Abstract
Inner ear hair cells are specialized sensory cells essential for auditory function. Previous studies have shown that the sensory epithelium is postmitotic, but it harbors cells that can behave as progenitor cells in vitro, including the ability to form new hair cells. Lgr5, a Wnt target gene, marks distinct supporting cell types in the neonatal cochlea. Here, we tested the hypothesis that Lgr5+ cells are Wnt-responsive sensory precursor cells. In contrast to their quiescent in vivo behavior, Lgr5+ cells isolated by flow cytometry from neonatal Lgr5EGFP-CreERT2/+ mice proliferated and formed clonal colonies. After 10 d in culture, new sensory cells formed and displayed specific hair cell markers (myo7a, calretinin, parvalbumin, myo6) and stereocilia-like structures expressing F-actin and espin. In comparison with other supporting cells, Lgr5+ cells were enriched precursors to myo7a+ cells, most of which formed without mitotic division. Treatment with Wnt agonists increased proliferation and colony-formation capacity. Conversely, small-molecule inhibitors of Wnt signaling suppressed proliferation without compromising the myo7a+ cells formed by direct differentiation. In vivo lineage tracing supported the idea that Lgr5+ cells give rise to myo7a+ hair cells in the neonatal Lgr5EGFP-CreERT2/+ cochlea. In addition, overexpression of β-catenin initiated proliferation and led to transient expansion of Lgr5+ cells within the cochlear sensory epithelium. These results suggest that Lgr5 marks sensory precursors and that Wnt signaling can promote their proliferation and provide mechanistic insights into Wnt-responsive progenitor cells during sensory organ development.
Keywords: regeneration, hearing, transdifferentiation, stem cells
Degeneration of sensory hair cells causes hearing loss and deafness, a sensory disorder affecting more than 48 million Americans (1). In the mature mammalian cochlea, hair cell loss is considered irreversible because no hair cell regeneration has been observed (2). Conversely, in nonmammalian vertebrates including birds and amphibians, supporting cells are capable of regenerating lost hair cells to restore hearing function (3, 4). Pulse–chase experiments found that supporting cells proliferated before differentiating into new hair cells in the avian cochlea, but they also could acquire a hair cell fate directly without dividing, a process termed “direct differentiation” (5).
The developing mammalian cochlear epithelium becomes mitotically quiescent between embryonic day (E) 12.5 and 14.5 and expresses the cell-cycle inhibitor p27(Kip1), which marks the prosensory region (6). Sensory hair cells emerge in this region and become organized in a checkerboard pattern and are interspersed with supporting cells. Although the postnatal sensory epithelium remains mitotically quiescent, several recent studies have reported isolation of cells with proliferative capacity from the neonatal cochlea. When isolated cochlear cells were cultured in growth factor or serum-supplemented conditions, they proliferated, demonstrated colony-formation capacity, and also generated new hair cell-like cells (7, 8). To begin identifying cells with progenitor cell potentials, Sinkkonen and colleagues (9) isolated CD326+/CD146low/CD271low supporting cells and found that these cells exhibit such progenitor behavior. These results corroborated with those of White et al. (7), who isolated supporting cells using the p27(Kip1)-GFP mouse line. When p75-nerve growth factor receptor was used as an enrichment marker, hair cell formation increased, raising the possibility that distinct supporting cell types are more capable of differentiating into hair cells in vitro. To date, the in vivo behavior of cochlear supporting cells in the postnatal period and the role of Wnt signaling in regulating their behavior remain unclear.
Wnt signaling plays a critical role in regulating tissue homeostasis, including the maintenance of somatic stem cells (10). Leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5), a Wnt target gene, has been shown to mark endogenous stem cells in rapidly proliferating organs (11, 12). In the postnatal cochlea we have shown that Lgr5 expression is Wnt dependent and limited to supporting cell subtypes (13). The current study demonstrates that Lgr5+ cells behave as hair cell precursors as supported by fate-mapping studies. In culture, they exhibited progenitor cell ability and formed clonal colonies and new hair cells. Moreover, both in vitro and in vivo, Wnt signaling enhanced proliferation of Lgr5+ cells. Together, these data indicate that Lgr5 marks Wnt-regulated sensory precursor cells in the postnatal cochlea.
