Abstract
One of the promises of nanoparticle (NP) carriers is the reformulation of promising therapeutics that have failed clinical development due to pharmacologic challenges. However, current nanomedicine research has been focused on the delivery of established and novel therapeutics. Here we demonstrate proof of the principle of using NPs to revive the clinical potential of abandoned compounds using wortmannin (Wtmn) as a model drug. Wtmn is a potent inhibitor of phosphatidylinositol 3′ kinase-related kinases but failed clinical translation due to drug-delivery challenges. We engineered a NP formulation of Wtmn and demonstrated that NP Wtmn has higher solubility and lower toxicity compared with Wtmn. To establish the clinical translation potential of NP Wtmn, we evaluated the therapeutic as a radiosensitizer in vitro and in vivo. NP Wtmn was found to be a potent radiosensitizer and was significantly more effective than the commonly used radiosensitizer cisplatin in vitro in three cancer cell lines. The mechanism of action of NP Wtmn radiosensitization was found to be through the inhibition of DNA-dependent protein kinase phosphorylation. Finally, NP Wtmn was shown to be an effective radiosensitizer in vivo using two murine xenograft models of cancer. Our results demonstrate that NP drug-delivery systems can promote the readoption of abandoned drugs such as Wtmn by overcoming drug-delivery challenges.
Keywords: nanotechnology, nanoparticle radiosensitizer
Nanoparticles (NPs) possess unique properties, such as preferential accumulation in tumors and reduced distribution in normal tissue, that make them ideally suited for the treatment of cancer. Consequently, there has been high interest in developing NP-based therapeutics for cancer treatment (1). Current research has been focused on developing NP formulations of widely used chemotherapeutics or newly discovered cancer agents, which are few in number (2). However, one of the promises and great potentials of NP drug-delivery carriers is the reformulation of abandoned cancer therapeutics that were initially promising but failed clinical development due to challenges in delivery. Although these challenges are difficult to overcome with traditional drug-delivery techniques, NP drug-delivery vehicles present an unprecedented opportunity. In this study, we aim to demonstrate proof of principle that NPs permit the reformulation of abandoned therapeutics into formulations appropriate for clinical use.
We used wortmannin (Wtmn) as a model drug for our study. Wtmn is a furanosteroid metabolite of the fungi Penicillium funiculosum and Talaromyces (Penicillium) wortmannii (3). It is an inhibitor of phosphatidylinositol 3′ kinases (PI3Ks) and phosphatidylinositol 3′ kinase-related kinases (PIKKs) such as DNA-dependent protein kinase (DNA-PK) (4). Although preclinical studies showed Wtmn was an extremely effective radiosensitizer, its clinical translation was limited by poor solubility, low stability, and high toxicity (3–8). Since its discovery more than four decades ago, many Wtmn analogs have been developed to improve on the stability and pharmacologic properties of Wtmn (9–11). Today, only one Wtmn analog, PX-866, remains under clinical investigation (12). Wtmn is now a commonly used laboratory research reagent for the inhibition of class I PI3Ks (13).
Because NP drug delivery has the ability to overcome poor solubility, low stability, and high toxicity, we hypothesized that NP formulation of Wtmn can renew the clinical translation potential of Wtmn. In this study, we report the development of a polymeric NP formulation of Wtmn. We demonstrated that NP formulation has improved Wtmn’s solubility, stability, and toxicity. NP Wtmn was shown to be a potent radiosensitizer in vitro and in vivo. Our study demonstrates that NP drug delivery can renew the clinical potential of abandoned therapeutics and that such strategy can emerge as a paradigm in drug development.
Results
NP Wtmn Characterization.
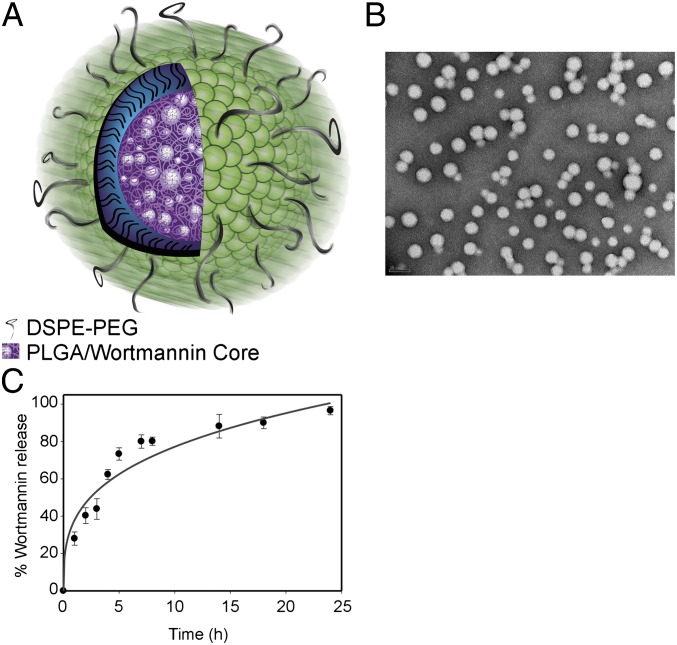
A NP formulation of Wtmn was developed using a biodegradable lipid-polymer NP platform (14, 15). These NPs contain a hydrophobic polymeric core and a lipid-polymer surface. The NP is stabilized by a monolayer of lipids (lecithin and the PEGylated lipids [1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-amino(polyethylene glycol) (DSPE-PEG)] (Fig. 1A). The polyethylene glycol (PEG) surface prevents serum protein adsorption. The solvent ratios and Wtmn:polymer ratio were optimized to achieve maximum Wtmn loading. A loading efficiency of 20% (wt/wt) was achieved, and the maximum loading attained was 4% of Wtmn by weight. Dynamic light scattering analysis revealed that the NP Wtmn possesses a narrow size distribution with polydispersity of 0.15 ± 0.03. These results were confirmed by transmission electron microscopy (TEM) analysis, which showed monodispersed NPs with a well-defined spherical shape and an average size of 40 nm (Fig. 1B). ζ-Potential analysis demonstrated that the NPs possess a negatively charged surface of −38 ± 5 mV.
