Abstract
The xenoestrogen bisphenol A (BPA) used in the manufacturing of various plastics and resins for food packaging and consumer products has been shown to produce numerous endocrine and developmental effects in rodents. Exposure to low doses of BPA during fetal mammary gland development resulted in significant alterations in the gland’s morphology that varied from subtle ones observed during the exposure period to precancerous and cancerous lesions manifested in adulthood. This study assessed the effects of BPA on fetal mammary gland development in nonhuman primates. Pregnant rhesus monkeys were fed 400 μg of BPA per kg of body weight daily from gestational day 100 to term, which resulted in 0.68 ± 0.312 ng of unconjugated BPA per mL of maternal serum, a level comparable to that found in humans. At birth, the mammary glands of female offspring were removed for morphological analysis. Morphological parameters similar to those shown to be affected in rodents exposed prenatally to BPA were measured in whole-mounted glands; estrogen receptor (ER) α and β expression were assessed in paraffin sections. Student's t tests for equality of means were used to assess differences between exposed and unexposed groups. The density of mammary buds was significantly increased in BPA-exposed monkeys, and the overall development of their mammary gland was more advanced compared with unexposed monkeys. No significant differences were observed in ER expression. Altogether, gestational exposure to the estrogen-mimic BPA altered the developing mammary glands of female nonhuman primates in a comparable manner to that observed in rodents.
Keywords: endocrine disruptor, perinatal exposure, morphogenesis, internal dose, mammogenesis
Bisphenol A (BPA) was first synthesized in 1891 (1). In 1936, after observing that ovariectomized rats injected with the chemical for three consecutive days stopped cycling and remained in an estrous phase, Dodds and Lawson described BPA as a “synthetic estrogenic agent” (2). Two years later, these scientists developed diethylstilbestrol (DES), a much more potent synthetic estrogen and, thus, BPA ceased to be considered for pharmacological use. Decades later, BPA became the building block of polycarbonate plastic and main component of epoxy resins used in many industries. Worldwide, ≈3 million metric tons of BPA are produced per year (3).
According to the Environmental Protection Agency (EPA), humans appear to be primarily exposed through food packaging (Bisphenol-A Action Plan, EPA, 2010, http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/bpa.html); however, it is worth noting that BPA is also used in some cash register receipts, medical devices, and other consumer products. As a result, humans are routinely exposed and most likely throughout life (4). A 2008 study by the Centers for Disease Control and Prevention (CDC) detected BPA in the urine of >90% of Americans sampled (5). To date, few epidemiological studies have examined the effects of BPA on humans. For instance, toddlers exposed to high maternal levels of BPA during pregnancy showed altered behavior (6). Also, urinary BPA levels have been shown to be associated with increased cardiovascular disease and diabetes in adults (7) and sexual dysfunction in men (8).
Pharmacokinetics studies in rodents and nonhuman primates showed that, once ingested, BPA is conjugated in the liver into BPA-glucuronide and BPA-sulfate. Although most BPA in serum is conjugated, unconjugated BPA was measured in these two species (9) and in humans (10). Unlike its conjugated version, unconjugated BPA is able to bind to estrogen receptors and trigger a response. Unconjugated BPA was also measured in some human tissues (e.g., fat and placenta) and fluids including blood, breast milk, and amniotic fluid. Human serum levels of unconjugated BPA were reported at ≈1 ng/mL (10). Importantly, studies have suggested that the pharmacokinetics appear to be similar across these species (9). The doses that produce similar levels in rodents and in nonhuman primates suggest that the exposure levels in humans are higher than expected. Although oral exposure is thought to be the main source of BPA, a recent human study showed that urinary levels of conjugated BPA do not decrease rapidly after fasting, suggesting that nonoral exposures may be significant (11). In fact, evidence of transdermal BPA exposure has recently been documented (12).
BPA binds to estrogen receptors α and β (13) and to membrane-bound estrogen receptors (14) and, thus, affects various functions at all levels of biological complexity in estrogen target organs (15–21). Animal studies showed that BPA exposure at or below the reference dose of 50 μg/kg per day (http://cfpub.epa.gov/ncea/iris/index.cfm?fuseaction=iris.showQuickView&substance_nmbr=0356) displayed a variety of alterations in the prostate, brain, ovary, thyroid, and uterus (reviewed in ref. 22) as well as diminished reproductive capacity in aging females (23). More importantly, BPA exposure during gestation and organogenesis produces effects that alter the structure and function of target organs (24). In particular, these low environmentally relevant developmental exposures caused changes in the tissue organization of the mouse mammary gland observed during fetal development (25), and further changes manifested long after the end of exposure at puberty (18, 26) and in adulthood (18, 26–30). Female mice exposed to BPA during gestation also displayed a heightened response to estrogen compared with unexposed controls (31) and developed intraductal hyperplasias, i.e., a precancerous lesion (28). Furthermore, rats exposed to BPA in utero developed precancerous and cancerous lesions (32). Also, perinatal exposure increased rodents’ sensitivity to chemical carcinogens (33–36). Although a small human study found no association between BPA concentrations and breast cancer incidence (37), exposure to the synthetic estrogen DES increased the risk of breast cancer (38). Additionally, women under 14 years of age who were exposed to the estrogenic pesticide DDT have five times greater risk of developing breast cancer (39), suggesting that mammary glands are organs sensitive to estrogenic chemicals across species.
