Abstract
The cellular events that cause ischemic neurological damage following aneurysmal subarachnoid hemorrhage (SAH) have remained elusive. We report that subarachnoid blood profoundly impacts communication within the neurovascular unit—neurons, astrocytes, and arterioles—causing inversion of neurovascular coupling. Elevation of astrocytic endfoot Ca2+ to ∼400 nM by neuronal stimulation or to ∼300 nM by Ca2+ uncaging dilated parenchymal arterioles in control brain slices but caused vasoconstriction in post-SAH brain slices. Inhibition of K+ efflux via astrocytic endfoot large-conductance Ca2+-activated K+ (BK) channels prevented both neurally evoked vasodilation (control) and vasoconstriction (SAH). Consistent with the dual vasodilator/vasoconstrictor action of extracellular K+ ([K+]o), [K+]o <10 mM dilated and [K+]o >20 mM constricted isolated brain cortex parenchymal arterioles with or without SAH. Notably, elevation of external K+ to 10 mM caused vasodilation in brain slices from control animals but caused a modest constriction in brain slices from SAH model rats; this latter effect was reversed by BK channel inhibition, which restored K+-induced dilations. Importantly, the amplitude of spontaneous astrocytic Ca2+ oscillations was increased after SAH, with peak Ca2+ reaching ∼490 nM. Our data support a model in which SAH increases the amplitude of spontaneous astrocytic Ca2+ oscillations sufficiently to activate endfoot BK channels and elevate [K+]o in the restricted perivascular space. Abnormally elevated basal [K+]o combined with further K+ efflux stimulated by neuronal activity elevates [K+]o above the dilation/constriction threshold, switching the polarity of arteriolar responses to vasoconstriction. Inversion of neurovascular coupling may contribute to the decreased cerebral blood flow and development of neurological deficits that commonly follow SAH.
Keywords: vasospasm, potassium channels, reactive astrogliosis, vascular smooth muscle cells, two-photon calcium imaging
Aneurysmal subarachnoid hemorrhage (SAH)—strokes involving cerebral aneurysms that rupture and release blood onto the brain surface—are associated with high rates of death and disability. Up to 50% of SAH patients die within the first 30 d after aneurysm rupture, and 30–50% of survivors are left with moderate to severe permanent disabilities (1). Cerebral aneurysms tend to rupture in relatively young individuals (age of peak rupture rate ∼55 y), compounding the societal and economic impact of SAH. Delayed ischemic neurological deficits (DINDs), developing several days after aneurysm rupture, are a significant contributor to morbidity and mortality in SAH patients (2).
Improving outcome for SAH patients has proven to be an elusive goal, and current pharmacological therapies remain ineffective (1, 3). For decades, delayed blood-induced vasospasm of brain surface arteries was considered the major underlying cause of SAH-induced DINDs (4, 5). Recently, this paradigm has been challenged, leading to a reevaluation of the causal relationship between angiographically defined vasospasm of surface conduit arteries and SAH-induced morbidity and mortality (5–7). Supporting this change in sentiment, clinical trials have found that the endothelin-1A receptor antagonist clazosentan significantly decreased the incidence of large-artery vasospasm but failed to improve outcome in SAH patients (8, 9). Additional mechanisms that may contribute to SAH-induced DINDs include early brain injury, inflammation, cortical spreading depression, and focal cortical infarcts arising from perfusion deficiencies within the microcirculation (5, 6, 10, 11). Perfusion deficiencies reflecting restricted blood flow through intracerebral (parenchymal) arterioles could result from microthrombi formation (12), vasoconstriction caused by extravascular blood or blood breakdown products (13, 14), or impaired neurovascular coupling (15).
Neurovascular coupling is the process by which local blood flow within the brain is regulated through the coordinated activity of neurons, astrocytes, and parenchymal arterioles (i.e., the neurovascular unit). Physiologically, neurovascular coupling forms the basis of functional hyperemia, whereby localized neuronal activity leads to a focal increase in blood flow that serves to ensure adequate delivery of oxygen and nutrients to active neurons. This neurovascular signaling cascade involves (i) neuronal activation and release of the neurotransmitter glutamate; (ii) activation of metabotropic glutamate receptors (mGluRs) on astrocytic processes, leading to a wave of elevated calcium (Ca2+) in astrocytes mediated by activation of inositol trisphosphate receptors; and (iii) Ca2+-dependent release of vasodilatory signals from astrocyte endfeet onto parenchymal arterioles (16–18). Prominent among released vasodilatory mediators are vasodilatory arachidonic acid metabolites and K+ efflux mediated by activation of large-conductance Ca2+-activated K+ (BK) channels on astrocyte endfeet (19, 20).
Ex vivo studies have reported that, paradoxically, neuronal activity and elevations in astrocytic endfoot Ca2+ also can lead to parenchymal arteriolar constriction (21–23). Because it decreases local cerebral blood flow and compromises neuronal viability, neurally evoked vasoconstriction likely represents a pathological phenomenon. Consistent with this interpretation, vasoconstrictive or inverted neurovascular coupling has been reported during ischemic depolarization in mice (24) and during cortical spreading depression in human SAH patients (25).
Here, we report that SAH causes a fundamental shift in neurovascular coupling from vasodilation to vasoconstriction. Our findings suggest that increased basal levels of perivascular K+ caused by an increase in the amplitude of spontaneous Ca2+ oscillations and enhanced BK channel activity in astrocyte endfeet underlie this pathology. This change in the polarity of the neurovascular response from vasodilation to vasoconstriction may promote the development of DINDs and contribute to the morbidity and mortality associated with cerebral aneurysm rupture.
Results
Neuronal Activation Causes Constriction, Not Dilation, in Brain Slices from SAH Model Rats.
In humans, ischemic neurological deficits frequently occur several days after cerebral aneurysm rupture and SAH. To mimic aneurysmal SAH, we surgically injected whole autologous arterial blood through the cisterna magna into the subarachnoid space of anesthetized rats (14). Sham-operated animals received injections of artificial cerebral spinal fluid (aCSF) rather than blood. Cortical brain slices obtained from sham-operated and SAH model rats were studied 4 d post surgery. In some brain slices from SAH animals, extravascular red blood cells were observed along parenchymal arterioles more than 500 μm into the cerebral cortex (Fig. S1). These observations confirm that subarachnoid blood can pass beyond the Virchow–Robin space and potentially interact with astrocytes and parenchymal arterioles to influence neurovascular coupling.