Fig. 1.
Characterization of NP Wtmn. (A) Cartoon of NP Wtmn depicting a PLGA core containing Wtmn surrounded by a lipid monolayer (green head groups) and a PEG shell. (B) TEM image of NP Wtmn. (C) Release profile of NP Wtmn in PBS at 37 °C. Error bars correspond to SD of three separate sample preparations with duplicate samples per data point.
Wtmn has a very low solubility of 4 mg/L in aqueous solutions. In comparison, the solubility of the NP Wtmn was 20 g/L. We were able to dissolve 20 mg of NP Wtmn (containing 4% Wtmn by weight) in 1 mL of saline, equivalent to 800 mg/L of Wtmn. This represents a 200-fold increase in free Wtmn solubility. Mass spectrometry illustrated that Wtmn does not react with components of the NP. High-performance liquid chromatography (HPLC) confirmed the release of intact Wtmn from the NPs. Fig. 1C shows the percentage of Wtmn released over a period of 24 h. Drug release studies revealed that NP Wtmn releases Wtmn in a controlled fashion.
NP Wtmn Has Significantly Lower Toxicity than Wtmn.
A major limitation of Wtmn as a cancer therapeutic is its toxicity (16). We hypothesized that NP formulation of Wtmn would significantly reduce Wtmn toxicity. As a control we solubilized Wtmn with cremophor, a well-established solvent for poorly soluble chemotherapeutics. From this point on in this article, “Wtmn” refers to the cremophor formulation of Wtmn unless otherwise specified. Given that toxicity evaluations can depend on the mouse strain, the maximum tolerated dose (MTD) of both NP Wtmn and Wtmn was determined in four strains of mice (NOD/SCID, C57BL/6, CB6F1, and CD1). As seen in Table 1, the MTD for NP Wtmn is three to five times higher than that of Wtmn. This indicates that the NP Wtmn has a three- to five-times lower overall toxicity profile compared with that of Wtmn. Furthermore, hepatotoxicity and hematologic toxicities (17), the dose-limiting toxicities of Wtmn, were compared between NP Wtmn and Wtmn. Because the MTD was similar between the mouse strains, we chose to evaluate these toxicities in CD1 mice only. The toxicities were compared at 1/5th and 1/10th of the Wtmn MTD. As shown in Table 2, mice treated with 0.14 mg/kg of Wtmn had a depressed granulocyte count of 0.8 ± 0.14 (103/μL) [normal 1.2–8 (103/μL)] and significantly elevated alanine transaminase (ALT) levels of 134 ± 17 U/L (normal 40–50 U/L). In comparison, mice treated with 7 mg/kg NP Wtmn (which contains 0.14 mg/kg of Wtmn) had granulocyte counts of 2.9 ± 0.07 (103/μL) and slightly elevated ALT levels at 82 ± 12 U/L. At 1/10th MTD dose, we did not observe any significant hepatotoxicity or hematologic toxicities.
Table 1.
MTD of Wtmn formulations in four different strains of mice
| Wtmn | NP Wtmn | |
| Mouse strain | MTD (mg/kg) | MTD (mg/kg) |
| NOD/SCID | 0.7 | 2.2 (110) |
| C57BL/6 (Black) | 0.7 | 2.4 (120) |
| CB6F1 (Brown) | 0.5 | 2.5 (125) |
| CD1 (White) | 0.7 | 2.4 (120) |
Numbers represent milligrams per kilograms of Wtmn, either in cremophor solution or encapsulated in NP (2% wt/wt). Numbers in parentheses represent milligrams per kilograms of the NP Wtmn including the weight of the NP.
Table 2.
Toxicity profile in CD1 mice post i.v. injection of Wtmn formulations
| Normal range | Wtmn | NP Wtmn | |
| 1/5th MTD of Wtmn (0.14 mg/kg) | |||
| Hematologic toxicity | |||
| WBC | 3.5–10 (103/μL) | 2.8 ± 0.28 | 5.1 ± 0.42 |
| Granulocytes | 1.2–8 (103/μL) | 0.8 ± 0.14 | 2.9 ± 0.07 |
| Lymphocytes | 0.5–5 (103/μL) | 0.4 ± 0.42 | 3.56 ± 0.21 |
| Monocytes | 0.1–1.5 (103/μL) | 0.55 ± 0.07 | 0.7 ± 0.14 |
| Hepatotoxicity | |||
| ALT U/L | 40–50 U/L | 134 ± 17 | 82 ± 12 |
| AST U/L | 40–50 U/L | 44 ± 3 | 46 ± 7 |
| 1/10th MTD of Wtmn (0.07 mg/kg) | |||
| Hematologic toxicity | |||
| WBC | 3.5–10 (103/μL) | 4.3 ± 0.13 | 4.9 ± 0.20 |
| Granulocytes | 1.2–8 (103/μL) | 2.4 ± 0.11 | 3.7 ± 0.12 |
| Lymphocytes | 0.5–5 (103/μL) | 0.9 ± 0.28 | 1.05 ± 0.21 |
| Monocytes | 0.1–1.5 (103/μL) | 0.8 ± 0.42 | 0.5 ± 0.14 |
| Hepatotoxicity | |||
| ALT U/L | 40–50 U/L | 64 ± 11 | 60 ± 9 |
| AST U/L | 40–50 U/L | 40 ± 7 | 42 ± 10 |
Mice were treated with indicated doses of Wtmn or NP Wtmn containing an equivalent dose of Wtmn (2% wt/wt). Single tail-vein i.v. injection was given, and a toxicity profile was taken 24 h post injection. Full profile is in Table S1.