Rodents are by far the most commonly used animals in toxicological studies. However, because of the potential implications to human health, there is a strong interest in performing BPA exposure studies in nonhuman primates. For instance, rodents are considered a “low-estrogen level” model because the highest level of estradiol in a female rodent is ≈40 pg/mL during proestrous, with very low levels detected after ovulation. In comparison, estradiol levels peak in humans at 400 pg/mL during ovulation and at ≈270 pg/mL in monkeys (40). Additionally, monkeys also resemble humans in that they have a true luteal phase characterized by the secretion of estrogen and progesterone by the corpus luteum (41). Monkeys thus provide a “high-estrogen level model” that closely resembles the human condition. Despite the differences in endogenous estrogen levels, similar effects of BPA were reported in the developing brains of rodents and nonhuman primates (42, 43).
By taking advantage of an ongoing study designed to assess the effect of BPA on ovarian morphogenesis and to determine the circulating levels of unconjugated BPA in female rhesus monkeys exposed orally, we assessed whether gestational exposure to BPA would affect the mammary gland of these animals. A quantitative analysis that compared the morphology of exposed and unexposed female neonatal mammary glands was conducted, and here we show that gestational exposure to BPA affected the development of the nonhuman primate mammary gland in a similar fashion as was observed in mice.
Results
The results presented here are part of a series of studies that aim at assessing the effects of BPA on organogenesis in nonhuman primates by using an oral dose that results in serum levels of unconjugated BPA similar to those observed in humans. The original experiments were designed to study ovarian development. Because of the expenses and ethical considerations of producing these animals, several independent laboratories from across the country were invited to analyze various tissues obtained from the Macaca mulatta female offspring.
Ultrasound screens were performed to confirm pregnancy and normal development. Only those animals carrying a female offspring were used (44). The duration of gestation in these monkeys is ≈160–165 d. Mammary glands were collected from newborn animals.
A previous pharmacokinetics study in nonpregnant female rhesus monkeys and CD-1 mice (9) showed that an oral dose of 400 μg of BPA per kg of body weight per day was necessary to reach circulating levels of unconjugated, biologically active, BPA similar to levels measured in humans (≈1 ng/mL). The average unconjugated BPA in serum was 0.5 ng/mL in monkeys and 0.7 ng/mL in mice. It is important to also measure the conjugate levels because they unequivocally represent BPA that was metabolized in the organism, ruling out the possibility of spurious contamination. Additionally, the pharmacokinetics of BPA in the rhesus monkey exposed orally is known (9) and, thus, these measurements provide a comparison with previous studies.
Maternal Serum Concentrations of BPA.
Pregnant monkeys were fed a small piece of fruit containing 400 μg of deuterated BPA per kg of body weight; the daily dose was administered from gestational day 100–165, which roughly corresponds to the third trimester of human gestation. Control animals received vehicle (ethanol) only, also delivered in a small piece of fruit. Maternal serum samples from exposed and control animals were taken near the time of delivery and were analyzed for conjugated and unconjugated BPA. Maternal serum levels were not available for one of the control and one of the BPA-treated animals.
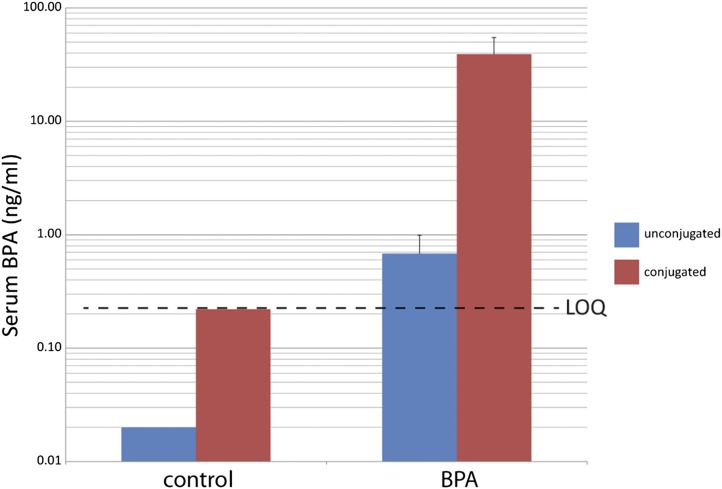
In the BPA-treated mothers, the average level of unconjugated BPA was 0.68 ± 0.312 ng/mL (range: 0.22–1.88 ng/mL), and the average level of conjugated BPA was 39.09 ± 15.71 ng/mL (range: 11.42–94.82 ng/mL) (Fig. 1). Conjugated and unconjugated BPA were quantifiable in all of the exposed animals analyzed. In the control mothers, the levels of conjugated and unconjugated BPA were below the level of quantification in three of four animals and four of four animals, respectively. Only one control mother had a quantifiable level of conjugated BPA (0.814 ng/mL); however, this level represents a mere 2% of the mean value found in exposed animals. The average ratio of conjugated to unconjugated BPA was 90.70 (range: 17.37–244.56 ng/mL) in the BPA-treated mothers, which is comparable to that found in orally exposed nonpregnant monkeys (9).
