Abstract
Localized pH changes have been suggested to occur in the brain during normal function. However, the existence of such pH changes has also been questioned. Lack of methods for noninvasively measuring pH with high spatial and temporal resolution has limited insight into this issue. Here we report that a magnetic resonance imaging (MRI) strategy, T1 relaxation in the rotating frame (T1ρ), is sufficiently sensitive to detect widespread pH changes in the mouse and human brain evoked by systemically manipulating carbon dioxide or bicarbonate. Moreover, T1ρ detected a localized acidosis in the human visual cortex induced by a flashing checkerboard. Lactate measurements and pH-sensitive 31P spectroscopy at the same site also identified a localized acidosis. Consistent with the established role for pH in blood flow recruitment, T1ρ correlated with blood oxygenation level-dependent contrast commonly used in functional MRI. However, T1ρ was not directly sensitive to blood oxygen content. These observations indicate that localized pH fluctuations occur in the human brain during normal function. Furthermore, they suggest a unique functional imaging strategy based on pH that is independent of traditional functional MRI contrast mechanisms.
Keywords: brain pH, functional magnetic resonance imaging, T1rho
To what degree pH changes during normal brain function is unclear (1). However, neuronal activity could cause transient, localized pH changes via several mechanisms. Increased neuronal activity enhances carbohydrate metabolism producing the pH-lowering by-products lactic acid and CO2 (2). Activity-evoked HCO3− transport can alter pH (3). Local field potentials produced by ion fluxes could change pH (4). In addition, acidic synaptic vesicles release protons during neurotransmission (5). Such dynamic pH fluctuations have the potential to dramatically alter physiology and behavior through a number of pH-sensitive receptors and channels (6). Acid-sensing ion channels, for example, play critical roles in synaptic plasticity, learning, memory, pain, and neurodegeneration (7–10). Superimposed on activity-dependent brain pH changes and the potential physiological effects are several buffering systems. Principal among these is the CO2/HCO3− system. In a reversible reaction, CO2 combines with water to form carbonic acid, which readily dissociates into HCO3− and H+. Raising HCO3− shifts the equilibrium away from H+ and increases pH. Conversely, raising CO2 shifts the equilibrium toward H+, thereby lowering pH. The ability to measure these pH changes in the functioning brain is key for gaining insight into this poorly understood dimension of CNS physiology and pathophysiology.
Routinely measuring pH in the brain would require novel noninvasive methods. Traditionally, 31P spectroscopy has been used to estimate brain pH (11); however, 31P is limited by poor spatial resolution (typically 10- to 30-cm3 volumes), long acquisition times (often 5–10 min for a single measurement), and the need for special hardware not typically available on clinical scanners. Recently, 1H MRI pulse sequences have been shown to detect H+ exchange between water and proteins and thus can be highly pH sensitive. These techniques include amide proton transfer (APT) and T1 in the rotating frame (T1ρ) (12–15). APT detects H+ exchange by taking advantage of differences in resonances between amide and water protons. The spin-lock preparation pulse used in T1ρ imaging sensitizes the magnetic resonance (MR) signal to relaxation effects arising from H+ exchange between free water protons and those bound to proteins and macromolecules. Here, we focused on T1ρ because of its pH sensitivity, high spatial and temporal resolution, and potential to detect dynamic pH changes during brain function.
Results
Validation of pH Sensitivity in Buffered Phantoms.
To evaluate T1ρ sensitivity to pH in the physiological range, we first studied phantoms [3.5% agar (wt/vol)], 8% BSA (wt/vol), 0.1 M phosphate buffered saline pH-adjusted with HCl and NaOH to values ranging from pH 6.0 to pH 8.0 (Fig. 1A). They were imaged by using a fast spin-echo sequence with a spin-locking preparation pulse, which created a B1 field of 1,000 Hz, and four spin-lock times (10, 20, 40, and 60 ms). T1ρ times were inversely proportional to the measured pH (R2 = 0.98) (Fig. 1A), suggesting that T1ρ is sensitive to pH in the physiological range.
