Abstract
Teleost fishes comprise approximately half of all living vertebrates. The extreme range of diversity in teleosts is remarkable, especially, extensive morphological variation in their jaws and dentition. Some of the most unusual dentitions are found among members of the highly derived teleost order Tetraodontiformes, which includes triggerfishes, boxfishes, ocean sunfishes, and pufferfishes. Adult pufferfishes (Tetraodontidae) exhibit a distinctive parrot-like beaked jaw, forming a cutting edge, unlike in any other group of teleosts. Here we show that despite novelty in the structure and development of this “beak,” it is initiated by formation of separate first-generation teeth that line the embryonic pufferfish jaw, with timing of development and gene expression patterns conserved from the last common ancestor of osteichthyans. Most of these first-generation larval teeth are lost in development. Continuous tooth replacement proceeds in only four parasymphyseal teeth, as sequentially stacked, multigenerational, jaw-length dentine bands, before development of the functional beak. These data suggest that dental novelties, such as the pufferfish beak, can develop later in ontogeny through modified continuous tooth addition and replacement. We conclude that even highly derived morphological structures like the pufferfish beak form via a conserved developmental bauplan capable of modification during ontogeny by subtle respecification of the developmental module.
Keywords: evolutionary developmental biology, morphological novelty, tooth development, replacement dentition, phenotypic diversity
Vertebrates offer an impressive range of morphological diversity, especially in dentitions. Morphological diversity in teleost fishes is unparalleled among vertebrates, exemplified by the bizarre forms assigned to the order Tetraodontiformes. Some members of this teleost order, the Gymnodontes, are known for their unusual jaws, superficially resembling the beak of a parrot (Fig. 1) (1). The members of one family of Gymnodontes, the pufferfishes (Tetraodontidae), possess a unique oral dentition of four teeth, two in the upper jaws and two in the lower jaws, extending laterally from the midline (Fig. 1 C and D). These teeth form paired opposing beak-shaped toothplates that can crush or slice prey items, different from most other teleost dentitions (Fig. 2 H and N) (2, 3). The ontogenetic and developmental mechanisms that form the unique tetraodontid dentition have been little studied; only a brief mention of larval teeth in pufferfishes (4) and descriptions of the adult dentition (5) have been published to date.
Fig. 1.
Adult freshwater pufferfish, (A) Monotrete abei male guarding eggs on the substrate. (B) Lateral view of the M. abei head showing the mouth with a partly exposed beak; the large lips cover most of the beak. (C) Lateral view of a typical pufferfish skull of tetraodon lineatus (skeletal preparation; scale bar: 2 cm) and (D) frontal view showing the extensive beak tissue fused with the bone of the articulated jaws. (Scale bar: 1 cm.)
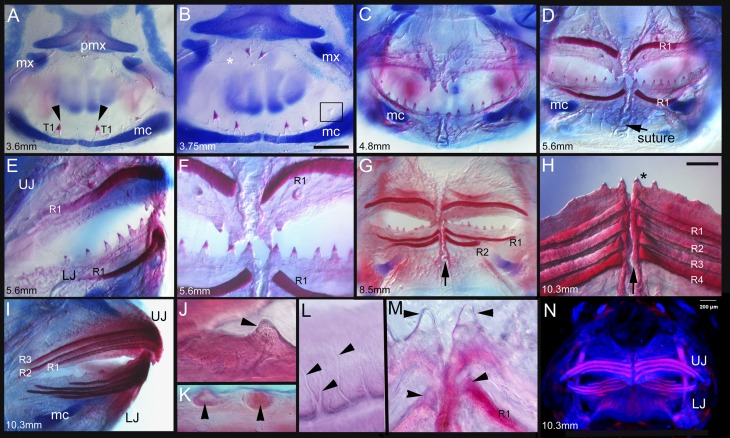
Fig. 2.
Developmental sequence from a conserved pattern of initial teeth to replacement dentine bands during the formation of the unique pufferfish beak. (A–D, F–H, M, and N) Frontal views (into the mouth) of the developing dentition (3.6 mm NL to 10.3 mm SL) of a pufferfish (Monotrete suvattii). (E, I, and L) Lateral views. (K) Medial view. A–M show specimens cleared and double- stained with alizarin red (staining calcium-rich tissues, e.g., bone and dentine) and alcian blue (staining mucopolysaccharides in cartilage). The first-generation dentition in pufferfish is composed of individual teeth with acrodin (enameloid) caps identical to those of other actinopterygians (A–H). From the youngest stage with 2 teeth in the lower jaw (LJ) (A, black arrowheads, T1), separate teeth are added along the jaws [B, 4 teeth plus a developing tooth (black box) in the LJ, 2 teeth plus a developing tooth in the upper jaw (UJ), denoted by an asterisk] with up to 14 (C) to 16 teeth (D) in the LJ and up to 6 teeth in the UJ (C and D). First-generation teeth and superficial intertooth dentine are retained until worn (H, asterisk). Strongly mineralized jaw length bands of dentine form from individual replacement teeth, stacked below the first-generation teeth in the UJ and LJ, increasing in number with size from one band in D to up to four bands in H and N, the largest stage that we studied (10.3 mm SL). These stacks of dentine bands (R1–R4) form as multigeneration replacement teeth of only the four most medial teeth (D–H). H shows a frontal view of the lower jaw beak, showing the four generations of replacement bands (R1–R4) of stacked dentine that will form the adult beak. The asterisk denotes the retained first-generation teeth at the beak surface; black arrows denote the symphysis between the left and right halves of the LJ (D, G, and H). Dentine tubules from living cells are present in first-generation teeth (J) and in replacement dentine bands (L, black arrowheads). (N) Optical projection tomography image of the juvenile M. suvattii beak in frontal view, showing the pink fluorescent bands of stacks of replacement dentine bands forming the beak. (Scale bar: 200 μm.) mc, Meckel’s cartilage; mx, maxillary; pmx, premaxillary. Lengths are provided as either NL or standard length SL in mm of embryonic and juvenile M. suvattii (A, 3.6 mm NL; B, 3.75 mm NL; C, 4.8 mm SL; D–F, 5.6 mm SL; G, 8.5 mm SL; H, I, and N, 10.3 mm SL; J–M, 5.6 mm SL).