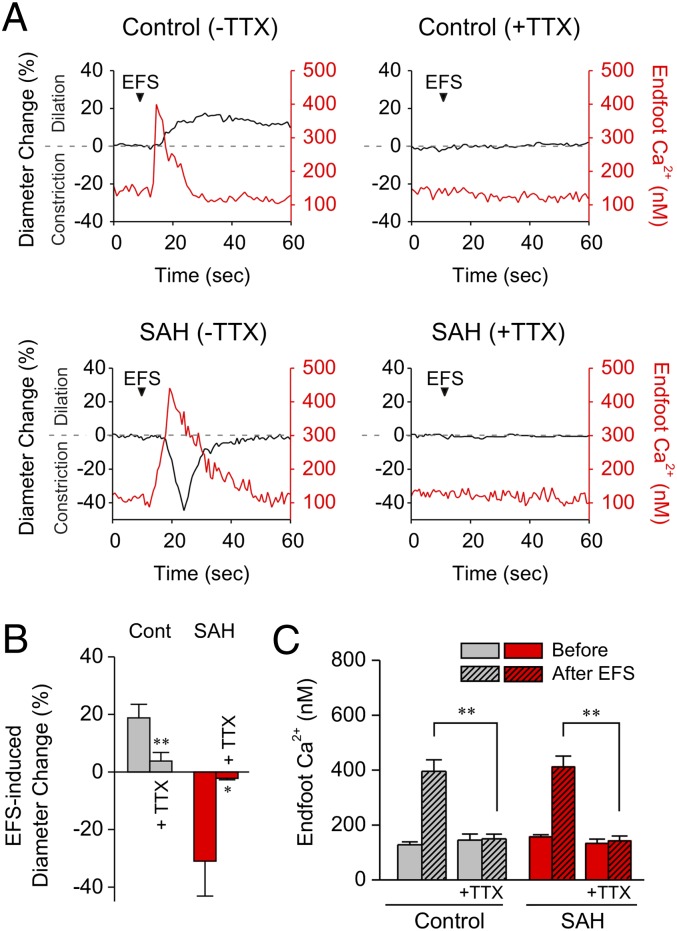
Under physiological conditions, localized increases in brain neuronal activity lead to elevated astrocytic endfoot Ca2+ and vasodilation (16, 19, 20, 26). To examine the impact of experimental SAH on neurovascular coupling, we simultaneously measured astrocytic endfoot Ca2+ and parenchymal arteriolar diameter in cortical brain slices of adult rats using two-photon and infrared-differential interference contrast (IR-DIC) microscopy. Electrical-field stimulation (EFS) was used to activate neurons and evoke changes in parenchymal arteriolar diameter. Because the vasculature in brain slices is unpressurized, arterioles in this preparation lack pressure-induced or myogenic tone. To induce a moderate level of arteriolar tone and create an experimental system that allows both dilatory and constrictor responses to be monitored, we superfused brain slices with the vasoconstrictor U46619 (100 nM) before EFS. The level of parenchymal arteriolar constriction in the presence of U46619 was similar in brain slices from unoperated control (14 ± 3% decrease in diameter), sham-operated (11 ± 2% decrease), and SAH (16 ± 4% decrease) animals. In brain slices from control and sham-operated animals, activation of neurons with moderate-intensity EFS induced the anticipated neurovascular-coupling outcome: a rise in astrocytic endfoot Ca2+ followed by vasodilation of the adjoining parenchymal arteriole (Fig. 1 and Movie S1). In marked contrast, EFS of similar intensity caused the opposite vascular response—i.e., vasoconstriction—in brain slices from SAH model rats (Fig. 1 and Movie S2). EFS caused vasoconstriction in 92% (54/59) of brain slices from SAH animals and vasodilation in 89% (47/53) and 100% (11/11) of brain slices from control and sham-operated animals, respectively. Importantly, the EFS-induced vasoconstriction observed in brain slices from SAH animals was similar in the absence or presence of U46619 (Fig. S2). As with EFS-induced vasodilation in control and sham-operated animals, EFS-induced vasoconstriction in SAH model animals was preceded by a rise in astrocytic endfoot Ca2+ (Fig. 1A). As illustrated in Fig. 2, EFS-evoked elevations in endfoot Ca2+ and the ensuing vascular responses in control (dilation) and SAH (constriction) animals were abolished by TTX (3 μM), an inhibitor of neuronal voltage-dependent sodium channels. These observations demonstrate a fundamental shift in EFS-induced neurovascular responses from dilation to constriction in brain slices from SAH model animals.
Fig. 1.
Conversion of neurovascular coupling from vasodilation to vasoconstriction after SAH. (A) (Upper) IR-DIC images from brain slices of control, sham-operated, and SAH model rats. Red dashes outline the intraluminal diameter of parenchymal arterioles. Overlapping pseudocolor-mapped Ca2+ levels in astrocyte endfeet were obtained simultaneously using the fluorescent Ca2+ indicator Fluo-4 and two-photon imaging. (Scale bars, 10 μm.) (Lower) Simultaneous recordings of EFS-induced changes in diameter and estimated endfoot Ca2+ concentrations obtained from brain slices depicted in upper images. (See also Movies S1 and S2.) (B–D) Summary of EFS-evoked changes in arteriolar diameter and astrocytic endfoot Ca2+ obtained from control (n = 53 brain slices from 23 animals), sham-operated (n = 11 brain slices from five animals), and SAH model (n = 59 brain slices from 26 animals) rats. Average diameters before EFS were 6.6 ± 0.3 μm (control), 7.8 ± 1.0 μm (sham), and 6.8 ± 0.3 μm (SAH). **P < 0.01 by one-way ANOVA followed by post hoc comparison of means using the Tukey test. Error bars indicate SEM.
Fig. 2.
TTX abolishes EFS-evoked vasodilation (Control) and vasoconstriction (SAH). (A) Simultaneous recordings of EFS-evoked changes in parenchymal arteriole diameter and astrocytic endfoot Ca2+ from brain slices in the absence and presence of TTX (3 μM), a blocker of neuronal voltage-dependent sodium channels. (B and C) Summary of the effects of TTX on EFS-evoked changes in arteriolar diameter and astrocytic endfoot Ca2+ obtained from control (n = 5 brain slices from three animals) and SAH model (n = 6 brain slices from four animals) rats. **P < 0.01, *P < 0.05 by Student’s t test. Error bars indicate SEM.
Similar Elevations in Astrocytic Endfoot Ca2+ Induce Opposing Vascular Responses in Brain Slices from Control and SAH Model Animals.
As shown above, increases in astrocytic endfoot Ca2+ evoked by EFS were followed by vasoconstriction, rather than vasodilation, in brain slices from SAH model animals. Further, EFS-evoked increases in endfoot Ca2+ and vasoconstriction were inhibited by TTX (Fig. 2) and the mGluR subtype 5 antagonist, 6-methyl-2-(phenylethynyl) pyridine (30 μM) (Fig. S2C), suggesting a causal link between neuronally released glutamate and subsequent elevation of endfoot Ca2+ and vasoconstriction. However, pathways independent of changes in endfoot Ca2+ [e.g., direct innervation of the vasculature via interneurons (27)] conceivably could contribute to the EFS-induced vasoconstriction observed after experimental SAH. To bypass neuronal activity and directly examine the role of endfoot Ca2+, we loaded brain slices with the caged Ca2+ compound, 1-[4,5-dimethoxy-2-nitrophenyl]-EDTA (DMNP-EDTA, 10 μM), for 1 h before uncaging and imaging. Under these conditions, two-photon photolysis of caged Ca2+ within an astrocyte endfoot (excitation volume <1 μm3) yielded a modest level of endfoot Ca2+ (200–500 nM) comparable to levels observed using EFS. As with EFS, elevating endfoot Ca2+ within this range via photolysis of caged Ca2+ caused arteriolar dilation in brain slices from control animals but constriction in brain slices from SAH animals (Fig. 3). These data demonstrate that directly increasing endfoot Ca2+ to moderate levels induces vasoconstriction in brain slices from SAH model rats, confirming a fundamental switch—from vasodilation to vasoconstriction—in the response of these animals to a physiological neuronal stimulus.