Results
Isolated Lgr5+ Cells Behave as Progenitor Cells in Vitro.
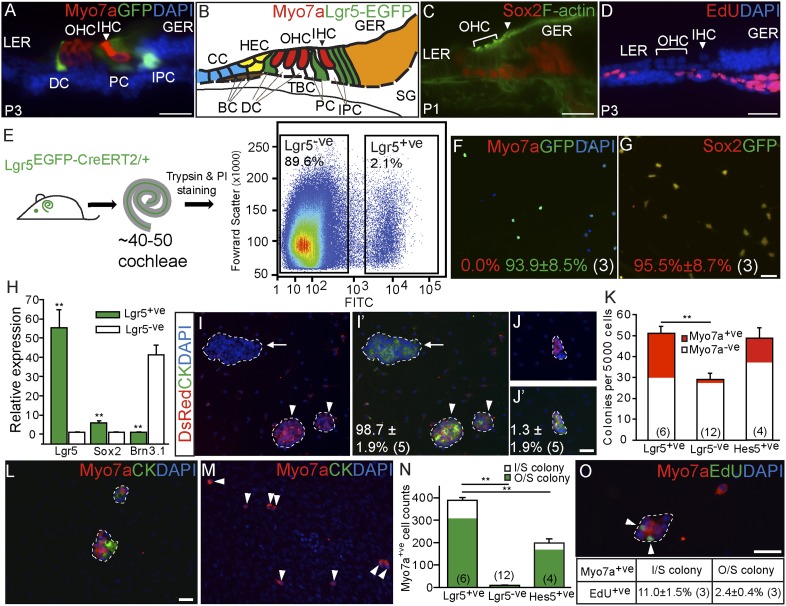
We previously characterized the Lgr5EGFP-CreERT2/+ mouse and found it to have normal cochlear morphology and hearing thresholds (11, 13). In the cochleae of neonatal Lgr5EGFP-CreERT2/+ mice, GFP is coexpressed with Sox2 in the third Deiters’ cells, inner pillar cells, inner phalangeal cells, and lateral greater epithelial ridge cells (Fig. 1 A–C). When the thymidine analog 5-ethynyl-2′-deoxyuridine (EdU) was injected on postnatal day (P) 0–2 (50 mg/kg two times/d), no labeling was noted in the sensory epithelium (Fig. 1D), confirming previous findings that supporting cells are mitotically quiescent (6, 14). Next, we dissociated P0–3 cochleae from Lgr5EGFP-CreERT2/+ mice and isolated GFP+ cells via flow cytometry; GFP+ cells constituted ∼2.1% of viable cells (Fig. 1E). Immunostained Lgr5+ cells were 94% GFP+, 96% Sox2+, and 0% myo7a+ (Fig. 1 F and G), whereas Lgr5− cells were 0% GFP+, 3% Sox2+, and 1% myo7a+ (Fig. S1 A and B). Quantitative RT-PCR showed that sorted Lgr5+ cells, in comparison with Lgr5− cells, expressed higher levels of Lgr5 and Sox2 and lower levels of the hair cell marker Brn3.1 (Fig. 1H and Table S1) (15). These data indicate that sorted Lgr5+ supporting cells were highly pure.
Fig. 1.