In Vitro Evaluation of NP Wtmn as a Radiosensitizer.
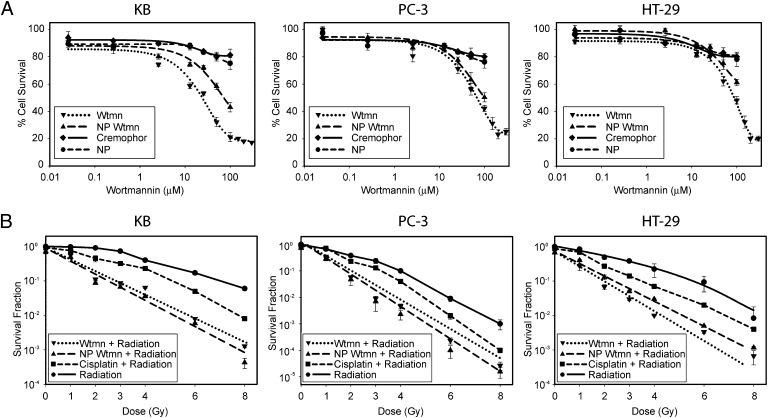
Unlike small molecules that permeate through the cell membrane, NPs enter cells through endocytosis. Therefore, NP Wtmn may have a therapeutic efficacy and/or mechanism of action different from that of Wtmn. Thus, we evaluated NP Wtmn as a radiosensitizer in vitro in three tumor-cell lines representing three different cancers: KB (head and neck cancer), PC-3 (prostate cancer), and HT-29 (colorectal cancer). NP Wtmn and Wtmn were found to have minimal cytotoxicity in these cells at concentrations lower than 10 μM (Fig. 2A). Radiation survival curves comparing NP Wtmn to Wtmn and cisplatin, one of the most common clinical radiosensitizers, showed that NP Wtmn is a potent radiosensitizer and is more effective than cisplatin (P = 0.05) (Fig. 2B). Furthermore, NP Wtmn eliminated the “shoulder region” of the survival curves. A shorter therapeutic incubation time of 1 h was also evaluated, and the clonogenic survival curves were similar to that of the 3-h incubation (Fig. S1).
Fig. 2.
In vitro efficacy of NP Wtmn. (A) Dose–response assay to measure cytotoxicity of NP Wtmn and Wtmn in three cancer cell lines: KB (head and neck cancer cell line), PC-3 (prostate cancer cell line), and HT-29 (colon cancer cell line). Cells were treated with indicated doses of Wtmn or NP Wtmn containing an equivalent dose of Wtmn. Cells were also treated with vehicle controls cremophor or empty NP. (B) Clonogenic assay of cancer cell lines after treatment with 25 μM Wtmn or NP Wtmn (containing 25 μM Wtmn) and radiation. Error bars correspond to the SD of repeated measurements (two NP preparations, duplicate measurements per preparation per data point). Wtmn + radiation and NP Wtmn + radiation curves are significantly different from cisplatin + radiation (P = 0.05) and radiation only (P = 0.05) curves.
The potency of a radiosensitizer can be quantified by the sensitizer enhancement ratio (SER) (18). As shown in Table S2, the SER of NP Wtmn is 3.68 and is similar to that of 3.51 for Wtmn in KB cells at 10% survival. In all three tumor-cell lines, the SER of NP Wtmn is nearly double that of the SER of cisplatin. These results indicate that NP Wtmn is a very potent radiosensitizer in vitro.
NP Wtmn Sensitizes Cancer Cells to Radiotherapy by Inhibiting DNA-PK.
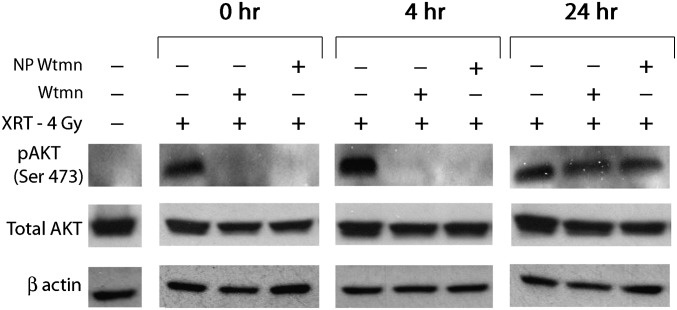
Wtmn is known to inhibit phosphorylation of Akt (p-Akt) (19). To determine if the mechanism of action of NP Wtmn is similar to Wtmn, p-Akt levels were determined in KB cells. As seen in Fig. 3, the level of p-Akt was increased in cells that received radiotherapy (XRT). In contrast, cells incubated with either NP Wtmn or Wtmn followed by radiotherapy had low levels of p-Akt until 24 h post drug treatment. This demonstrates that NP Wtmn functions similarly to Wtmn with respect to inhibition of Akt phosphorylation.
Fig. 3.
Effect of Wtmn formulations and radiation on phosphorylation of Akt. KB cells were pretreated with 25 μM Wtmn or NP Wtmn (containing 25 μM Wtmn) for 3 h and subsequently irradiated (4 Gy). Cell lysates were collected at indicated time points.
