Abstract
Aristolochic acid, a potent human carcinogen produced by Aristolochia plants, is associated with urothelial carcinoma of the upper urinary tract (UUC). Following metabolic activation, aristolochic acid reacts with DNA to form aristolactam (AL)-DNA adducts. These lesions concentrate in the renal cortex, where they serve as a sensitive and specific biomarker of exposure, and are found also in the urothelium, where they give rise to a unique mutational signature in the TP53 tumor-suppressor gene. Using AL-DNA adducts and TP53 mutation spectra as biomarkers, we conducted a molecular epidemiologic study of UUC in Taiwan, where the incidence of UUC is the highest reported anywhere in the world and where Aristolochia herbal remedies have been used extensively for many years. Our study involves 151 UUC patients, with 25 patients with renal cell carcinomas serving as a control group. The TP53 mutational signature in patients with UUC, dominated by otherwise rare A:T to T:A transversions, is identical to that observed in UUC associated with Balkan endemic nephropathy, an environmental disease. Prominent TP53 mutational hotspots include the adenine bases of 5′AG (acceptor) splice sites located almost exclusively on the nontranscribed strand. A:T to T:A mutations also were detected at activating positions in the FGFR3 and HRAS oncogenes. AL-DNA adducts were present in the renal cortex of 83% of patients with A:T to T:A mutations in TP53, FGFR3, or HRAS. We conclude that exposure to aristolochic acid contributes significantly to the incidence of UUC in Taiwan, a finding with significant implications for global public health.
Keywords: upper urinary tract carcinoma, traditional Chinese medicine, transitional cell carcinoma, DNA damage, herbal medicine
Aristolochic acid (AA), a constituent of all Aristolochia plants, is a powerful nephrotoxin and human carcinogen associated with chronic kidney disease (CKD) and upper urinary tract urothelial carcinomas (UUC) (1). These dual toxicities and target tissues were revealed when a group of otherwise healthy Belgian women developed renal failure and UUC after ingesting Aristolochia herbs in conjunction with a weight-loss regime (2, 3). Reports of this syndrome, now known as aristolochic acid nephropathy (AAN), called attention to the serious toxicities associated with the use of Aristolochia sp. as herbal remedies. Subsequently, sporadic cases of AAN were reported in countries throughout the world (4, 5).
AA was shown recently to be the causative agent of Balkan endemic nephropathy (EN) (6, 7). This devastating environmental disease, affecting residents of rural farming villages in Bosnia and Herzegovina, Bulgaria, Croatia, Romania, and Serbia, is associated with a high incidence of UUC (8, 9). In EN, exposure to AA occurs via consumption of bread prepared from flour contaminated with seeds of Aristolochia clematitis (10).
Following metabolic activation, AA reacts with DNA to form covalent aristolactam (AL)-DNA adducts (11). These lesions persist for years in the renal cortex, providing a robust, internal biomarker of exposure to AA (7). AL-DNA adducts are found also in urothelial tissues (6), where they initiate tumors bearing a characteristic pattern of mutations in the TP53 tumor-suppressor gene, thereby creating a biomarker specific for AA-induced UUC (6, 7, 12).
In Taiwan, the remarkably high incidence of UUC (13), coupled with widespread use of Aristolochia herbal remedies, suggested that AA might play a central role in the etiology of this disease. The high level of exposure to AA in Taiwan has been documented by a systematic analysis of prescriptions filled by a 200,000 person random sample of the entire insured population of Taiwan between 1997 and 2003, revealing that approximately one-third of these individuals consumed herbs containing, or likely to contain, AA (14). Moreover, consumption of AA is associated, in a dose-dependent manner, with an increased risk of developing end-stage renal disease or urothelial carcinoma (13, 15). Importantly, 43% of these carcinomas were located in the upper urinary tract (13).
In this molecular epidemiologic study, we used AL-DNA adducts and TP53 mutational spectra as biomarkers of exposure and effect to establish the contribution of AA to the prevalence of UUC in Taiwan. We then extended our identification of AA-induced mutations to include the oncogenes FGFR3 and HRAS. Results of our study support strongly the hypothesis that AAN/UUC represents a long-overlooked iatrogenic disease in Taiwan and, by extension, in China and other countries worldwide, where Aristolochia herbal remedies traditionally have been used for medicinal purposes.
Results
Demographics.
Subjects of this study include 151 patients (82 men) with urothelial (transitional cell) carcinomas of the renal pelvis or ureter, and 25 patients (18 men) with renal cell carcinomas (RCC). Demographics and other relevant data for patients with UUC are summarized in Table 1. The mean age at diagnosis for patients with UUC and RCC was 66 and 63 y, respectively.
Table 1.
Demographic and other characteristics of UUC subjects (n = 151)
| Age at diagnosis (y), median (range) | 67 (35–91) |
| Demographics | n |
| Females | 69 (45.7%) |
| Males | 82 (54.3%) |
| Smokers | |
| Females | 5 (7.2%) |
| Males | 28 (34.1%) |
| CKD stage | |
| 0–2 | 59 (39.1%) |
| 3 | 57 (37.7%) |
| 4 | 12 (7.9%) |
| 5 | 23 (15.2%) |
| Tumor site | |
| Renal pelvis | 73 (48.3%) |
| Ureter | 58 (38.4%) |
| Renal pelvis and ureter | 10 (6.6%) |
| Renal pelvis and bladder | 7 (4.6%) |
| Renal pelvis, ureter, and bladder | 3 (2.0%) |
TP53 Mutational Spectra.
