Carbon, a vital element in our daily lives, is still fascinating us at the nanoscale. In PNAS, Geng et al. (1) manage to synthesize ordered arrays of atom-thick hexagonal microplatelets consisting of sp2 hybridized carbon. Since the discovery of C60 (Buckminsterfullerene; an icosahedral carbon cage molecules with diameter of approximately 0.7 nm) (2), carbon nanoscience emerged, and it was witnessed that nanoscale carbon materials possessed different physicochemical properties compared with the well known bulk allotropes of carbon: graphite and diamond. During the past 20 y, other novel carbon materials have been synthesized and intensively studied: carbon nanotubes (3–5) and graphene (6, 7).
Graphene consists of an atom-thick sp2 hybridized carbon sheet, in which each carbon atom is bonded to three carbon atoms, thus forming a hexagonal framework with bond lengths of 1.42 Å. This 2D hexagonal sheet has shown outstanding properties compared with other forms of carbon. Graphene exhibits a room-temperature quantum Hall effect (8), it is a semimetal, and it has an extremely high room temperature carrier mobility (at least two orders of magnitude greater than that of silicon) (9). Graphene could also exhibit extremely high thermal conductivity values ranging from (2.50 ± 0.44)×103 to (5.30 ± 0.48)×103 W/m⋅K (10, 11). From the mechanical standpoint, graphene is very robust and exhibits a Young modulus of approximately 1 TPa (12). It is noteworthy that the aforementioned properties of graphene systems strongly depend on, for example, the degree of crystallinity (e.g., domain size of crystalline domains and types/number of defects), edge morphology (atomically smooth edges or rough edges), and number of stacking layers (e.g., bilayer, trilayer).
Individual graphene sheets were first isolated by using a repeated peeling “scotch-tape” method (6). However, alternative methods involving the thermal decomposition of SiC (13), and chemical vapor deposition (CVD) of CH4 on Ni (14) and Cu (15) films, have been used to synthesize large area graphene sheets. During CVD growth, the carbon precursor (e.g., CH4) decomposes primarily on metallic crystalline imperfections that act as nucleation sites, and these are also responsible for diffusing carbon on the metal surface. During growth, carbon atoms keep precipitating on the growing domains (Fig. 1A), and these graphene domains eventually meet (Fig. 1A) and form a uniform single layer exhibiting grain boundaries containing pentagons and heptagons (16). Parameters such as substrate type (Cu, Pt, Ni), the carrier gas flow (Ar, H2), and the carbon source (e.g., CH4) are crucial to control the number of stacking layers and the morphologies of graphene domains.
Fig. 1.
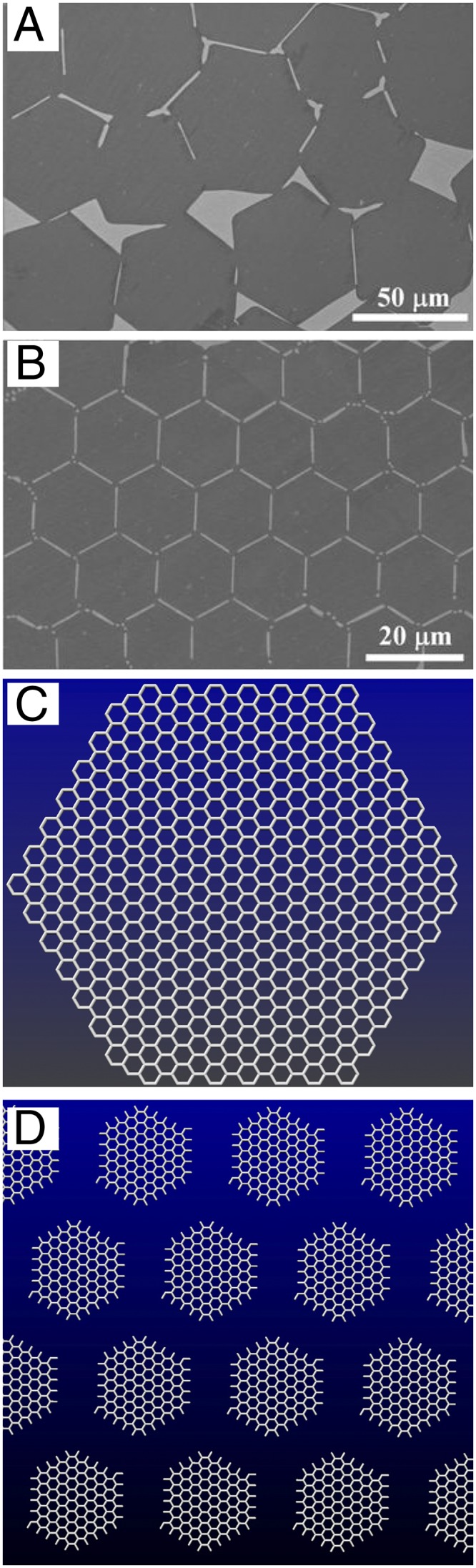
(A and B) SEM images in which dark and bright parts represent the graphene platelets and the Cu surface, respectively. Changing the synthesis temperature and CH4 flow rates (A) shows an average plate size of approximately 50 μm in diameter, approaching a more perfect packing. [Reproduced from Geng, et al. (1)]. (B) Nearly perfect 2D lattice of graphene platelets obtained by Geng et al. (1). [Reproduced from Geng, et al. (1)]. (C) Molecular model of a hexagonal graphene platelet and (D) molecular model of an ordered array of hexagonal graphene platelets (courtesy of F. López-Urías).