Fig. 3.
Ca2+ uncaging in astrocyte endfeet evokes opposing vascular responses in brain slices from control and SAH model animals. (A) Examples of two-photon photolysis of caged Ca2+ within an astrocyte endfoot. Ca2+ uncaging was achieved in brain slices loaded with DMNP-EDTA (10 μM, 60 min) by scanning a region of interest within an endfoot (excitation volume <1 μm3) at a higher (10×) laser intensity for ∼1 s. The resulting elevations in endfoot Ca2+ (range, 200–500 nM) caused parenchymal arteriole dilation in brain slices from control animals but constriction in brain slices from SAH animals. (Scale bars, 10 μm.) (B–D) Summary data of changes in arteriolar diameter and astrocytic endfoot Ca2+ evoked during Ca2+ uncaging in brain slices from control (n = 8 brain slices from four animals) and SAH model (n = 11 brain slices from five animals) rats. **P < 0.01 by Student’s t test. Error bars indicate SEM.
Astrocytic Endfoot BK Channels and Elevated Perivascular K+ Are Necessary for Constriction to Elevated Endfoot Ca2+ in Post-SAH Brain Slices.
On the basis of current knowledge, we explored two models that conceivably might link modest elevations in endfoot Ca2+ to arteriolar constriction in brain slices from SAH model animals.
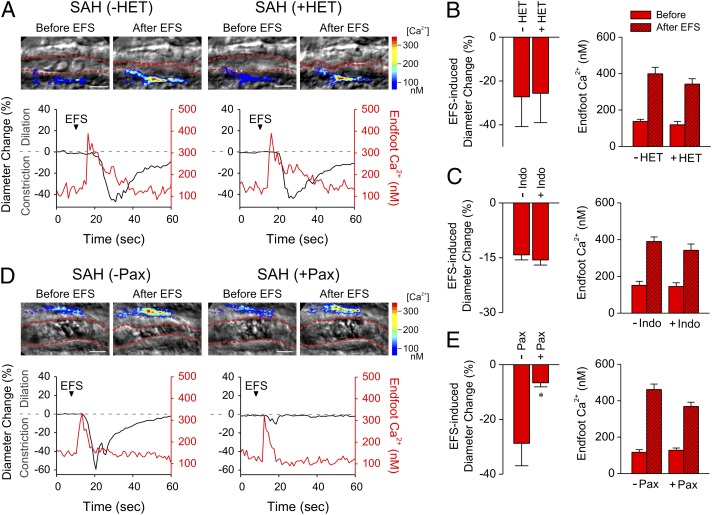
The first model (hereafter, “Model 1”) involves production of the arachidonic acid metabolite 20-hydroxyeicosatetraenoic acid (20-HETE) and inhibition of arteriolar smooth muscle BK channels. This model is based on studies demonstrating that, under certain conditions, vessel constrictions in response to evoked increases in astrocytic soma Ca2+ were prevented by high concentrations of an inhibitor of cytochrome P450 enzyme (CYP4a), which is responsible for the conversion of arachidonic acid to 20-HETE (23, 28). Previous work also has demonstrated that 20-HETE can inhibit BK channels in renal and pial artery smooth muscle cells (29), and increased 20-HETE synthesis has been implicated in vascular pathologies associated with SAH (30). To examine the involvement of 20-HETE in EFS-induced vasoconstriction after SAH, we exposed brain slices to the CYP4a inhibitor, N-hydroxy-N′-(4-n-butyl-2-methylphenyl)formamidine (HET0016, 100 nM) (31, 32), before EFS. In brain slices from SAH animals, HET0016 did not change the magnitude or time course of EFS-induced elevations in endfoot Ca2+ or vasoconstriction (Fig. 4 A and B). Further, inhibition of cyclooxygenase-mediated metabolism of arachidonic acid to vasoactive prostaglandins or thromboxane A2 with indomethacin (10 μM) had no effect on EFS-induced constriction or dilation in brain slices from SAH or control animals, respectively (Fig. 4C and Fig. S3A). These data argue against Model 1 and suggest that elevated astrocytic endfoot Ca2+ and parenchymal arteriolar constriction in brain slices from SAH model rats are independent of arachidonic acid metabolism via CYP4a or cyclooxygenase.
Fig. 4.
BK channel are involved in neurally evoked vasoconstriction in brain slices from SAH animals. (A) Simultaneous recordings of EFS-evoked changes in parenchymal arteriole diameter and astrocytic endfoot Ca2+ before and after incubation of SAH brain slices with HET0016 (HET; 100 nM), a blocker of CYP4A and 20-HETE synthesis. (B) Summary of the effects of HET0016 on EFS-evoked changes in arteriolar diameter and astrocytic endfoot Ca2+ obtained from SAH model rats (n = 6 brain slices from three animals). (C) Summary data demonstrating that the cyclooxygenase inhibitor, indomethacin (Indo; 10 μM) did not alter EFS-induced changes in diameter or endfoot Ca2+ in SAH model rats (n = 4 brain slices from two animals). (D) Simultaneous recordings of EFS-evoked changes in parenchymal arteriolar diameter and astrocytic endfoot Ca2+ before and after a 10-min incubation of brain slices with the BK channel blocker paxilline (Pax; 1 μM). (Scale bars, 10 μm.) (See also Movies S3 and S4.) (E) Summary data demonstrating that paxilline did not alter EFS-evoked changes in astrocytic endfoot Ca2+ but did greatly reduce EFS-induced constriction in SAH model rats (n = 5 brain slices from three animals). *P < 0.05 by Student’s t test. Error bars indicate SEM.