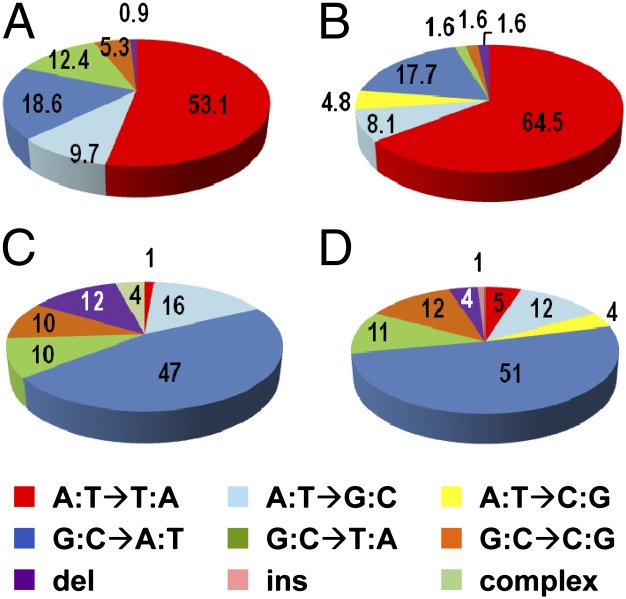
TP53 mutation data for individual patients are presented in Table S1. A total of 113 TP53 mutations were detected in 84 (55.6%) of 151 patients with UUC. All but one of these mutations, a deletion of T:A, consisted of single-base substitutions in exons 2–11, resulting in missense, nonsense, or splice-site mutations. Mutations at A:T pairs (63.7%) consisted of A:T to T:A transversions (53.1%), A:T to G:C transitions (9.7%), and a single A:T pair deletion (0.9%) (Fig. 1A). Mutations at G:C pairs (36.3%) consisted of G:C to A:T transitions (18.6%), G:C to T:A transversions (12.4%), and G:C to C:G transversions (5.3%).
Fig. 1.
TP53 mutational spectra in urothelial carcinomas. (A) TP53 mutations in DNA obtained from UUC in Taiwan (113 mutations). (B) TP53 mutations in DNA obtained from UUC in endemic regions of Bosnia, Croatia, and Serbia (62 mutations) (12). (C) TP53 mutations in urothelial carcinomas of the renal pelvis and ureter, worldwide (73 mutations) (16). (D) TP53 mutations in urothelial carcinomas of the renal pelvis, ureter, bladder, and nonspecified urinary organs, worldwide (696 mutations) (16).
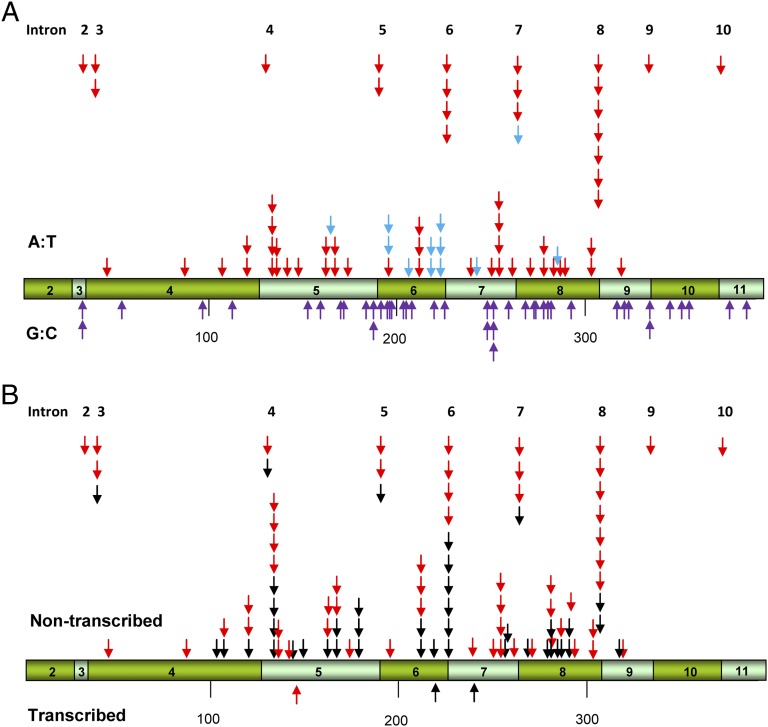
Among patients with UUC, 60% (30 of 50) of women and 50% (17 of 34) of men with TP53 mutations displayed the characteristic A:T to T:A transversions (Table 2). Remarkably, 37% (22 of 60) of all A:T to T:A transversions occurred at splice sites, mainly at introns 6 and 8 (Fig. 2A). All but 1 of 60 adenine bases involved in A:T transversions were located on the nontranscribed strand (Fig. 2B). G:C pairs at splice sites were not mutated (Fig. 2A). We identified mutational “hotspots” for A:T to T:A transversions at introns 6, 7, and 8 and at codons 131, 209, and 249, all of which contain three or more of these mutations (Fig. 2A). C or T is the 5′-neighboring base for 44 of 60 A:T to T:A transversions and G or A is the dominant 3′-neighboring base (53 of 60, 88%). Thus, in this group, 5′-(C/T)A(G/A) appears to be a consensus mutational hotspot for A→T transversions.