Fig. 1.
T1ρ and T2* sensitivity to pH and pO2 manipulations. (A) T1ρ maps for agar phantoms with pH adjusted to different levels (6.0–8.0). The relationship between T1ρ times and pH was linear within this range. The estimated T1 times were calculated within a central 5 × 5 region. (B) T1ρ maps in sheep blood phantoms (Upper) with corresponding mean and SD in a central 5 × 5 region of interest plotted in Lower. The sheep blood phantoms are arranged from left to right as follows: unaltered (control), acidified, and hyperoxygenated. (C) Corresponding T2* maps in same sheep blood phantoms (Upper) with mean and SD in a central 5 × 5 region of interest plotted in Lower.
T1ρ Insensitivity to Oxyhemoglobin Content.
Because current blood oxygenation level-dependent (BOLD) imaging relies on T2* contrast to detect changes in blood oxyhemoglobin content, we compared the specificity of T1ρ and T2* for pH and oxygen content in fresh sheep blood. Blood pH and O2 content were varied in three test conditions: (i) unaltered, (ii) acidified (25 mM HCl), and (iii) oxygenated with 100% O2. Data from a single axial slice were collected by using a T1ρ fast spin-echo sequence and T2*-weighted gradient-echo sequence. We found that T1ρ was sensitive to pH, but not O2 content (Fig. 1B). Conversely, T2* was sensitive to O2 content, but not pH (Fig. 1C). These data suggest that T1ρ changes are unlikely due to changes in oxyhemoglobin content.
pH Detection in Mouse Brain.
To assess pH sensitivity of T1ρ in vivo, measurements were simultaneously obtained with T1ρ and a custom-made MRI-compatible pH sensor (pHOptica; World Precision Instruments; detection range pH 5–9) implanted into the amygdala of mice. Data were collected from anesthetized mice under three conditions: (i) room air inhalation, (ii) 20% CO2 inhalation, and (iii) following HCO3− injection (5 mmol/kg, i.p.). Fig. 2A shows examples of T1ρ maps obtained from a single mouse brain. Direct pH measurements varied from animal to animal, most likely due to differences in respiratory suppression from anesthesia. In all cases, CO2 inhalation lowered pH relative to air and prolonged T1ρ times throughout the brain, including at the pH-sensing probe tip. HCO3− injection produced the opposite effect, raising pH and shortening T1ρ times. Fig. 2B shows the relationship between T1ρ at the sensor tip and pH measured from the sensor across all mice and conditions (R2 = 0.77).
Fig. 2.
T1ρ detects pH changes in the anesthetized mouse brain evoked by CO2 inhalation or i.p. HCO3− injection. (A) T1ρ maps from a mouse brain after given bicarbonate (Left), exposed to room air (Center), and exposed to 10% CO2 (Right). The position of the fiber-optic pH sensor is shown by the white arrow, and the open square indicates the location of the ROI used for estimating the T1ρ times. (B) The relationship between the mean T1ρ times and the corresponding direct pH measurements obtained from the fiber-optic sensor across four mice. Each mouse is represented by a different symbol.
Systemic pH Changes in Human Brain Induced by CO2 and Hyperventilation.