However, when dental development is examined in detail, this adult morphological novelty shows greater similarity and structural conservation in initial development to that of osteichthyans than was previously appreciated (Fig. 2). The pufferfishes are a morphologically derived group of teleosts with numerous reductive characteristics, including a lack of pelvic fins, ribs, and lower pharyngeal jaws, a reduced number of vertebrae, and absence of various cranial bones (6–9). Moreover, members of the family Tetraodontidae possess some of the most concise vertebrate genomes known (10–13). In the present study, we focused on the southern Asian freshwater pufferfish genus Monotrete and examined embryos of several closely related species (M. abei, M. cochinchinensis, M. leiurus, and M. suvattii) (Fig. 1). Our initial hypothesis was that the pufferfish “beak” (Fig. 2 I, N) represents a unique dental structure from an unknown developmental genetic bauplan. We expected this genetic bauplan to be unique not only among teleosts, but also among vertebrates.
To test this hypothesis, we investigated how this highly derived beak-like pufferfish dentition forms developmentally. Specifically, we examined how the spatial and temporal pattern of gene expression unfolds, as related to tooth initiation and development, during sequential ontogenetic stages of the embryonic and hatchling dentitions (Fig. 2). Gene expression associated with developmental phases during formation of the pufferfish dentition has received little attention so far (14). Thus, we have taken advantage of this unique dentition to address more general questions regarding genetic control related to the developmental origins of teleost morphological diversity and the evolution of these patterns. Here we document the morphogenetic progression from initial stages of formation of the first-generation dentition through to transitional stages of beak initiation.
Results
We examined the expression of a subset of highly conserved genes (expressed similarly across many taxa) known to be active during all similarly studied stages of tooth development in several teleosts, reptiles, and mammals (15–17) for comparison. We chose the genes bmp4, pax9, pitx2, and shh for this study because they include some of the most studied gene representatives across taxa for tooth development from fish to mammals, allowing for generalizations across vertebrates (15, 16, 18–24). For this study, we generated riboprobes from recently developed genomic resources for closely related pufferfishes, Tetraodon (http://www.genoscope.cns.fr/externe/tetranew/) and Fugu (http://www.fugu-sg.org/), to examine temporal and spatial expression patterns by whole-mount in situ hybridization (Figs. 3 and 4) and monitor how gene expression changed during ontogenetic formation of the beak. We aimed to test whether developmental mechanisms common to other teleost dentitions were present in the pufferfish dentition.
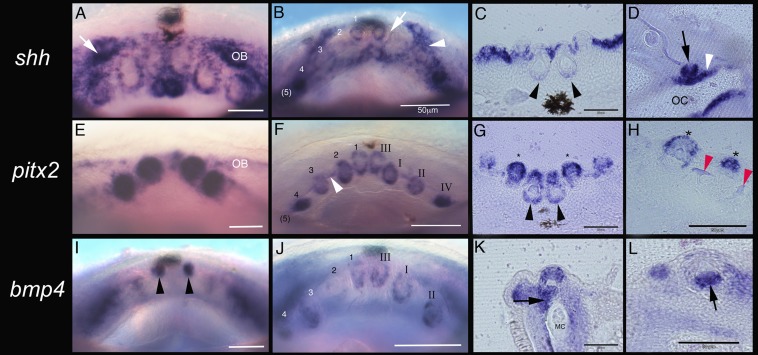
Fig. 3.
