Abstract
rRNA genes (rDNA) exist in two distinct epigenetic states, active promoters being unmethylated and marked by euchromatic histone modifications, whereas silent ones are methylated and exhibit heterochromatic features. Here we show that the nucleosome remodeling and deacetylation (NuRD) complex establishes a specific chromatin structure at rRNA genes that are poised for transcription activation. The promoter of poised rRNA genes is unmethylated, associated with components of the preinitiation complex, marked by bivalent histone modifications and covered by a nucleosome in the “off” position, which is refractory to transcription initiation. Repression of rDNA transcription in growth-arrested and differentiated cells correlates with elevated association of NuRD and increased levels of poised rRNA genes. Reactivation of transcription requires resetting the promoter-bound nucleosome into the “on” position by the DNA-dependent ATPase CSB (Cockayne syndrome protein B). The results uncover a unique mechanism by which ATP-dependent chromatin remodeling complexes with opposing activities establish a specific chromatin state and regulate transcription.
Keywords: poised chromatin, cell differentiation, epigenetic dynamics, NoRC
In vertebrates, the rDNA promoter is preceded by a terminator sequence, known as T0, which is recognized by the transcription factor TTF-I (1). Binding of TTF-I to the T0 element mediates ATP-dependent nucleosome remodeling, which correlates with efficient transcription initiation on repressed nucleosomal rDNA (2). TTF-I interacts with CSB (Cockayne syndrome protein B), a DNA-dependent ATPase that recruits the histone methyltransferase G9a to rDNA and promotes transcription elongation (3). TTF-I also interacts with the nucleolar remodeling complex (NoRC), a SNF2h-containing chromatin remodeling complex that targets histone-modifying enzymes and DNA methyltransferase to rDNA, establishing a heterochromatic state that is incompatible with transcription complex formation (4–6). In addition, NoRC alters the position of the promoter-bound nucleosome, placing it into the “off” position, which is unfavorable for transcription complex formation (7). The finding that TTF-I interacts with both the activating chromatin modifier CSB/G9a and the repressive remodeling complex NoRC, emphasizes the key role of TTF-I in establishing a characteristic chromatin structure at active or silent rRNA genes.
In this study, we have uncovered another chromatin-based mechanism that regulates Pol I transcription. We found that CSB is associated with NuRD (nucleosome remodeling and deacetylation complex), a complex that integrates ATP-dependent chromatin remodeling and histone modifying activities. NuRD contains class I histone deacetylases (HDACs), the ATPases/helicases CHD3 and CHD4 (chromodomain, helicase and DNA-binding 3 and 4), methyl-CpG binding domain proteins MBD2 and -3, and nonenzymatic proteins, such as metastasis-associated proteins (MTAs) and RbAp46 and -48 (8–11). The association with histone deacetylases and transcriptional repressors is consistent with a function of NuRD in gene silencing. However, NuRD is also associated with active genes, suggesting a versatile use of NuRD in transcriptional regulation (12–15). Here we demonstrate that CHD4/NuRD establishes a distinct chromatin state at rRNA genes that are transcriptionally inactive but are poised for transcription activation. The results reveal a unique mechanism by which ATP-dependent remodeling complexes regulate transcription, demonstrating that DNA methylation, nucleosome positioning, and histone modifications act in a concerted manner to create an epigenetic “landscape” that is recognized by multiprotein complexes that modify the chromatin structure and affect transcription.
Results
NuRD Interacts with CSB and TTF-I.