Table 2.
AL-DNA adducts and TP53 mutations in UUC cases from Taiwan
| Cases | All subjects | Males | Females | Males vs. females |
| Cases with AL-DNA adducts | 89/148 (60%) | 45/82 (55%) | 44/66 (67%) | χ2 = 2.12 P = 0.1454 |
| Cases with TP53 mutations | 84/151 (56%) | 34/82 (41%) | 50/69 (72%) | χ2 = 14.59 P = 0.0001 |
| Cases with TP53 A→T transversions | 47/151 (31%) | 17/82 (21%) | 30/69 (43%) | χ2 = 9.04 P = 0.0026 |
| Cases with AL-DNA adducts and TP53 A→T transversions | 38/148 (26%) | 14/82 (17%) | 24/66 (36%) | χ2 = 7.13 P = 0.0076 |
| AL-DNA adduct-positive cases with TP53 A→T transversions | 38/89 (43%) | 14/45 (31%) | 24/44 (55%) | χ2 = 4.99 P = 0.0254 |
| TP53 A→T transversion cases with AL-DNA adducts | 38/45 (84%) | 14/17 (82%) | 24/28 (86%) | χ2 = 0.091 P = 0.7629 |
Fig. 2.
TP53 base substitution mutations in DNA from urothelial UUCs. (A) Arrows above and below the bar representing TP53 cDNA indicate the position of mutations at A:T and G:C (purple arrows) pairs, respectively, in tumor DNA from Taiwanese subjects. Red arrows indicate A:T→T:A transversions; blue arrows show other types of mutations at A:T pairs. (B) Positions of A:T→T:A transversions on transcribed and nontranscribed strands of DNA in UUC patients from Taiwan (red arrows) and in residents of endemic regions of Bosnia, Croatia, and Serbia (black arrows) (12).
The TP53 mutational spectra for patients with UUC (Fig. 1A) is dominated by A:T to T:A transversions (53.1% of the total), a class of mutations found only in 5.1% of all human cancers, 4.8% of all urothelial carcinomas (Fig. 1D) and 1.4% of all carcinomas of the renal pelvis and ureter (Fig. 1C), based on an analysis of 23,544 TP53 mutations collected in version R11 of an international database (16). Remarkably, the overall distribution of mutations and positions of TP53 A:T to T:A transversions in UUC samples from Taiwan are almost identical to those observed for UUC in residents of endemic regions of Bosnia, Croatia, and Serbia (compare Fig. 1 A with B, and see Fig. 2B). Three of 25 (12%) patients with RCC displayed mutations in TP53. These mutations consisted of two G:C to T:A transversions and one G:C to A:T transition; mutations involving A:T base pairs in RCC were not observed.
Oncogene-Activating Mutations.
We determined the frequencies of A→T transversion events at the second nucleotide of the HRAS codon 61 (5′CAG) and at the first nucleotide of the FGFR3 codon 373 (5′AGT), nonsynonymous mutations that activate these oncogenes in urothelial tumors (17, 18). These frequencies (Table S2) were compared with those for urothelial carcinomas in the COSMIC database (http://www.sanger.ac.uk/genetics/CGP/cosmic). Because mutational data for urothelial carcinomas of the renal pelvis and ureter in this database are limited, we included mutational data for urothelial carcinomas of the bladder in this comparison. For HRAS codon 61, the frequency of A→T transversions in our samples was 4.7% (7 of 150) compared with 1.1% (12 of 1,123) for urothelial carcinomas in the COSMIC database [χ2(1) = 11.65; P < 0.006]. For FGFR3 codon 373, the frequency of A→T transversions in our samples was 4.0% (6 of 150) and only 0.78% (28 of 3,574) for all urothelial carcinomas [χ2(1) = 16.46; P < 0.0001]. Thus, the frequencies of A→T transversions in these oncogene-activating mutations are four- to fivefold higher in these samples than in the population at large. Importantly, all 13 adenine bases involved in A→T transversions are located in the nontranscribed strand. Moreover, deoxyadenosine (dA)-AL adducts were detected in the majority (10 of 13, 76.9%) of patients carrying A→T transversions in HRAS or FGFR3. Taken together, these findings suggest that A→T transversions observed in HRAS and FGFR3 were induced by dA-AL.
AL-DNA Adducts as Biomarkers of Exposure.