Lgr5+ cochlear supporting cells act as progenitor cells in vitro. (A) Cryosection of P3 Lgr5EGFP-CreERT2/+ cochlea showed GFP expression in the third Deiters’ cells (DC), inner pillar cells (PC), inner phalangeal cells (IPC), and the lateral greater epithelial ridge (GER). IHC, inner hair cells; LER, lesser epithelial ridge; OHC, outer hair cells. (B) Schematic depicting cell types in the P0–3 cochlea. BC, Boettcher cells; CC, Claudius cells; HEC, Hensen’s cells; SG, spiral ganglia; TBC, tympanic border cells. (C) Sox2 is expressed in Lgr5+ cells and other supporting cell types. (D) Cochlea from P3 mouse injected with EdU (P0–2) demonstrated no EdU uptake in the sensory epithelium. (E) Lgr5EGFP-CreERT2/+ cochleae were dissociated, and GFP+ and GFP− cells were isolated using flow cytometry. (F–H) Immunostaining and quantitative PCR showed that isolated GFP+ cells did not contain myo7a+ cells and robustly expressed Lgr5 and Sox2 but not brn3.1. (I and J) Lgr5+ cells isolated from Lgr5EGFP-CreERT2/+; Actin-DsRed mice were mixed (1:1) with those from Lgr5EGFP-CreERT2/+ mice and cultured. After 10 d, the majority of cytokeratin-positive colonies formed were monochromatic. I and J show DsRed and DAPI labeling only. I′ and J′ show DsRed, cytokeratin (CK), and DAPI labeling. (K) Lgr5+ and Hes5+ cells formed more colonies than Lgr5− cells. Forty percent of colonies from Lgr5+ cells contained myo7a+ cells. (L–N) Lgr5+ cells generated more myo7a+ cells than Lgr5− or Hes5+ cells, most of which were outside (O/S) colonies. I/S, inside colonies. (O) In the presence of EdU, only a minority of myo7a+ cells were EdU+; double-positive cells were noted more commonly inside colonies. Data are represented as mean ± SD. **P < 0.01. In F, G, I–K, N, and O, n is shown in parentheses. (Scale bars, 25 μm.)
To study the behavior of Lgr5+ cells, we cocultured 5,000 Lgr5+ cells with mitomycin-inactivated feeder cells derived from embryonic chicken utricle mesenchyme (Fig. S2). Inner ear-derived mesenchymal tissues have been shown to foster differentiation of cochlear supporting cells (7, 9). These mesenchymal cells do not express hair cell or supporting cell markers (16). After 10 d in serum-free medium, Lgr5+ cells formed epithelial colonies (consisting of at least five DAPI+ cells), which were immunostained with the pan-cytokeratin antibody (Fig. 1 I and J). When Lgr5+ cells from Lgr5EGFP-CreERT2/+;Actin-DsRed mice were mixed (1:1) with those from Lgr5EGFP-CreERT2/+ animals, 99% of colonies were monochromatic, suggesting that they were clonally derived from single cells (Fig. 1 I and J and Fig. S2B). To determine if Lgr5 serves as a marker for sensory progenitor cell enrichment, we compared it with Hes5, which is a Notch target gene expressed in most cochlear supporting cell types (Fig. S3 A and B) (17). We isolated and cultured Hes5+ cells from cochleae of P0–3 Hes5-GFP transgenic mice (18) and found that the colony counts from Hes5+ and Lgr5+ cells were comparable and significantly higher than in Lgr5− cells (Fig. 1K).
Myo7a is a well-established specific marker of hair cells (9, 16). To investigate Lgr5+ cells’ potential for hair cell differentiation, we immunostained and characterized two classes of myo7a+ cells: those residing inside or outside epithelial colonies. On average, the majority of myo7a+ cells (308 of 389) resided outside colonies (Fig. 1 L–N). In comparison with Hes5+ and Lgr5− cells, Lgr5+ cells generated significantly more myo7a+ cells. With EdU supplementation, 11.0% of myo7a+ cells within epithelial colonies were labeled. We observed a correlation between myo7a+ cells residing outside colonies and decreased EdU labeling (Fig. 1O), suggesting that the majority of hair cells were generated via direct differentiation. In support of their hair cell identity, myo7a+ cells also expressed other hair cell markers: calretinin, parvalbumin, and myo6 (Fig. S4 A–C). In addition to myo7a+ cells, many Sox2+ and GFP− cells were found both inside and outside colonies (Fig. S4 C and G). Seventy-one percent of myo7a+ cells exhibited polarized protrusions expressing F-actin and the actin-bundling protein espin (Fig. S4 D–F) (19). These protrusions occasionally are V-shaped and resemble stereocilia of nascent hair cells. These results show that Lgr5+ cells can act as sensory progenitors and led us to investigate their in vivo behavior.