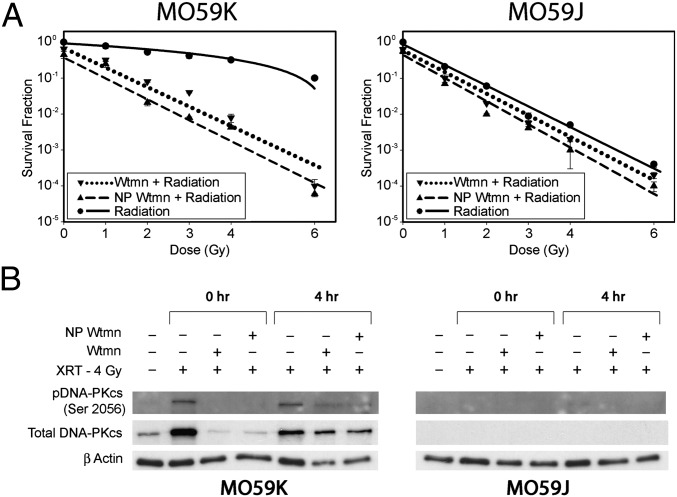
Wtmn’s proposed mechanism of function as a radiosensitizer is the inhibition of radiation-induced autophosphorylation of DNA-PKcs (DNA-dependent protein kinase catalytic subunit) (5, 6). To validate NP Wtmn function as a radiosensitizer through inhibition of DNA-PKcs, we used the M059K (DNA-PK intact) and M059J (DNA-PK deficient) glioblastoma-derived cell lines (20). Fig. 4A shows that M059K cells treated with either Wtmn or NP Wtmn had survival curves similar to that of M059J cells. On the other hand, neither NP Wtmn nor Wtmn could sensitize the DNA-PK–deficient M059J further to radiotherapy. These results suggest that the mechanism of action for NP Wtmn radiosensitization is indeed through inhibition of DNA-PKcs. We further confirmed the findings by examining the phosphorylated DNA-PKcs (p-DNA-PKcs) levels in irradiated M059K cells. As seen in Fig. 4B, untreated M059K cells demonstrated increased levels of p-DNA-PKcs after radiotherapy, whereas cells first treated with NP Wtmn or Wtmn and then irradiated showed only a moderate increase in p-DNA-PKcs.
Fig. 4.
NP Wtmn inhibits DNA-PK phosphorylation. (A) Effect of Wtmn formulations and radiation on DNA-PK phosphorylation. Clonogenic assay with glioma cancer cell lines M059K (DNA-PKcs positive) and M059J (DNA-PKcs negative) after treatment with Wtmn formulations and radiation. Error bars correspond to SD of repeated measurements (two nanoparticle preparations, duplicate measurements per preparation per data point). (B) M059K and M059J cells were pretreated with 25 μM Wtmn or NP Wtmn for 3 h and subsequently irradiated (4 Gy). Cell lysates were collected at the indicated time points.
In Vivo Evaluation of NP Wtmn as a Radiosensitizer.
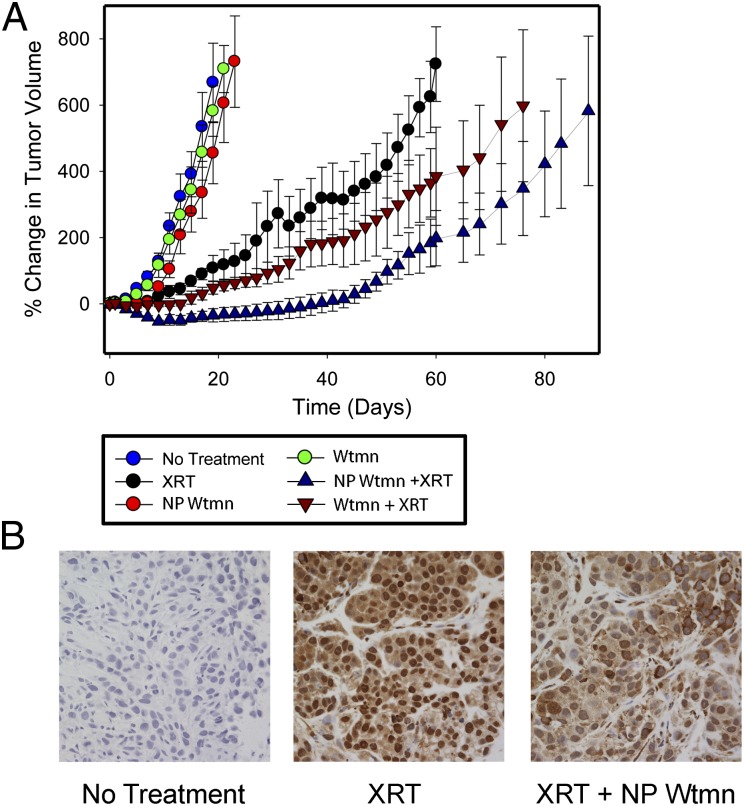
To evaluate the potential clinical translation of NP Wtmn as a radiosensitizer, we studied its radiosensitization efficacy in vivo using a murine xenograft model of cancer. Immune-deficient NOD/SCID mice bearing KB cell xenografts were treated with a single dose of Wtmn or NP Wtmn followed by radiotherapy, and the tumor volume was measured (Fig. 5A). Mice that received Wtmn or NP Wtmn with no radiotherapy had similar tumor growth rates to that of mice that received no treatment, indicating that Wtmn and NP Wtmn alone had minimal effects. In comparison, mice treated with Wtmn or NP Wtmn plus radiotherapy had significant delay in tumor growth, indicating excellent radiosensitization effects. When the tumor growth delay curves were compared between mice receiving NP Wtmn and Wtmn, the difference in delay was found to be statistically significant (P < 0.01). Histological samples from the tumors post treatment revealed a decrease in nuclear staining of p-DNA-PKcs from NP Wtmn-treated mice compared with tumors from mice that received only radiotherapy (Fig. 5B). This further validates the mechanism of action of NP Wtmn in vivo. We confirmed the in vivo findings using mice bearing HT-29 tumor-cell xenografts. NP Wtmn was compared with 5-flurouracil (5-FU), a commonly used radiosensitizer in chemoradiotherapy for gastrointestinal cancers. Using three times the initial tumor volume as an end point, we generated survival curves and showed that NP Wtmn is an effective radiosensitizer and is more effective than 5-FU (P < 0.05) (Fig. S2A). Tumor growth curves also demonstrated that NP Wtmn is significantly more effective as a radiosensitizer than 5-FU (P < 0.02) (Fig. S2B).