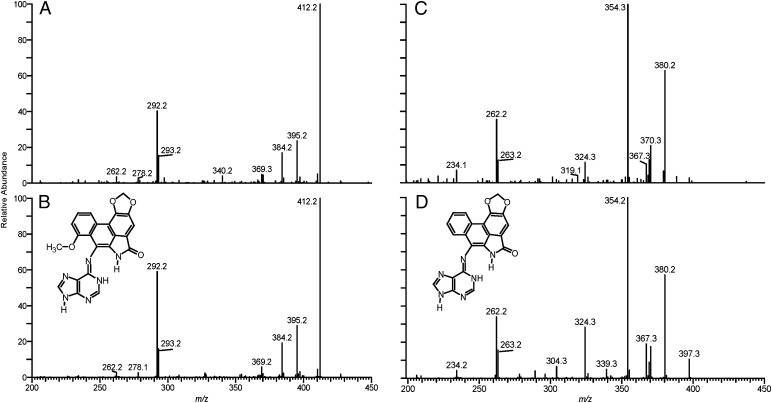
AA-I and AA-II are the principal forms of AA in all Aristolochia sp. Enzymatic nitroreduction of AAs in target and nontarget tissues leads to the formation of dA- and deoxyguanosine (dG)-adducts containing the corresponding aristolactams, AL-I and AL-II (11). The major adduct found in renal cortex DNA was identified by MS analysis as 7-(deoxyadenosin-N6-yl) aristolactam-I (dA-AL-I) (Fig. 3 and Fig. S1). dA-AL-II also was detected in this sample in smaller amounts, but not by 32P-postlabeling, and dG-AL adducts were not observed either by MS or 32P-postlabeling analysis. Similar adduct spectra were obtained in eight other patients.
Fig. 3.
Characterization of AL-DNA adducts, using liquid chromatography electrospray ionization/multistage mass spectrometry (LC-ESI/MS/MS3), in the renal cortex of a representative Taiwanese subject with UUC. (A) The product ion spectrum of the protonated base adduct [BH2]+ for the dA-AL-I adduct identified in renal cortex; (B) product ion spectrum of synthetic dA-AL-I; (C) product ion spectrum of the dA-AL-II adduct identified in renal cortex; and (D) product ion spectrum of synthetic dA-AL-II.
dA-AL-I adducts were found in 60% of UUC and in 60% of RCC cases. Stratified by sex (Table 2), these adducts were present in 67% (44 of 66) and 100% (5 of 5) of women, and in 55% (45 of 82) and 47% (7 of 15) of men, with UUC and RCC, respectively. Adduct levels ranged between 1.4 and 234 adducts per 108 nucleotides, as estimated by 32P-postlabeling assays. The limit of detection of adducts by this method is three adducts in 109 bases when using 10–20 μg of DNA.
Eighty-nine of 148 (60%) UUC patients harbor dA-AL adducts, and 38 (43%) of these patients exhibited A→T transversions in TP53 (Table 2). Conversely, 38 of 45 (84%) patients with A:T to T:A transversions in TP53 were adduct-positive (Table 2), underscoring the close association between exposure to AA and its carcinogenic effect. Logistic regression analysis, after adjusting for age, sex, and smoking, indicates that UUC patients with dA-AL adducts were significantly more likely to carry the signature A→T mutation than were those without adducts [odds ratio = 4.86 (1.94–12.20); P = 0.001].
Definitive evidence for AA exposure is provided by the detection of AL-DNA adducts. AL-DNA adducts in renal cortical DNA and A→T transversions in urothelial tumor DNA were both found in 26% of our subjects, occurring more frequently in females (24 of 66; 36%) than in males (14 of 82; 17%) (Table 2). Of the 34 males lacking both AL-DNA adducts and A→T transversions in TP53, 15 (44%) were smokers, an established risk factor for the development of UUC (19, 20). None of the 18 females who tested negative for AL-DNA adducts and A→T transversions in TP53 smoked.
AA Exposure and Renal Disease.
The Modification of Diet in Renal Disease (MDRD) study equation (21) was used to estimate renal function (22) by CKD stage (Table 1). Severe CKD (stage 5) was present in 26% (10 of 38) of UUC subjects who exhibited both AL-DNA adducts and A→T transversions, compared with only 12% (6 of 52) of subjects lacking both biomarkers [χ2(1) = 3.28; P = 0.07].
Discussion
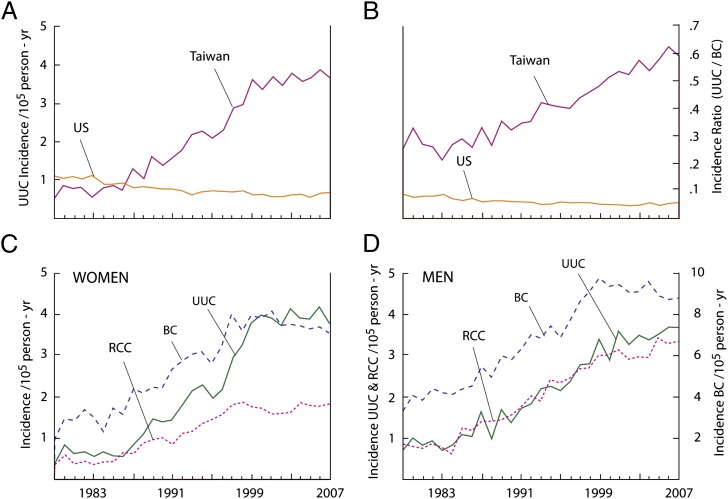
This molecular epidemiologic study was undertaken to explore the proposition that AA, a powerful nephrotoxin and established human carcinogen, and an intrinsic component of all Aristolochia herbal remedies, contributes significantly to the high incidence of UUC in Taiwan (Fig. 4) (13). This hypothesis is supported strongly by our demonstration that all components of the AA signature TP53 mutational spectrum, established in the context of UUC associated with EN (12), are recapitulated in Taiwanese patients with UUC (Figs. 1 and 2). The sites of A→T transversions in humans are in concordance with those observed in AA-I exposed fibroblasts prepared from Hupki (human TP53 knock in) mice (23).