Our second conceptual model supposes activation of astrocytic endfoot BK channels rather than inhibition of smooth muscle BK channels, as proposed in Model 1. In brain slices from healthy animals, elevation of endfoot Ca2+ to <550 nM activates BK channels, increasing perivascular K+ to cause vasodilation (19, 33). Moderate levels of extracellular K+ (<20 mM) cause arteriolar dilation through membrane potential hyperpolarization caused by activation of smooth muscle inward rectifier K+ (Kir) channels (19, 33, 34). Notably, however, levels of extracellular K+ greater than ∼20 mM cause arteriolar constriction as the result of a depolarizing shift in the K+ equilibrium potential and activation of voltage-dependent Ca2+ channels. In this context, Girouard et al. (21) recently have demonstrated that the activity of BK channels in astrocyte endfeet also can mediate vasoconstriction if either the magnitude of the evoked increase in endfoot Ca2+ or the preexisting level of perivascular K+ is increased sufficiently. These investigators observed that elevating bath K+ from 3 mM to 8 mM in brain slices from control animals caused an inversion of the neurovascular response—from vasodilation to vasoconstriction—to stimulated increases in endfoot Ca2+, consistent with the summation of bath K+ and BK-mediated efflux of K+ from astrocyte endfeet. To assess BK channel involvement in neurovascular constriction after SAH, we examined EFS-induced responses in the presence of paxilline (1 μM), a specific blocker of BK channels. As shown in Fig. 4 D and E, paxilline significantly decreased EFS-induced arteriolar constriction in brain slices from SAH animals (see also Movies S3 and S4). Paxilline did not directly alter parenchymal arteriolar diameter (34, 35) or influence EFS-induced increases in endfoot Ca2+ (Fig. 4E), indicating that paxilline’s action on these brain slices likely reflects blocking of BK channels on astrocyte endfeet rather than a direct effect on neurons or arteriolar myocytes. The lack of a direct effect of paxilline on parenchymal arteriolar diameter also argues against the inhibition of the smooth muscle BK channel by enhanced 20-HETE production after SAH (see conceptual Model 1, above).
Taking these observations into consideration, we hypothesized that, after SAH, perivascular K+ is elevated abnormally so that “normal” EFS-induced increases in astrocytic endfoot Ca2+ and BK channel-mediated K+ efflux raise local K+ concentration in the restricted space between the endfoot and smooth muscle membranes to a level sufficient to induce vasoconstriction rather than vasodilation. To test this concept, vascular responses were examined after increasing K+ from 3 mM to 10 mM in the aCSF used to bathe brain slices (Fig. 5 A and B). As expected, increasing extracellular K+ from 3 mM to 10 mM in brain slices from control animals caused parenchymal arteriolar dilation, analogous to the effect of activation of endfoot BK channels by moderate increases in astrocytic Ca2+. In contrast, increasing extracellular K+ from 3 mM to 10 mM in brain slices from SAH animals caused modest constriction of parenchymal arterioles, consistent with the idea that abnormally elevated basal levels of perivascular K+ sum with bath levels of K+ to exceed the dilation/constriction threshold. This result argues that a change in the K+ environment of arterioles in SAH animals, rather than altered responsiveness of arterioles to their environment, is responsible for the paradoxical contractile effects of astrocyte activation. In support of this interpretation, parenchymal arterioles isolated from the brains of SAH animals and pressurized in vitro dilated to modest increases in extracellular K+ in a manner similar to arterioles isolated from control animals (Fig. 5C). Further, Kir current density was similar in parenchymal arteriolar myocytes isolated from control and SAH animals (Fig. 5 D and E). Collectively, these data demonstrate that vascular Kir channel function is maintained after SAH and support the hypothesis that basal perivascular K+ is elevated following SAH.
Fig. 5.
K+-induced dilations are absent in brain slices from SAH model animals despite the presence of functional smooth muscle Kir channels. (A) Elevation of K+ from 3 mM to 10 mM in aCSF superfusate dilated parenchymal arterioles in brain slices from control (n = 5 brain slices from two animals) but not in brain slices from SAH model (n = 5 brain slices from three animals) rats. (B) Brain slices were treated with the BK channel blocker paxilline (Pax; 1 μM) for 10 min before superfusion with aCSF containing 10 mM K+ and paxilline. Paxilline restored K+-induced dilations in brain slices from SAH model rats (n = 8 brain slices from four animals). K+-induced dilations were similar in the absence and presence of paxilline in brain slices from control animals (n = 7 brain slices from four animals). (C) Diameter changes obtained from isolated pressurized parenchymal arterioles from control (n = 4 arterioles from four animals) and SAH model (n = 3 arterioles from three animals) rats. Diameter changes are expressed relative to the initial diameter in aCSF containing 3 mM K+. (D) Ba2+-sensitive Kir currents measured in 140 mM extracellular K+ during 500-ms voltage ramps from −100 mV to +40 mV. (E) Summary data of current density recorded at −100 mV in parenchymal arteriolar myocytes isolated from control (n = 12 cells from three animals) and SAH (n = 9 cells from three animals) rats. *P < 0.05 by Student’s t test. Error bars indicate SEM.
Although up-regulation of BK channels conceivably could increase BK channel activity at a given level of endfoot Ca2+, quantitative real-time RT-PCR (Fig. S4A, Table S1) showed no changes in cortical levels of mRNA for the BK channel pore-forming α1-subunit or the neuronal/astrocytic β4 accessory subunit (36, 37), suggesting that an increase in perivascular K+ concentration rather than changes in BK expression accounts for SAH-induced neurally evoked vasoconstriction. However, these results do not exclude an elevation of BK channel expression specifically in the astrocytic endfoot plasma membrane.
Increased Amplitude of Spontaneous Ca2+ Oscillations in Astrocytic Endfeet May Contribute to Enhanced Endfoot BK Channel Activity in Brain Slices After SAH.
