Abstract
In the mammalian retina, life-long renewal of light-sensitive photoreceptor outer segments (POS) involves circadian shedding of distal rod POS tips and their subsequent phagocytosis by the adjacent retinal pigment epithelium (RPE) every morning after light onset. Molecular mechanisms that promote or synchronize POS tip shedding have thus far remained unknown. Here we examined plasma membrane asymmetry of living POS by quantifying surface exposure of the membrane phospholipid phosphatidylserine (PS) using antibodies, annexin V, and pSIVA (polarity-sensitive indicator of viability and apoptosis), an annexin-based biosensor with switchable states of fluorescence. We found that isolated POS particles possess externalized PS, whose blockade or removal reduces their binding and engulfment by RPE in culture. Imaging of live photoreceptors in freshly dissected mouse retina detected PS externalization restricted to POS tips with discrete boundaries. In wild-type mice, frequency of rod tips exposing PS and length of tips with exposed PS peak shortly after light onset. In contrast, PS-marked POS tips do not vary in mice lacking the diurnal phagocytic rhythm of the RPE due to loss of either the phagocytosis receptor αvβ5 integrin, expressed by the RPE but not by photoreceptors, or its extracellular ligand milk fat globule-EGF factor 8 (MFG-E8). These data identify a molecular distinction, localized PS exposure, that is specific to the surface of rod POS tips. Enhanced PS exposure preceding rod shedding and phagocytosis suggests that surface PS promotes these processes. Moreover, our results demonstrate that the diurnal rhythm of PS demarcation of POS tips is not intrinsic to rod photoreceptors but requires activities of the RPE as well.
In the mammalian retina, life-long renewal of photoreceptor outer segments (POS) involves daily shedding of distal POS tips and their phagocytosis by the adjacent retinal pigment epithelium (RPE) (1, 2). POS renewal is under circadian control, with a burst of rod shedding and phagocytosis occurring in the morning, shortly after light onset (3). RPE cells use a molecular mechanism for POS tip phagocytosis that is highly similar to mechanisms used by other phagocytic cells for clearance of apoptotic cells. In these pathways, integrin receptors αvβ3 (in macrophages) or αvβ5 (in RPE and dendritic cells) recognize extracellular, soluble bridge proteins that opsonize phagocytic particles and that display an arginyl-glycyl-aspartic acid tripeptide integrin receptor-binding motif (4–6). In the retina, secreted milk fat globule-EGF factor 8 (MFG-E8) in the subretinal space fulfills this role in promoting clearance of shed POS tips by ligating αvβ5 receptors that localize specifically to the apical, phagocytic surface of RPE cells (5). αvβ5 integrin ligation stimulates cytosolic signaling toward focal adhesion kinase and Mer tyrosine kinase (MerTK), both of which must be activated for particle engulfment (7, 8). Lack of either MFG-E8 ligand or αvβ5 receptors is sufficient to abolish the diurnal burst of RPE phagocytosis in knockout mice, but basal levels of POS particle uptake continue to maintain retinal integrity (9, 10). Unlike the pathways used by RPE cells to phagocytose spent POS tips, mechanisms that designate POS tips for shedding and removal have thus far remained obscure.
Externalized phosphatidylserine (PS), an anionic phospholipid normally restricted to the cytosolic leaflet of the plasma membrane, is the main “eat me” signal displayed by cells undergoing apoptosis (11, 12). Phagocytic integrin ligands, including MFG-E8, possess PS binding domains, through which they designate apoptotic cells for clearance (13). Using both traditional annexin V (A5) or antibody-based PS binding reagents and a PS biosensor allowing real-time imaging of externalized PS in living, dissected tissue, we demonstrate increased frequency of PS exposure and elongation of precisely PS-marked tips by POS immediately preceding the peak of diurnal RPE phagocytosis in mouse retina. These results identify a molecular change, PS exposure, that distinguishes the plasma membrane of photoreceptor POS tips at the time of POS shedding. Moreover, we found that these synchronized changes of PS externalization are completely absent in mice lacking either the RPE receptor αvβ5 integrin or its extracellular ligand MFG-E8. Thus, the RPE via its phagocytic machinery contributes to stimulation of PS exposure by POS tips rather than photoreceptor rods controlling this process autonomously.