Fig. 1.
Mean conjugated and unconjugated BPA concentrations in maternal serum. The values of the controls are not given with SE bars because amounts of BPA measured were below the level of quantification (LOQ) of this assay (indicated by the dashed line).
BPA Alters the Development of the Rhesus Monkey Mammary Gland.
Nonhuman primate mammary gland morphology.
The nonhuman primate gland contains 5–7 lactiferous ducts converging to the nipple (Fig. 2); each of these ducts drain a lobe (suggesting 5–7 lobes per mammary gland) formed by multiple ductal-lobular units. This structure closely resembles the human breast, which consists of 15–25 lobes, each drained by one lactiferous duct, and both are known as multilobular glands. This arrangement contrasts with that of the mouse mammary gland that has a single lactiferous duct that branches and develops as a ductal tree and is considered a unilobular gland.
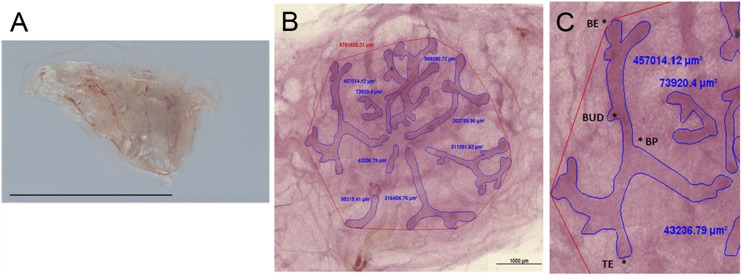
Fig. 2.
Morphometric parameters used for whole-mount analysis. (A) A newborn mammary gland dissected for whole mount analysis. (B) The quantification of the ductal area; the epithelial ducts are outlined in blue, and their respective area is indicated by the abutting blue figures. The red polygon indicates area subtended by the ductal structures (total mammary gland area). (C) The four main structural parameters measured. BE, bifurcating end; BP, branching point; BUD, bud; TE, terminal end. (Scale bars: A, 1 cm; B, 1 mm.)
Mammary gland size and quantification of morphometric structures.
The mammary gland architecture and developmental patterns are best visualized by using the technique known as whole-mounting, in which the entire tissue is spread on a glass and stained while preserving the 3D structures and allowing for quantitative measurements. Using comparable morphometric parameters to those applied to the analysis of rodent fetal mammary glands, we measured the total mammary gland area, ductal area, and number of buds, terminal ends, bifurcating ends, and branching points (Fig. 2).
We also calculated the relative abundance of various epithelial structures and the density of ductal structures within the mammary gland and the relative abundance of these structures per lobe. These measurements allowed us to directly compare previously published data on the mouse’s unilobular with the monkey’s multilobular mammary gland.
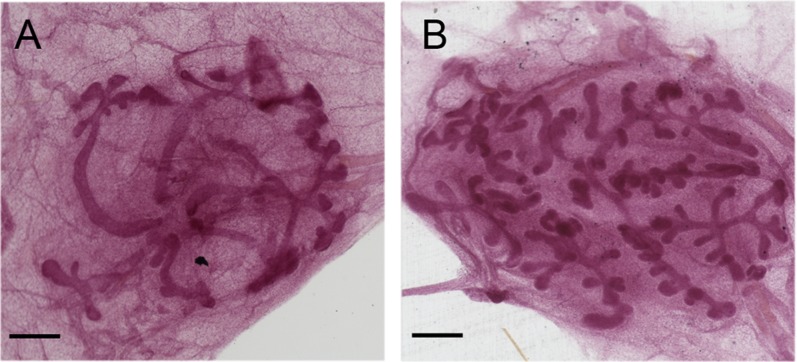
Table 1 summarizes the measurements for the control and BPA-treated animals. The mammary glands of the BPA-exposed animals were more developed for every parameter assessed, including the number of buds, terminal ends, branching points, bifurcating ends, as well as the total mammary gland area, the ductal area, and the number of ductal units (namely, the number of lactiferous ducts that define the number of lobes) compared with controls. Some endpoints showed striking differences between these two groups. For instance, there was a 1.46-fold increase in total area, a 2.02-fold increase in the number of buds, and a 1.54-fold increase in the number of buds and terminal ends combined in the BPA group compared with controls. These differences, however, were not statistically significant, most likely due to the low number of samples (five controls and four BPA). Fig. 3 shows two representative images of whole-mounted neonatal mammary glands in which the BPA-exposed gland appears clearly more complex overall and contains a higher number of buds than the control mammary gland.