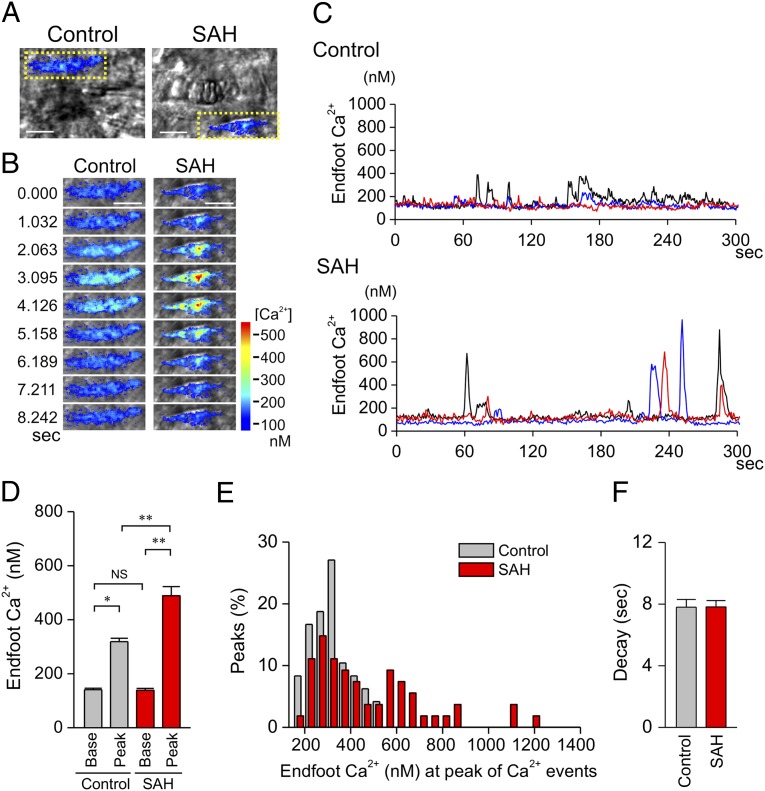
The foregoing experimental results raise the question of how basal perivascular K+ levels are raised in SAH animals and maintained at an elevated level in brain slices equilibrated in aCSF containing 3 mM K+. One possible explanation lies in an analysis of spontaneous Ca2+ oscillations observed in astrocyte endfeet in the absence of evoked neuronal activity (Fig. 6 A–C, Movies S5 and S6). In brain slices from control animals, these events occurred at a relatively low frequency (0.03 ± 0.005 Hz, n = 9) and increased Ca2+ to a mean peak level of 318.6 ± 12.8 nM. In brain slices from SAH animals, the frequency of these events was similar (0.02 ± 0.002 Hz, n = 10), but their amplitudes were increased markedly, reaching an average peak Ca2+ level of 488.9 ± 33.5 nM (Fig. 6 D and E). The durations of spontaneous Ca2+ oscillations were similar (Fig. 6F), as was the basal Ca2+ concentration measured in the absence of spontaneous oscillations (control, 140.9 ± 5.3 nM; SAH, 140.3 ± 5.9 nM). The spontaneous Ca2+ oscillations observed after SAH were higher in amplitude than the EFS-evoked elevations in endfoot Ca2+ that led to activation of endfoot BK channels (Figs. 1–3). We therefore hypothesized that the increased amplitude of these spontaneous Ca2+ oscillations in SAH animals increases endfoot BK channel activity and K+ efflux to elevate basal levels of perivascular K+ abnormally. If enhanced basal endfoot BK channel activity contributes to increased perivascular K+ concentration after SAH, the prediction is that inhibition of BK channels would “normalize” perivascular K+ concentration and restore vasodilation to aCSF containing 10 mM K+. Consistent with this hypothesis, the BK channel blocker paxilline (1 μM) restored the dilatory response to 10 mM extracellular K+ in brain slices from SAH animals (Fig. 5B). On average, paxilline had no net effect on arteriolar diameter in brain slices from SAH animals bathed in 3 mM K+. However, paxilline caused modest constriction in three brain slices and modest dilation in three others (out of a total of eight SAH brain slices). This variability in the paxilline response in 3 mM K+ may reflect variations in the basal elevation in perivascular K+ in brain slices from SAH animals. Taken together, our data strongly suggest that the increased amplitude of spontaneous endfoot Ca2+ oscillations and the ensuing K+ efflux through endfoot BK channels elevate the K+ concentration in the restricted perivascular space and account for the inverted vascular response to neuronal activation observed in brain slices from SAH model animals.
Fig. 6.
Increased amplitude of spontaneous Ca2+ oscillations in astrocyte endfeet following SAH. (A and B) Representative images of spontaneous Ca2+ oscillations in astrocyte endfeet in brain slices from control and SAH model animals. (Scale bars, 10 μm.) (B) Time-lapse images from the area within the yellow dotted box in A. (C) Spontaneous Ca2+ oscillations recorded from 1.2 × 1.2-μm regions of interests placed on distinct astrocyte endfeet in a brain slice from a control (Upper) and SAH model (Lower) animal. (See also Movies S5 and S6.) (D and E) Summary data of spontaneous Ca2+ oscillations in astrocyte endfeet illustrating that the amplitude of spontaneous Ca2+ oscillations was increased in brain slices from SAH animals. Data were obtained from 5-min recordings of spontaneous Ca2+ oscillations in control (n = 9 endfeet in four brain slices from four animals) and SAH model (n = 10 endfeet in three brain slices from three animals) rats. **P < 0.01 by one-way ANOVA followed by post hoc comparison of means using the Tukey test. Error bars indicate SEM. (F) Summary of decay time of spontaneous Ca2+ oscillations in astrocyte endfeet in brain slices from control and SAH model animals. Error bars indicate SEM.
Discussion
This study demonstrates that neurovascular coupling is fundamentally changed in brain slices from SAH model animals. Rather than evoking the dilatory response typically associated with functional hyperemia, neuronal activation in these animals causes vasoconstriction, a pathological response that could limit the delivery of oxygen and nutrients to brain cortex. Although the polarity of the vascular response is inverted after SAH, both neurally evoked dilation (control) and constriction (SAH) involve the same mechanistic elements: increased endfoot Ca2+ and K+ efflux via endfoot BK channels. These responses reflect the dual vasodilator/vasoconstrictor potential of increased [K+]o (21, 38). Further, our present work indicates that SAH increases the amplitude of spontaneous Ca2+ oscillations in astrocyte endfeet, leading to enhanced BK channel activity and elevated basal [K+]o within the perivascular microdomain of brain cortex. Our evidence suggests that abnormally elevated basal perivascular K+ combined with the additional K+ efflux via astrocytic endfoot BK channels stimulated by neuronal activity elevates [K+]o above the dilation/constriction threshold, inducing smooth muscle membrane potential depolarization and parenchymal arteriolar constriction (Fig. 7).
Fig. 7.
Schematic model linking SAH to inversion of neurovascular coupling. In control animals, EFS causes elevated cytoplasmic Ca2+ in astrocytes leading to increased BK channel activity and modest (<20 mM) increases in perivascular K+, promoting vasodilation. SAH increases the magnitude of spontaneous astrocytic Ca2+ oscillations and basal activity of BK channels, elevating K+ in the restricted perivascular space. The summation of increased basal perivascular K+ and “normal” nerve-evoked astrocyte BK activity results in extracellular K+ concentrations that exceed the dilation/constriction threshold (∼20 mM), inducing vasoconstriction. BK, large-conductance Ca2+-activated K+ channel; EFS, electrical field stimulation; Glu, glutamate; Kir, inward rectifier K+ channel; mGluR, metabotropic glutamate receptor; VDCC, voltage-dependent Ca2+ channel.
Our work supports a growing body of evidence suggesting that SAH-induced perfusion deficits originate from the brain microvasculature rather than arising as a consequence of blood-induced narrowing of large-diameter conduit arteries on the brain surface (i.e., angiographic vasospasm). Unlike the brain surface, the brain cortex lacks significant collateral sources of blood supply (39, 40). The relative abundance of localized cortical infarcts after SAH supports the concept that vascular events occurring beyond the point of redundant blood supply contribute to the manifestation of delayed ischemic neurological deficits after SAH (41–43). In one study (42), it was reported that approximately half the cortical infarcts observed in SAH patients involved a single territory and were near the site of aneurysm rupture. However, the remainder of patients had multiple infarcts that often were distant from the site of rupture. Further, the presence or absence of angiographic large-artery vasospasm failed to predict the pattern of cortical infarction. The exact cause of these infarcts and their relationship to impaired neurovascular coupling remains to be determined. Conversion of the neurovascular response from vasodilation to vasoconstriction may act in concert with other actions of SAH, such as direct constrictor effects on parenchymal arterioles (14), microthrombi formation (12), and cortical spreading depolarization (7, 25), to compromise cortical blood flow. For example, pressurized parenchymal arterioles isolated from SAH animals exhibit depolarization of smooth muscle membrane potential and enhanced constriction in vitro (14). Direct vasoconstrictor effects of blood components such as oxyhemoglobin include suppression of smooth muscle K+ channels (44–46), up-regulation of voltage-dependent Ca2+ channels (47) or transient receptor potential channels (48), and scavenging of nitric oxide (49).