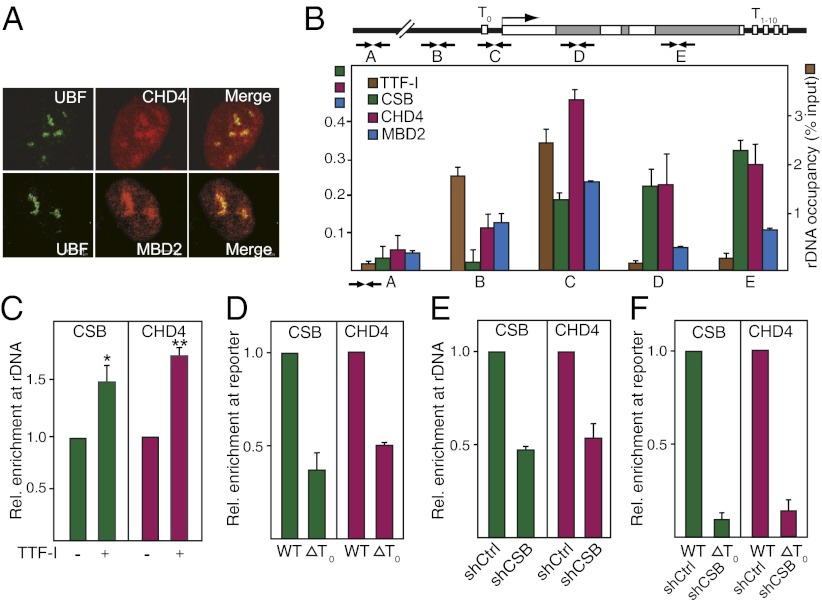
Identification of CSB-associated proteins by tandem affinity purification followed by mass spectrometry revealed that all components of NuRD, i.e., HDAC1 and -2, MBD2 and -3, MTA1–3, p66, and CHD3 and CHD4, are associated with CSB (Table S1 and Fig. S1A). Consistent with a role in rDNA transcription, CHD4/NuRD colocalizes with the upstream binding factor UBF in nucleoli (Fig. 1A) and is enriched in the transcribed part of rDNA (Fig. 1B). CSB is recruited to rDNA by interaction with TTF-I bound to the promoter-proximal terminator T0 (3). Coimmunoprecipitation and pulldown experiments demonstrate that CHD4 interacts with TTF-I in vivo and in vitro, indicating that TTF-I guides both CSB and CHD4 to rDNA (Fig. S1B). Consistent with this result, overexpression of TTF-I augmented rDNA occupancy of both CSB and CHD4 (Fig. 1C and Fig. S1C). In accord with NuRD being targeted to rDNA by interaction with TTF-I, both CSB and CHD4 bound to a reporter plasmid harboring the TTF-I target site T0 (WT) but not to a plasmid lacking T0 (ΔT0, Fig. 1D). Knockdown of CSB decreased rDNA occupancy of CHD4 (Fig. 1E). Depletion of CSB and deletion of T0 (shCSB+ΔT0) further impaired the association of CHD4 with rDNA (Fig. 1F), reinforcing that NuRD is recruited to rDNA by interaction with TTF-I and CSB.
Fig. 1.
TTF-I recruits CHD4/NuRD to the rDNA promoter. (A) CHD4 and MBD3 localize in nucleoli. Immunofluorescent images of NIH 3T3 cells stained with antibodies against UBF, CHD4, and MBD2. (B) CHD4 is associated with rDNA. ChIP measuring rDNA occupancy of the indicated proteins in NIH 3T3 cells is shown. Bars show enrichment of CSB, CHD4, and MBD2 (Left y axis) and TTF-I (Right y axis) normalized to input DNA. Scheme indicates the position of primers used to amplify different regions of rDNA. White boxes mark the position of the terminators T0 and T1–10. Error bars denote SD (n = 3). (C) Overexpression of TTF-I increases CHD4 occupancy at rDNA. ChIP assay shows binding of CHD4 and CSB to the rDNA promoter after overexpression of EGFP-tagged TTF-I. *P value < 0.05, **P value < 0.01. (D) Binding of TTF-I to the promoter-proximal terminator T0 is required for targeting CHD4 to rDNA. NIH 3T3 cells were transfected with plasmids encoding Flag-tagged CHD4 and HA-tagged CSB together with the reporter plasmid pMr-1930-BH (WT) or pMr-1930ΔT0 (ΔT0). The association of CHD4 and CSB with the reporter plasmids was assayed by ChIP (n = 3). (E) Depletion of CSB impairs CHD4 binding to rDNA. ChIP shows the occupancy of CHD4 and CSB at the rDNA promoter in NIH 3T3 cells infected with retroviruses encoding shRNA against CSB compared with control shRNA (n = 3). (F) Recruitment of CHD4/NuRD to rDNA requires both TTF-I and CSB. NIH 3T3 cells infected with retroviruses encoding shRNA against CSB (shCSB) or control shRNA (shCtrl) were transfected with the reporter plasmid pMr-1930-BH (WT) or pMr1930ΔT0 (T0). The association of CHD4 and CSB with the reporter plasmids was assayed by ChIP (n = 3).
CHD4/NuRD and CSB Cooperate in Pol I Transcription Activation.
Next we monitored pre-rRNA synthesis after shRNA-mediated knockdown of CHD4. Depletion of CHD4 repressed rDNA transcription shown by decreased fluorouridine incorporation into nucleolar RNA (Fig. 2A and Fig. S1E) and reduced pre-rRNA synthesis (Fig. 2B). Moreover, promoter occupancy of the Pol I transcription machinery, e.g., Pol I, SL1, and TIF-IA, was decreased, indicating that CHD4 depletion impaired transcription complex formation (Fig. 2C). Overexpression of CHD4 led to a slight increase in pre-rRNA, coexpression of CSB further augmenting transcription (Fig. 2D). Moreover, overexpression of CHD4 increased MBD2 binding to rDNA, supporting that NuRD rather than CHD4 alone augments transcription (Fig. S1F).
Fig. 2.