A conserved gene expression program initiates the first-generation teeth in pufferfish. (A–H) shh and pitx2 are coexpressed in the developing tooth bud epithelium of the first- generation dentition in M. abei embryonic lower jaws. (A, B, E, F, I, and J) Dorsal view of the embryonic lower jaw. Tooth position number along the jaw (B) does not reflect the time order in which the teeth develop; position 2 is the first tooth germ initiated (see the Roman numerals in the right quadrant of F), then 3, then 1 as shown (nos. 2, 3, 1, 4, and 5 in the left quadrant of F). This represents an osteichthyan-specific pattern for first tooth initiation order (the 5 in parentheses is not in the focal plane). Superficial tooth buds (2–5 in B and F) expressing shh (black arrow in D) and pitx2 (asterisks in G and H) are never replaced. OB, extended odontogenic band (white arrow in A and white arrowhead in B). The lack of shh around later-stage first tooth development (white arrow in B) marks the loss of surface shh expression in the odontogenic band as shh expression transfers to tooth morphogenesis (C, black arrowheads). Note the deep invagination of the two parasymphyseal teeth in C and G (black arrowheads); only these will be replaced with dentine bands to become the beak. (H) Intertooth dentine (red arrowheads) occurs between each first-generation tooth. Dentine forms and joins all teeth together as an extension of durable dentine, an attachment mechanism. Expression of pitx2 (white arrowhead in F) might define the initial region for intertooth dentine secretion associated with the remaining OB. (I–L) bmp4 expression present in both epithelium and mesenchyme of the developing first-generation tooth (black arrowhead in I). bmp4 expression is extended labially into deep mesenchyme of the first tooth (parasymphyseal) positions (black arrow in K) associated with continued invagination of the parasymphyseal teeth and interdentine production as opposed to the superficial mesenchymal expression (black arrow in L) in teeth 2–5 that are not replaced, and are not related to the beak formation. OC, oral cavity; OB, odontogenic band; MC, Meckel’s cartilage. (Scale bars: 20 μm in A, C, E, G, and I; 50 μm in B, F, H, J, K, and L.)
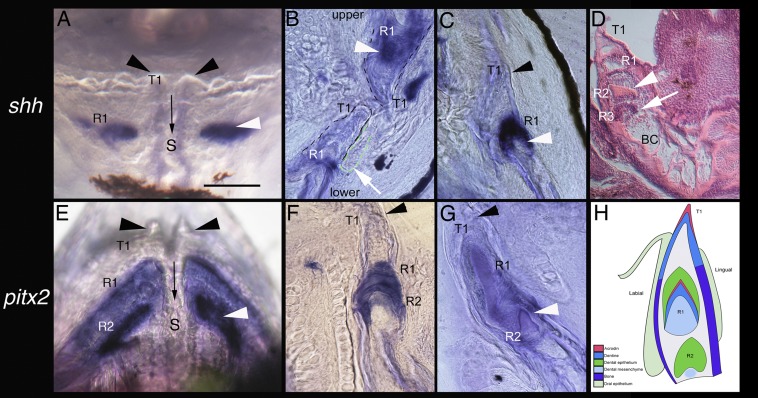
Fig. 4.
The unique pufferfish beak develops as a result of replacement from only the four parasymphyseal teeth. (A and E) Frontal whole-mount views of lower jaw beak. (B–D and F–H) Sagittal thin sections. A–C and E–G show epithelial expression of shh and pitx2, respectively (white arrowheads). These genes are redeployed for replacement tooth morphogenesis, forming the extended dentine bands that provide units parallel to the oral surface that compose the beak in M. abei. (A and E) Frontal view of gene expression in the beak after in situ hybridization. S, symphyseal suture (black arrow). (B) Epithelial (labial) downgrowth (with green dotted outline, white arrow, and black dashes marking the dentine beak boundary) provides odontogenic epithelial cells for continued replacement bands (R1, white arrowhead; upper and lower beak sections together). (D) H&E-stained coronal section through the early beak. Seen are the developing dentine band (R2, white arrowhead) and underlying cluster of odontogenic cells for the newest successive dentine band (R3, white arrow) forming within the bone cavity (BC) of the beak. Medial first teeth are still present as T1 (black arrowheads in A–C and E–G). (H) Schematic drawing of the pufferfish beak in sagittal section, showing pale-green oral epithelium and labial downgrowth (as in B), acrodin cap (red) with dentine of a functional tooth (T1, dark blue), and bone of the cavity wall (purple). Expression of shh, pitx2, and bmp4 would be within the dental epithelium (dark green) surrounding the successive developing dentine bands R1 (already showing mineralized acrodin in red and dentine in dark blue) and R2 bud stage with condensing mesenchyme (pale blue), where bmp4 expression would occur (Fig. S2), underlying the dental epithelium (dark green), where shh, pitx2, and bmp4 would be expressed. The cavity mesenchyme is in light gray. (Scale bar in A: 200 μm.)
Pufferfish First-Generation Dentition Originates from a Conserved Osteichthyan Pattern.