Table 1.
Incidence of various epithelial structures
| Control (n = 5) |
BPA (n = 4) |
|||
| Morphometric parameter | Mean ± SEM | (Range) | Mean ± SEM | (Range) |
| Total area, mm2 | 7.80 ± 1.73 | (1.90–12.17) | 11.44 ± 5.27 | (3.45–26.96) |
| Ductal area, mm2 | 2.04 ± 0.45 | (0.58–3.44) | 2.80 ± 0.71 | (1.19–4.68) |
| No. of ductal units | 6.00 ± 1.00 | (3–9) | 6.75 ± 0.75 | (5–8) |
| No. of ductal units/total area | 0.92 ± 0.18 | (0.51–1.58) | 0.94 ± 0.31 | (0.30–1.74) |
| Buds | 14.20 ± 5.50 | (5–35) | 28.75 ± 6.01 | (16–45) |
| Terminal ends | 20.20 ± 4.00 | (8–30) | 24.50 ± 6.22 | (13–40) |
| Branching points | 13.80 ± 2.95 | (5–21) | 17.75 ± 5.58 | (7–32) |
| Bifurcating ends | 5.60 ± 0.87 | (3–8) | 6.00 ± 2.27 | (2–12) |
Data are expressed as mean ± SEM.
Fig. 3.
Comparison between whole mounts of neonatal mammary glands of control and BPA dosed animals. (A) Control. (B) BPA dosed. (Scale bars: 500 μm.)
Density of epithelial structures.
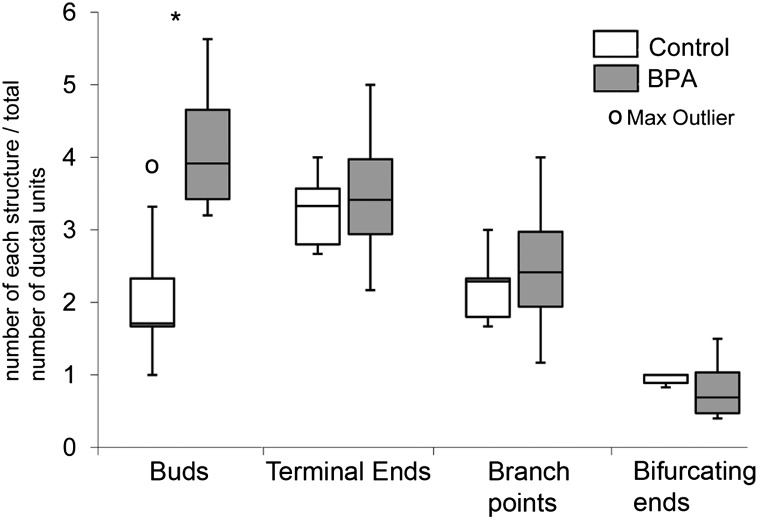
The mammary gland density was defined as the number of buds, terminal ends, branching points, and bifurcating ends, normalized for the number of ductal units present. These results are shown in Fig. 4. The BPA group had significantly more buds per ductal unit than the control group (P = 0.027).
Fig. 4.
Number of morphological features normalized by the number of ductal units. Data in figures presented as stem-and-leaf plots were created in Microsoft Excel. In these graphs, the central line marks the 50th percentile (median), the outer bounds of the box represent the 25th and 75th percentiles, and the stems represent 1.5 × (75th − 25th percentiles). Asterisk denotes significance (P = 0.027).
Expression of steroid hormone receptors and markers of epithelial development.