Fig. 4.
Incidence rates of UUC and RCC in Taiwan and the United States. (A) The age-adjusted incidence rate of UUC in Taiwan and the United States. (B) UUC/bladder cancer (BC) incidence ratio in Taiwan and the United States. (C) Age-adjusted incidence rates of UUC, BC, and RCC in Taiwanese women. (D) Age-adjusted incidence rates of UUC, BC, and RCC in Taiwanese men. All incidence results were adjusted according to the world population proposed by Segi et al. (40). Data for the United States were derived from the International Agency for Research on Cancer database (32), and for Taiwan, from the Taiwan Cancer Registry (33).
Unequivocal evidence of exposure to AA is provided by our finding that 60% of all cases of UUC and 84% of patients with A:T to T:A mutations in TP53 contained dA-AL-I adducts in their renal cortex. dA-AL-I adducts also are present in 60% of patients with RCC; however, in this control group, A:T to T:A mutations were not detected in TP53. Our data, based on these robust biomarkers of internal exposure and disease, are consistent with epidemiologic studies showing that, in recent years, approximately one-third of the population of Taiwan has been exposed to Chinese medicinal herbs containing, or likely to contain, AA (13–15), and that a linear dose–response relationship exists between consumption of herbal remedies containing AA and the risk of developing urinary tract cancer. These epidemiologic studies likely underestimate AA exposure because they consider only Aristolochia-based prescriptions written by physicians over a period of 7 y, and Aristolochia herbs have long been available in Taiwan from alternative sources.
Fifty-seven percent of patients with AL-DNA adducts do not harbor A-to-T mutations in TP53. This observation may reflect heterogeneity of tissues used for this analysis and the relative sensitivity of biomarker assays used. Alternatively, AA could induce UUC via mutations in genes such as RB, a tumor-suppressor gene that is frequently inactivated in invasive urothelial carcinomas (24, 25). Indeed, in our study, the oncogenes FGFR3 and HRAS appear to be activated by mutations induced by AA. Thus, the frequency of A→T transversions at codon 373 and codon 61 of FGFR3 and HRAS, respectively, is increased fivefold in the Taiwanese cohort compared with urothelial carcinomas worldwide (www.sanger.ac.uk/genetics/CGP/cosmic). This comparison is based on similar relative frequencies among all FGFR3 and HRAS activating mutations in UUC and carcinomas of the bladder combined (26). Additionally, as in TP53, adenine residues mutated in FGFR3 codon 373 and HRAS codon 61 are found invariably on the nontranscribed strand. Moreover, dA-AL adduct analyses revealed that the majority of these patients have been exposed to AA.
Several recently characterized molecular events, including the intrinsic miscoding properties of dA-AL, in which dAMP is inserted preferentially opposite this lesion during translesion DNA synthesis (27), combine to create the unusual pattern of mutations observed in AA-induced UUC. As with most bulky adducts, dA-AL adducts normally would be excised by global genomic nucleotide excision repair. However, the structure of dA-AL in adducted DNA (28) precludes recognition by XPC/R23B, the initial step in global genomic repair, while transcription-coupled repair of this lesion is unaffected (29). As a result, dA-AL adducts persist in human tissues for many years (3, 6, 7). Selective transcription-coupled repair results in a mutational pattern with a marked strand bias, reflected by the position of adenine bases in mutated TP53, FGFR3, and HRAS.
An unusual large number of splice-site mutations are observed in AA-associated UUC (Fig. 2) (12). Thus, when TP53 mutational data for patients with Balkan EN are combined with those with UUC from Taiwan (Fig. 2B), all 5′AG acceptor splice sites in TP53 were found to be mutated at least once, with introns 6 and 8 identified as prominent hotspots. Remarkably, in the combined spectrum, mutations at splice sites reflect 37% (27 of 60) of all A-to-T mutations, in contrast to only 1.8% recorded for all splice sites in the TP53 database (16). This observation is consistent with the preferential targeting of adenine residues in DNA by AA (11).