Fig. 5.
In vivo efficacy of NP Wtmn. (A) Tumor growth inhibition efficacy of Wtmn formulations on s.c. tumor xenograft. Mice received single tail-vein i.v. injections of 0.07 mg/kg of Wtmn or 3.5 mg/kg of NP Wtmn (contains the equivalent dose of 0.07 mg/kg Wtmn). Tumors were radiated locally once 3 h post injection (6 Gy). (B) pDNA-PKcs immunohistochemistry of untreated, irradiated only, or NP Wtmn and irradiated tumors collected 24 h post treatment. Nuclei are stained with hematoxylin.
Discussion
Before the development of NP drug-delivery vehicles, the strategy to overcome pharmacologic challenges of a promising small-molecule therapeutic was the modification of molecular structures. However, these modifications frequently led to diminished therapeutic efficacy and increased toxicity. As a result, many promising therapeutics failed clinical translation due to drug-delivery challenges and are no longer considered clinically viable. Wtmn is one such example. First discovered four decades ago, it was highly promising as a chemotherapeutic and a radiosensitizer. Unfortunately, its poor solubility, low stability, and high toxicity prevented its clinical translation. Many analogs of Wtmn have been formulated since its discovery, but none have succeeded thus far. The development of NP drug-delivery vehicles is a disruptive technology that has the potential to renew the clinical potential of “abandoned” drugs like Wtmn. Therefore, we aimed to demonstrate the proof-of-principle of this approach using Wtmn as a model drug.
An NP formulation of Wtmn was developed using a biodegradable polymeric NP platform. Wtmn is a highly hydrophobic molecule, and as a result the hydrophobic polymeric core of NPs can easily encapsulate Wtmn and, at the same time, protect it from environmental degradation. We found that Wtmn is unreactive with the NP and is released from the NP in a controlled fashion. We also showed that NP Wtmn is highly soluble in saline. Thus, NP delivery of Wtmn indirectly improved the stability and solubility of Wtmn by protecting it from an aqueous environment. Characterization of NP Wtmn’s physical properties showed that its size and surface charge are generally considered excellent for in vivo cancer applications (21). Although the NPs were able to encapsulate only 4% (wt/wt) of Wtmn, this loading capacity was determined to be more than sufficient for potential clinical applications due to the high potency of Wtmn.
One of the major barriers to the clinical translation of Wtmn is its systemic toxicity. Our data showed that NP Wtmn’s MTD is three to five times higher than that of Wtmn, indicating a significantly lower toxicity profile for NP Wtmn. In a detailed analysis of the dose-limiting hepatotoxicity and hematologic toxicities, NP Wtmn was found to have fewer toxic effects on the liver and bone marrow than Wtmn. Together, these results show that the NP formulation of Wtmn has dramatically reduced Wtmn’s toxicities and renewed the potential for its clinical translation.
Wtmn’s greatest therapeutic potential lies in its ability to potentiate the effects of radiotherapy. Wtmn inhibits DNA-PK, a key protein involved in the repair of double-stranded DNA damage. Because double-stranded DNA damage is the principal mechanism of action for radiotherapy, Wtmn has been determined to be a highly potent radiosensitizer. Furthermore, improving the therapeutic index of radiotherapy has been a major research focus in oncology (22–25). Our in vitro radiosensitization results demonstrated that NP Wtmn is a potent radiosensitizer. Furthermore, it is able to reduce the “shoulder region” in the radiation survival curves. The elimination of the shoulder region is highly significant, as daily clinical radiotherapy doses (1.5–3 Gy) generally fall into the shoulder region. The shoulder region, which reflects less effective cell killing by radiotherapy, is thought to be caused by the ability of tumor cells to repair their double-stranded DNA damage. Most radiosensitizers, like cisplatin, shifted the cell survival curves downward but did not reduce the shoulder region. In contrast, NP Wtmn not only shifted the cell survival curves downward, but also eliminated the shoulder region. Because the difference between cell survival is compounded each day as clinical radiotherapy is generally administered daily for several weeks, improved therapeutic efficacy in the shoulder region can lead to a potentially dramatic clinical treatment benefit. As an example, in the more radiosensitive HT-29 cell line, a single fraction of 2 Gy of radiotherapy with NP Wtmn is equivalent to approximately three fractions of 2 Gy of radiotherapy alone or two fractions of 2 Gy of radiotherapy with cisplatin (Fig. S3). However, in a radioresistant cell line like KB, a single fraction of 2 Gy of radiotherapy with NP Wtmn is equivalent to approximately 26 fractions of 2 Gy of radiotherapy alone or three fractions of 2 Gy of radiotherapy with cisplatin. Another method to assess radiosensitization efficacy is through the SER. Effective clinical radiosensitizers have SERs of nearly 2 at 10% cell survival. SERs of 3 or greater have been shown in vitro for very potent radiosensitizers, including Wtmn, but have never been translated into clinical practice (18). NP Wtmn was able to achieve a SER of 2.7–3.7 in this study. Together, the reduction in the shoulder region and the increase in the SER demonstrate NP Wtmn to be a potent radiosensitizer with very high therapeutic potential.