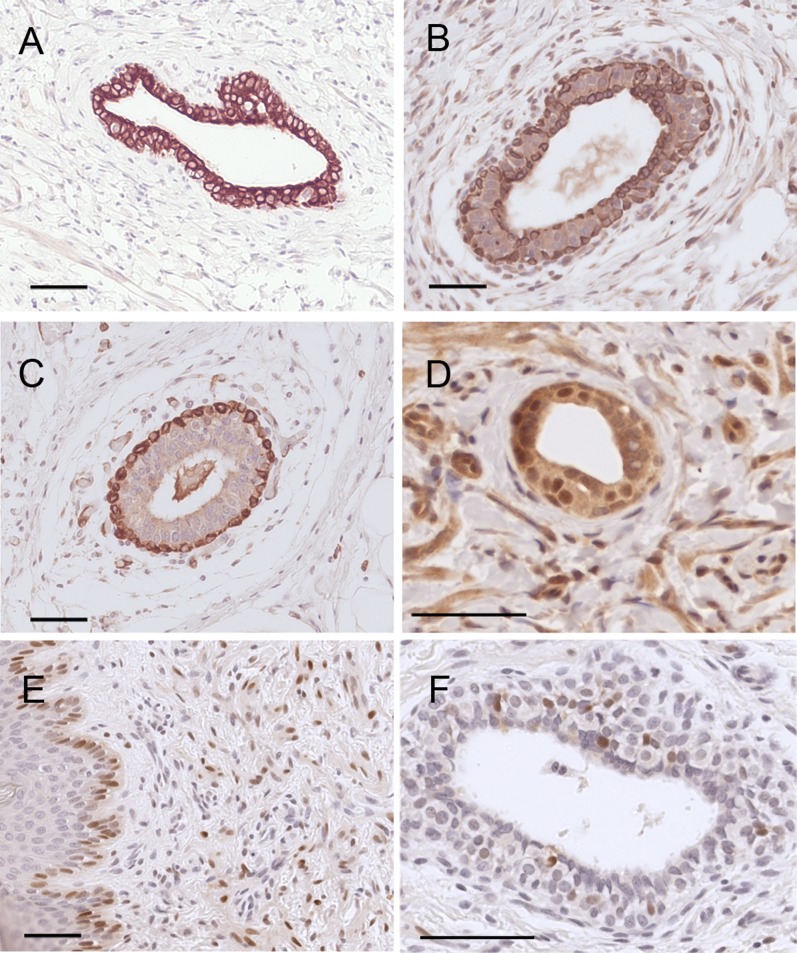
In rodents, estrogen receptors first appear in the stroma surrounding the mammary gland; epithelial localization is first observed at embryonic day 18 (25), whereas epithelial localization in the human mammary gland is first observed around the 30th week in a few cells (45). We observed that both ER α and β are expressed in epithelial cells of the rhesus monkey at birth. As in our previous rodent study, no differences in both the expression of ER α and β were observed between BPA-treated animals and controls. In mice, the differentiation of the myoepithelial cell layer occurs mostly postnatally (46), whereas in humans, the differentiation occurs mostly prenatally (45). Smooth muscle actin (SMA) starts to be expressed by week 22, and differentiation of basal and luminal cells expressing keratin 18 (K18) was first detected at the same time (45). We observed a strong basal expression of SMA and strong K18 expression in luminal cells, whereas keratin 14 (K14), a marker for myoepithelial cells, is expressed both basally and luminally, as reported in mice of peripubertal age (47); complete cytodifferentiation is achieved comparatively later in mice than in humans, and the data here show that the timing of cell differentiation is comparable in humans and macaques (Fig. 5).
Fig. 5.
Immunolocalization of markers of epithelial differentiation. (A) Keratin 18 (luminal cell marker). (B) Keratin 14 (myoepithelial cell marker). (C) SMA (myoepithelial cell marker). (D) ERβ. (E) ERα (epidermal epithelium). (F) ERα (glandular epithelium). (Scale bars: 50 μm.)
Discussion
The aim of this study was to determine whether maternal circulating levels of unconjugated BPA similar to those found in human serum affected the development of the mammary glands of female rhesus monkey offspring. Because very little is known about the fetal and neonatal development of the mammary gland in rhesus monkeys, we explored the presence of markers of epithelial differentiation and hormone receptors and found that the neonate monkey mammary gland is comparable to the human in the last trimester and to the neonatal mouse. Our results show that the mammary glands of nonhuman primates are sensitive to BPA exposure during fetal development, and this sensitivity is manifested as increased complexity of the ductal system compared with unexposed animals. This study is particularly valuable because it provides mammary gland data regarding fetal exposure to BPA in nonhuman primates.
BPA exposure in mothers resulted in detectable serum levels of unconjugated BPA of 0.68 ± 0.312 ng/mL. These data are important for the following reasons. First, these levels of unconjugated BPA are similar to those measured in humans, i.e., ≈1 ng/mL (10), making the results of this study very relevant to human exposure. Second, the oral dose given to the mother is 8 times higher than the reference dose of 50 μg/kg per day, suggesting that humans and the monkeys in this study are routinely exposed to levels above the “safe dose.” And third, similar levels of unconjugated BPA have been shown to trigger biological effects in vitro and in vivo (48), some of which are mediated by the classical ERs as demonstrated in experiments comparing wild-type and receptor null mice (21). Furthermore, these levels were within the range observed in nonpregnant female rhesus monkeys and nongestating CD-1 mice, both of which had an average 24-h unconjugated BPA concentration of ≈0.5 ng/mL (9).