Other chemical carcinogens, including certain analgesic agents, arsenic, and tobacco smoke, have been associated with UUC (20). Our study design excludes residents of areas in southwestern and northeastern Taiwan, where the drinking water is known to contain high concentrations of arsenic (30). In addition, all subjects enrolled in this study denied excessive use of analgesics. Moreover, tobacco smoke carcinogens are unlikely to be confounders in this study as 64 of 69 women enrolled, and 32 of 45 men for whom exposure to AA was documented by the presence of adducts, had never smoked (Table 1). Also, our logistic regression models included an adjustment for smoking. Finally, in principle, herbal remedies might contain genotoxins other than AA. This finding seems unlikely to confound our results, because the predominant DNA adduct detected in the renal cortex invariably was identified as dA-AL-I using the ultra-sensitive 32P-postlabeling technique and confirmed by quantitative MS analysis, in both cases, using chemically defined standards.
In 1995, Taiwan established a national health insurance program through which more than 96% of residents are reimbursed for the cost of prescribed medicines, including herbal remedies. This national reimbursement database has been used to conduct large-scale, population-based, case-control studies designed to examine the association between AA-containing herbal products and the risk of developing urothelial cancer and chronic renal disease. Using this approach, more than half of the 23 million residents of Taiwan were estimated to have used Chinese herbal remedies between 1997 and 2003, with more than one-third of the entire population using herbs containing, or likely to contain, AA (14). Additionally, these population-based studies documented a linear dose–response relationship between exposure to AA-containing herbs and the risk of developing urinary tract cancers (13) or end-stage renal failure (15). Importantly, among the 15–54 age subgroup, more than 60% of all Chinese herb prescriptions were written for women. Specifically, the widely used multiherb formula, Longdan Xiegan Tang, which contains Guanmutong (Aristolochia manchuriensis), was frequently prescribed for this population, comprising a male-to-female ratio of 44/55 (31). Thus, the higher incidence of UUC among Taiwanese women (Fig. 4C) may reflect, in part, the more extensive exposure of women to Aristolochia herbal remedies.
The vast majority of sporadic urothelial carcinomas are located in the urinary bladder, with less than 5% of these tumors involving the renal pelvis and ureter (32). Worldwide, UUC occurs predominantly in men; for example, in the United States, the male to female ratio is 3:1 (32). In Taiwan, however, statistics for urothelial carcinomas present a different demographic, one that mimics that associated with EN in Balkan countries (8, 9). Thus, 35% of urothelial carcinomas in Taiwan involve the renal pelvis or ureter, while the incidence of UUC in men and women is approximately equal (33). Additionally, Taiwanese men and women who undergo dialysis or renal transplantation for end-stage renal disease are at high risk of developing UUC; until now, the basis for this association has been unclear (34).
We attribute the progressive increase in the incidence of UUC in Taiwan over the past 25 y, especially among women (Fig. 4), in part to the systematic replacement of traditionally used Mutong and Fangchi herbs with Aristolochia manchuriensis and Aristolochia fangchi, respectively (35, 36). In mainland China, this practice appears to have begun in the 1930s, becoming universal by 1950 and continuing until 2003, when these substitutions were prohibited by the Chinese government (37). The presence of AA in Mutong and Fangchi exported to Taiwan between 1995 and 2003, as well as to other Asian countries, Great Britain, and the Netherlands, has been documented by chemical analysis (38, 39). Thus, assuming a latency period of 20–40 y, an estimate based on the development of AA-associated urothelial carcinomas in Balkan countries (8, 9), the carcinogenic effects of AA would be expected to have become increasingly manifest in Taiwan by 1985 (Fig. 4).
In conclusion, this study provides compelling evidence for the primary role of AA in the etiology of UUC in Taiwan. Importantly, the traditional practice of Chinese herbal medicine in Taiwan mirrors that in China and other Asian countries. Thus, it appears likely that UUC and its attendant AAN also are prevalent in these and in other countries where Aristolochia herbs have long been used for treatment and prevention of disease (1, 5). Because of the lifelong persistence of mutagenic DNA-AL-I adducts in target tissues and irreversible damage to the proximal renal tubules caused by AA, persons treated with Aristolochia herbal preparations at any time in their life are at significant risk of developing UUC or chronic renal disease, thereby creating an international public health problem of considerable magnitude.
Materials and Methods
All subjects of this study are long-term residents of Taiwan. Following nephroureterectomy for UUC, fresh tumor and renal cortex tissues were snap-frozen and stored at –80 °C at the National Taiwan University Hospital (NTUH) tissue bank. Portions of these tissues were fixed in formalin and used for histopathologic review. Samples deposited in the tissue bank from 1994 to 2011 were used in the present study; two patients living in arsenic endemic areas were excluded. The protocol for this study was approved by the Institutional Review Boards of NTUH and Stony Brook University.
A full medical history and clinical laboratory data were available for all patients. Histopathologic diagnoses were confirmed independently by pathologists in Taiwan and the United States. Creatinine levels in serum samples collected before surgery were used to calculate estimated glomerular filtration rates using the MDRD equation (21), which, in turn, was used to stage CKD (22).
Procedures used to isolate DNA from frozen tissues, to sequence the TP53 gene using the AmpliChip p53 mutation detection algorithm (Roche), to identify DNA-AL adducts by MS, and to quantify the level of dA-AL-I adducts in renal cortical tissues using 32P-postlabeling methods have been previously described (6, 7, 12). Methods used for pyrosequencing analysis of FGFR3 (epithelial form IIIb; codon 373) and HRAS (codon 61) are described in SI Materials and Methods and Table S3, along with details of MS analyses.