Geng et al. (1) use a modified CVD approach that is able to produce symmetric arrays of single-layered hexagonal graphene microplatelets by using liquid Cu as a substrate, instead of solid Cu. In particular, the authors used high temperatures (1,020–1,080 °C) to convert a 25-μm-thick Cu foil (i.e., solid) into liquid droplets and observed that these hexagonal platelets were mainly monolayers and tended to arrange symmetrically on the droplet upon specific flow rates and CVD time. For example, as time increased, the interplatelet distance decreased and the platelet arrays became more ordered, with an almost perfect edge-to-edge alignment (Fig. 1B). The authors claim that translations and rotations of the growing graphene platelets (Fig. 1C) occur because of the presence of the molten Cu surface, responsible for achieving the self-assembly of the hexagonal platelets (Fig. 1 B and D). The authors propose a mechanism based on the fast precipitation of carbon atoms and the quick assembly of the growing hexagonal platelets on molten droplets. However, the mechanism is still far from clear and further experiments are required to fully understand the process. For example, it may be possible to have the coexistence of solid and liquid Cu phases, and this coexistence would cause instabilities that could trigger nucleation and growth of the graphene platelets. Alternatively, it might be possible that a very fast cooling rate results in the self-fracture of the graphene precipitated on the molten Cu, and the observed patterns are related to the cooling conditions and the crack propagation (17).
The method Geng et al. (1) describe appears to be a single-step CVD method able to control the growth of hexagonal graphene micropatterns. In this context, it is important to mention that other authors have used other top-down routes to produce graphene arrays that include the patterning of regular patterned arrays using e-beam lithography in conjunction with of oxygen plasma etching (18). In addition, bottom-up approaches have demonstrated that it is possible to generate triangular graphene nanoplatelets by using cyclodehydrogenation of polyphenylene on Cu(111) (19). The reduction of graphene-oxide materials could also lead to hexagonal graphene platelets (20); however, additional self-assembly methods and research need to be used to achieve symmetric patterns of graphene platelets.
Although controlling the packing of the hexagonal platelets is very important, other important issues need to be considered in the development of future technologies that include controlling the platelet size, the precise number of layers, and the edge terminations (preferentially zigzag or “armchair”). In this context, Geng and coworkers (1) note that, by increasing the growth temperature and by decreasing the CH4 flow rate, it is possible to increase the average size of the platelets from 20 to 30 μm to 50 to 120 μm. Regarding the edge termination of these graphene systems, it is important to emphasize that zigzag edges tend to exhibit metallic characteristics as well as an intense density of states at the Fermi level (21), indicative of high chemical reactivity and possible magnetic states that could be advantageous in spintronics. However, for armchair edges, the electronic properties may change from metallic to semiconducting behavior, depending on the geometric size, for example for graphene nanoribbons (7). At this stage, detailed high-resolution transmission EM observations of the edges of these platelets need to be carried out to elucidate their nature and confirm if edges are atomically smooth. These edge states within platelets
The paper by Geng et al. constitutes a step forward in the growth and assembly of graphene platelets.
may also exhibit important catalytic activity that could allow them to serve as nucleation sites for growing nanorods of different materials, and could also exhibit efficient molecular sensing properties.
The micrometer sizes of these platelets (20–150 μm) suggest they could also be used for biological applications. For example, cells’ sizes are on the order of microns; therefore, these flat platelets could be well dispersed in water solutions and be used, for example, as biomarkers, drug deliverers, biosensors, scaffolds for tissue regeneration, or cancer treatments. Further work needs to be carried out along this direction, but the size control and reactivity of the graphene hexagons make them important in medical applications. Regarding the electronic properties of these platelets Geng et al. (1) produce, the authors noted they could display high current densities of the order of 0.96 ± 0.15 mA/μm, which are higher than those observed for CVD graphenes (0.44 mA/μm) (22). These results suggest that these platelets could eventually be used as highly transparent conducting films and conducting paints.
The paper by Geng et al. (1) constitutes a step forward in the growth and assembly of graphene platelets, and there are still numerous challenges that need to be overcome. (i) Is it possible to reduce the size of the hexagonal platelets to be on the order of nanometers via CVD so they could behave as quantum dots useful in the fabrication of light-emitting diodes? (ii) Is it possible to grow a single millimeter-size crystalline platelet without grain boundaries and structural defects? (iii) Is it possible to control the edge termination at will (i.e., armchair or zigzag)? (iv) Is it feasible to bulk-produce these platelets in kilogram quantities to fabricate robust polymer and highly conducting composites? (v) Is it possible to control the CVD growth so that the morphology of graphene platelets is transformed into arrays of, for example, triangles or star-like features?