Timed development of the earliest Monotrete dentition can be seen in cleared and double-stained skeletal preparations (Fig. 2) and also before morphological emergence via gene expression patterns (Fig. 3). Both of these developmental patterns show that a row of separate first-generation teeth forms in a timed sequence along the jaw margin, quite unlike in the adult beak and before any stages of beak initiation. These first stages of tooth development reiterate those of other teleosts studied to date. Importantly, the timing and spacing of tooth initiation along the jaw follows the same pattern that is well conserved across osteichthyan fishes (Fig. 3) (15, 18, 19). As in other bony fishes, initial tooth germs on the lower jaw appear in a temporal sequence not matched by tooth position along the jaw. The first tooth germ develops in position 2, followed first by position 3, then position 1 (roman numerals in Fig. 3 represent the positional order along the jaw), and then by successive proximal addition of tooth germs in positions 4–9 (Figs. 2 and 3). There is a different arrangement of separate teeth in the upper jaw comprising an oblique anteroposterior row with fewer teeth (between four and six; Fig. 2 C–F), compared with a total of 14–18 on the lower jaw. Comparable data have been reported in the rainbow trout Oncorhynchus mykiss (25, 26). Later in both jaws, two prominent parasymphyseal teeth at tooth position 1 in each quadrant (Fig. 2 E, H, and M) are retained for, and involved in, subsequent continuous tooth replacement (see below) during beak formation (Fig. 2 H, I, M, and N).
The first indication of tooth initiation is in restricted gene expression in Monotrete spp. at 6 d postfertilization, localized to a dentally competent epithelial strip, the odontogenic band coexpressing pitx2 and shh (Fig. 3 A–G). Underlying this epithelial expression are aggregated mesenchymal cells showing restricted expression of well-known “odontogenic-related” genes bmp4 (Fig. 3 I–L) and pax9 (Fig. S1) in underlying tooth fields, presumed to be reciprocal activity (18, 19, 27–29). However, pax9 is later restricted to those mesenchymal cells surrounding each tooth unit (Fig. S1), following the conserved gene expression domains of other teleosts (15, 18, 22). For example, identical patterns of gene expression have been documented in the rainbow trout, a phylogenetically basal teleost relative to pufferfishes (30), suggesting a high degree of conservation of developmental regulation despite highly disparate morphological structures. We examined the expression of several highly conserved genes including bmp4, pax9, pitx2, and shh, all of which exhibited location and timing of expression during first-generation tooth patterning in pufferfish identical to that of other studied teleosts (18, 19, 22, 31).
There are subtle differences between initial tooth development in pufferfish and other known teleosts; for example, the first-generation tooth germs (Fig. 3) appear to develop entirely within the surface epithelial layer rather than through invagination of the entire tooth germ. This invaginated dental epithelium is considered essential for tooth replacement in all other known vertebrate dentitions (17, 21, 32, 33). The first-generation parasymphyseal tooth germs in Monotrete spp., which also begin their development in a superficial position, later invaginate deep into the underlying mesenchyme. Thus, pufferfish acquire a deep dental epithelium only in the four parasymphyseal teeth. Tooth replacement can occur only from this invaginated, extended dental epithelium (Fig. 3), as seen in all other dentition studied to date (34).
Once at least five first-generation teeth have developed along the Monotrete lower jaw, other changes occur that demonstrate the uniqueness of this dental system. The pulp cavities of the first-generation marginal teeth fill with dentine and fuse with adjacent teeth at their bases (Fig. 2 J and K). All are surrounded by a superficial but extensive layer of “intertooth” dentine (Fig. 2 C–H and L) (35), providing a solid and robust functional biting surface with firm tooth attachment. This dentition of first-generation teeth plus their intertooth dentine consolidation is functional in the first few weeks after hatching (Fig. 2 C–H and L) (35). But although composed of dentine, this surface differs significantly from that of the adult beak, with a minimal covering of dentine around the teeth, far less mineralized than the substantial and highly mineralized, repeated bands of beak dentine. Although the pufferfish first-generation teeth represent the conserved pattern of osteichthyan odontogenesis, the stage of consolidation of the superficial teeth is different, transitory, and not comparable to the beak structure seen in adults.
Replacing Initial Teeth in Pufferfish with a Unique Beak via Continuous Replacement.
The first signs of pufferfish beak formation appear at 5.6 mm standard length (SL) as a highly mineralized band in a cavity of the jaw bone below the first-generation teeth. In the upper and lower jaws, new successional replacement teeth are initiated in each quadrant exclusively below the parasymphyseal teeth (Fig. 2 D–I). Only these develop an epithelial invagination (as a type of successional lamina) extending from these first-generation tooth germs and the first-generation superficial odontogenic band. The first-generation parasymphyseal teeth extend into the underlying jaw mesenchyme and are likely regulated by cells expressing the epithelial and mesenchymal gene bmp4, a marker for where the beak will arise (Figs. 3 and 5). Thus, the first-generation dentition is respecified from the common pattern in osteichthyans. However, tooth replacement proceeds only in the four successive parasymphyseal teeth with separate cusps of acrodin, below the first ones (Fig. 2M, arrows). For all other first-generation tooth positions (from position 2 along the jaw proximally), the process of tooth replacement is inhibited. Another unique feature is the extension of dentine proximally from these parasymphyseal replacement teeth in a continuous growth mode for each tooth to form the distinctive jaw quadrant length, stacked dentine bands characteristic of phylogenetically derived pufferfishes such as Monotrete (Figs. 2 D–I and N and 5 C and D). These vertical stacks of dentine bands serve as the replacement tissue for the first-generation tooth set embedded in superficial intertooth dentine. Each dentine band represents one replacement tooth with proximally extended growth (Fig. 4) occurring simultaneously in each quadrant. New bands are added continuously throughout life, and through the process of wear each dentine band eventually becomes the functional biting (tritural) surface as many stacked dentine bands form to replace a single tooth position (a system known as many-for-one replacement). As noted earlier, the replacement teeth are initiated where coexpression of key regulators of tooth initiation, shh, pitx2 (Fig. 4), and bmp4 (Fig. S2), are redeployed to form these dentine bands during beak morphogenesis.