×2 tests were used to evaluate associations. Logistic regression analysis was used to evaluate the association between DNA adducts and the risk of A:T to T:A transversions, adjusting for age and smoking, and age, smoking, and sex, respectively. A P value of 0.05 was considered statistically significant for all analyses.
Supplementary Material
Acknowledgments
We thank Shih-Chen Tang and Hui-Chun Chien (National Taiwan University), Sim Truong (Roche), and Gyongyi Mihalyne (Stony Brook University) for expert technical assistance; Katherine W. Snapinn (University of Washington) for statistical analysis; and Jung-Der Wang (National Cheng Kung University) and Jacques Ferlay (International Agency for Research on Cancer) for analyzing data from the Taiwan Cancer Registry and International Agency for Research on Cancer GLOBOCAN databases, respectively. This research was supported in part by Grant P01ES004068 from the National Institute of Environmental Health Sciences (to A.P.G.); Grant PTH9807 from Taoyuan General Hospital (to C.-H.C.); Grant DOH100-TD-C-111-001 from the Department of Health, Taiwan (to Y.-S.P.); and Grant R01ES019564 from the National Institute of Environmental Health Science (to R.J.T.). K.G.D. was the recipient of a Zickler Translational Research Scholar Award funded by the Zickler Family Foundation.
Footnotes
Conflict of interest statement: L.W. is an employee of Roche Molecular Systems, where the Amplichip p53 test used in this study is currently in product development.
*This Direct Submission article had a prearranged editor.
See Commentary on page 7955.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119920109/-/DCSupplemental.
References
- 1.Grollman AP, Scarborough J, Jelaković B. Aristolochic acid nephropathy: An environmental and iatrogenic disease. In: Fishbein JC, editor. Advances in Molecular Toxicology. 3rd Ed. Amsterdam: Elsevier; 2009. pp. 211–222. [Google Scholar]
- 2.Vanherweghem JL, Debelle F, Muniz-Martinez MC, Nortier JL. Aristolochic acid nephropathy after Chinese herb remedies. In: De Broe ME, Porter GA, Bennett WM, Verpooten GA, editors. Clinical Nephrotoxins. 2nd Ed. Dordrecht: Kluwer; 2003. pp. 588–601. [Google Scholar]
- 3.Nortier JL, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) N Engl J Med. 2000;342:1686–1692. doi: 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- 4.National Toxicology Program Aristolochic acids. Rep Carcinog. 2011;12:45–49. [PubMed] [Google Scholar]
- 5.Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008;74:158–169. doi: 10.1038/ki.2008.129. [DOI] [PubMed] [Google Scholar]
- 6.Grollman AP, et al. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci USA. 2007;104:12129–12134. doi: 10.1073/pnas.0701248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jelaković B, et al. Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid. Kidney Int. 2012;81:559–567. doi: 10.1038/ki.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petronić V. Tumors of the upper urothelium and endemic nephropathy. In: Radovanović Z, Sindić M, Polenaković M, Djukanović L, Petronić V, editors. Endemic Nephropathy. Belgrade, Serbia: Institute for Textbook Publishing; 2000. pp. 350–439. [Google Scholar]
- 9.Nikolić J. Izdavačko preduzeće. Belgrade, Serbia: Beograd AD; 2006. Epidemic nephropathy and upper urothelial tumors. [Google Scholar]
- 10.Hranjec T, et al. Endemic nephropathy: The case for chronic poisoning by Aristolochia. Croat Med J. 2005;46:116–125. [PubMed] [Google Scholar]
- 11.Arlt VM, Stiborova M, Schmeiser HH. Aristolochic acid as a probable human cancer hazard in herbal remedies: A review. Mutagenesis. 2002;17:265–277. doi: 10.1093/mutage/17.4.265. [DOI] [PubMed] [Google Scholar]
- 12.Moriya M, et al. TP53 Mutational signature for aristolochic acid: An environmental carcinogen. Int J Cancer. 2011;129:1532–1536. doi: 10.1002/ijc.26077. [DOI] [PubMed] [Google Scholar]
- 13.Lai MN, Wang SM, Chen PC, Chen YY, Wang JD. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl Cancer Inst. 2010;102:179–186. doi: 10.1093/jnci/djp467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh SC, Lin IH, Tseng WL, Lee CH, Wang JD. Prescription profile of potentially aristolochic acid containing Chinese herbal products: An analysis of National Health Insurance data in Taiwan between 1997 and 2003. Chin Med. 2008;3:13. doi: 10.1186/1749-8546-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai MN, et al. Risks of kidney failure associated with consumption of herbal products containing Mu Tong or Fangchi: A population-based case-control study. Am J Kidney Dis. 2010;55:507–518. doi: 10.1053/j.ajkd.2009.10.055. [DOI] [PubMed] [Google Scholar]