Considerable recent attention has focused on the role of cortical spreading depolarization in the pathologies associated with SAH (7, 11, 15, 25, 50). In vivo, episodes of cortical spreading depolarization associated with prolonged cerebral hypoperfusion have been reported in both human SAH patients and SAH model rats (41). Interestingly, in non-SAH patients, cortical spreading depolarization is associated with increased cerebral blood flow and vasodilation. Our data suggest that the modest elevations in basal perivascular [K+]o (≤20 mM) that follow SAH are sufficient to induce a polarity shift in the neurovascular response from vasodilation to vasoconstriction. Unlike the large increases in [K+]o associated with cortical spreading depression (15), which likely are of neuronal origin, our evidence suggests that subarachnoid blood has the effect of modestly elevating cortical perivascular [K+]o via effects on subcellular Ca2+ release events in astrocytes.
We documented a marked increase in the amplitude of spontaneous Ca2+ oscillations in astrocyte endfeet of SAH animals (Fig. 6). The peak increase in Ca2+ during these spontaneous oscillations was ∼490 nM in brain slices from SAH animals, or ∼170 nM higher than spontaneous events in brain slices of control animals and ∼90 nM greater than EFS-evoked increases in endfoot Ca2+ in either group. Considering that EFS-induced increases in endfoot Ca2+ have been shown to increase K+ efflux through BK channels (19, 21, 33), it is reasonable to suppose that these high-amplitude spontaneous events occurring after SAH enhance BK-channel activity to increase basal [K+]o in the perivascular space between astrocyte endfeet and parenchymal arteriolar myocytes. The narrow gap between astrocyte endfeet and arteriolar smooth muscle membrane prohibits the accurate use of K+-sensing electrodes or dyes to measure [K+]o directly in this restricted perivascular space. Parenchymal arterioles are completely encased by astrocytic endfeet, with a distance on the order of 20 nm separating endfeet and the vascular wall (51, 52). With the assumption that the average length of the astrocyte endfoot is 30 μm (53, 54), it has been estimated that the opening of a single astrocytic endfoot BK channel for 0.2 s (efflux of ∼106 K+ ions) would elevate perivascular K+ from 3 mM to 10 mM (19). Our data suggest that after SAH the concentration of K+ in the restricted perivascular space (between arteriolar smooth muscle and astrocytic endfeet) remains elevated above the K+ concentration in the bulk extracellular (bath: 3 mM or 10 mM K+) solution. This elevated concentration would be consistent with limited diffusion of K+ released from astrocytic endfeet out of the perivascular space and into the bulk extracellular solution. Mechanisms responsible for removing K+ from the perivascular space also might contribute to abnormally elevated basal levels of perivascular K+. Using cortical extracts, we did not detect differences in the levels of mRNA encoding Na+/K+-ATPase subunits, the K+/Cl− cotransporters KCC1-4, or Kir channels Kir4.1 and Kir5.1 (Fig. S4, Table S1); however, it remains possible that changes in the expression of one or more of these elements in cells of the neurovascular unit may have gone undetected.
Spontaneous Ca2+ oscillations in the astrocyte soma occur independently of neuronal activity and reflect inositol trisphosphate-mediated intracellular Ca2+ release from the sarcoplasmic reticulum (55). It is possible that blood components such as oxyhemoglobin (13) or their breakdown products [e.g., reactive oxygen species generated by the autooxidation of oxyhemoglobin (56)] act directly to alter Ca2+ signaling in astrocyte endfeet. Alternatively, subarachnoid blood may act indirectly by inducing inflammation (57) or reactive astrogliosis (58). Thrombin, for example, has been shown to induce reactive astrogliosis (59). The impact of astrogliosis on astrocyte Ca2+ signaling and neurovascular coupling is not known, although it has been associated with brain pathologies, including brain cancers, Alzheimer’s disease, and stroke (60). Interestingly, Nedergaard and colleagues (61) reported an increase in the frequency of spontaneous astrocytic Ca2+ in vivo in association with vascular abnormalities in the cortex of transgenic mouse models of Alzheimer’s disease. Further work, including time-course studies, clearly is needed to elucidate the underlying mechanisms of the pathological changes in astrocyte Ca2+ signaling after SAH and to pinpoint specific blood components involved in this response. However, our evidence indicates that astrocytic endfoot BK channels are important in transducing altered astrocytic Ca2+ signaling into changes in arteriolar diameter and blood flow within the brain, perhaps contributing to the development of neurological deficits following SAH.
Materials and Methods
Rat SAH Model.
All experiments used male Sprague–Dawley rats (10–12 wk of age) and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (62) and followed protocols approved by the Institutional Animal Use and Care Committee of the University of Vermont. Aneurysmal SAH was mimicked by injecting autologous, unheparinized arterial blood (500 μL) into the cisterna magna of anesthetized animals using a surgical approach (14). Twenty-four hours later, animals received a second intracisternal injection of blood. Sham-operated animals underwent identical procedures, except that aCSF (500 μL), rather than blood, was injected into the cisterna magna. After each injection, anesthetized animals were placed head down at a 45° angle for 20 min before recovery from anesthesia. Buprenorphine (0.05 mg/kg) was given every 12 h for 36 h as an analgesic. Surgical survival rates of SAH model and sham-operated animals were >95%. For ex vivo and in vitro studies, anesthetized rats were killed by decapitation 4 d after the initial surgical procedure.
Brain Slice Studies.
Cortical brain slices (160-μm-thick coronal sections) were loaded with the Ca2+ indicator dye, Fluo-4-AM (10 μM) (Invitrogen) in oxygenated aCSF containing 0.05% pluronic acid (Invitrogen) at 29 °C for 1 h. Arteriolar diameter and astrocytic endfoot Ca2+ were measured simultaneously using a BioRad Radiance multiphoton imaging system coupled to a Chameleon Ti:Sapphire laser (Coherent) and an Olympus BX51WI upright microscope (21). Brain slices were superfused with aCSF (aerated with 5% CO2/95% O2, pH ∼7.35) maintained at 37 °C. Fluo-4 was excited at 820 nm, and fluorescence emission was collected using a 575/150-nm bandpass filter. Simultaneous arteriole diameter images were acquired using IR-DIC microscopy. Both fluorescent and IR-DIC images were obtained at a frequency of ∼1 Hz. EFS (50 Hz; 0.3-ms alternating square pulse; 3-s duration) was applied using a pair of platinum wires connected to a Grass S48 Stimulator. For photolysis of caged Ca2+ in astrocyte endfeet, brain slices were loaded for 1 h with Fluo-4-AM (10 μM) and DMNP-EDTA-AM (10 μM) (Interchim) in oxygenated aCSF containing 0.05% pluronic acid at 29 °C. For uncaging studies, the laser was tuned to 730 nm to allow simultaneous excitation of Fluo-4 and photolysis of caged Ca2+. Photolysis was induced by scanning a 1.2 × 1.2 μm region of interest within an endfoot at 10-fold higher laser intensity for ∼1 s. Ca2+ concentrations in astrocyte endfeet were determined using the maximal fluorescence method (21, 63). Arteriolar diameter was determined by averaging diameter measurements obtained from three points on the same image and was expressed as percent change from the diameter recorded from the first image.