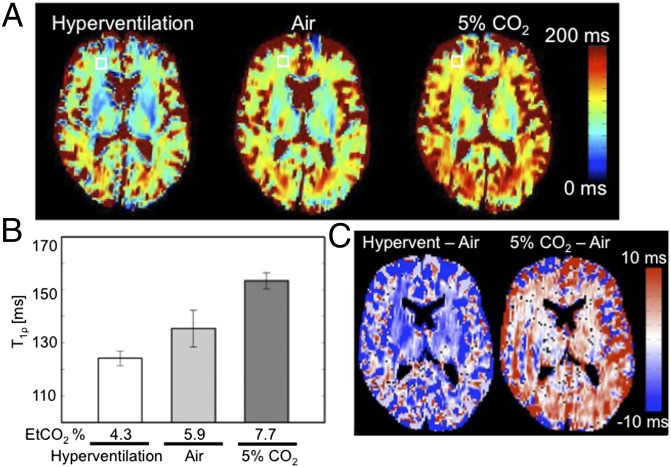
Qualitatively similar T1ρ responses were observed in the human brain when pH was manipulated through breathing. A research participant was imaged on a Siemens 3T scanner under three conditions: (i) normal breathing of room air, (ii) breathing 5% CO2, and (iii) paced hyperventilation of room air (27 breaths per minute). These manipulations changed average end-tidal CO2 (EtCO2) measurements from 4.3% to 7.7% CO2 (Fig. 3B). Consistent with hypercarbic acidosis, CO2 inhalation produced a widespread increase in T1ρ relaxation time (Fig. 3 A and B). Paced hyperventilation produced the opposite effect, and consistent with a respiratory alkalosis reduced T1ρ times (Fig. 3 A and B). Fig. 3C shows the subtracted T1ρ maps between hyperventilation and room air, and between 5% CO2 and room air, suggesting that these systemic manipulations change pH throughout the brain.
Fig. 3.
T1ρ measurements throughout the human brain are responsive to EtCO2 manipulation. (A) T1ρ maps of the human brain varied with EtCO2 concentration during hyperventilation, breathing room air, and 5% CO2 challenge. The open box identifies a 7 × 7 region of interest in white matter where T1ρ time estimates were obtained. (B) The estimated T1ρ times in white matter varied with measured EtCO2 concentrations (module CO2100C; Biopac). (C) T1ρ subtraction images between the hyperventilation and air conditions (Left) and 5% CO2 and air condition (Right).
Localized pH Changes Induced by Flashing Checkerboard.
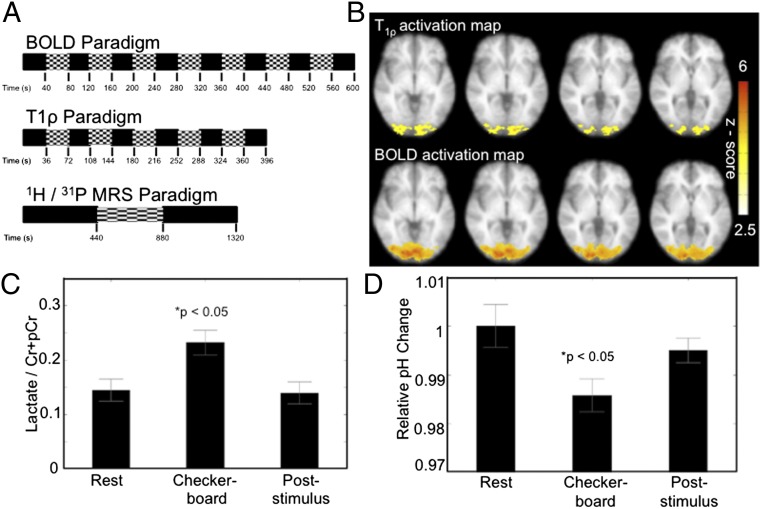
With the above data suggesting that T1ρ is sensitive to brain pH, we hypothesized that T1ρ might detect localized pH changes evoked by brain activation. To test this hypothesis, we used a visual flashing checkerboard task (Fig. 4A). Six participants were presented with a visual flashing checkerboard (22 × 22 squares) alternating at 8 Hz in a block design (Fig. 4A) (16). For comparison, two runs of BOLD were interleaved with three runs of T1ρ. Fig. 4B shows the group activation maps for the T1ρ and BOLD imaging overlaid on the average T1-weighted anatomical image. Voxels exhibiting significant activation (P < 0.05, corrected) are shown. The flashing checkerboard significantly increased T1ρ times in the occipital cortex. A similar activation region was measured by functional MRI (fMRI) BOLD. Consistent with the observation that T1ρ and BOLD detect mutually independent phenomena, a difference was observed between the size of the T1ρ- and BOLD-responsive areas. The reason for this apparent difference is not clear. It is possible that T1ρ detects more focal changes than BOLD, given that the vascular tree underlying the hemodynamic response is larger than regions of neural activity. For example, venous drainage of brain areas can extend the BOLD signal tens of millimeters from the activation site (17). The well-established effect of acidic pH on blood flow (18) suggests localized acidosis might even help drive the BOLD response.