We previously showed that fetal exposure to BPA affected mammary morphology in a mouse model; these alterations manifested during the period of exposure and throughout postnatal life (18, 25, 26, 28). Although the mammary glands of mice exposed s.c. to 250 ng of BPA per kg of body weight per day during fetal development showed increased epithelial area, ductal extension, and branching points at gestational day 18 (25), monkeys born to mothers orally exposed to 400 μg of BPA per kg of body weight per day had a significant increase in the number of buds per ductal unit. Because buds are incipient branches, these data point to an increased epithelial area and branching in the nonhuman primate gland similar to those observed in BPA-exposed mice. Because the morphological alterations observed at birth in the mammary glands of rodents and nonhuman primates are comparable, we conclude that BPA exposure during gestation can be detrimental to mammary gland development across species. To determine whether the mammary glands from BPA-exposed nonhuman primates follow similar altered patterns as those displayed by rodents at puberty and adulthood, such as precancerous and cancerous lesions, further studies are required.
From what is known about the role of endogenous hormones in the development of the mammary gland, and from previous studies in rodents showing that the effects of fetal estrogens typically manifest after puberty (49, 50), we also conclude that BPA affects several developmental parameters of the mammary gland of rhesus monkeys, including some that are relevant to breast cancer risk in humans, such as epithelial density (51). From the similarity of mammary gland alterations observed perinatally in mice and monkeys as a result of BPA exposure, we infer that BPA will have comparable effects throughout the lifespan of nonhuman primates. Our current studies reinforce the concept that the rodent mammary gland is a reliable model to study developmental exposures to chemicals with estrogenic activity.
Materials and Methods
Animals.
Pregnant adult female rhesus macaques (M. mulatta) were housed at the California National Primate Research Center. All procedures for maintenance and handling of the animals were reviewed and approved in advance by the Institutional Animal Use and Care Administrative Advisory Committee at the University of California at Davis. Animals were housed individually in cages made of stainless steel with a light cycle at 0600–1800 hours and at a temperature maintained at 25–27 °C. Animals were fed a diet of Purina Monkey Chow and provided water ad libitum. Water was delivered to each cage via rigid PVC pipes and a “lixit” device. Additionally, seasonal produce, seeds, and cereal were offered as supplements for environmental enrichment. Gravid rhesus monkeys were sonographically screened early in gestation to confirm normal development. To identify the sex of fetuses, a PCR analysis targeting the Y chromosome was performed in maternal serum by 40 d of gestation (44); only female fetuses were used in this study.
Oral Administration of BPA.
Deuterated BPA (CDN Isotopes) was prepared as described (9). Briefly, a daily dose of 400 μg of BPA per kg of body weight or vehicle (100 μL of ethanol) was administered per os from gestational day 100–165. All animals were trained to accept small pieces of fruit before beginning the BPA treatment period. Fruit was cut small enough that animals would take the fruit in one bite and would not try to pull it into smaller pieces before consuming. Preferences of each animal were noted. The BPA dose for each animal was calculated based on body weight at weekly intervals. The BPA solution (100–150 μL) was measured with a Hamilton 200-μL syringe and delivered into the center of fruit pieces, such as grapes, banana slices, dates or dried apricots, so that the animal could grasp the fruit and place it in her mouth without touching the BPA. The BPA doses were prepared by laboratory personnel and kept in a secure place before dosing and were then administered to each animal for immediate consumption, which was confirmed. All offspring were delivered naturally via vaginal birth within 1–2 d of full-term gestation (165 d). Tissues were collected at necropsy within 1–3 d after birth. Maternal serum samples were taken near the time of spontaneous birth, ≈4 h after oral dosing. These samples were analyzed for conjugated and unconjugated BPA on a fee-for-service basis at the University of Missouri as described (9). The limit of quantification for this method is reported as 0.2 ng/mL.
Sample Processing and Whole-Mount Staining.
The neonatal mammary glands were surgically removed; one mammary gland of each neonate was whole-mounted onto a slide and the other was processed for histological analysis. Both were fixed overnight in 10% phosphate-buffered formalin. The whole mounts were then washed twice with PBS, dehydrated in an ethanol series, cleared in toluene, and rehydrated before staining with carmine alum (52). After staining, the whole mounts were dehydrated in an ethanol series, cleared in xylene, and mounted with Permount (Fisher Scientific). The fixed tissue was processed, paraffin-embedded, and sectioned by following procedures described (25).
Morphometric Analysis.