We confirmed that the NP Wtmn’s mechanism of action is the same as Wtmn. Both Akt and DNA-PK are involved in the cellular response to DNA damage and are phosphorylated as a result of radiotherapy (5, 6, 19, 26). Our results clearly demonstrated that no phosphorylation of either Akt or DNA-PK ensued in cancer cells incubated with NP Wtmn or Wtmn plus radiation. The similarity in the response confirms that the NP formulation of Wtmn does not alter the mechanism of action of Wtmn.
To demonstrate the therapeutic potential of NP Wtmn in vivo, murine xenograft models of cancer were used. Our study used a low dose of radiotherapy (6 Gy) and low doses of Wtmn and NP Wtmn (0.07 mg/kg of Wtmn). Our results demonstrated that NP Wtmn is indeed a powerful radiosensitizer. The fact that NP Wtmn is significantly more effective than Wtmn supports the hypothesis that the NP formulation has both improved Wtmn’s efficacy and reduced its toxicity. Histological studies confirmed that the mechanism of radiosensitization in vivo is through DNA-PK inhibition.
In conclusion, we report the development of a NP formulation of Wtmn and demonstrate its potential as a radiosensitizer. NP Wtmn was found to be soluble and stable, and it possesses significantly lower toxicity than Wtmn. We also showed that NP Wtmn is a highly effective radiosensitizer, both in vitro and in vivo, even at doses significantly below MTD. Our study demonstrates the potential for clinical use of NP Wtmn that employs a NP therapeutic carrier to renew the clinical translation potential of an “abandoned” drug. This illustrates that abandoned therapeutics, such as Wtmn, can be brought into the clinic with NP formulations. Our work also addresses, at least in part, some of the major barriers to bringing otherwise promising small molecules from the laboratory to the clinic. Most importantly, this strategy can be applied to many other therapeutics that have the potential to impact the treatment of many human diseases in addition to cancer, the clinical translation of which has been hindered by toxicity.
Materials and Methods
Materials.
Wtmn was purchased from Sigma-Aldrich and LC Laboratories. Poly(d,l-lactide-coglycolide) (PLGA) with a 50:50 monomer ratio, ester-terminated, and viscosity of 0.72–0.92 dL/g was purchased from Durect. Soybean lecithin consisting of 90–95% (wt/wt) phosphatidylcholine was obtained from MP Biomedicals. DSPE–PEG2000–COOH [1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-carboxy (polyethylene glycol) 2000] was obtained from Avanti Polar Lipids.
Formulation and Characterization of NP Wtmn.
PLGA–lecithin–PEG core–shell NPs were synthesized from PLGA, soybean lecithin, and DSPE–PEG–COOH using a previously reported nanoprecipitation technique (14). Wtmn was dissolved at a dosage of 10% (wt/wt) of the polymer into the PLGA/acetonitrile solution (1 mg/mL) before nanoprecipitation. NP size (diameter in nm), polydispersity, and surface charge (ζ-potential in mV) were obtained with a ZetaPALS dynamic light-scattering detector (Brookhaven Instruments). TEM images were obtained at the Microscopy Services Laboratory Core Facility at the University of North Carolina School of Medicine.
Nanoparticle Wtmn Release.
Three-milliliter NP solutions at a concentration of 0.5 mg/mL were split equally into 30 Slide-ALyzer MINI dialysis microtubes with a molecular weight cutoff of 10 kDa (Pierce) and subjected to dialysis against 4 L phosphate buffer saline (PBS) with gentle stirring at 37 °C. PBS was changed periodically during the dialysis process. At the indicated times, 0.2 mL of solution from two microtubes was removed and mixed with an equal volume of acetonitrile to dissolve the NPs. Wtmn content was subjected to quantitative analysis using an Agilent 1100 HPLC equipped with a C18 chromolith flash column (Merck KGaA). Wtmn concentration was measured using the Ultraviolet/Visible detector at 245 nm. Wtmn retention time was 1.6 min in 50:50 acetonitrile/water nongradient mobile phase at 0.25 mL/min.
Cell Culture.
Cancer cell lines KB, HT-29, PC3, M059J, and M059K were obtained from ATCC. KB cells were cultured in Eagle’s Minimum Essential Medium supplemented with 10% (vol/vol) FBS (Mediatech) and penicillin/streptomycin (Mediatech). PC3 and HT29 were cultured in a 1:1 mixture of DMEM and Ham’s F-12 medium (Gibco, Invitrogen) supplemented with 10% (vol/vol) FBS and penicillin/streptomycin. M059J and M059K were maintained in a 1:1 mixture of DMEM and Ham’s F-12 medium supplemented with 0.05 mM nonessential amino acids, penicillin/streptomycin, and 10% FBS.
Cell Viability Assay.
Cells were plated on 96-well plates at a density of 30,000 cells per well. NP Wtmn, free Wtmn (dissolved in cremophor), NP, and cremophor were added to cells in a total final volume of 0.1 mL per well and were incubated for 3 h. At the end of incubation, cells were gently washed twice with sterile PBS and were further incubated with sterile fresh complete medium for 48 h at 37 °C and 5% CO2. To evaluate cell viability, cells were washed with sterile PBS, and an MTS [(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)] assay was performed according to manufacturer’s instructions (Promega).
Clonogenic Survival Assay.
Cells (2 × 106) were seeded in 4 mL of culture medium in 25-mL flasks before treatment. Cells were treated with therapeutics for 3 h and washed two times with sterile PBS after incubation. Cells were trypsinized and seeded at various densities ranging from 100 to 100,000 cells in 4 mL of culture medium in 25-mL flasks and radiated with 0, 2, 4, 6, and 8 Gy from an X-RAD 320 (Precision X-Ray) machine operating at 320 kVp and 12.5 mA. The dose rate at a source-subject distance of 50 cm was 2.07 Gy/min. Cells were incubated for 12 d after irradiation and then fixed in 1:1 acetone/methanol and stained with trypan blue. All colonies with over 50 cells were counted. The relative cell-surviving fraction was calculated by dividing the number of colonies of radiated cells by the cells plated, with a correction for the plating efficiency.