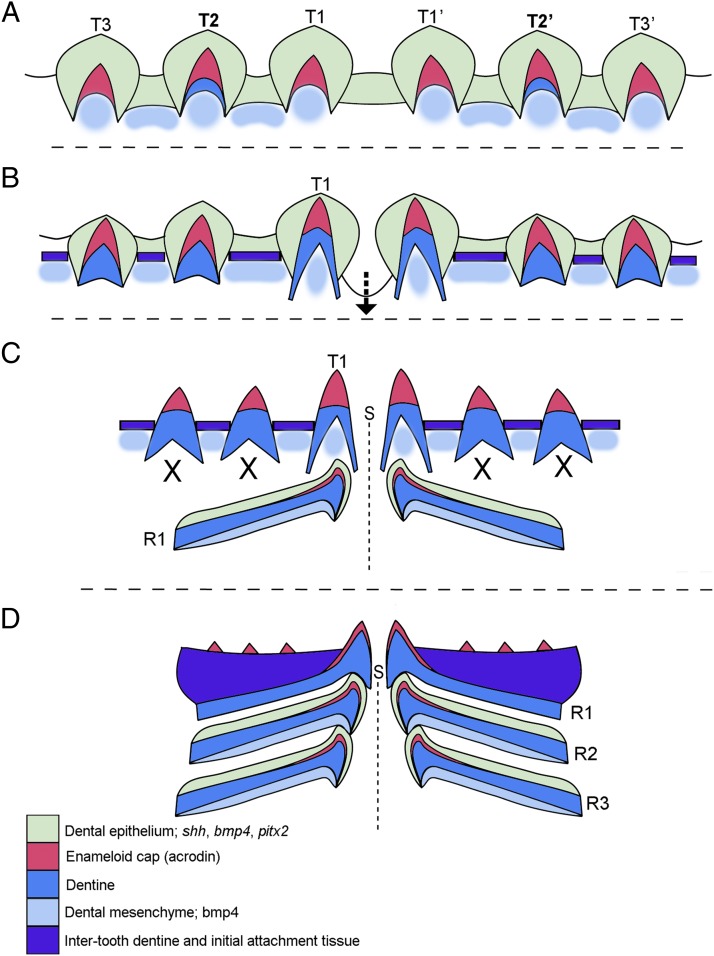
Fig. 5.
Conserved developmental origins in pufferfish with teeth in order before appearance of the unique beak represented by schematic drawings of the transitional stages forming the beak constructed via tooth replacement (representing only the lower jaw dentition). (A) In stage A, first-generation superficial tooth development exemplifies the osteichthyan pattern. The order of initiation is different from the order along the jaw in a conserved pattern: T2, T3, and T1, with the number referring to position along the jaw from most proximal (parasymphyseal), T1. In addition, expression of key “tooth” genes (bmp4, shh, and pitx2) is conserved during this phase of development. All teeth form a cap of acrodin (red), but parasymphyseal teeth (T1) are larger. (B) In stage B, development of the parasymphyseal (medial, T1) teeth progresses to provide a deep extension (arrow) of dental epithelium from the tooth germ for initiation of beak morphogenesis. At this time, new intertooth dentine (purple) develops to surround first-generation teeth and join bases together for a functional surface, the “first bite.” (C) In stage C, restricted replacement teeth form (R1) only for the parasymphyseal teeth. Proximal tooth positions (e.g., T2, T3) are lost (X) and never replaced. Replacement teeth (R1) are structurally very different from the first- generation teeth, as growth extends to the length of the jaw and each dentine band forms in the cavity within the bone of the jaws. First and future generations of replacement teeth are formed of single bands of dentine for each replacement round, as successive events within the bone. Expression of pitx2, shh, and bmp4 continues to be redeployed for further morphogenesis of the replacement teeth into the characteristic bands of dentine (dark blue) to form the beak structure. The medial tips of each replacement band (stage D, R1–R3) contain an acrodin cap (red). (D) In stage D, further rounds of replacement (R1–R3) continue before the stacked dentine bands become a fully functional and novel structure as the beak. Each quadrant of the beak is separated by a complex sutured symphysis (S, dashed line). Dental epithelium (green) expresses bmp4, pitx2, and shh during both tooth initiation and continued replacement and initiation of dentine bands. Dental mesenchyme (light blue) expresses bmp4 at all stages of tooth initiation and replacement band initiation and development.