Lgr5+ Cells Behave as Precursors to Hair Cells and Supporting Cells in Vivo.
Prior in situ hybridization experiments had shown that Lgr5 expression is restricted to supporting cell subtypes (13). Cochleae from P3 Lgr5EGFP-CreERT2/+ also showed this expression pattern with no apical-to-basal gradient (Fig. 2A). However, we noticed rare pairings of GFP+, myo7a+ cells (two pairs in eight cochleae), with one located at the outer hair cell and the other at the third Deiters’ cell position (Fig. 2B). These pairs of double-positive cells also were rare at P5 (two pairs in four cochleae), and none were found at P9 (Fig. 2 D–F). In light of Lgr5+ cells’ ability to differentiate into hair cells in vitro, we postulate that GFP+, myo7a+ cells may represent differentiating hair cells. To probe this possibility further, we performed lineage-tracing experiments using Lgr5EGFP-CreERT2/+; R26RtdTomato/+ mice (20). Tamoxifen administration at P3 activated tdTomato labeling of Lgr5+ cells at P5 (Fig. 2 C–E and Fig. S5 A and B). In the P5 cochleae, traced cells included rare GFP+, myo7a+ cells but also occasional GFP− hair cells and other supporting cells (Fig. S5 A and B). It is likely that GFP+, myo7a+ cells at both the hair cell and the third Deiters’ cell positions from the P3 cochlea contributed, at least in part, to tdTomato+, myo7a+ cells in the P5 and P9 cochleae.
Fig. 2.
Lineage tracing of Lgr5+ cells in the postnatal cochlea. (A and B) P3 Lgr5EGFP-CreERT2/+ cochlea showed GFP signals in supporting cell subtypes. GFP was rarely detected in myo7a+ cells and always was adjacent to a myo7a+, GFP+ cell at the third Deiters' cell position (arrowheads in B). (B′--B′′) Side views of reconstructed confocal images from B. B′ corresponds to the ′ position and B′′ to the ′′ position in B. (C) Tamoxifen was administered to P3 Lgr5EGFP-CreERT2/+; R26RtdTomato/+ mice, and cochleae were examined 2 and 6 d later. (D and E) Two days later, traced cells included the third Deiters' cells, inner pillar cells, inner phalangeal cells, and greater epithelial ridge cells. Traced GFP+, myo7a+ cells were rarely noted, and all were associated with myo7a+ cells at the the third Deiters' cell position (arrow in E′). (E′–E′′′′) Reconstructed z-stack images from E, showing merged labeling (E′), GFP and myo7a (E′′), tdTomato and myo7a (E′′′), and myo7a only (E′′′′). HC, hair cell. (F) Counts of GFP+ cells per cochlea. n is stated in parentheses. (G) At P9, traced cells included Hensen's cells, the second Deiters' cells (arrowheads), and hair cells (arrows). (H) GFP and myo7a labeling only. H′–H′′′′ are reconstructed z-stack images from G, showing merged labeling (H′), GFP and myo7a (H′′), tdTomato and myo7a (H′′′), and myo7a only (H′′′′). (I) Corn oil controls showed no tdTomato labeling. (J and K) Traced cells among outer and inner hair cells (OHC and IHC). (J′ and J′′) Side view and 3D reconstruction of confocal images from J′′. (K′–K′′′′) Reconstructed z-stack images from K, showing merged labeling (K′), GFP and myo7a (K′′), tdTomato and myo7a (K′′′), and myo7a only (K′′′′). (L) Counts of labeled hair cells and supporting cells from Lgr5EGFP-CreERT2/+; R26RtdTomato/+ mice. n is shown in parentheses. (Scale bars, 25 μm.)