Western Blot.
Cells were treated with 25 μM Wtmn, NP Wtmn, or vehicle for 3 h, washed with PBS, and incubated with fresh media before 4 Gy irradiation. Cell lysates were collected in HNTG (20 mM Hepes; 150 mM NaCl; 10% glycerol; 0.1% Triton-X100; 1mM Na3VO4) buffer at the indicated times post irradiation. Protein concentration was measured by bicinchoninic acid protein assay (Pierce). Primary antibodies used were Akt (Cell Signaling), p-Akt (Ser473) (Cell Signaling), DNA PKcs (Abcam), DNA-PKcs (phospho S2056) (Abcam), and β-actin (13E5) (Cell Signaling) at dilutions indicated by the manufacturers. Secondary antibodies were α-mouse IgG HRP-linked antibody (Cell Signaling) or α-rabbit IgG HRP-linked antibody (Cell Signaling).
MTD.
MTD—defined as the highest possible dose resulting in no animal deaths and less than 20% weight loss—was evaluated for Wtmn formulations. Non-tumor–bearing mice (NOD/SCID, C57BL/6, CB6F1, and CD1) (Charles River Laboratories) were injected with varying amounts of NP Wtmn and Wtmn normalized to milligram of Wtmn injected/kilogram of mice body weight. The animals were weighed and observed daily for any change in physical activity.
Mice were housed in an American Association for the Accreditation of Laboratory Animal Care-accredited facility in sterile housing at the University of North Carolina at Chapel Hill, and the work was approved and monitored by the University of North Carolina Animal Care and Use Committee.
Toxicity.
CD1 mice were given tail-vein i.v. injection with different Wtmn formulations (NP Wtmn or Wtmn in cremophor) at the indicated doses (1/5th the MTD or 1/10th the MTD). After 24 h, submandibular bleeding was performed. Blood (100–200 μL) was collected in heparin-coated tubes to prevent coagulation. Whole-blood samples were immediately submitted for blood toxicity to the Animal Clinical Laboratory Core Facility at the University of North Carolina. For liver toxicity studies, blood samples were centrifuged (15,000 × g for 10 min) to separate blood cells from the plasma, and samples were submitted for analysis.
Tumor Efficacy.
KB cells (1 × 106 cells in 200 μL 1:1 RPMI-1640 and matrigel) were s.c. inoculated into the left flank of 6- to 8-wk-old male NOD/SCID mice. Ten days after inoculation, the mice were randomly distributed into different groups for subsequent treatment. We aimed to achieve tumor volume of 50–75 mm3. NP Wtmn (3.5 mg/kg) or free Wtmn in cremophor formulation (0.07 mg/kg) was tail-vein i.v. injected. The dose of NP Wtmn contains an equivalent of 0.07 mg/kg of Wtmn. Three hours post injection, the tumors were subjected to a dose of 6 Gy with XRAD 320. Mice were shielded with a specially designed lead shield allowing radiation of the tumor site and minimal radiation to other organs. Tumor volumes were calculated by measuring two perpendicular diameters with a caliper and by using the formula of V = 0.5 × a × b2 where, a and b are the larger and smaller diameters, respectively. The tumor volumes were measured every 2–4 d, and the relative percentage change in tumor volume was calculated using the relation 100 * (Vi − Vo)/Vo, where Vi is the volume calculated and Vo is the initial volume on day 1.
HT-29 cells (1 × 106 cells in 200 μL 1:1 RPMI-1640 and matrigel) were s.c. inoculated into the upper dorsal region of 6- to 8 wk-old male Nu/Nu mice. Ten days after inoculation, mice were randomly distributed into different groups for subsequent treatment. Average tumor volume before treatment was ∼200 mm3. NP Wtmn (3.5 mg/kg) or 5-flurouracil (25 mg/kg) was tail-vein i.v. injected. Three hours post injection, tumors were subjected to a dose of 6 Gy with XRAD 320. Mice were shielded with a specially designed lead shield allowing radiation of the tumor site and minimal radiation to other organs. Tumor volumes were calculated as above. The change in tumor volume was monitored by taking the ratio Vi/Vo, where Vi is the volume calculated and Vo is the volume on day 0. Tumor growth for the different treatment modalities was monitored until this ratio value reached 3, at which point the animals were euthanized.
Tumor Histology.
KB cells (1 × 106 cells in 200 μL 1:1 RPMI-1640 and matrigel) were s.c. inoculated into left flank of 6- to 8-wk-old male NOD/SCID mice. Ten days after inoculation, mice were given treatments. Twenty-four hours post irradiation, animals were euthanized and tumors were removed and fixed in 10% neutral buffered formalin (vol/vol) (Thermo Scientific). Immunohistochemistry was performed on paraffin-embedded sections of tumor using DNA-PKcs (phospho S2056) (Abcam) and hematoxylin.
Statistics.
To statistically compare the in vitro clonogenic assays, we chose the area under the growth curve (AUC). Wilcoxon rank sum test was performed to compare the survival fraction of the different groups. The exact one-sided P value of the Wilcoxon rank sum test is presented.
To statistically compare the percentage change in KB tumor growth rates of Wtmn + XRT and NP Wtmn + XRT-treated mice, we chose the AUC over the largest common observation period as an indicator of growth rate. The largest common observation period of the two groups is from time 0 to day 76. We used the trapezoidal rule to approximate AUC. On the basis of the AUCs, the Wilcoxon rank sum test was performed to compare the growth rates between the two groups. The exact one-sided P value of the Wilcoxon rank sum test is presented. To statistically compare the change in HT-29 tumor growth rates of Wtmn + XRT and 5-flurouracil + XRT-treated mice, we recorded the time needed for the tumors to reach three times the initial volume and generated a Kaplan–Meier curve. One-sided P value of the Wilcoxon rank sum test is presented. Also, AUC was measured as above to compare the change in tumor growth for d 0–12.