CHD4 is required for rDNA transcription. (A) Knockdown of CHD4 impairs nucleolar transcription. NIH 3T3 cells infected with retroviruses encoding CHD4-specific shRNA (shCHD4) were mixed with untransfected cells (ratio 4:1), labeled for 30 min with fluorouridine (FUrd), and stained with α-CHD4 and α-BrdU antibodies. CHD4-depleted cells are encircled; bar diagram represents quantification of FUrd staining of 300 CHD4-depleted cells compared with untransfected cells. (B) Depletion of CHD4 inhibits pre-rRNA synthesis. Northern blot (NB) shows the level of pre-rRNA in NIH 3T3 cells infected with retroviruses encoding CHD4-specific shRNA (shCHD4) or control shRNA (shCtrl). Ethidium bromide (EtBr)-stained cellular rRNA and CHD4 levels (WB) are shown below. (C) Knockdown of CHD4 impairs transcription complex formation. ChIPs show the occupancy of Pol I (RPA116), TIF-IA, SL1, and UBF at the rDNA promoter in NIH 3T3 cells expressing control shRNA (gray bars) or CHD4-specific shRNA (red bars, n = 3). Western blots show the levels of CHD4, CSB, UBF, Pol I, SL1, and tubulin upon CHD4 knockdown. (D) CHD4 and CSB cooperate in transcription activation. NIH 3T3 cells were infected with retroviruses encoding Flag/HA-tagged CHD4 and Flag/HA-tagged CSB. Pre-rRNA was monitored by qRT-PCR and normalized to GAPDH mRNA (n = 3). (E) CHD4 affects CpG methylation of the rDNA promoter. qPCR data show the level of HpaII-sensitive (gray bars) and HpaII-resistant (colored bars) rDNA promoter in control and CHD4 overexpressing cells, and in cells infected with retroviruses encoding off-target shRNA (shCtrl) and CHD4-specific shRNA (shCHD4, n = 3). (F) Depletion of CHD4 increases CpG methylation at the rDNA promoter. Bisulfite sequencing data show methylation of rDNA (−164/+50) from control (shCtrl) and CHD4-depleted NIH 3T3 cells (shCHD4). Methylated CpGs are represented by black circles, unmethylated CpGs by white ones.
Active and silent rDNA repeats can be distinguished by the degree of DNA methylation, the promoter of active rRNA genes being unmethylated and sensitive to digestion by HpaII, whereas at silent ones CpG residues at positions −143 and −133 within the upstream control element (UCE) are methylated. Overexpression of CHD4 decreased the level of HpaII-resistant methylated rDNA, whereas shRNA-mediated depletion of CHD4 increased methylation of the rDNA promoter (Fig. 2E). Methylation in CHD4-deficient cells was restricted to CpG-143 and CpG-133, whereas methylation of CpG+8 was not affected (Fig. 2F). Thus, CHD4/NuRD protects the rDNA promoter from being methylated, allowing UBF binding to the UCE, an important step in transcription complex formation. Depletion of CHD4 did not affect cell cycle progression, demonstrating that inhibition of rDNA transcription was not caused by cell cycle arrest (Fig. S2 A–C).
CHD4 Is Associated with Poised rRNA Genes.
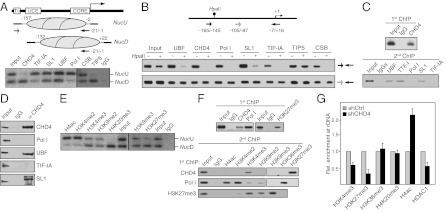
Active and silent rRNA genes also exhibit different histone modifications and nucleosome positions (5–7). At active rDNA promoters, the 3′-end of the promoter-bound nucleosome extends to nucleotide −2, whereas the nucleosome at silent rRNA genes is positioned 24 nucleotides further downstream, placing the nucleosome into a translational position that is unfavorable to transcription complex assembly. To examine whether CHD4/NuRD exhibits preference to nucleosomes in the upstream or downstream position, we precipitated mononucleosomes with antibodies against TIP5, CSB, Pol I, UBF, SL1, TIF-IA, and CHD4 and analyzed coprecipitated rDNA by ligation-mediated PCR (LM-PCR). The two promoter-associated nucleosomes yielded PCR products of different length, the longer one corresponding to the “upstream” nucleosome (NucU) in the active position, the shorter one representing the “downstream” one (NucD) in the repressive position (Fig. 3A). TIP5, the large subunit of the silencing complex NoRC, was almost exclusively associated with NucD, whereas CSB and Pol I, TIF-IA, SL1, and UBF were associated with NucU. Intriguingly, a fraction of SL1, UBF, and CSB was also associated with NucD, indicating that preinitation complexes are assembled at NucD. Even more surprising was the observation that CHD4/NuRD was almost exclusively associated with NucD. This result was validated with a different method by which immunoprecipitated mononucleosomal DNA was amplified with two different promoter-specific primer pairs (Fig. S3 A and B). The fact that CHD4/NuRD co-occupies with components of the preinitiation complex at NucD indicates that the NucD position is not restricted to silent genes established by NoRC but also includes a subfraction of rDNA, which is associated with CHD4/NuRD and is permissive for transcription.
Fig. 3.