Two glands were not analyzed because one had an abnormal polycystic phenotype and the other was from a stillborn animal with many dilated blood vessels that hindered the analysis. Whole-mounted mammary glands were used to quantify area, budding, and branching. A total of five control and four BPA samples were analyzed. First, images of whole-mounted mammary glands were captured by using a Zeiss Stemi 2000-C dissection microscope with a Zeiss AxioCam HRc digital camera (Carl Zeiss). These images were analyzed by using the Zeiss AxioVision program version 4.4. Mammary glands were coded and analyzed in a treatment-blind manner. Once analysis was complete but before the code was revealed, the results were used to sort the glands into two distinct morphological categories. Once the code was broken, it was evident that all glands had been sorted correctly by treatment. For each sample, the total mammary gland area was quantified by drawing tangent lines from the outermost point of one epithelial structure and moving clockwise to the next furthest point of that structure, or a new structure, that could be reached without excluding any of the epithelium (Fig. 2). The ductal area was quantified by outlining the epithelial ducts of each gland. The four main structural parameters measured in each gland were: number of terminal ends, buds, bifurcating ends, and branching points (Fig. 2). Terminal ends included the end of a duct and newly developed ducts branching from a main duct that were >200 μm in length; buds were defined as branches that were <200 μm in length. If one point of a ductal structure clearly split into two separate ducts, it was deemed to be a branching point, whereas bifurcating ends, where an invagination and thus a soon-to-be-branching point was present, were counted as a terminal end. The number of buds, terminal ends, branching points, and bifurcating ends were analyzed. In addition, the relative abundance of these structures was calculated via a correction for the number of ductal units (each unit corresponds to a lobe) present in the mammary gland. This measurement, referred to as density of epithelial structures allowed a direct comparison between the unilobular mouse mammary gland with the multilobar rhesus monkey mammary gland.
Immunohistochemical Analysis.
Immunohistochemistry was performed for smooth muscle actin (SMA) (Abcam), keratin 14 (Thermo), keratin 18 (Sigma), ERα (Novocastra), and ERβ (Thermo). Sections were treated with xylene to remove paraffin and were rehydrated. An antigen-retrieval method using microwave pretreatment and 0.01 M sodium citrate buffer (pH 6) was performed. Nonspecific binding was blocked with normal goat serum before overnight incubation in a humidity chamber with primary antibody. Secondary antibody incubation was followed by visualization using the streptavidin–peroxidase complex, with diaminobenzidine tetrahydrochloride (Sigma-Aldrich) as the chromogen. Counterstaining was performed with Mayer’s hematoxylin. Images were captured by using a Zeiss Axioscope 2 plus microscope (Carl Zeiss MicroImaging). Primary and secondary antibody concentrations are as follows: SMA, 1:100/1:200; keratin 14, 1:100/1:200; keratin 18, 1:500/1:500; ERα, 1:50/1:100; ERβ, 1:50/1:100.
Statistics.
SPSS software package 15.0 (SPSS) was used for all statistical analyses. Significance between groups was determined by using Student's t tests for equality of means. For all statistical tests, results were considered significant at P < 0.05. All results are presented as mean ± SEM.
Acknowledgments
We thank David Damassa for assistance with statistical analysis and Cheryl Schaeberle and Laura Vandenberg for their editorial assistance. This work was supported by the Passport Foundation, National Center for Research Resources Grant RR00169 and National Institute on Environmental Health Sciences Grants ES08314 and ES016770.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Dianin AP. Products of ketone condensation with phenols (translated from Russian) 1891;23:488–506. [Google Scholar]
- 2.Dodds EC, Lawson W. Synthetic estrogenic agents without the phenanthrene nucleus. Nature. 1936;137:996. [Google Scholar]
- 3.Burridge E. Chemical profile: Bisphenol A. ICIS Chemical Business. 2008;274:48. [Google Scholar]
- 4.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun JM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang IA, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. J Am Med Assoc. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 8.Li DK, et al. Relationship between urine bisphenol-A level and declining male sexual function. J Androl. 2010;31:500–506. doi: 10.2164/jandrol.110.010413. [DOI] [PubMed] [Google Scholar]
- 9.Taylor JA, et al. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: Relevance for human exposure. Environ Health Perspect. 2011;119:422–430. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandenberg LN, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect. 2009;117:784–789. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalko D, Jacques C, Duplan H, Bruel S, Perdu E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere. 2011;82:424–430. doi: 10.1016/j.chemosphere.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 13.Kuiper GGJM, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 14.Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114:106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: An estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 16.Shioda T, et al. Importance of dosage standardization for interpreting transcriptomal signature profiles: Evidence from studies of xenoestrogens. Proc Natl Acad Sci USA. 2006;103:12033–12038. doi: 10.1073/pnas.0605341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howdeshell KL, et al. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect. 2003;111:1180–1187. doi: 10.1289/ehp.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markey CM, Luque EH, Munoz De Toro MM, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65:1215–1223. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 19.