When the tracing period was extended to P9, significantly more traced myo7a+ hair cells were found. Traced cells were found in a subset of outer and inner hair cells and supporting cells (first and second Deiters’ cells and outer pillar cells; Fig. 2 J and K, Fig. S5 C and D, and Movies S1 and S2). Parallel lineage-tracing experiments using Lgr5EGFP-CreERT2/+; R26RlacZ/+ mice (21) similarly found traced (lacZ+) cells among hair cells and supporting cells (Fig. S5 E–H). In both tracing paradigms, corn oil alone did not alter GFP expression or induce Cre-reporter activity (Fig. 2I and Fig. S5E). In addition, traced cells increased significantly from 2 to 6 d after tamoxifen administration (Fig. 2L). The increase in traced cells can represent fully differentiated cells derived from Lgr5+ cells, but we cannot rule out the possibility of a delayed accumulation of Cre reporter activity. In sum, these results support the notion that Lgr5+ cells in the neonatal cochlea behave as precursors to hair cells and supporting cells in vivo.
Active Wnt Signaling Induces Proliferation in Lgr5+ Cells.
In self-renewing organs, overexpression of β-catenin/Wnt signaling expands Lgr5+ cell populations and causes tumor formation (22, 23). To understand how Wnt signaling regulates the mitotically quiescent Lgr5+ cochlear cells, we characterized the Lgr5EGFP-CreERT2/+; Catnbflox(exon3)/+ mice (24), in which tamoxifen-responsive Cre recombinase initiates overexpression of β-catenin in Lgr5+ cells. Following tamoxifen injection at P0–1, we observed the formation of multiple GFP+ foci at P8 adjacent to inner hair cells and lateral to outer hair cells (Fig. 3A). Because Lgr5 expression can be an indication of active Wnt signaling in the cochlea (13), these foci represent expanded clusters of Wnt-activated cells that normally become down-regulated and are most detectable in the third Deiters’ cells at this age (13).
Fig. 3.
Wnt signaling induces proliferation of Lgr5+ cells. (A–C) Tamoxifen was given to P0–1 Lgr5EGFP-CreERT2/+; Catnbflox(exon3)/+ mice. Foci of GFP+ cells were noted 7 d later, abutting the inner hair cells and laterally in the lesser epithelial ridge. The medial foci (arrowheads) shifted adjacent to inner hair cells and disappeared by P21. (D) Pulse–chase experiments showed BrdU uptake (arrowheads) among formed foci. (D′ and D′′) High magnification and side view of foci. (E) Corn oil injection did not induce foci formation. (F) Foci counts per cochlea. (G and H) Tamoxifen was administered to P0–1 Lgr5EGFP-CreERT2/+; Catnbflox(exon3)/+; R26RtdTomato/+ mice. All GFP+ foci expressed tdTomato and Sox2 but not myo7a. G′–G′′′′ Side view of reconstructed images from G. (H′–H′′′′) Individual foci with tdTomato, GFP, Sox2, and Hoescht (H′), Sox2 only (H′′), tdTomato and Hoescht (H′′′), and GFP and Hoescht labeling (H′′′′). (I–O) Purified Lgr5+ cells were cultured (10 d) with Wnt3a or R-spondin1. Treatment cells with Wnt3a or R-spondin1 increased cytokeratin-positive colonies, myo7a+ cells inside colonies, and EdU+, myo7a+ cells, but the total counts of myo7a+ cells were unaffected. *P < 0.05; **P < 0.01. In F and M–O, n is shown in parentheses. (Scale bars, 25 μm in A–E, G, H, and J–L; 10 μm in H′–H′′′′.)