Results
Blocking Exposed PS Reduces RPE Cell Phagocytosis of Experimental POS Fragments.
RPE cells in culture retain avid phagocytic activity via the MFG-E8–αvβ5–MerTK pathway. MFG-E8 binds to POS fragments and possesses a PS binding site. To assess whether PS exposure may be relevant for phagocytosis, we incubated experimental, isolated POS fragments with a monoclonal antibody specific to PS (αPS) or with recombinant A5. A5 is well characterized to bind specifically to PS (14). Fig. 1A shows that both PS-binding reagents coisolate with POS particles. Coincubation reduced binding of both reagents, indicating that they compete for POS particle binding. This competition was specific: incubation with rhodopsin antibody resulted in its binding to POS particles at levels similar to αPS without effect on A5 binding. Recombinant β-galactosidase applied at the same concentration as A5 had no effect on αPS binding. Fluorescence microscopy revealed that FITC-A5 labeled unfixed POS particles in a patchy distribution (Fig. S1A). αPS showed a similar distribution labeling paraformaldehyde-fixed, unpermeabilized POS (Fig. S1B). POS particle preincubation with αPS or A5 reduced their binding to RPE-J cells by 46% and 45% compared with their respective control (Fig. 1B). Internalization was also decreased, albeit to lesser extent, possibly as a consequence of reduced availability of bound particles (Fig. 1C). In a separate experiment, we determined that predigestion with phosphatidylinositol-specific phospholipase C was sufficient to increase PS recognition on unfixed POS particles by A5 (Fig. S2 A and B). RPE-J cells bound and engulfed such PS-enhanced POS particles more efficiently than untreated POS particles (Fig. S2 C and D). In contrast, decreasing A5-recognizable PS by treatment with non–phospholipid-specific phospholipase C diminished POS particle binding and engulfment (Fig. S2). Thus, levels of externalized PS on POS particles correlate with their binding and thus phagocytosis by RPE cells.
Fig. 1.
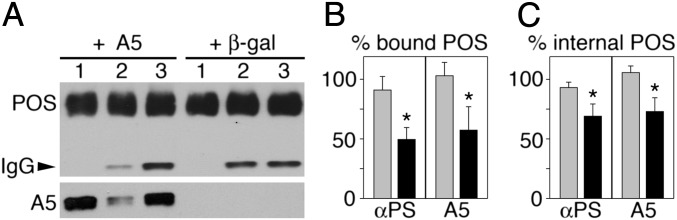
Competitive binding and inhibition of phagocytosis of purified POS fragments by αPS and A5. (A) Detection of opsin POS load (Upper, POS) and bound mouse IgG light chains (Upper, IgG, arrowhead) and bound A5 (Lower, A5) in lysates of POS particles after incubation with nonimmune IgG (lanes 1), αPS (lanes 2), or rhodopsin antibody (lanes 3) in the presence of recombinant A5 (+ A5) or β-galactosidase (+ β-gal), which served as negative control. One representative blot is shown of four independent experiments performed with similar results. (B and C) POS particle binding (B) and internalization (C) by RPE cells of particles preincubated with αPS or A5 (black bars, αPS, A5), rhodopsin antibody (gray bar, αPS), or β-galactosidase (gray bar, A5). Bars show relative values compared with levels of bound or internalized POS particles preincubated with nonimmune IgG (set as 100%), displayed as mean ± SD of three independent experiments, each with duplicate samples. *P < 0.05 of αPS and A5 samples compared with the respective control by Student t test.
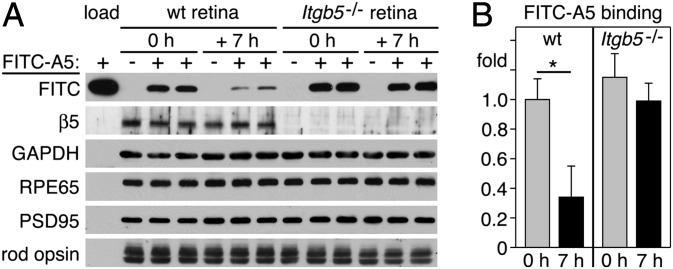
Both WT and Itgb5−/− POS in Vivo Expose PS, but only WT PS Exposure Follows a Diurnal Rhythm.