Diameter Measurements of Isolated Parenchymal Arterioles.
Cortical parenchymal arterioles were isolated from the middle cerebral artery territory at a depth of at least 500 μm from the brain surface (14, 34). Arterioles were cannulated using glass pipettes, and intravascular pressure was adjusted to 40 mmHg using a pressure servo-controller (Living Systems Instruments). Arterioles were superfused continuously with oxygenated aCSF warmed to 37 °C, and diameter was recorded continuously using a CCD camera and edge-detection software (Dataq Instruments). The composition of aCSF was (in mM): 125 NaCl, 3 KCl, 18 NaHCO3, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 5 glucose aerated with 5% CO2, 20% O2, 75% N2. Bath pH was monitored closely and maintained at 7.30–7.35. Bath solutions containing K+ concentrations >3 mM were made by iso-osmotic replacement of NaCl with KCl.
Kir Current Measurements in Parenchymal Arteriolar Myocytes.
Parenchymal arteriolar myocytes were isolated enzymatically using a previously described protocol (45). Cells used for patch-clamp studies were kept on ice for up to 6 h. Membrane K+ currents were measured with conventional whole-cell patch-clamp electrophysiology using a holding potential of −70 mV. Cell voltage was stepped to −100 mV for 100 ms and then was ramped from −100 mV to +40 mV over a period of 500 ms. Pipette solutions contained (in mM): 20 KCl, 87 potassium aspartate, 1 MgCl2, 1 CaCl2, 10 Hepes, and 10 EGTA (pH 7.2); bath solutions contained (in mM): 140 KCl, 0.1 CaCl2, 1 MgCl2, 10 Hepes, and 10 glucose (pH 7.4). Kir currents were defined as 100 μM Ba2+-sensitive currents. Cell capacitance was similar in myocytes from control (8.7 ± 0.8 pF; n = 12 cells from three animals) and SAH model (8.9 ± 0.9 pF; n = 9 cells from three animals) animals.
Statistical Analysis.
Data are expressed as mean ± SEM, and the number of observations and the number of animals used in each experimental series are included in the figure legends. Differences among more than two groups were analyzed by one-way ANOVA followed by post hoc comparison of means using the Tukey test. Student’s t tests (paired or unpaired) were used to compare data between two groups.
Reagents.
The thromboxane agonist U46619 was obtained from Calbiochem. Indomethacin and HET0016 were purchased from Cayman Chemical. Papain was obtained from Worthington Biochemical. All other chemicals were purchased from Sigma-Aldrich.
Supplementary Material
Acknowledgments
We thank Dr. David C. Hill-Eubanks, Dr. Richard D. Murray, Ms. Sheila R. Russell, and Mr. Kevin P. O’Connor for their assistance with this study and we also thank the University of Vermont Neuroscience Center of Biomedical Research Excellence (COBRE) molecular biology and imaging core facilities. This work was supported by the Totman Medical Research Trust; the Peter Martin Brain Aneurysm Endowment; National Institutes of Health Grants P01 HL095488, R01 HL078983, R01 HL078983-05S1, R01 HL044455, R37 DK053832, R01 DK065947, and R01 HL077378; and National Center for Research Resources Grant P20 RR16435.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 7968 (volume 109, number 21).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121359109/-/DCSupplemental.
References
- 1.Bederson JB, et al. American Heart Association Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 2.Biller J, Godersky JC, Adams HP., Jr Management of aneurysmal subarachnoid hemorrhage. Stroke. 1988;19:1300–1305. doi: 10.1161/01.str.19.10.1300. [DOI] [PubMed] [Google Scholar]
- 3.Etminan N, Vergouwen MD, Ilodigwe D, Macdonald RL. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J Cereb Blood Flow Metab. 2011;31:1443–1451. doi: 10.1038/jcbfm.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- 5.Pluta RM, et al. Cerebral vasospasm following subarachnoid hemorrhage: Time for a new world of thought. Neurol Res. 2009;31:151–158. doi: 10.1179/174313209X393564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen-Schwartz J, Vajkoczy P, Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm: Looking beyond vasoconstriction. Trends Pharmacol Sci. 2007;28:252–256. doi: 10.1016/j.tips.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Leng LZ, Fink ME, Iadecola C. Spreading depolarization: A possible new culprit in the delayed cerebral ischemia of subarachnoid hemorrhage. Arch Neurol. 2011;68:31–36. doi: 10.1001/archneurol.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macdonald RL, et al. CONSCIOUS-1 Investigators Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): Randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald RL, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: A randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2) Lancet Neurol. 2011;10:618–625. doi: 10.1016/S1474-4422(11)70108-9. [DOI] [PubMed] [Google Scholar]
- 10.Iadecola C. Bleeding in the brain: Killer waves of depolarization in subarachnoid bleed. Nat Med. 2009;15:1131–1132. doi: 10.1038/nm1009-1131. [DOI] [PubMed] [Google Scholar]
- 11.Lauritzen M, et al. Clinical relevance of cortical spreading depression in neurological disorders: Migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:17–35. doi: 10.1038/jcbfm.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergouwen MD, Vermeulen M, Coert BA, Stroes ES, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: An additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1761–1770. doi: 10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]
- 13.Nishizawa S, Laher I. Signaling mechanisms in cerebral vasospasm. Trends Cardiovasc Med. 2005;15:24–34. doi: 10.1016/j.tcm.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Nystoriak MA, et al. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am J Physiol Heart Circ Physiol. 2011;300:H803–H812. doi: 10.1152/ajpheart.00760.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 16.Attwell D, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 18.Straub SV, Nelson MT. Astrocytic calcium signaling: The information currency coupling neuronal activity to the cerebral microcirculation. Trends Cardiovasc Med. 2007;17:183–190. doi: 10.1016/j.tcm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filosa JA, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 20.Takano T, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 21.Girouard H, et al. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 24.Shin HK, et al. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- 25.Dreier JP, et al. COSBID study group Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:1866–1881. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- 27.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 28.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: A mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou AP, et al. 20-HETE is an endogenous inhibitor of the large-conductance Ca2+-activated K+ channel in renal arterioles. Am J Physiol. 1996;270:R228–R237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]