Although most foci were adjacent to inner hair cells, a few also were observed in the region of pillar cells and lateral to outer hair cells (Fig. S6 G–I). BrdU injected at P4 was incorporated into these foci, all of which were Sox2+ and myo7a− (Fig. 3 D, G, and H), suggesting proliferation without further differentiation. When Lgr5+ cells were traced simultaneously using the R26R-tdTomato Cre reporter allele, all GFP+ foci expressed tdTomato, implying that they arose from Lgr5+ cells overexpressing β-catenin. Interestingly, we observed a decrease in both the number and size of foci from P8 to P15, and no foci were detected at P21 (Fig. 3 A–C and F and Fig. S6 E and F). This result highlights the transient nature of these foci and suggests that cells constituting these foci underwent cell death. A subset of Lgr5+ cells did not degenerate, because many tdTomato+ cells remained at P21 (Fig. S6F). These results support our hypothesis that active Wnt signaling stimulates the proliferation of Lgr5+ cells. To test this theory further, we next examined the effects of Wnt agonists on isolated Lgr5+ cells.
Isolated Lgr5+ cells formed more colonies when cultured with Wnt3a (25) or R-spondin1 (Fig. 3 I–L and N) (23), but colony size was variable and was not affected by either treatment. Myo7a+ cell counts were comparable in drug-treated cells and vehicle-only controls; however, EdU+, myo7a+ cells doubled when treated with either Wnt agonist (P < 0.01 and <0.001, respectively) (Fig. 3 M and O). In support of the notion that more hair cells differentiated following a mitotic division, Wnt agonists increased the number of myo7a+ cells residing inside colonies and decreased the number residing outside colonies (Fig. 3O).
Wnt Inhibition Decreases Proliferation in Lgr5+ Cells.
IWP-2, a small-molecule Wnt inhibitor (26), significantly decreased Lgr5+ cells’ colony-forming capacity and EdU uptake (P < 0.001 for both) (Fig. 4 B–F). In comparison with vehicle-treated controls, IWP-2, reduced EdU, myo7a double-labeled cells, myo7a+ cells inside colonies, and EdU-labeled colonies. As a positive control, we treated Lgr5+ cells with the DNA polymerase inhibitor aphidocilin and found similar results. The number of myo7a+ cells outside colonies did not change significantly with aphidocilin but increased slightly with IWP-2 treatment (Fig. 4G). These results show that Wnt inhibition diminishes the proliferative capacity of Lgr5+ cells, whose ability to differentiate into hair cells does not require proliferation. Similarly, IWP-2 and aphidocilin decreased the colony-forming capacity of and EdU uptake in isolated Lgr5− cells (Fig. S7 C and D).
Fig. 4.
Wnt inhibition reduced proliferation of Lgr5+ cells in vitro. (A) Isolated Lgr5+ cells were cultured (10 d) with IWP-2 or aphidocilin. (B–G) IWP-2 or aphidocilin decreased colony formation and EdU+ colonies. Drug treatment also reduced EdU+, myo7a+ cells and myo7a+ cells inside colonies but not outside colonies. In E–G, n is shown in parentheses. **P < 0.01. (Scale bar, 25 μm in B–D.) (H) Hypothetical model of Wnt-dependent proliferation and differentiation of Lgr5+ cells.
Discussion
Multiple studies have demonstrated that the neonatal cochlea harbors cells capable of proliferating and regenerating hair cells in vitro. Here we show that Lgr5-marked supporting cells exhibit similar progenitor cell behavior in vitro. When cochlear sensory epithelial cells were isolated and cultured, others have observed a transition from mitotic quiescence to proliferation (7–9). Although their colony-forming capacity is comparable to that of Hes5-expressing supporting cells, Lgr5+ cells are enriched hair cell precursors. On average, 7.8% of cultured Lgr5+ cells versus 3.9% of Hes5+ cells generated myo7a+ cells.