Discussion
Our developmental and gene expression data obtained at early ontogenetic stages of Monotrete spp. demonstrate that the first-generation set of marginal teeth is induced in a spatiotemporal series that represents the typical teleost and likely a general osteichthyan pattern (Fig. 3 A–C), the latter being conserved since the actinopterygian/sarcopterygian split at least 416 Mya (36). Thus, the pufferfish beak is not developed de novo, but rather emerges ontogenetically as a modification of the program for tooth replacement. Our new data show how, after the conserved tooth program has unfolded in time and space as in most osteichthyan developmental programs (Figs. 2 and 3), the dental module is modified during the first replacement phase via respecification of continuous rounds of tooth addition and replacement to produce a unique beak capable of crushing or slicing prey (Figs. 1, 2, 4, and 5). Only the four parasymphyseal teeth form replacement teeth, and through the activity of odontoblasts extend dentine growth to form the beak within cavities (intramedullary bone spaces) of both the upper and lower jaws (Figs. 2 D–I and M and 5 B and C). As a new testable hypothesis, we suggest that despite their highly diverse and divergent dental structure, teleost dentitions will be initiated in ontogeny with the same conserved program, and that dental diversity and novelty will be manifested only during cyclical replacement. This offers insight into another question: Is a complete initial tooth row necessary for correct pufferfish beak formation? We suggest that pufferfish require only the formation of two parasymphyseal teeth (on the upper and lower jaws) in the correct position for the beak to initiate and form continuous replacement tooth bands for each jaw. It is in this medial location that the dental epithelium extends (invaginates) ventrally from the superficial tooth germs to initiate parasymphyseal replacement teeth. This parasymphyseal location is essential for both the sutured bony linkage of the two upper and two lower jaw halves and for the addition of their replacement dentine bands for a functional beak. Thus, it appears that no teeth of the first-generation dentition except the four parasymphyseal teeth play an obvious role in the development of the adult dental structure. However, these teeth are required for earlier larval feeding and are lost, essentially through the process of wear, as they become the first trituration (biting) surface of the jaw.
The beak-like dentition in the Monotrete pufferfish (Fig. 1) differs from that of other Gymnodontes, including the phylogenetically basal family Triodontidae (4). In the Triodontidae, the beak results from the coalescence of numerous separate tooth units, whereas the Monotrete beak develops from compaction of consecutive replacement dentine bands (5) that continue to form via modified tooth replacement established in the second generation of tooth development. In contrast, in the Triodontidae the separate tooth units develop within separate small cavities on the lateral surface of the jaw bones. This indicates that tooth replacement has shifted to a cavity inside the jaw bones (37, 38) during pufferfish evolution, although replacement mechanisms have undergone further significant changes with diversification of the group.
Our results indicate that the highly modified dentition of pufferfishes is derived by tinkering with a developmental plan of a generalized osteichthyan dentition, itself retained over 400 million years, highlighting the gradual nature of evolutionary change and the principle of natura non facit saltus (“nature does not make jumps”). Future research should consider how highly distinctive vertebrate morphological structures are derived developmentally and how individual developmental modifications facilitate evolution away from a conserved bauplan. We have demonstrated how respecification of continuous tooth replacement can be reorganized to form a specialized, unique mechanism for dental tissues. The unique morphological structure of pufferfishes should promote their use as a new evo-devo model for the study of developmental modification and evolutionary novelty.
Materials and Methods
Fish Husbandry.
Embryos and larvae of Monotrete pufferfish were raised to the required stage in a recirculating aquarium system at 20–23 °C at the Natural History Museum, London. Lengths given refer to either notochord length (NL) before flexion or SL after flexion of larvae.
cDNA and Riboprobes.
Cloned cDNA sequences used to generate digoxigenin-labeled antisense riboprobes from Monotrete spp. were identified through partial genome assemblies of Fugu and Tetraodon spp. (http://www.genoscope.cns.fr/externe/tetranew/; http://www.fugu-sg.org/) and cloned from M. abei cDNA libraries. The following primers were used to generate the M. abei cDNA fragments for cloning and riboprobe synthesis from exact matching sequences from the foregoing databases: bmp4 forward, CCTTAGCAGCATTCCAGAGG; bmp4 reverse, CCCAGGCTCTTGGTGTAAAG; pax9 forward, GCACATTCGGACATACAAGC; pax9 reverse, TTGGAGGTCGGGTAGGAGTA; pitx2 forward, TTGGTTCAAGAACCGGAGAG; pitx2 reverse, AGTGCTGCTTGGCTTTCAGT; shh forward, GTCGGTCTCCTCTGCTTGTC; shh reverse, CACCGGTGTTCTCTTCATCC.
In Situ Hybridization.