Supplementary Material
Acknowledgments
We thank Amber Cummings for assistance with graphics in this manuscript and the Microscopy Services Laboratory, Animal Studies Core, and Animal Histopathology Core at the University of North Carolina for their assistance with procedures in this manuscript. This work was supported by grants from the University Cancer Research Fund from the University of North Carolina. A.Z.W. is also supported by National Institutes of Health/National Cancer Institute K12 Career Development Award 5-K12-CA120780-01-05 and National Institutes of Health Center for Nanotechnology Excellence Grant 1-U54-CA151652-01.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120508109/-/DCSupplemental.
References
- 1.Heath JR, Davis ME. Nanotechnology and cancer. Annu Rev Med. 2008;59:251–265. doi: 10.1146/annurev.med.59.061506.185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang AZ, Langer RS, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63(1):185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 3.Workman P, Clarke PA, Raynaud FI, van Montfort RL. Drugging the PI3 kinome: From chemical tools to drugs in the clinic. Cancer Res. 2010;70(6):2146–2157. doi: 10.1158/0008-5472.CAN-09-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenzweig KE, Youmell MB, Palayoor ST, Price BD. Radiosensitization of human tumor cells by the phosphatidylinositol3-kinase inhibitors wortmannin and LY294002 correlates with inhibition of DNA-dependent protein kinase and prolonged G2-M delay. Clin Cancer Res. 1997;3(7):1149–1156. [PubMed] [Google Scholar]
- 5.Hashimoto M, et al. DNA-PK: The major target for wortmannin-mediated radiosensitization by the inhibition of DSB repair via NHEJ pathway. J Radiat Res. 2003;44(2):151–159. doi: 10.1269/jrr.44.151. [DOI] [PubMed] [Google Scholar]
- 6.Sarkaria JN, et al. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998;58(19):4375–4382. [PubMed] [Google Scholar]
- 7.Yuan H, Luo J, Weissleder R, Cantley L, Josephson L. Wortmannin-C20 conjugates generate wortmannin. J Med Chem. 2006;49(2):740–747. doi: 10.1021/jm050699p. [DOI] [PubMed] [Google Scholar]
- 8.Yuan H, Barnes KR, Weissleder R, Cantley L, Josephson L. Covalent reactions of wortmannin under physiological conditions. Chem Biol. 2007;14(3):321–328. doi: 10.1016/j.chembiol.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27(41):5511–5526. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 10.Yu K, et al. PWT-458, a novel pegylated-17-hydroxywortmannin, inhibits phosphatidylinositol 3-kinase signaling and suppresses growth of solid tumors. Cancer Biol Ther. 2005;4(5):538–545. doi: 10.4161/cbt.4.5.1660. [DOI] [PubMed] [Google Scholar]
- 11.Zhu T, et al. Pegylated wortmannin and 17-hydroxywortmannin conjugates as phosphoinositide 3-kinase inhibitors active in human tumor xenograft models. J Med Chem. 2006;49(4):1373–1378. doi: 10.1021/jm050901o. [DOI] [PubMed] [Google Scholar]
- 12.Howes AL, et al. The phosphatidylinositol 3-kinase inhibitor, PX-866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures. Mol Cancer Ther. 2007;6(9):2505–2514. doi: 10.1158/1535-7163.MCT-06-0698. [DOI] [PubMed] [Google Scholar]
- 13.Kong D, Yamori T. Phosphatidylinositol 3-kinase inhibitors: Promising drug candidates for cancer therapy. Cancer Sci. 2008;99(9):1734–1740. doi: 10.1111/j.1349-7006.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, et al. Nanoparticles in medicine: Therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, et al. Self-assembled lipid–polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano. 2008;2(8):1696–1702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ihle NT, et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol Cancer Ther. 2004;3(7):763–772. [PubMed] [Google Scholar]
- 17.Schultz RM, et al. In vitro and in vivo antitumor activity of the phosphatidylinositol-3-kinase inhibitor, wortmannin. Anticancer Res. 1995;15(4):1135–1139. [PubMed] [Google Scholar]
- 18.Hall EJ. Radiobiology for the Radiologist. 5th Ed. Philadelphia: Lippincott Williams & Wilkins; 2000. pp xi, 588 pp. [Google Scholar]
- 19.Ng SS, Tsao MS, Nicklee T, Hedley DW. Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clin Cancer Res. 2001;7(10):3269–3275. [PubMed] [Google Scholar]
- 20.Lees-Miller SP, et al. Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science. 1995;267(5201):1183–1185. doi: 10.1126/science.7855602. [DOI] [PubMed] [Google Scholar]
- 21.Gref R, et al. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 22.Stupp R, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 23.Seiwert TY, Salama JK, Vokes EE. The concurrent chemoradiation paradigm: General principles. Nat Clin Pract Oncol. 2007;4(2):86–100. doi: 10.1038/ncponc0714. [DOI] [PubMed] [Google Scholar]
- 24.Willett CG, Czito BG. Chemoradiotherapy in gastrointestinal malignancies. Clin Oncol. 2009;21(7):543–556. doi: 10.1016/j.clon.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Eifel PJ. Chemoradiotherapy in the treatment of cervical cancer. Semin Radiat Oncol. 2006;16(3):177–185. doi: 10.1016/j.semradonc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Chan DW, et al. Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes Dev. 2002;16(18):2333–2338. doi: 10.1101/gad.1015202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.