CHD4 is associated with poised rRNA genes. (A) CHD4, SL1, UBF, and CSB are associated with poised nucleosomes. Mononucleosomes from cross-linked NIH 3T3 cells were incubated with the indicated antibodies, precipitated DNA was subjected to LM-PCR, and PCR products were analyzed on 6% PAA gels. Scheme illustrates the positions of NucU and NucD at the murine rDNA promoter and the primers used for LM-PCR. (B) CHD4 is associated with hypomethylated rDNA. Chromatin-immunoprecipitated DNA was digested with HpaII before PCR amplification. Data refer to 10% of input chromatin. Scheme shows the position of primers and the HpaII site at −143 (lollipop). (C) Sequential ChIP showing co-occupancy of CHD4 with the preinitiation complex. Cross-linked mononucleosomes were immunoprecipitated with CHD4 antibody (first ChIP), followed by precipitation with antibodies against UBF, TTF-I, Pol I, (RPA116), SL1 (TAFI95), and TIF-IA (second ChIP). Coprecipitated DNA was analyzed by PCR. (D) Coimmunoprecipitation assay showing interaction of CHD4 with UBF and SL1. A total of 10% of input and 50% of precipitated proteins were analyzed. (E) Histone modifications associated with NucD. Cross-linked mononucleosomes were immunoprecipitated and precipitated DNA was analyzed by LM-PCR. (F) Sequential ChIP showing colocalization of H3K4me3, H3K27me3, and CHD4 at NucD. Mononucleosomes were immunoprecipitated with antibodies against CHD4, Pol I, and H3K27me3 (first ChIP) before precipitation with the indicated histone antibodies (second ChIP). A total of 20% of input is shown. (G) ChIP showing the relative occupancy of specific histone modifications at the rDNA promoter in CHD4-depleted (dark bars) and control cells (light bars). ChIP data for histone modifications were normalized to histone H3 (n = 3).
To examine whether NucD associated with CHD4 is distinct from NucD bound by NoRC, we assayed the methylation state of CHD4-associated rDNA by chromatin immunoprecipitation (ChIP)-chop assays. As expected, TIP5/NoRC occupied methylated, HpaII-resistant rDNA, whereas CSB, CHD4/NuRD, and components of the Pol I transcription machinery, e.g., Pol I, TIF-IA, SL1, and UBF, were preferentially associated with unmethylated rRNA genes (Fig. 3B and Fig. S3C). Thus, NucD associated with CHD4 is distinct from NucD associated with NoRC. Sequential ChIP assays revealed that UBF and SL1, factors that form the preinitiation complex, but neither Pol I nor the transcription initiation factor TIF-IA, co-occupied with CHD4 (Fig. 3C). The same result was obtained by re-ChIP assays using antibodies against Pol I and basal Pol I transcription factors followed by immunoprecipitation of CHD4 (Fig. S3D). Consistently, CHD4 was found to interact with UBF and SL1 but not with Pol I and TIF-IA (Fig. 3D). Thus, CHD4/NuRD is associated with rRNA genes that are in a transcriptionally permissive “poised” nucleosomal conformation, suggesting that the association of Pol I and TIF-IA with the preinitiation complex requires movement of NucD to NucU.
Active genes carry euchromatic histone modifications, such as hyperacetylated histone H4 (H4ac) and H3K4me2 and -3, whereas silent ones carry heterochromatic marks, such as H3K9me2, H4K20me3, and H3K27me3 (5). To examine whether NucU and NucD are marked by different histone modifications, we performed ChIP and ChIP-chop assays on purified mononucleosomes. Consistent with NucU marking the promoter of active rRNA genes and NucD marking silent ones, hyperacetylated histone H4 (H4ac) and H3K4me2 were preferentially associated with NucU, whereas H4K20me3 and H3K27me3 were associated with NucD. H3K4me3, H3K9me2, and H3K9me3, however, were associated with both NucU and NucD, indicating that these histone modifications are not restricted to active or silent repeats (Fig. 3E). Hyperacetylated histone H4 and H3K4me3 were associated with unmethylated rRNA genes, H4K20me3 with methylated ones, and H3K27me3 and H3K9me2 were bound to both unmethylated and methylated rDNA (Fig. S3C). Consecutive ChIPs on mononucleosomes, first precipitating CHD4, Pol I, or H3K27me3, followed by precipitation with antibodies recognizing specific histone modifications, revealed that CHD4/NuRD is associated with nucleosomes carrying both the active histone mark H3K4me3 and the inactive mark H3K27me3, a bivalent chromatin signature found at genes that are maintained at a low transcriptional state (16) (Fig. 3F). Consistent with this result, NuRD has been shown to interact with the H3K4 and H3K27 methyltransferases ALL-1 and PRC2, supporting its role in the establishment or maintenance of bivalent histone marks (17). Pol I, on the other hand, colocalized with euchromatic histone modifications, e.g., H4ac, H3K4me2, and H3K36me3. Depletion of CHD4 decreased promoter occupancy of H3K4me3, H3K27me3, and HDAC1, a subunit of NuRD, leading to hyperacetylation of histone H4 (Fig. 3G).
Histone Binding and ATPase Activity of CHD4 Are Required for Transcription Activation.