To ensure that the embryos of comparison were of equivalent stages (especially during gene expression comparisons), specimens used from the same brood were stage-matched based on external features, including pectoral and caudal fin development and eye development and maturity. Specimens for whole-mount in situ hybridization were anesthetized in tricaine methanesulfonate (MS-222; Argent Chemical Laboratories) and fixed overnight in 4% (wt/vol) paraformaldehyde (PFA) in PBS at 4 °C. Whole-mount in situ hybridization experiments were based on published protocols (15), modified as follows. Embryos were transferred to methanol for dehydration and stored at −20 °C. Specimens were rehydrated through to PBS with Tween-20 and digested with 4–10 μg/mL of proteinase K. After hybridization, embryos were washed in 10 mM NaCl, 10 mM Tris-HCl, and Tween-20 in diethylpyrocarbonate-treated H2O. During the color reaction stage of the protocol, all embryos were allowed to fully develop the color. Thus, embryos were continuously transferred into fresh NBT/BCIP solution (Roche) in NTMT until full staining was complete; this was determined after multiple regions of known expression became positive. All in situ hybridization experiments were performed with multiple specimens (with multiple individuals fixed at regular intervals, within single broods, then repeated at least twice with alternative broods), to fully characterize the expression patterns. After color reaction (NBT/BCIP; Roche), embryos were washed in PBS and fixed again in 4% (wt/vol) PFA in PBS before whole-mount imaging using a Leica Microsystems M205 stereomicroscope. Embryos were embedded in gelatin and chick albumin with 2.5% gluteraldehyde. The gelatin–albumin blocks were postfixed in 4% PFA before sectioning. Thin sections (15–25 μm) were cut with a Leica Microsystems VT1000 vibratome.
Skeletal Staining.
The embryonic and juvenile stages of pufferfishes were cleared and double-stained with alizarin red (bone and dentine) and alcian blue (cartilage) according to the protocol of Taylor and Van Dyke (39). Specimens were photographed with a Zeiss Discovery V20 stereomicroscope using Zeiss Axiovision z-stacking software.
Supplementary Material
Acknowledgments
We thank Alasdair Edgar for the optical projection tomography imaging and Rolf Ericsson, Patricia Dyal, Juan Galindo, Chris Healy, and Liam Rasch for laboratory assistance. We also thank Greg Edgecombe, George Mattox, and Robert Knight for their comments on earlier drafts of the manuscript; Noboru Tazoe for providing a copy of Fujita’s 1962 hard-to-find paper (4); Ai Nonaka for translating relevant sections of Fujita’s paper; and Peter Konstantinidis for preserving some of the embryonic stages used in this study. This work was funded by the Natural History Museum, London (Z.J. and R.B.), the University of Sheffield (G.J.F.), and the Leverhulme Trust (G.J.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119635109/-/DCSupplemental.
References
- 1.Cuvier G. Leçons d’Anatomie Comparee: La Première Partie des Organes de la Digestion. Vol III. Paris: Crochard; 1805. [Google Scholar]
- 2.Konstantinidis P, Harris MP. Same but different: Ontogeny and evolution of the musculus adductor mandibulae in the Tetraodontiformes. J Exp Zoolog B Mol Dev Evol. 2011;316:10–20. doi: 10.1002/jez.b.21375. [DOI] [PubMed] [Google Scholar]
- 3.Turingan RG. Ecomorphological relationships among Caribbean tetraodontiform fishes. J Zool (Lond) 1994;233:493–521. [Google Scholar]
- 4.Fujita S. Nagasaki Prefectural Research Station Report. Vol 2. Japan: NPRS; 1962. Studies on the life history and culture of common pufferfishes in Japan; pp. 1–121. [Google Scholar]
- 5.Andreucci RD, Britski HA, Carneiro J. Structure and evolution of tetraodontoid teeth: An autoradiographic study (Pisces, Tetraodontiformes) J Morphol. 1982;171:283–292. doi: 10.1002/jmor.1051710304. [DOI] [PubMed] [Google Scholar]
- 6.Amores A, et al. Developmental roles of pufferfish Hox clusters and genome evolution in ray-fin fish. Genome Res. 2004;14:1–10. doi: 10.1101/gr.1717804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brainerd EL, Patek SN. Vertebral column morphology, C-start curvature, and the evolution of mechanical defenses in Tetraodontiform fishes. Copeia. 1998;(4):971–984. [Google Scholar]
- 8.Tanaka M, et al. Developmental genetic basis for the evolution of pelvic fin loss in the pufferfish Takifugu rubripes. Dev Biol. 2005;281:227–239. doi: 10.1016/j.ydbio.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Tyler JC. Osteology, Phylogeny, and Higher Classification of the Fishes of the Order Plectognathi (Tetraodontiformes) Washington, DC: National Oceanic and Atmospheric Administration, National Marine Fisheries Service; 1980. NOAA Tech Rep NMFS Circ 434:1–422. [Google Scholar]
- 10.Venkatesh B, Gilligan P, Brenner S. Fugu: A compact vertebrate reference genome. FEBS Lett. 2000;476:3–7. doi: 10.1016/s0014-5793(00)01659-8. [DOI] [PubMed] [Google Scholar]
- 11.Brenner S, et al. Characterization of the pufferfish (Fugu) genome as a compact model vertebrate genome. Nature. 1993;366:265–268. doi: 10.1038/366265a0. [DOI] [PubMed] [Google Scholar]