- 16.Olivier M, et al. The IARC TP53 database: New online mutation analysis and recommendations to users. Hum Mutat. 2002;19:607–614. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- 17.Wu XR. Urothelial tumorigenesis: A tale of divergent pathways. Nat Rev Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 18.Kompier LC, et al. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS ONE. 2010;5:e13821. doi: 10.1371/journal.pone.0013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin JK, et al. Cigarette smoking and cancers of the renal pelvis and ureter. Cancer Res. 1992;52:254–257. [PubMed] [Google Scholar]
- 20.Colin P, et al. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int. 2009;104:1436–1440. doi: 10.1111/j.1464-410X.2009.08838.x. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, et al. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 23.Nedelko T, Arlt VM, Phillips DH, Hollstein M. TP53 mutation signature supports involvement of aristolochic acid in the aetiology of endemic nephropathy-associated tumours. Int J Cancer. 2009;124:987–990. doi: 10.1002/ijc.24006. [DOI] [PubMed] [Google Scholar]
- 24.Mitra AP, Birkhahn M, Cote RJ. p53 and retinoblastoma pathways in bladder cancer. World J Urol. 2007;25:563–571. doi: 10.1007/s00345-007-0197-0. [DOI] [PubMed] [Google Scholar]
- 25.Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Oers JM, et al. FGFR3 mutations indicate better survival in invasive upper urinary tract and bladder tumours. Eur Urol. 2009;55:650–657. doi: 10.1016/j.eururo.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Attaluri S, et al. DNA adducts of aristolochic acid II: Total synthesis and site-specific mutagenesis studies in mammalian cells. Nucleic Acids Res. 2010;38:339–352. doi: 10.1093/nar/gkp815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukin M, Zaliznyak T, Johnson F, de Los Santos C. Structure and stability of DNA containing an aristolactam II-dA lesion: Implications for the NER recognition of bulky adducts. Nucleic Acids Res. 2012;40:2759–2770. doi: 10.1093/nar/gkr1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidorenko VS, et al. Lack of recognition by global-genome nucleotide excision repair accounts for the high mutagenicity and persistence of aristolactam-DNA adducts. Nucleic Acids Res. 2012;40:2494–2505. doi: 10.1093/nar/gkr1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CL, et al. Arsenic in drinking water and risk of urinary tract cancer: A follow-up study from northeastern Taiwan. Cancer Epidemiol Biomarkers Prev. 2010;19:101–110. doi: 10.1158/1055-9965.EPI-09-0333. [DOI] [PubMed] [Google Scholar]
- 31.Wang JD. Establishment of an active surveillance system for Chinese herbal medicine. Yearbook Chinese Medicine and Pharmacy. 2006;24:157–280. [Google Scholar]
- 32.Ferlay J, et al. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 (International Agency for Research on Cancer, Lyon, France. 2010. Available from: http://globocan.iarc.fr. Accessed October 18, 2010.
- 33.Bureau of Health Promotion, Deptartment of Health, Taiwan. The incidence of bladder, renal pelvic and ureteral tumor in Taiwan, https://cris.bhp.doh.gov.tw/pagepub/Home.aspx?itemNo=cr.q.10. Accessed March 15, 2011.
- 34.Chen CY, Liao YM, Tsai WM, Kuo HC. Upper urinary tract urothelial carcinoma in eastern Taiwan: High proportion among all urothelial carcinomas and correlation with chronic kidney disease. J Formos Med Assoc. 2007;106:992–998. doi: 10.1016/S0929-6646(08)60074-1. [DOI] [PubMed] [Google Scholar]
- 35.Wu KM, Farrelly JG, Upton R, Chen J. Complexities of the herbal nomenclature system in traditional Chinese medicine (TCM): Lessons learned from the misuse of Aristolochia-related species and the importance of the pharmaceutical name during botanical drug product development. Phytomedicine. 2007;14:273–279. doi: 10.1016/j.phymed.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Zhu YP. Toxicity of the Chinese herb mu tong (Aristolochia manshuriensis). What history tells us. Adverse Drug React Toxicol Rev. 2002;21:171–177. doi: 10.1007/BF03256194. [DOI] [PubMed] [Google Scholar]
- 37.China State Food and Drug Administration Suspension of Traditional Chinese Medicine Standard on Guan Mutong, April 1 announcement, Notice no. 121. 2003. Available at http://www.zhong-yao.net/shi/24631.htm. Accessed December 1, 2011.
- 38.Chuang MS, Hsu YH, Chang HC, Lin JH, Liao CH. Studies on adulteration and misuage of marketed Akebiae caulis. Ann Rept NLFD Taiwan ROC. 2002;20:104–119. [Google Scholar]
- 39.Martena MJ, et al. Enforcement of the ban on aristolochic acids in Chinese traditional herbal preparations on the Dutch market. Anal Bioanal Chem. 2007;389:263–275. doi: 10.1007/s00216-007-1310-3. [DOI] [PubMed] [Google Scholar]
- 40.Segi M, Fujisaku S, Kurihara M, Narai Y, Sasajima K. The age-adjusted death rates for malignant neoplasms in some selected sites in 23 countries in 1954–1955 and their geographical correlation. Tohoku J Exp Med. 1960;72:91–103. doi: 10.1620/tjem.72.91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.