CHD4 contains two PHD (plant homeodomain) fingers and chromodomains (CD), motifs known to bind to methylated histone tails (18, 19). In pulldown assays, PHD1 and CD2 bound to bulk histones (Fig. 4A). A point mutation (Y649E) in the CD2 domain, which mediates binding to methylated histone tails, impaired binding to rDNA, whereas mutations in the PHD finger (D386A) or the ATPase/helicase domain (K757R) did not affect the association of CHD4 with rDNA, indicating that CD2 is required for CHD4 binding (Fig. 4B). Depletion of WDR5, an essential subunit of the histone H3K4 methyltransferase MLL/Set1 (20), decreased the level of H3K4me3 and impaired the association of CHD4 with rDNA, indicating that H3K4me3 is required for CHD4/NuRD binding to chromatin (Fig. 4C and Fig. S3E). Overexpression of wild-type CHD4, but none of the mutants activated rDNA transcription (Fig. 4D). Consistently, none of the mutants was capable of rescuing rDNA transcription in CHD4-depleted cells (Fig. 4E), demonstrating that all three domains are required for transcriptional activation.
Fig. 4.
Transcription activation requires the histone binding and ATPase activity of CHD4. (A) The PHD1 and CD2 domains bind histones. (Top) CHD4 truncations comprising the PHD fingers (PHD1 and PHD2) and chromodomains (CD1 and CD2) were incubated with histones and associated histones were visualized on stained PAA gels (Top) and on immunoblots with α-histone H3 antibody (Middle). (Bottom) Amount of histones. Scheme illustrates the position of PHD fingers (PHD), chromodomains (CD), and ATPase/helicase domain. Point mutations are indicated by asterisks. (B) CD2 is required for CHD4 binding to rDNA. ChIP monitoring rDNA promoter occupancy of wild-type CHD4 and the indicated mutants is shown. Binding of mutant proteins was normalized to wild-type CHD4 (n = 3). Western blot shows the expression level of wild-type and mutant CHD4. (C) H3K4me3 is required for CHD4 binding to chromatin. ChIP comparing rDNA occupancy of CHD4, UBF, and H3K4me3 in WDR5-depleted (siWDR5) and control (siCtrl) cells (n = 3). (D) Chromatin remodeling and histone binding activity of CHD4 are required for transcriptional activation. Pre-rRNA levels in NIH 3T3 cells expressing wild-type or mutant CHD4 compared with mock transfected cells are shown (n = 3). Western blot shows the level of Flag-tagged wild-type and mutant CHD4. (E) Wild-type but not mutant CHD4 rescues pre-rRNA synthesis in CHD4-depleted cells. CHD4-deficient NIH 3T3 cells were infected with retroviruses expressing wild-type CHD4 or the indicated mutants. Bars represent the relative levels of pre-rRNA normalized to GAPDH mRNA (n = 3). (F) Depletion of CHD4 shifts NucD to the position of NucU. Nucleosome positions were analyzed in NIH 3T3 cells expressing shRNA against CSB, TIP5, or CHD4 (lanes 1–4). To rescue nucleosome positions, CHD4-depleted cells were infected with retroviruses expressing wild-type or mutant CHD4 (lanes 5–9). Bar diagram shows the ratio of NucU (gray) to NucD (dark).
The finding that the chromatin remodeling activity of CHD4 is indispensable for transcription activation suggests that CHD4/NuRD establishes the NucD position at transcriptionally permissive genes. Indeed, knockdown of CHD4/NuRD enhanced the ratio of NucU to NucD, supporting that CHD4/NuRD is associated with NucD at rRNA genes that are poised for transcription activation (Fig. 4F). Overexpression of wild-type CHD4 in CHD4-depleted cells reestablished the ∼1:1 ratio of NucU to NucD and activated rDNA transcription, whereas none of the mutants was capable of restoring the position of NucD and rescuing transcription (Fig. 4 E and F). Depletion of CSB had the opposite effect, leading to loss of NucU. These results demonstrate that CHD4/NuRD-dependent activation of rDNA transcription requires the activity of both CHD4 and CSB, CHD4 shifting the promoter-bound nucleosome to the position of NucD, whereas CSB moves the nucleosome into the NucU position that favors transcription complex assembly.
The Fraction of Poised rRNA Genes Is Increased in Growth-Arrested Cells.