Fig. 4.
Flashing checkerboard alters T1ρ, BOLD, lactate, and 31P measurements in the visual cortex. (A) Flashing checkerboard paradigms for functional T1ρ, BOLD, and MRS studies. For the T1ρ/BOLD studies, three runs of the T1ρ block sequence where interleaved with two runs of BOLD. (B) T1ρ and BOLD functional activation maps (P < 0.05, corrected) resulting from the visual flashing checkerboard stimulus. Four contiguous slices are shown. (C) Lactate to total creatine ratio increased significantly during visual stimulation relative to both baseline and the poststimulus recovery phase. *P < 0.05, paired t test. (D) 31P spectroscopy estimates of pH in the visual cortex were significantly reduced during flashing checkerboard presentation relative to both baseline and recovery. *P < 0.05, paired t test.
Because lactic acid is one potential source of localized pH change, we measured lactate by 1H MR spectroscopy (MRS) in a voxel positioned at the BOLD site. Consistent with a previous report (19), we found that the flashing checkerboard significantly increased the lactate-to-creatine ratio (Fig. 4C). If the T1ρ and lactate responses indeed reflect a localized acidosis, we hypothesized that we should be able to detect the pH change with an alternative method. We chose 31P spectroscopy, a widely accepted measure of pH, and used the T1ρ data to guide voxel positioning. In six additional subjects, the flashing checkerboard altered visual cortex 31P estimates of pH (Fig. 4D). Like T1ρ, these changes indicate a transient, activity-dependent localized acidosis.
Discussion
These results indicate that neuronal activity can change local pH in the human brain during normal function. Neuronal activation may lower pH through a variety of processes (20) acting individually or together to produce the acidosis observed here; further study will be required to identify the mechanisms underlying this pH change. Because monocarboxylate transporters cotransport protons with lactate (21), our studies suggest that lactate production and transport might contribute to the localized acidosis.
The localized acidosis observed here might have a number of consequences for brain physiology and pathophysiology (6, 8). The reduced pH could activate some ion channels and receptors and inhibit others, thereby influencing brain function and behavior (5, 7, 8, 22). A reduced pH has also been implicated in ischemic stroke, neurodegenerative disease, seizures, and respiratory control (9, 10, 23–27). Interestingly, in patients with panic disorder, lactate induction by the flashing checkerboard was abnormally elevated (19). Others have also suggested lactate and pH-buffering abnormalities in panic disorder (28). These observations coupled with our findings support the possibility that abnormal pH dynamics may contribute to panic disorder (2).
Additional advances in our knowledge of brain pH dynamics might come from the improved spatial and temporal resolution provided by T1ρ. The T1ρ sequence used here has an isotropic spatial resolution of ∼4 mm and temporal resolution of 6 s, whereas the spatial and temporal resolutions of 31P spectroscopy are 30 mm and several minutes, respectively. Further improvements in the T1ρ scan time may be possible by acquiring only two spin-lock times and by using parallel imaging. Comparable temporal and spatial resolution might also be possible with APT in an echo-planar sequence if only two frequency-offset pulses were applied about the center imaging frequency.