- 30.Roman RJ, Renic M, Dunn KM, Takeuchi K, Hacein-Bey L. Evidence that 20-HETE contributes to the development of acute and delayed cerebral vasospasm. Neurol Res. 2006;28:738–749. doi: 10.1179/016164106X152016. [DOI] [PubMed] [Google Scholar]
- 31.Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol. 2008;294:H2855–H2863. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra A, Hamid A, Newman EA. Oxygen modulation of neurovascular coupling in the retina. Proc Natl Acad Sci USA. 2011;108:17827–17831. doi: 10.1073/pnas.1110533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunn KM, Nelson MT. Potassium channels and neurovascular coupling. Circ J. 2010;74:608–616. doi: 10.1253/circj.cj-10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SKCa and IKCa channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab. 2011;31:1175–1186. doi: 10.1038/jcbfm.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dabertrand F, Nelson MT, Brayden JE. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ Res. 2012;110:285–294. doi: 10.1161/CIRCRESAHA.111.258145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wynne PM, Puig SI, Martin GE, Treistman SN. Compartmentalized beta subunit distribution determines characteristics and ethanol sensitivity of somatic, dendritic, and terminal large-conductance calcium-activated potassium channels in the rat central nervous system. J Pharmacol Exp Ther. 2009;329:978–986. doi: 10.1124/jpet.108.146175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebremedhin D, et al. Metabotropic glutamate receptor activation enhances the activities of two types of Ca2+-activated K+ channels in rat hippocampal astrocytes. J Neurosci. 2003;23:1678–1687. doi: 10.1523/JNEUROSCI.23-05-01678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knot HJ, Zimmermann PA, Nelson MT. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J Physiol. 1996;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci USA. 2007;104:365–370. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimura N, Rosidi NL, Iadecola C, Schaffer CB. Limitations of collateral flow after occlusion of a single cortical penetrating arteriole. J Cereb Blood Flow Metab. 2010;30:1914–1927. doi: 10.1038/jcbfm.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dreier JP, et al. Focal laminar cortical MR signal abnormalities after subarachnoid hemorrhage. Ann Neurol. 2002;52:825–829. doi: 10.1002/ana.10383. [DOI] [PubMed] [Google Scholar]
- 42.Rabinstein AA, Weigand S, Atkinson JL, Wijdicks EF. Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2005;36:992–997. doi: 10.1161/01.STR.0000163090.59350.5a. [DOI] [PubMed] [Google Scholar]
- 43.Weidauer S, et al. Focal laminar cortical infarcts following aneurysmal subarachnoid haemorrhage. Neuroradiology. 2008;50:1–8. doi: 10.1007/s00234-007-0294-1. [DOI] [PubMed] [Google Scholar]
- 44.Ishiguro M, et al. Oxyhemoglobin-induced suppression of voltage-dependent K+ channels in cerebral arteries by enhanced tyrosine kinase activity. Circ Res. 2006;99:1252–1260. doi: 10.1161/01.RES.0000250821.32324.e1. [DOI] [PubMed] [Google Scholar]
- 45.Koide M, Penar PL, Tranmer BI, Wellman GC. Heparin-binding EGF-like growth factor mediates oxyhemoglobin-induced suppression of voltage-dependent potassium channels in rabbit cerebral artery myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H1750–H1759. doi: 10.1152/ajpheart.00443.2007. [DOI] [PubMed] [Google Scholar]
- 46.Koide M, et al. Reduced Ca2+ spark activity after subarachnoid hemorrhage disables BK channel control of cerebral artery tone. J Cereb Blood Flow Metab. 2011;31:3–16. doi: 10.1038/jcbfm.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishiguro M, et al. Emergence of a R-type Ca2+ channel (CaV 2.3) contributes to cerebral artery constriction after subarachnoid hemorrhage. Circ Res. 2005;96:419–426. doi: 10.1161/01.RES.0000157670.49936.da. [DOI] [PubMed] [Google Scholar]
- 48.Xie A, et al. Novel mechanism of endothelin-1-induced vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2007;27:1692–1701. doi: 10.1038/sj.jcbfm.9600471. [DOI] [PubMed] [Google Scholar]
- 49.Pluta RM. Delayed cerebral vasospasm and nitric oxide: Review, new hypothesis, and proposed treatment. Pharmacol Ther. 2005;105:23–56. doi: 10.1016/j.pharmthera.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Piilgaard H, Lauritzen M. Persistent increase in oxygen consumption and impaired neurovascular coupling after spreading depression in rat neocortex. J Cereb Blood Flow Metab. 2009;29:1517–1527. doi: 10.1038/jcbfm.2009.73. [DOI] [PubMed] [Google Scholar]
- 51.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 52.Price DL, Ludwig JW, Mi H, Schwarz TL, Ellisman MH. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res. 2002;956:183–193. doi: 10.1016/s0006-8993(02)03266-3. [DOI] [PubMed] [Google Scholar]
- 53.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Straub SV, Bonev AD, Wilkerson MK, Nelson MT. Dynamic inositol trisphosphate-mediated calcium signals within astrocytic endfeet underlie vasodilation of cerebral arterioles. J Gen Physiol. 2006;128:659–669. doi: 10.1085/jgp.200609650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parri HR, Crunelli V. The role of Ca2+ in the generation of spontaneous astrocytic Ca2+ oscillations. Neuroscience. 2003;120:979–992. doi: 10.1016/s0306-4522(03)00379-8. [DOI] [PubMed] [Google Scholar]
- 56.Asano T. Oxyhemoglobin as the principal cause of cerebral vasospasm: A holistic view of its actions. Crit Rev Neurosurg. 1999;9:303–318. doi: 10.1007/s003290050147. [DOI] [PubMed] [Google Scholar]
- 57.Prunell GF, Svendgaard NA, Alkass K, Mathiesen T. Inflammation in the brain after experimental subarachnoid hemorrhage. Neurosurgery. 2005;56:1082–1092, discussion 1082–1092. [PubMed] [Google Scholar]
- 58.Murakami K, et al. subarachnoid hemorrhage induces gliosis and increased expression of the pro-inflammatory cytokine high mobility group box 1 protein. Transl Stroke Res. 2011;2:72–79. doi: 10.1007/s12975-010-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shirakawa H, et al. Transient receptor potential canonical 3 (TRPC3) mediates thrombin-induced astrocyte activation and upregulates its own expression in cortical astrocytes. J Neurosci. 2010;30:13116–13129. doi: 10.1523/JNEUROSCI.1890-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 61.Takano T, Han X, Deane R, Zlokovic B, Nedergaard M. Two-photon imaging of astrocytic Ca2+ signaling and the microvasculature in experimental mice models of Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:40–50. doi: 10.1196/annals.1379.004. [DOI] [PubMed] [Google Scholar]
- 62.Committee on Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. Bethesda: Natl Inst Health; 1985. DHHS Publ No (NIH) 85–23. [Google Scholar]
- 63.Maravall M, Mainen ZF, Sabatini BL, Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys J. 2000;78:2655–2667. doi: 10.1016/S0006-3495(00)76809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]