To examine whether down-regulation of pre-rRNA synthesis in differentiated cells is accompanied by an increase in poised genes, we compared rDNA transcription, histone modifications, DNA methylation, and nucleosome positioning in undifferentiated and differentiated C2C12 cells (Fig. S4). As expected, decreased rDNA transcription in terminally differentiated cells correlated with hypoacetylation of histone H4 and reduced levels of Pol I and TIF-IA (Fig. 5 A and B). Whereas promoter occupancy of CSB, SL1, and UBF remained unaffected, the association of CHD4, H3K4me3, and H3K27me3 was increased in differentiated cells. Significantly, the ratio of NucU to NucD was altered in differentiated cells, the level of NucD increasing at the expense of NucU (Fig. 5C). Moreover, binding of CHD4 to rDNA was enhanced, indicating that repression of rDNA transcription in differentiated cells is accompanied by a CHD4/NuRD-dependent increase of poised genes. Similarly, down-regulation of Pol I transcription in serum-deprived NIH 3T3 cells correlated with increased binding of CHD4/NuRD and enhanced promoter occupancy of histone H3K4me3 and H3K27me3 (Fig. S5A). Again, the ratio of NucU to NucD decreased, changing from ∼1:1 in growing cells to 1:9 in starved cells (Fig. S5B). The ∼1:1 ratio was reestablished after refeeding the cells with serum. Reactivation of transcription did not occur in CHD4-depleted cells (Fig. S5C), underscoring the role of CHD4/NuRD in transcriptional regulation. Together, the results suggest that transcription of rRNA genes involves dynamic and reversible changes of nucleosome positions and histone modifications at the rDNA promoter. CHD4/NuRD establishes the transcriptional permissive poised state in growth-arrested cells and transcriptional activation requires the interplay of CHD4/NuRD and CSB.
Fig. 5.
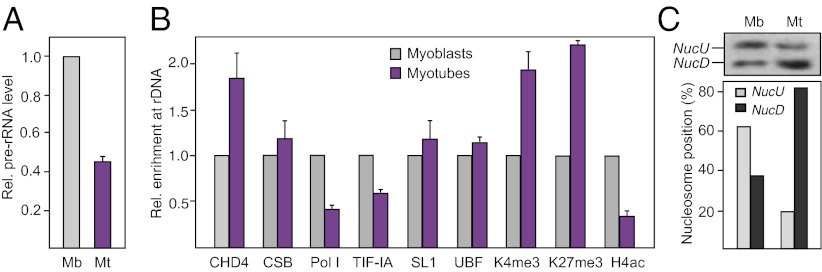
Number of poised rRNA genes is increased in differentiated cells. (A) Pre-rRNA synthesis is inhibited in differentiated C2C12 cells. Level of pre-rRNA in undifferentiated (Mb) and differentiated C2C12 cells (Mt) was monitored by RT-PCR and normalized to GAPDH mRNA (n = 3). Microscopic images showing the morphology of undifferentiated C2C12 cells (myoblasts) and differentiated C2C12 cells (myotubes) are shown on the Left. (B) ChIP comparing promoter occupancy of the indicated proteins in myoblasts and myotubes. ChIPs for histone modifications were normalized to histone H3 (n = 3). (C) Nucleosome positions are altered in differentiated cells. Nucleosome positions were analyzed in myoblasts (Mb) and myotubes (Mt) by LM-PCR. Bar diagram represents quantification of NucU and NucD.
Discussion
rRNA genes exist in two distinct epigenetic states: the promoter of active rRNA genes being unmethylated and exhibiting euchromatic features, whereas silent genes are methylated and exhibit a more condensed, heterochromatic conformation. The active configuration of rRNA genes is mediated by CSB, a DNA-dependent ATPase that interacts with the histone methyltransferase G9a and localizes at sites of active transcription (3). The silent state of rRNA genes is established by NoRC, a SNF2h-containing chromatin remodeling complex that recruits chromatin-modifying enzymes to the rDNA promoter and triggers promoter methylation, heterochromatin formation, and transcriptional silencing (5, 6, 21). Although the relative amounts of active and silent rDNA repeats are stably maintained through cell cycle progression (22), long-term changes in the ratio of genes in an “open” euchromatic versus a “closed” heterochromatic structure have been observed. Hypomethylation of rRNA genes, reflecting an increase in the number of active genes at the expense of silent ones, has been observed in several tumors (23). Conversely, our data demonstrate that in differentiated and growth-arrested cells, the number of poised genes is increased. Thus, active rRNA genes undergo dynamic changes upon growth arrest, suggesting a mechanism that establishes long-term down-regulation of rDNA transcription.
The results of this study demonstrate the existence of a third chromatin configuration of rDNA that marks genes that are poised for transcription activation. Poised rRNA genes exhibit a chromatin structure that is accessible for binding of transcription factors, but they are not transcribed. Such potentially active rRNA genes are associated with NuRD, a protein complex that contains HDACs and CHD4. Like active rRNA genes, the promoter of potentially active genes is unmethylated and is associated with components of the preinitiation complex. Unlike active genes, however, there is no Pol I and TIF-IA at the rDNA promoter and the promoter-associated nucleosome is in the position of NucD. Moreover, poised rRNA genes are marked with nucleosomes that carry both euchromatic (H3K4me3) and heterochromatic histone modifications (H3K27me3). These results demonstrate that NucD comprises two distinct fractions of rRNA genes: methylated ones that are silenced by NoRC and unmethylated ones that are associated with CHD4/NuRD and are permissive for transcription activation. Of note, down-regulation of rDNA transcription in differentiated C2C12 cells was not accompanied by decreased UBF occupancy as in murine granulocytes (24), suggesting that the chromatin state of rRNA genes may vary between cell types, reflecting lineage-specific or developmental mechanisms of transcriptional repression.