One limitation of both T1ρ and APT is that they depend on H+ exchange with proteins and amide groups. Thus, either T1ρ or APT could be affected by appreciable changes in local protein concentration as well as pH. The observation that T1ρ correlated closely with direct pH measurements in the mouse brain and with secondary pH imaging methods in human brain argues that the T1ρ changes observed here were due at least in part to pH.
In addition to improving the ability to measure brain pH, this study has unique implications for functional imaging in general. Because T1ρ showed a linear response to pH, T1ρ may be more quantifiable than BOLD fMRI, which is not quantifiable other than percent change and does not address baseline conditions. In addition, the spatial resolutions of BOLD fMRI and 15O-water positron emission tomography depend on blood flow and oxyhemoglobin content and are thus limited by the vascular anatomy. Although T1ρ provided a similar pattern of activation as BOLD, T1ρ changes were independent of blood oxygenation. Thus, by measuring functional pH changes, T1ρ MRI might provide a means for more precisely localizing brain activity.
Methods
Sheep Blood Phantom Imaging.
All animal care met National Institutes of Health standards, and the University of Iowa Animal Care and Use Committee approved all procedures. T1ρ data were collected by using a fast spin-echo sequence with four spin-lock times (10, 20, 40, and 60 ms) and B1 = 400 Hz. T2*-weighted imaging, which is sensitive to blood oxygenation, was obtained from a single axial slice by using a gradient-echo sequence with eight echo-times (1.7, 2, 3, 6, 9, 12, 14, and 16 ms). pH, pO2, and pCO2 levels in the phantoms were confirmed with a blood gas analyzer before and after imaging (Radiometer ABL 5).
Mouse Brain pH Measurements.
pH sensors (pHOptica) were custom-clad in MRI-compatible PEEK tubing (PlasticsOne) and assembled by World Precision Instruments and PreSens Inc. Sensors were implanted into the amygdala as described (7). Twenty-four hours postimplantation, mice were anesthetized with ketamine/xylazine and imaged on a Varian 4.7-T scanner. CO2 (10% and/or 20%) was administered by nasal cannula to lower brain pH as described (7), and NaHCO3 (5 mmol/kg, i.p.) was administered to raise pH as described (7, 29). T1ρ images were collected by using a fast spin-echo sequence [time to echo (TE) = 12 ms, time to repetition (TR) = 2,000 ms, field of view (FOV) = 30 × 30 mm, imaging matrix size = 256 × 128, slice thickness = 1 mm] with spin-lock durations of 10, 20, 40, and 60 ms and B1 = 1,000 Hz. T1ρ maps were generated for each condition, and a 5 × 5 region of interest was placed at the tip of the fiber-optic probe to study the relationship between T1ρ times and pH measured via the fiber optic sensor.
Functional Brain Imaging (BOLD, T1ρ, and 1H MRS).
All human research protocols were approved by the University of Iowa Institutional Review Board. Multimodal functional imaging was performed on six subjects (four males and two females, age 28–35 y). Functional T1ρ images were collected by using an echo-planar spin-echo sequence (TE = 12 ms, TR = 2,200 ms, FOV = 220 × 220 mm, matrix size = 64 × 64, and slice thickness/gap = 4/1.0 mm) with three spin-lock pulses (10, 30, and 50 ms) and a B1 frequency of 400 Hz. This sequence had a temporal resolution of 6.6 s per T1ρ measurement. BOLD imaging was performed by using a T2* weighted echo-planar gradient-echo sequence (TE = 30 ms, TR = 2,000 ms, FOV = 220 × 220 mm, matrix size = 64 × 64, and slice thickness/gap = 4.0/1.0 mm). For BOLD imaging, seven cycles of flashing checkerboard and visual fixation were presented with an 80-s period. For functional T1ρ imaging, five cycles were collected with a 72-s period. The 1H MRS data were acquired by using a single-voxel point-resolved spin-echo sequence with water suppression. For functional 1H spectroscopic imaging, the task began in the baseline task followed by the visual activation condition and returning to the baseline condition. For all activation studies, attention was ensured by asking subjects to press a button in response to a red square presented in the center of the screen every 4 s.