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung E, Genco MC, Megrelis L, Ruderman JV. Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proc Natl Acad Sci USA. 2011;108:17732–17737. doi: 10.1073/pnas.1115187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soriano S, et al. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: Role of estrogen receptor β. PLoS ONE. 2012;7:e31109. doi: 10.1371/journal.pone.0031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter CA, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabaton NJ, et al. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ Health Perspect. 2011;119:547–552. doi: 10.1289/ehp.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.vom Saal FS, et al. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenberg LN, et al. Exposure to the xenoestrogen bisphenol-A alters development of the fetal mammary gland. Endocrinology. 2007;148:116–127. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz-de-Toro MM, et al. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146:4138–4147. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: Exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev. 2003;5:1–9. doi: 10.1046/j.1525-142x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 28.Vandenberg LN, et al. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol. 2008;26:210–219. doi: 10.1016/j.reprotox.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fenton SE. Endocrine-disrupting compounds and mammary gland development: Early exposure and later life consequences. Endocrinology. 2006;147(Suppl):S18–S24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 30.Ayyanan A, et al. Perinatal exposure to bisphenol a increases adult mammary gland progesterone response and cell number. Mol Endocrinol. 2011;25:1915–1923. doi: 10.1210/me.2011-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wadia PR, et al. Perinatal bisphenol A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains. Environ Health Perspect. 2007;115:592–598. doi: 10.1289/ehp.9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol. 2007;23:383–390. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durando M, et al. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect. 2007;115:80–86. doi: 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins S, et al. Oral exposure to bisphenol a increases dimethylbenzanthracene-induced mammary cancer in rats. Environ Health Perspect. 2009;117:910–915. doi: 10.1289/ehp.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Betancourt AM, Eltoum IA, Desmond RA, Russo J, Lamartiniere CA. In utero exposure to bisphenol A shifts the window of susceptibility for mammary carcinogenesis in the rat. Environ Health Perspect. 2010;118:1614–1619. doi: 10.1289/ehp.1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moral R, et al. Effect of prenatal exposure to the endocrine disruptor bisphenol A on mammary gland morphology and gene expression signature. J Endocrinol. 2008;196:101–112. doi: 10.1677/JOE-07-0056. [DOI] [PubMed] [Google Scholar]
- 37.Yang M, Ryu JH, Jeon R, Kang D, Yoo KY. Effects of bisphenol A on breast cancer and its risk factors. Arch Toxicol. 2009;83:281–285. doi: 10.1007/s00204-008-0364-0. [DOI] [PubMed] [Google Scholar]
- 38.Palmer JR, et al. Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1509–1514. doi: 10.1158/1055-9965.EPI-06-0109. [DOI] [PubMed] [Google Scholar]
- 39.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: New data on the significance of age at exposure. Environ Health Perspect. 2007;115:1406–1414. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monfort SL, et al. Comparison of serum estradiol to urinary estrone conjugates in the rhesus macaque (Macaca mulatta) Biol Reprod. 1987;37:832–837. doi: 10.1095/biolreprod37.4.832. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz NB. In: Endocrinology: Basic and Clinical Principles. Melmed S, Conn PM, editors. Totowa, NJ: Humana; 2010. pp. 367–374. [Google Scholar]
- 42.MacLusky NJ, Hajszan T, Leranth C. The environmental estrogen bisphenol a inhibits estradiol-induced hippocampal synaptogenesis. Environ Health Perspect. 2005;113:675–679. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci USA. 2008;105:14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jimenez DF, Tarantal AF. Fetal gender determination in early first trimester pregnancies of rhesus monkeys (Macaca mulatta) by fluorescent PCR analysis of maternal serum. J Med Primatol. 2003;32:315–319. doi: 10.1046/j.1600-0684.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 45.Friedrichs N, Steiner S, Buettner R, Knoepfle G. Immunohistochemical expression patterns of AP2alpha and AP2gamma in the developing fetal human breast. Histopathology. 2007;51:814–823. doi: 10.1111/j.1365-2559.2007.02887.x. [DOI] [PubMed] [Google Scholar]
- 46.Moumen M, et al. The mammary myoepithelial cell. Int J Dev Biol. 2011;55:763–771. doi: 10.1387/ijdb.113385mm. [DOI] [PubMed] [Google Scholar]
- 47.Sun P, Yuan Y, Li A, Li B, Dai X. Cytokeratin expression during mouse embryonic and early postnatal mammary gland development. Histochem Cell Biol. 2010;133:213–221. doi: 10.1007/s00418-009-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prins GS, Ye SH, Birch L, Ho SM, Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reprod Toxicol. 2011;31:1–9. doi: 10.1016/j.reprotox.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soto AM, Vandenberg LN, Maffini MV, Sonnenschein C. Does breast cancer start in the womb? Basic Clin Pharmacol Toxicol. 2008;102:125–133. doi: 10.1111/j.1742-7843.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamartiniere CA, Jenkins S, Betancourt AM, Wang J, Russo J. Exposure to the endocrine disruptor Bisphenol A alters susceptibility for mammary cancer. Horm Mol Biol Clin Investig. 2011;5:45–52. doi: 10.1515/HMBCI.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li T, et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 52.Maffini MV, Soto AM, Calabro JM, Ucci AA, Sonnenschein C. The stroma as a crucial target in rat mammary gland carcinogenesis. J Cell Sci. 2004;117:1495–1502. doi: 10.1242/jcs.01000. [DOI] [PubMed] [Google Scholar]