To test whether POS in vivo also expose PS at the time of phagocytosis, we incubated whole mouse retina immediately after excision with FITC-A5 and quantified bound FITC-A5 by immunoblotting. We found that WT retina at light onset bound ∼threefold more FITC-A5 than WT retina isolated 7 h later (Fig. 2). The same experiments showed that there was no difference in FITC-A5 binding by Itgb5−/− retina at the same two time points (Fig. 2). As a complementary approach, we examined the localization of exposed PS on POS in live, excised retina by fluorescence microscopy after labeling with Alexa Fluor 488-conjugated A5 (488-A5). 488-A5 stained POS in WT retina harvested at light onset (0 h) and even more intensely 1 h later but much less 7 h after light onset (Fig. 3 A–D). In contrast, 488-A5 staining of Itgb5−/− POS was as intense as staining of WT retina at light onset, regardless of time of tissue harvest (Fig. 3 E–H). αPS yielded similar staining pattern and diurnal variation when applied to paraformaldehyde-fixed WT retina (Fig. S3). These results show that POS in vivo expose PS only at times of active RPE phagocytosis.
Fig. 2.
Increased binding of A5 to WT but not Itgb5−/− retina at light onset. (A) Representative immunoblot membrane sequentially probed for proteins as indicated comparing FITC signal detecting of FITC-A5 in working solution used for labeling (load) and in individual neural retinas excised from eyes from two different WT or Itgb5−/− mice at light onset (0 h) or 7 h later (+ 7 h) after incubation with FITC-A5 (lanes +). Lanes - show neural retina incubated with buffer without FITC-A5. (B) Quantification of blots as in A. Bars show relative binding of FITC-A5 to WT and Itgb5−/− retina at time points as indicated, with binding at 0 h to WT retina set as 1 (mean ± SD of six retinas of six mice tested in three independent experiments. *P < 0.05 relative to value for WT at 0 h by Student t test. For each sample, FITC-A5 values were normalized to rod opsin to account for differences in tissue yield.
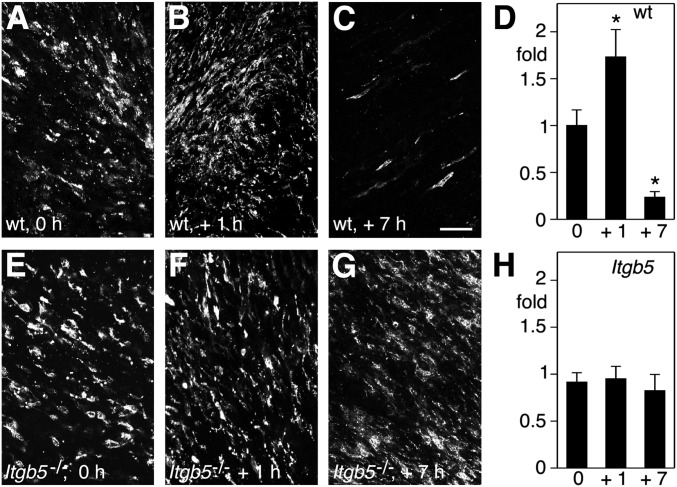
Fig. 3.
Diurnal variation in POS binding of 488-A5 in WT but not in Itgb5−/− retina. (A–C, E–G) Representative whole mounts of retina from WT and Itgb5−/− mice harvested at times of day as indicated, labeled live with 488-A5 followed by washing and fixation before imaging. Maximal projections are shown. (Scale bar, 25 μm.) (D and H) Quantification of labeling intensity per area for WT retina (D) and Itgb5−/− retina (H) relative to labeling of WT retina at 0 h, which was set as 1. Bars show mean ± SEM, n = 6 retinas of six mice. *P < 0.05 relative to labeling in WT at 0 h (Student t test).
Imaging of Live, Dissected Retina Reveals Precise Restriction of PS Externalization to POS Tips.