In vivo, overactive Wnt signaling was sufficient for Lgr5+ cells to overcome cycle arrest and proliferate, albeit temporarily. Proliferation foci were observed more commonly in the medial region and could have resulted from heterogeneity of Lgr5+ cells and/or their corresponding microenvironments. Although the precise mechanism underlying the disappearance of these proliferation foci currently is unknown, it likely involves activation of the cell-death pathway, a phenomenon occurring when supporting cells are induced to proliferate via retinoblastoma deletion (27). In vitro, Wnt agonists promoted and Wnt antagonist suppressed proliferation in Lgr5+ cells. Together, these data led us to conclude that the degree of Wnt signaling dictates proliferation of Lgr5+ cochlear supporting cells, a relationship observed in other tissue stem/progenitor cells (22, 23).
The mitotic quiescence of the mammalian cochlear sensory epithelium contrasts starkly with that of the nonmammalian vertebrates. However, several lines of evidence show that both mammals and nonmammalian vertebrates are capable of regenerating hair cells by direct differentiation (5, 28). In cultures of Lgr5+ cells, most myo7a+ hair cells were outside epithelial colonies, were EdU−, and formed despite aphidocilin treatment. These results raise the possibility that Lgr5+ cells can differentiate into hair cells via two routes: mitotic or direct differentiation, in which active Wnt signaling promotes proliferation before hair cell differentiation (Fig. 4H). In the mitotically quiescent cochlear epithelium, hair cell formation by direct differentiation may explain the results of lineage-tracing experiments in which labeling of Lgr5+ cells subsequently yielded labeled hair cells. The low level of labeled hair cells may be related to residual Cre recombinase activity, compounded by a delayed clearance of tamoxifen or its active metabolites. However, we cannot rule out the possibility that the increase in traced hair cells also may be attributed to a delayed accumulation of Cre reporter activity. Although our control experiments indicate tight regulation of Cre recombinase activity, we acknowledge these limitations when interpreting the lineage-tracing results.
During development, sensory hair cells first arise in the prosensory region in the basal turn of the cochlea at E13.5, and hair cell formation was presumed complete by E16.5 (29). Our data raise the possibility of a low level of differentiating hair cells in the neonatal cochlea. Postnatal addition of hair cells has been observed in other mammalian species (30, 31); whether this time course is similar in the mouse cochlea will be of interest in future studies. It is important to point out that although Lgr5EGFP-CreERT2/+ cochleae have normal morphology and function (13), it is possible that Lgr5 haploinsufficiency can affect progenitor cell behavior. Recent studies show that Lgr5, like Lgr4 and Lgr6, functions as a receptor for R-spondins and may play a role in fine-tuning Wnt signals (32). It would be of interest to explore whether Lgr5 functions similarly with complementary homologs in the cochlea.
In summary, we report that Lgr5-marked cochlear supporting cells behave as sensory precursor cells in vitro and in vivo. Wnt signals function to regulate proliferation of these cells, which have an intrinsic ability to generate new hair cells. We therefore propose Lgr5+ cells and Wnt signaling as candidate targets in future hair cell regeneration efforts.
Materials and Methods
Further details are given in SI Materials and Methods.
Mice.
Actin-DsRed (33), Rosa26R-lacZ (21), Rosa26R-tdTomato (20), and Lgr5EGFP-CreERT2/+ (11) (all from Jackson Laboratory), Hes5-GFP (provided by Verdon Taylor, University of Sheffield, Sheffield, United Kingdom) (18), and Catnb1-flox(exon3) (24) in C57BL/6 background were used. Primers used for genotyping are shown in Table S2. All protocols were approved by the institutional Animal Care and Use Committees.
Flow Cytometry.
Cochleae from P0–3 Lgr5EGFP-CreERT2/+ or Hes5-GFP mice were isolated under sterile conditions as described previously (13).