All BOLD fMRI data were analyzed by using standard preprocessing steps, including motion correction, slice timing correction, and spatial smoothing. A general linear model was used to generate individual statistical maps and calculate signal change. T1ρ data were preprocessed by first performing motion correction followed by T1ρ map generation. T1ρ data were spatially smoothed, statistical maps were generated by using a general linear model, and estimates of T1ρ time changes were computed. BOLD percent signal change and T1ρ time changes were mapped to MNI space where a t test was performed across the subjects and corrected for multiple comparisons by using a false discovery rate analysis.
The two 1H spectroscopic measurements obtained for each condition were frequency and phase corrected and averaged, and the resulting spectral data were analyzed by using LCModel. Ratios of Lactate/Cr and Lac/NAA were obtained and compared between (i) baseline, (ii) activation, and (iii) recovery periods using ANOVA.
31P Spectroscopic Functional Measures.
Functional data were acquired in six subjects (male/female = 4/2; ages = 22–33 y). A 2D 31P spectroscopic sequence used a free induction decay acquisition (TE = 2.3 ms, TR = 4,000 ms, FOV = 240 × 240 mm, matrix = 8 × 8, thickness = 30 mm, averages = 16, vector size = 1,024). This acquisition was repeated three times (baseline, activation, baseline) with each measurement taking 10 min 24 s. 31P data were analyzed by using the Siemens Syngo software to determine the chemical shift of the inorganic phosphate (Pi) and phosphocreatine (PCr) peaks in the 31P spectra. The analysis included frequency filtering, frequency and phase correction, baseline correction, and curve fitting with prior knowledge. Brain pH was estimated by using the proposed equation (30):
where δ is the chemical shift between in ppm between Pi and PCr. The pH estimates for the baseline, activated, and recovery phases were compared by using ANOVA.
Acknowledgments
This work was supported by grants from the McKnight Foundation and the Dana Foundation and by a University of Iowa Clinical and Translational Science Award (to V.A.M. and J.A.W.). M.J.W. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
References
- 1.Kauppinen RA, Williams SR. Use of NMR spectroscopy in monitoring cerebral pH and metabolism during systemic and focal Acid-Base Disturbances. In: Kaila K, Ransom BR, editors. pH and Brain Function. New York: Wiley-Liss; 1998. p. 605. [Google Scholar]
- 2.Esquivel G, Schruers KR, Maddock RJ, Colasanti A, Griez EJ. Acids in the brain: A factor in panic? J Psychopharmacol. 2010;24:639–647. doi: 10.1177/0269881109104847. [DOI] [PubMed] [Google Scholar]
- 3.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 4.Stewart PA. Independent and dependent variables of acid-base control. Respir Physiol. 1978;33:9–26. doi: 10.1016/0034-5687(78)90079-8. [DOI] [PubMed] [Google Scholar]
- 5.Traynelis SF, Chesler M. Proton release as a modulator of presynaptic function. Neuron. 2001;32:960–962. doi: 10.1016/s0896-6273(01)00549-9. [DOI] [PubMed] [Google Scholar]
- 6.Tresguerres M, Buck J, Levin LR. Physiological carbon dioxide, bicarbonate, and pH sensing. Pflugers Arch. 2010;460:953–964. doi: 10.1007/s00424-010-0865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziemann AE, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: Advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Xiong ZG, et al. Neuroprotection in ischemia: Blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Friese MA, et al. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 11.Gillies RJ, Raghunand N, Garcia-Martin ML, Gatenby RA. pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Mag. 2004;23:57–64. doi: 10.1109/memb.2004.1360409. [DOI] [PubMed] [Google Scholar]