The process of POS renewal involves diurnal shedding of only the most distal tips of POS. However, in our hands, 488-A5 incubation followed by washing and fixation before imaging did not result in distinct labeling of POS tips. Technical obstacles such as the length of incubation time at 37 °C required for effective A5 binding and numerous tissue rinses needed before fixation likely explain that bound A5 may have in part diffused or been internalized by the time of tissue imaging. To solve these issues we replaced our multistep labeling protocol that used conventional recombinant fluorescent A5 with imaging of live retinas immediately after dissection in the presence of the polarity-sensitive annexin-based biosensor (“polarity-sensitive indicator of viability and apoptosis,” pSIVA) (15). Like A5, pSIVA is highly specific for PS. Unlike 488-A5, pSIVA does not fluoresce unless bound to PS, allowing application at high concentration and immediate imaging of live samples without washes or fixation. Indeed, application of pSIVA to dissected, live retina followed by immediate imaging revealed clearly demarcated POS tips (Fig. 4). In WT retina, pSIVA stained more POS tips at light onset and 1 h later compared with 1 h before and 7 h after light onset (Fig. S4). In Itgb5−/− retina, frequency of pSIVA-labeled POS tips did not vary among the time points tested (Fig. S4). Thus, pSIVA staining patterns confirmed and extended our prior results quantifying A5 binding in fixed or lysed tissues. Furthermore, fewer labeled POS tips 1 h before light further confirmed that POS expose PS at their distal tips only at the time of tip shedding and RPE phagocytosis.
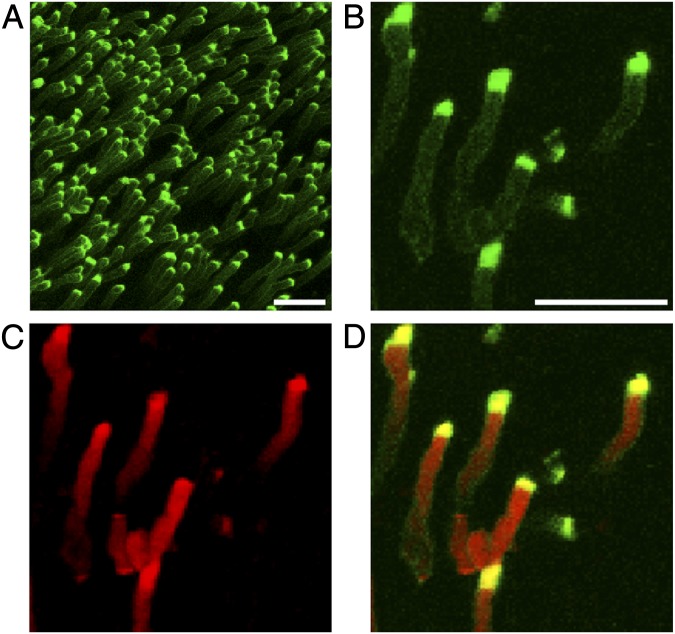
Fig. 4.
Imaging of PS exposed at POS tips in live, dissected mouse retina. Images show maximal projections of WT mouse retina harvested 15 min after light onset and imaged immediately while incubating in pSIVA. (B–D) Field shows costain of pSIVA (B), CellMask membrane stain (C), and overlay of both (D). (Scale bar in A, 10 μm; in B for B–D, 5 μm.)
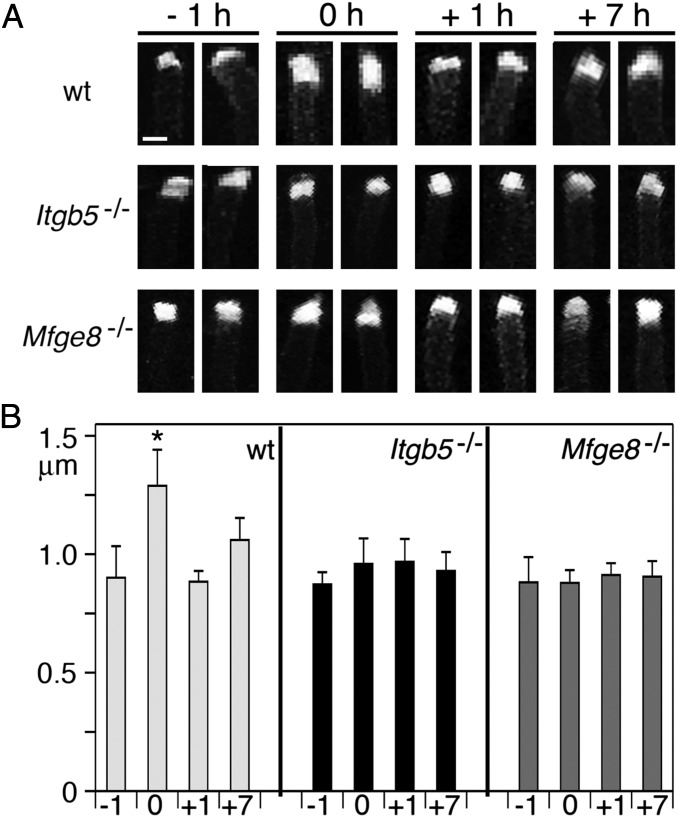
PS-Marked POS Tips Elongate at Light Onset in WT but Not Itgb5−/− or Mfge8−/− Retina.