Supplementary Material
Acknowledgments
We thank members of our laboratories for fruitful discussions; V. Nookala, Z. Sayyid, J. Kim, and K. Harnish for excellent technical assistance; and V. Taylor for providing the Hes5-GFP mouse line. This work was supported by a Stanford University Dean’s Fellowship (to R.C.), National Institute on Deafness and Other Communication Disorders/National Institutes of Health Grant P30DC010363; American Lebanese Syrian Associated Charities Grant CA21765; Office of Naval Research Grants N000140911014 and R01DC06471; the Hartwell Foundation (to J.Z.), the American Otological Society, Triological Society Grant K08DC011043, and the Akiko Yamazaki and Jerry Yang Faculty Scholar Fund (to A.G.C.). R.N. is a Howard Hughes Medical Institute investigator.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202774109/-/DCSupplemental.
References
- 1.Lin FR, Niparko JK, Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med. 2011;171:1851–1852. doi: 10.1001/archinternmed.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermingham-McDonogh O, Reh TA. Regulated reprogramming in the regeneration of sensory receptor cells. Neuron. 2011;71:389–405. doi: 10.1016/j.neuron.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987;113:1058–1062. doi: 10.1001/archotol.1987.01860100036017. [DOI] [PubMed] [Google Scholar]
- 4.Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 5.Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007;236:156–170. doi: 10.1002/dvdy.21023. [DOI] [PubMed] [Google Scholar]
- 6.Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 7.White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 8.Oshima K, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinkkonen ST, et al. Intrinsic regenerative potential of murine cochlear supporting cells. Sci Rep. 2011;1:26. doi: 10.1038/srep00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 11.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 12.Jaks V, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 13.Chai R, et al. Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J Assoc Res Otolaryngol. 2011;12:455–469. doi: 10.1007/s10162-011-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruben RJ. Development of the inner ear of the mouse: A radioautographic study of terminal mitoses. Acta Otolaryngol Suppl. 1967;220:221–244. [PubMed] [Google Scholar]
- 15.Erkman L, et al. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–606. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- 16.Oshima K, et al. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–716. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartman BH, et al. Hes5 expression in the postnatal and adult mouse inner ear and the drug-damaged cochlea. J Assoc Res Otolaryngol. 2009;10:321–340. doi: 10.1007/s10162-009-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basak O, Taylor V. Identification of self-replicating multipotent progenitors in the embryonic nervous system by high Notch activity and Hes5 expression. Eur J Neurosci. 2007;25:1006–1022. doi: 10.1111/j.1460-9568.2007.05370.x. [DOI] [PubMed] [Google Scholar]
- 19.Zheng L, et al. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell. 2000;102:377–385. doi: 10.1016/s0092-8674(00)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 22.Barker N, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Ootani A, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 26.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Y, et al. In vivo proliferation of postmitotic cochlear supporting cells by acute ablation of the retinoblastoma protein in neonatal mice. J Neurosci. 2010;30:5927–5936. doi: 10.1523/JNEUROSCI.5989-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin V, et al. Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J Neurosci. 2011;31:15329–15339. doi: 10.1523/JNEUROSCI.2057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley MW. Cellular commitment and differentiation in the organ of Corti. Int J Dev Biol. 2007;51:571–583. doi: 10.1387/ijdb.072388mk. [DOI] [PubMed] [Google Scholar]
- 30.Kaltenbach JA, Falzarano PR. Postnatal development of the hamster cochlea. I. Growth of hair cells and the organ of Corti. J Comp Neurol. 1994;340:87–97. doi: 10.1002/cne.903400107. [DOI] [PubMed] [Google Scholar]
- 31.Mu MY, Chardin S, Avan P, Romand R. Ontogenesis of rat cochlea. A quantitative study of the organ of Corti. Brain Res Dev Brain Res. 1997;99:29–37. doi: 10.1016/s0165-3806(96)00194-0. [DOI] [PubMed] [Google Scholar]
- 32.de Lau W, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 33.Vintersten K, et al. Mouse in red: Red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis. 2004;40:241–246. doi: 10.1002/gene.20095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.