescence-activated cell sorter. Ann N Y Acad Sci. 1975 Jun 30;254:163–171. doi: 10.1111/j.1749-6632.1975.tb29166.x. [DOI] [PubMed] [Google Scholar]
- Maino V. C., Hayman M. J., Crumpton M. J. Relationship between enhanced turnover of phosphatidylinositol and lymphocyte activation by mitogens. Biochem J. 1975 Jan;146(1):247–252. doi: 10.1042/bj1460247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Olden K., Willingham M., Pastan I. Cell surface myosin in cultured fibroblasts. Cell. 1976 Jul;8(3):383–390. doi: 10.1016/0092-8674(76)90150-1. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlés B., Flanagan M. T., Auger J., Crumpton M. J. Mechanism of lymphocyte activation: the binding of phytohemagglutinin to the lymphocyte surface. Eur J Immunol. 1977 Sep;7(9):613–619. doi: 10.1002/eji.1830070907. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Puszkin E. G., Maldonado R., Spaet T. H., Zucker M. B. Platelet myosin. Localization of the rod myosin fragment and effect of its antibodies on platelet function. J Biol Chem. 1977 Jun 25;252(12):4371–4378. [PubMed] [Google Scholar]
- Schreiner G. F., Fujiwara K., Pollard T. D., Unanue E. R. Redistribution of myosin accompanying capping of surface Ig. J Exp Med. 1977 May 1;145(5):1393–1398. doi: 10.1084/jem.145.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow M. P., Maxwell L. C., Ruegg J. C., Bohr D. F. Preparation and properties of a calcium ion-sensitive actomyosin from arteries. Am J Physiol. 1970 Nov;219(5):1366–1372. doi: 10.1152/ajplegacy.1970.219.5.1366. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Sundqvist K. G., Ehrnst A. Cytoskeletal control of surface membrane mobility. Nature. 1976 Nov 18;264(5583):226–231. doi: 10.1038/264226a0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Hyman R., Mazauskas C. Surface molecules of cultured human lymphoid cells. Eur J Immunol. 1976 Nov;6(11):777–782. doi: 10.1002/eji.1830061105. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Ralph P., Bevan M. J. Differences in the surface proteins of mouse B and T cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):157–161. doi: 10.1073/pnas.72.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Crumpton M. J. Orientation of cell-surface antigens in the lipid bilayer of lymphocyte plasma membrane. Nature. 1977 Sep 22;269(5626):307–311. doi: 10.1038/269307a0. [DOI] [PubMed] [Google Scholar]