PS exposure at POS tips likely designates tips for removal. We thus hypothesized that individual PS-marked POS tips may be of different quality at light onset compared with other times. Close-up imaging and quantification revealed that PS-labeled POS tips were significantly longer in WT retina at light onset, by 43% on average, than at 1 h before or after (Fig. 5, WT). Strikingly, we did not find elongated tips in retina of Itgb5−/− or Mfge8−/− mice, regardless of time of day (Fig. 5, Itgb5−/− and Mfge8−/−). These results indicate that rod POS in vivo extend tips exposing PS just in time for shedding and subsequent phagocytosis. In retina whose RPE fails to phagocytose in a diurnal rhythm, rod POS tips also lack rhythmic PS exposure. These results suggest that interactions of RPE and photoreceptor rods are required to regulate this process.
Fig. 5.
Elongation of PS-exposing tips in WT but not Itgb5−/− or Mfge8−/− retina after light onset. Retinas were harvested at time points and from mice of different genotypes as indicated and imaged immediately during incubation with pSIVA. (A) Fields show maximal projections of two representative tips from the same retina for each sample type as indicated. (Scale bar, 1 μm.) (B) Quantification of PS-positive POS tip length. Bars show mean ± SD, n = 4–5 independent experiments each analyzing both eyes of the same mouse. *P < 0.05 relative to labeling in WT at 0 h (Student t test).
Discussion
Continuous POS renewal relies on a strict balance of POS growth and clearance phagocytosis of shed POS tips by RPE cells. Unlike for RPE phagocytosis, there are no cell cultures or in vitro models for the process of POS tip shedding. Triggering shedding in frog retina with light exposure, Matsumoto and Besharse (16) showed increased entry (possibly by membrane fusion events) of low-molecular-weight fluorescence dyes such as Lucifer yellow specifically into disks at POS tips. Molecular–cellular mechanisms through which mammalian photoreceptors promote circadian POS tip shedding have not yet been reported. Our experiments reveal PS exposure specifically at POS tips in a diurnal rhythm of rod photoreceptors in mammalian retina. We propose that PS exposure by spent POS tips serves to recruit the extracellular integrin ligand MFG-E8, which is available in the subretinal space that surrounds POS. Indeed, like pSIVA, fluorescent recombinant MFG-E8 binds specifically to POS tips, supporting such a hypothesis (Fig. S5). MFG-E8 decoration of tips may suffice to stimulate αvβ5 integrin signaling toward the engulfment receptor MerTK, inducing the swift and efficient POS tip clearance characteristic and essential for healthy retina.
Mechanistically, the swift onset of PS exposure we observed leads us to speculate that PS exposure at rod tips likely requires a decrease in enzymatic outside-in PS flipping that normally ensures cytosolic leaflet PS and/or active enzymatic scrambling of PS at rod tips. The flippase P4-ATPa82 is expressed in POS in bovine retina and functions in flipping PS to the cytosolic leaflet of plasma membranes (17). However, it may localize mainly to internal disks rather than to the rod plasma membrane. PS-specific scramblase function has recently been attributed to TMEM16F, a member of the TMEM16/anoctamin family of proteins with tissue-specific expression patterns. Some TMEM16 proteins are chloride channels, whereas others remain to be characterized (18, 19). Further studies will be necessary to identify the enzymes that contribute to POS tip turnover.