Our results indicate that NuRD/CHD4 cooperates with CSB, a DNA-dependent ATPase that localizes at active rRNA genes and is part of a protein complex comprising TFIIH and components of the Pol I transcription machinery (25). CSB, in turn, interacts with G9a, a histone H3K9 methyltransferase that augments Pol I transcription through chromatin (3). Consistent with specific combinations of different chromatin regulators colocalizing in characteristic patterns at distinct genes, we find that CSB and NuRD co-bind to the rDNA promoter and both activities are required for transcription. Knockdown of either CSB or CHD4 impaired the association of Pol I with rDNA and inhibited pre-rRNA synthesis. Surprisingly, although CSB and CHD4 interact with each other and cooperate in transcription activation, both proteins are associated with differently modified and positioned nucleosomes. Whereas CHD4 is bound to poised NucD, a position that prevents assembly of the transcription initiation complex, CSB was found to interact with both poised NucD and with NucU. This suggests that the combinatorial utilization of CSB and CHD4 is required for transcriptional activation, and the functional interplay of CSB and CHD4/NuRD ensures proper chromatin regulation. Accordingly, knockdown of CHD4 increased the position of poised NucU at the expense of NucD, whereas knockdown of CSB triggered the shift of NucU to the position of NucD. Down-regulation of rDNA transcription in G0-arrested cells correlated with increased levels of NucD and enhanced rDNA promoter occupancy of CHD4. Reactivation of transcription after serum addition was accompanied by transition of NucD to NucU. We propose that CHD4/NuRD establishes the transcriptionally permissive state of rRNA genes, whereas CSB mediates the transition from the permissive to the active state. Thus, CSB and CHD4 cooperate to establish distinct nucleosome positions at the rDNA promoter, the functional interplay of both chromatin modifiers being required for activation of rDNA transcription. A variation of this model would be that CHD4 and CSB cooperate in the assembly and disassembly of NucU and NucD during each round of transcription, CSB promoting dissociation and CHD4 mediating reassembly of NucD. In this scenario, cyclic assembly and disassembly is required for transcription initiation. Although our data are consistent with either model, further studies are needed to unequivocally prove the postulated dynamics and flexibility of nucleosomal architecture at the rDNA promoter.
Experimental Procedures
ChIP, Mononucleosome Precipitation, and DNA Methylation Assays.
ChIPs were performed as described (6). To immunoprecipitate mononucleosomes, cross-linked chromatin was digested with micrococcal nuclease (Roche) and isolated nucleosomes were incubated with the respective antibodies. DNA methylation was monitored by digestion with HpaII before qPCR amplification. The relative amount of HpaII-resistant DNA was normalized to mock-digested DNA. Alternatively, CpG methylation was monitored by bisulfite treatment, followed by cloning and sequencing of rDNA.
Transfections, Retroviral Infections, and RNA Analysis.
A total of 3 × 105 NIH 3T3 cells were transfected with 2 μg of reporter plasmid and different amounts of expression vectors. For knockdown of proteins, NIH 3T3 cells were infected with retroviruses encoding the respective shRNAs. To rescue CHD4 expression, cells expressing shRNA–CHD4 were cultured for 4–5 d in the presence of puromycin (3 μg/mL) before infection with retroviruses expressing wild-type or mutant CHD4.
Analysis of Nucleosome Positions.
Analysis of nucleosome positions at the rDNA promoter has been described (7). Briefly, cross-linked cells were permeabilized with 0.05% (mass/vol) lysolecitin (1 min, 37 °C) in 150 mM sucrose, 80 mM KCl, 35 mM Hepes (pH 7.4), 5 mM K2HPO4, 5 mM MgCl2, and 0.5 mM CaCl2. Chromatin was digested with MNase (100 units/mL) in 150 mM sucrose, 50 mM Tris-HCl (pH 7.4), 50 mM NaCl, and 2 mM CaCl2 (20 min, 37 °C). After reversal of the cross-link and gel electrophoresis, mononucleosome-sized DNA was isolated (Fig. S3A), ligated to phosphorylated oligonucleotides (linker S: 5′-gaattcagatc-3′; linker L: 5′-gcggtgacccgggagatctgaattc-3′) and amplified using linker L and a radiolabeled rDNA-specific primer (−21/−1).
Supplementary Material
Acknowledgments
Work in I.G.’s laboratory has been supported by the Deutsche Forschungsgemeinschaft, the Bundesministerium für Bildung und Forschung (“EpiSys”), and an Advanced Grant from the European Research Council (ERC). W.T. has been supported by National Natural Science Foundation of China Grants 30871377, 31171255, and 31071116.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201262109/-/DCSupplemental.