- 12.Elgar G, et al. Small is beautiful: Comparative genomics with the pufferfish (Fugu rubripes) Trends Genet. 1996;12:145–150. doi: 10.1016/0168-9525(96)10018-4. [DOI] [PubMed] [Google Scholar]
- 13.Brainerd EL, Slutz SS, Hall EK, Phillis RW. Patterns of genome size evolution in tetraodontiform fishes. Evolution. 2001;55:2363–2368. doi: 10.1111/j.0014-3820.2001.tb00750.x. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki K, Suzuki T, Weiss KM. Phenogenetic drift in evolution: The changing genetic basis of vertebrate teeth. Proc Natl Acad Sci USA. 2005;102:18063–18068. doi: 10.1073/pnas.0509263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser GJ, Graham A, Smith MM. Conserved deployment of genes during odontogenesis across osteichthyans. Proc Biol Sci. 2004;271:2311–2317. doi: 10.1098/rspb.2004.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67:111–123. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 17.Fraser GJ, Berkovitz BK, Graham A, Smith MM. Gene deployment for tooth replacement in the rainbow trout (Oncorhynchus mykiss): A developmental model for evolution of the osteichthyan dentition. Evol Dev. 2006;8:446–457. doi: 10.1111/j.1525-142X.2006.00118.x. [DOI] [PubMed] [Google Scholar]
- 18.Fraser GJ, Bloomquist RF, Streelman JT. A periodic pattern generator for dental diversity. BMC Biol. 2008;6:32. doi: 10.1186/1741-7007-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser GJ, Graham A, Smith MM. Developmental and evolutionary origins of the vertebrate dentition: Molecular controls for spatio-temporal organisation of tooth sites in osteichthyans. J Exp Zoolog B Mol Dev Evol. 2006;306:183–203. doi: 10.1002/jez.b.21097. [DOI] [PubMed] [Google Scholar]
- 20.Handrigan GR, Richman JM. Autocrine and paracrine Shh signaling are necessary for tooth morphogenesis, but not tooth replacement in snakes and lizards (Squamata) Dev Biol. 2010;337:171–186. doi: 10.1016/j.ydbio.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Handrigan GR, Richman JM. A network of Wnt, hedgehog and BMP signaling pathways regulates tooth replacement in snakes. Dev Biol. 2010;348:130–141. doi: 10.1016/j.ydbio.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Jackman WR, Draper BW, Stock DW. Fgf signaling is required for zebrafish tooth development. Dev Biol. 2004;274:139–157. doi: 10.1016/j.ydbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, et al. Conservation of early odontogenic signaling pathways in Aves. Proc Natl Acad Sci USA. 2000;97:10044–10049. doi: 10.1073/pnas.160245097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berkovitz BK. The order of tooth development and eruption in the Rainbow trout (Salmo gairdneri) J Exp Zool. 1977;201:221–226. doi: 10.1002/jez.1401930211. [DOI] [PubMed] [Google Scholar]
- 26.Berkovitz BK. Tooth ontogeny in the upper jaw and tongue of the rainbow trout (Salmo gairdneri) J Biol Buccale. 1978;6:205–215. [PubMed] [Google Scholar]
- 27.Peters H, Balling R. Teeth: Where and how to make them. Trends Genet. 1999;15:59–65. doi: 10.1016/s0168-9525(98)01662-x. [DOI] [PubMed] [Google Scholar]
- 28.Peters H, Neubüser A, Balling R. Pax genes and organogenesis: Pax9 meets tooth development. Eur J Oral Sci. 1998;106(Suppl 1):38–43. doi: 10.1111/j.1600-0722.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 29.Peters H, Neubüser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12:2735–2747. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson JS. Fishes of the World. Hoboken, NJ: Wiley; 2006. p. 601. [Google Scholar]
- 31.Fraser GJ, et al. An ancient gene network is co-opted for teeth on old and new jaws. PLoS Biol. 2009;7:e31. doi: 10.1371/journal.pbio.1000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handrigan GR, Leung KJ, Richman JM. Identification of putative dental epithelial stem cells in a lizard with life-long tooth replacement. Development. 2010;137:3545–3549. doi: 10.1242/dev.052415. [DOI] [PubMed] [Google Scholar]
- 33.Buchtová M, et al. Initiation and patterning of the snake dentition are dependent on sonic hedgehog signaling. Dev Biol. 2008;319:132–145. doi: 10.1016/j.ydbio.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Smith MM, Fraser GJ, Mitsiadis TA. Dental lamina as source of odontogenic stem cells: Evolutionary origins and developmental control of tooth generation in gnathostomes. J Exp Zoolog B Mol Dev Evol. 2009;312B:260–280. doi: 10.1002/jez.b.21272. [DOI] [PubMed] [Google Scholar]
- 35.Britski HA, Andreucci RD, Menezes NA. Coalescence of teeth in fishes. Rev Brasil de Zool. 1985;2:459–484. [Google Scholar]
- 36.Hurley IA, et al. A new time-scale for ray-finned fish evolution. Proc Biol Sci. 2007;274:489–498. doi: 10.1098/rspb.2006.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huysseune A, Thesleff I. Continuous tooth replacement: The possible involvement of epithelial stem cells. Bioessays. 2004;26:665–671. doi: 10.1002/bies.20039. [DOI] [PubMed] [Google Scholar]
- 38.Trapani J. Position of developing replacement teeth in teleosts. Copeia. 2001;2001:35–51. [Google Scholar]
- 39.Taylor WR, van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985;9:107–119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.