Exposed PS remains at POS tips, generating PS-marked tips with precise borders, suggesting mechanisms that limit PS diffusion. PS exposure by cells that do not progress to cell death in other tissues may be a result of extracellular interactions. For example, mouse myoblasts in developing embryos transiently externalize PS specifically at cell contact sites (20). In peritoneal macrophages, the PS receptor Tim-4 relocalizes to punctate caps at sites of contact with apoptotic cells, suggesting that PS is specifically localized to regions of cell contact on the surface of apoptotic cells (21). Because PS on POS tips remains localized after RPE detachment, similar direct RPE–POS cell–cell contacts seem unlikely. However, it is possible that specific extracellular matrix components of the subretinal space maintain PS at tips.
Both WT and mutant retinas we studied possess mechanisms for externalizing PS and for precisely restricting PS exposure to POS tips. In addition, our experiments revealed that both frequency of tips exposing PS as well as the length of individual PS-marked tips follow a strict diurnal rhythm in WT mouse retina that matches the daily burst of RPE phagocytosis. These synchronized changes of PS exposure are completely lost in mice lacking either αvβ5 integrin receptors or MFG-E8, its ligand in the retina. These findings are logical given that lack of αvβ5 or MFG-E8 cause a complete loss of RPE phagocytosis rhythm without obvious distortion or elongation of POS, indicating that these mutant retinas maintain an overall balance of POS growth, tip shedding, and tip clearance. However, it is intriguing that RPE cells but not photoreceptors express αvβ5 integrin receptors. Thus, photoreceptors are not directly altered in Itgb5−/− mice. Taken together, our results imply that photoreceptors do not act autonomously in externalizing PS at POS tips in a diurnal rhythm. Rather, RPE cells also participate in synchronizing PS exposure.
Our results show that PS exposure by POS particles directly affects their phagocytosis by RPE cells in culture. In intact retina, the precise causal relationship of PS exposure and phagocytic activity needs further study. Diurnal PS exposure may precede RPE phagocytosis or follow activation of the RPE’s phagocytic activity. Interactions of POS with components of the active phagocytic machinery of RPE cells, including possibly αvβ5 itself, may stimulate PS externalization by POS. Alternately, RPE cells may secrete or display at their apical surface signals unrelated to phagocytosis that remain to be identified and that activate a mechanism in photoreceptors for discrete PS externalization of POS tips.
Materials and Methods
Reagents were from Invitrogen or Sigma unless otherwise indicated.
Cell Culture Experiments.
Porcine POS fragments isolated and FITC labeled as previously described (5, 22) were incubated on a rocker for 30 min at 37 °C with 20 μg/mL αPS or nonimmune IgG or 10 μg/mL rhodopsin antibody 1D4 (all from Millipore) in the presence of 3 μg/mL A5 (AbD Serotec) or β-galactosidase (Abcam) in A5 binding buffer [10 mM Hepes (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2]. After five washes in A5 binding buffer, POS particles were lysed or used in phagocytosis assays. Polarized RPE-J cells were raised in DMEM with 4% (vol/vol) FBS and fed with particles for 2.5 h (5). Bound and internal POS particles were quantified by fluorescence scanning as described previously (23).
Retinal Dissections, Labeling, and Imaging.
All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and reviewed and approved by the Fordham University Institutional Animal Care and Use Committee. WT 129T2/SvEms, Itgb5−/−, and Mfge8−/− mice in the same background characterized previously (9, 10, 24, 25) were housed under cyclic 12 h light/12 h dark conditions, fed ad libitum, and killed by CO2 asphyxiation at 3 to 4 mo of age. Retinas were immediately dissected and incubated with PS detection reagents. Dissection and labeling at 1 h before light onset were performed in complete darkness using Dark Invader Owl infrared goggles and adaptors (B. F. Meyers). αPS or nonimmune IgG (50 μg/mL) in PBS or 488- or FITC-A5 (1:10) in A5 binding buffer were applied for 20 min at 37 °C, followed by five washes in PBS and lysis or fixation with 4% (wt/vol) paraformaldehyde. Fixed, antibody-labeled tissues were further incubated in donkey anti mouse secondary antibody conjugated to Alexa Fluor 488. Fixed retinas were mounted photoreceptor side up in Vectashield (Vector Labs). pSIVA (10 μg/mL) in Hank’s buffered saline was applied and tissues imaged live within 20 min. For double labeling, dissected tissue was first incubated with 5 μg/mL in CellMask Deep Red in Hank’s buffered saline for 20 min at 37 °C before addition of pSIVA and imaging. Live or fixed tissues were placed on glass slides photoreceptor side up. Image stacks were obtained using a Leica TSP5 laser scanning confocal microscopy system.