References
- 1.Grummt I, Rosenbauer H, Niedermeyer I, Maier U, Öhrlein A. A repeated 18 bp sequence motif in the mouse rDNA spacer mediates binding of a nuclear factor and transcription termination. Cell. 1986;45:837–846. doi: 10.1016/0092-8674(86)90558-1. [DOI] [PubMed] [Google Scholar]
- 2.Längst G, Blank TA, Becker PB, Grummt I. RNA polymerase I transcription on nucleosomal templates: The transcription termination factor TTF-I induces chromatin remodeling and relieves transcriptional repression. EMBO J. 1997;16:760–768. doi: 10.1093/emboj/16.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan X, Feng W, Imhof A, Grummt I, Zhou Y. Activation of RNA polymerase I transcription by cockayne syndrome group B protein and histone methyltransferase G9a. Mol Cell. 2007;27:585–595. doi: 10.1016/j.molcel.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Strohner R, et al. NoRC—a novel member of mammalian ISWI-containing chromatin remodeling machines. EMBO J. 2001;20:4892–4900. doi: 10.1093/emboj/20.17.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santoro R, Li J, Grummt I. The nucleolar remodeling complex NoRC mediates heterochromatin formation and silencing of ribosomal gene transcription. Nat Genet. 2002;32:393–396. doi: 10.1038/ng1010. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Santoro R, Grummt I. The chromatin remodeling complex NoRC targets HDAC1 to the ribosomal gene promoter and represses RNA polymerase I transcription. EMBO J. 2002;21:4632–4640. doi: 10.1093/emboj/cdf460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Längst G, Grummt I. NoRC-dependent nucleosome positioning silences rRNA genes. EMBO J. 2006;25:5735–5741. doi: 10.1038/sj.emboj.7601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, et al. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13:1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Q, Zhang Y. The NuRD complex: Linking histone modification to nucleosome remodeling. Curr Top Microbiol Immunol. 2003;274:269–290. doi: 10.1007/978-3-642-55747-7_10. [DOI] [PubMed] [Google Scholar]
- 10.Le Guezennec X, et al. MBD2/NuRD and MBD3/NuRD, two distinct complexes with different biochemical and functional properties. Mol Cell Biol. 2006;26:843–851. doi: 10.1128/MCB.26.3.843-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirose F, Ohshima N, Kwon EJ, Yoshida H, Yamaguchi M. Drosophila Mi-2 negatively regulates dDREF by inhibiting its DNA-binding activity. Mol Cell Biol. 2002;22:5182–5193. doi: 10.1128/MCB.22.14.5182-5193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams CJ, et al. The chromatin remodeler Mi-2beta is required for CD4 expression and T cell development. Immunity. 2004;20:719–733. doi: 10.1016/j.immuni.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Murawska M, et al. dCHD3, a novel ATP-dependent chromatin remodeler associated with sites of active transcription. Mol Cell Biol. 2008;28:2745–2757. doi: 10.1128/MCB.01839-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miccio A, et al. NuRD mediates activating and repressive functions of GATA-1 and FOG-1 during blood development. EMBO J. 2010;29:442–456. doi: 10.1038/emboj.2009.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimono K, Shimono Y, Shimokata K, Ishiguro N, Takahashi M. Microspherule protein 1, Mi-2beta, and RET finger protein associate in the nucleolus and up-regulate ribosomal gene transcription. J Biol Chem. 2005;280:39436–39447. doi: 10.1074/jbc.M507356200. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, et al. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol Cell. 2002;10:1119–1128. doi: 10.1016/s1097-2765(02)00740-2. [DOI] [PubMed] [Google Scholar]
- 18.Sims RJ, 3rd, et al. Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. J Biol Chem. 2005;280:41789–41792. doi: 10.1074/jbc.C500395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flanagan JF, et al. Double chromodomains cooperate to recognize the methylated histone H3 tail. Nature. 2005;438:1181–1185. doi: 10.1038/nature04290. [DOI] [PubMed] [Google Scholar]
- 20.Wysocka J, et al. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature. 2006;442:86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- 21.Mayer C, Schmitz KM, Li J, Grummt I, Santoro R. Intergenic transcripts regulate the epigenetic state of rRNA genes. Mol Cell. 2006;22:351–361. doi: 10.1016/j.molcel.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 22.Conconi A, Widmer RM, Koller T, Sogo JM. Two different chromatin structures coexist in ribosomal RNA genes throughout the cell cycle. Cell. 1989;57:753–761. doi: 10.1016/0092-8674(89)90790-3. [DOI] [PubMed] [Google Scholar]
- 23.Ghoshal K, et al. Role of human ribosomal RNA (rRNA) promoter methylation and of methyl-CpG-binding protein MBD2 in the suppression of rRNA gene expression. J Biol Chem. 2004;279:6783–6793. doi: 10.1074/jbc.M309393200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanij E, et al. UBF levels determine the number of active ribosomal RNA genes in mammals. J Cell Biol. 2008;183:1259–1274. doi: 10.1083/jcb.200805146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradsher J, et al. CSB is a component of RNA pol I transcription. Mol Cell. 2002;10:819–829. doi: 10.1016/s1097-2765(02)00678-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.