It is clear that this is the tip of the iceberg regarding growth control and assembly of graphene platelets. It appears that bottom-up approaches (e.g., CVD, chemical approach) could be eventually used to produce bulk quantities of platelets, and further experimental and theoretical research is now waiting to be conducted.
Footnotes
The author declares no conflict of interest.
See companion article on page 7992.
References
- 1.Geng D, et al. Uniform hexagonal graphene flakes and films grown on liquid copper surface. Proc Natl Acad Sci USA. 2012;109:7992–7996. doi: 10.1073/pnas.1200339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroto HW, Heath JR, Obrien SC, Curl RF, Smalley RE. C-60 -Buckminsterfullerene. Nature. 1985;318:162–163. [Google Scholar]
- 3.Oberlin A, Endo M, Koyama T. Filamentous growth of carbon through benzene decomposition. J Cryst Growth. 1976;32:335–349. [Google Scholar]
- 4.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- 5.Terrones M. Carbon nanotubes: Synthesis and properties, electronic devices and other emerging applications. Int Mater Rev. 2004;49:325–377. [Google Scholar]
- 6.Novoselov KS, et al. Electric field effect in atomically thin carbon films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 7.Terrones M, et al. Graphene and graphite nanoribbons: Morphology, properties, synthesis, defects and applications. Nano Today. 2010;5:351–372. [Google Scholar]
- 8.Novoselov KS, et al. Room-temperature quantum Hall effect in graphene. Science. 2007;315:1379–1379. doi: 10.1126/science.1137201. [DOI] [PubMed] [Google Scholar]
- 9.Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007;6:183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 10.Balandin AA, et al. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008;8:902–907. doi: 10.1021/nl0731872. [DOI] [PubMed] [Google Scholar]
- 11.Cai WW, et al. Thermal transport in suspended and supported monolayer graphene grown by chemical vapor deposition. Nano Lett. 2010;10:1645–1651. doi: 10.1021/nl9041966. [DOI] [PubMed] [Google Scholar]
- 12.Lee C, Wei XD, Kysar JW, Hone J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008;321:385–388. doi: 10.1126/science.1157996. [DOI] [PubMed] [Google Scholar]
- 13.Berger C, et al. Electronic confinement and coherence in patterned epitaxial graphene. Science. 2006;312:1191–1196. doi: 10.1126/science.1125925. [DOI] [PubMed] [Google Scholar]
- 14.Reina A, et al. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009;9:30–35. doi: 10.1021/nl801827v. [DOI] [PubMed] [Google Scholar]
- 15.Li XS, et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science. 2009;324:1312–1314. doi: 10.1126/science.1171245. [DOI] [PubMed] [Google Scholar]
- 16.Huang PY, et al. Grains and grain boundaries in single-layer graphene atomic patchwork quilts. Nature. 2011;469:389–392. doi: 10.1038/nature09718. [DOI] [PubMed] [Google Scholar]
- 17.Yakobson BI. Morphology and rate of fracture in chemical decomposition of solids. Phys Rev Lett. 1991;67:1590–1593. doi: 10.1103/PhysRevLett.67.1590. [DOI] [PubMed] [Google Scholar]
- 18.Shi ZW, et al. Patterning graphene with zigzag edges by self-aligned anisotropic etching. Adv Mater (Deerfield Beach Fla) 2011;23:3061–3065. doi: 10.1002/adma.201100633. [DOI] [PubMed] [Google Scholar]
- 19.Treier M, et al. Surface-assisted cyclodehydrogenation provides a synthetic route towards easily processable and chemically tailored nanographenes. Nat Chem. 2011;3:61–67. doi: 10.1038/nchem.891. [DOI] [PubMed] [Google Scholar]
- 20.Varela-Rizo H, Rodriguez-Pastor I, Merino C, Terrones M, Martin-Gullon I. Graphene oxide nanoplatelets of different crystallinity synthesized from helical-ribbon carbon nanofibers and multiwall carbon nanotubes. J Mater Res. 2011;26:2632–2641. [Google Scholar]
- 21.Nakada K, Fujita M, Dresselhaus G, Dresselhaus MS. Edge state in graphene ribbons: Nanometer size effect and edge shape dependence. Phys Rev B Condens Matter. 1996;54:17954–17961. doi: 10.1103/physrevb.54.17954. [DOI] [PubMed] [Google Scholar]
- 22.Bai JW, et al. Top-gated chemical vapor deposition grown graphene transistors with current saturation. Nano Lett. 2011;11:2555–2559. doi: 10.1021/nl201331x. [DOI] [PMC free article] [PubMed] [Google Scholar]