Immunoblotting.
Samples were lysed in 50 mM Hepes (pH 7.4), 150 mM NaCl, 10% (vol/vol) glycerol, 1.5 mM MgCl2, 1% Triton-X100, 1% protease inhibitor mixture before separation by reducing SDS/PAGE, immunoblotting, and enhanced chemiluminescence detection. Antibodies used recognized A5, RPE65 (both Genetex), rod opsin (26), FITC, β5 integrin (Santa Cruz), GAPDH (Rockland), or PSD95 (Cell Signaling). Band intensities were quantified by densitometry.
Quantification of PS Labeling.
Pixel intensities per area in images of 488-A5–labeled retinas were analyzed using Adobe Photoshop CS3. For each retina six images were averaged. pSIVA-labeled POS tips were measured using ImageJ. For each retina, 100–300 tips were measured.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants EY13295 (to S.C.F.), EY12155 (to J.C.), and GM063915 (to R.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121101109/-/DCSupplemental.
References
- 1.Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaVail MM. Rod outer segment disk shedding in rat retina: Relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- 4.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 5.Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires α(v)β5 integrin for binding but not for internalization. Proc Natl Acad Sci USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert ML, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem. 2002;277:17016–17022. doi: 10.1074/jbc.M107876200. [DOI] [PubMed] [Google Scholar]
- 8.Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003;22:4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nandrot EF, et al. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med. 2004;200:1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nandrot EF, et al. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci USA. 2007;104:12005–12010. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 12.Krahling S, Callahan MK, Williamson P, Schlegel RA. Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 1999;6:183–189. doi: 10.1038/sj.cdd.4400473. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: A view to a kill. Trends Cell Biol. 2006;16:189–197. doi: 10.1016/j.tcb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Martin SJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YE, Chen J, Langen R, Chan JR. Monitoring apoptosis and neuronal degeneration by real-time detection of phosphatidylserine externalization using a polarity-sensitive indicator of viability and apoptosis. Nat Protoc. 2010;5:1396–1405. doi: 10.1038/nprot.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto B, Besharse JC. Light and temperature modulated staining of the rod outer segment distal tips with Lucifer yellow. Invest Ophthalmol Vis Sci. 1985;26:628–635. [PubMed] [Google Scholar]
- 17.Coleman JA, Kwok MC, Molday RS. Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J Biol Chem. 2009;284:32670–32679. doi: 10.1074/jbc.M109.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 19.Duran C, Hartzell HC. Physiological roles and diseases of Tmem16/Anoctamin proteins: Are they all chloride channels? Acta Pharmacol Sin. 2011;32:685–692. doi: 10.1038/aps.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Eijnde SM, et al. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J Cell Sci. 2001;114:3631–3642. doi: 10.1242/jcs.114.20.3631. [DOI] [PubMed] [Google Scholar]
- 21.Wong K, et al. Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc Natl Acad Sci USA. 2010;107:8712–8717. doi: 10.1073/pnas.0910929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molday RS, Hicks D, Molday L. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci. 1987;28:50–61. [PubMed] [Google Scholar]
- 23.Finnemann SC, Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: Apoptotic cells and photoreceptors compete for alphavbeta3 and alphavbeta5 integrins, and protein kinase C regulates alphavbeta5 binding and cytoskeletal linkage. J Exp Med. 1999;190:861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Griffiths M, Wu J, Farese RV, Jr, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in β5-deficient mice. Mol Cell Biol. 2000;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atabai K, et al. Mfge8 is critical for mammary gland remodeling during involution. Mol Biol Cell. 2005;16:5528–5537. doi: 10.1091/mbc.E05-02-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamus G, et al. Anti-rhodopsin monoclonal antibodies of defined specificity: characterization and application. Vision Res. 1991;31:17–31. doi: 10.1016/0042-6989(91)90069-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.