- 12.Mäkelä HI, Gröhn OH, Kettunen MI, Kauppinen RA. Proton exchange as a relaxation mechanism for T1 in the rotating frame in native and immobilized protein solutions. Biochem Biophys Res Commun. 2001;289:813–818. doi: 10.1006/bbrc.2001.6058. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9:1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 14.Jones CK, et al. Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med. 2006;56:585–592. doi: 10.1002/mrm.20989. [DOI] [PubMed] [Google Scholar]
- 15.Jokivarsi KT, et al. Estimation of the onset time of cerebral ischemia using T1rho and T2 MRI in rats. Stroke. 2010;41:2335–2340. doi: 10.1161/STROKEAHA.110.587394. [DOI] [PubMed] [Google Scholar]
- 16.Kwong KK, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duvernoy HM, Delon S, Vannson JL. Cortical blood vessels of the human brain. Brain Res Bull. 1981;7:519–579. doi: 10.1016/0361-9230(81)90007-1. [DOI] [PubMed] [Google Scholar]
- 18.Kontos HA, Wei EP, Raper AJ, Patterson JL., Jr Local mechanism of CO2 action of cat pial arterioles. Stroke. 1977;8:226–229. doi: 10.1161/01.str.8.2.226. [DOI] [PubMed] [Google Scholar]
- 19.Maddock RJ, Buonocore MH, Copeland LE, Richards AL. Elevated brain lactate responses to neural activation in panic disorder: A dynamic 1H-MRS study. Mol Psychiatry. 2009;14:537–545. doi: 10.1038/sj.mp.4002137. [DOI] [PubMed] [Google Scholar]
- 20.Kaila K, Chesler M. Activity-evoked changes in extracellular pH. In: Kaila K, Ransom BR, editors. pH and Brain Function. New York: Wiley-Liss; 1998. p. 309. [Google Scholar]
- 21.Nedergaard M, Goldman SA. Carrier-mediated transport of lactic acid in cultured neurons and astrocytes. Am J Physiol. 1993;265:R282–R289. doi: 10.1152/ajpregu.1993.265.2.R282. [DOI] [PubMed] [Google Scholar]
- 22.Wemmie JA, Zha X, Welsh MJ. Acid-sensing ion channels (ASICs) and pH in synapse physiology. In: Hell JW, Ehlers MD, editors. Structural and Functional Organization of the Synapse. New York: Springer; 2008. [Google Scholar]
- 23.Katsura K, Siesjo B. Acid-base metabolism in ischemia. In: Kaila K, Ransom BR, editors. pH and Brain Function. New York: Wiley-Liss; 1998. p. 563. [Google Scholar]
- 24.Siesjö BK, von Hanwehr R, Nergelius G, Nevander G, Ingvar M. Extra- and intracellular pH in the brain during seizures and in the recovery period following the arrest of seizure activity. J Cereb Blood Flow Metab. 1985;5:47–57. doi: 10.1038/jcbfm.1985.7. [DOI] [PubMed] [Google Scholar]
- 25.Ziemann AE, et al. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richerson GB. Cellular mechanisms of sensitivity to pH in the mammalian respiratory system. In: Kaila K, Ransom BR, editors. pH and Brain Function. New York: Wiley-Liss; 1998. pp. 509–533. [Google Scholar]
- 27.Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman SD, Mathis CM, Hayes C, Renshaw P, Dager SR. Brain pH response to hyperventilation in panic disorder: Preliminary evidence for altered acid-base regulation. Am J Psychiatry. 2006;163:710–715. doi: 10.1176/ajp.2006.163.4.710. [DOI] [PubMed] [Google Scholar]
- 29.Schuchmann S, et al. Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nat Med. 2006;12:817–823. doi: 10.1038/nm1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel N, Forton DM, Coutts GA, Thomas HC, Taylor-Robinson SD. Intracellular pH measurements of the whole head and the basal ganglia in chronic liver disease: A phosphorus-31 MR spectroscopy study. Metab Brain Dis. 2000;15:223–240. doi: 10.1007/BF02674531. [DOI] [PubMed] [Google Scholar]