Abstract
Impairments of spatial awareness and decision making occur frequently as a consequence of parietal lesions. Here we used event-related functional MRI (fMRI) in monkeys to investigate rapid reorganization of spatial networks during reversible pharmacological inactivation of the lateral intraparietal area (LIP), which plays a role in the selection of eye movement targets. We measured fMRI activity in control and inactivation sessions while monkeys performed memory saccades to either instructed or autonomously chosen spatial locations. Inactivation caused a reduction of contralesional choices. Inactivation effects on fMRI activity were anatomically and functionally specific and mainly consisted of: (i) activity reduction in the upper bank of the superior temporal sulcus (temporal parietal occipital area) for single contralesional targets, especially in the inactivated hemisphere; and (ii) activity increase accompanying contralesional choices between bilateral targets in several frontal and parieto-temporal areas in both hemispheres. There was no overactivation for ipsilesional targets or choices in the intact hemisphere. Task-specific effects of LIP inactivation on blood oxygen level-dependent activity in the temporal parietal occipital area underline the importance of the superior temporal sulcus for spatial processing. Furthermore, our results agree only partially with the influential interhemispheric competition model of spatial neglect and suggest an additional component of interhemispheric cooperation in the compensation of neglect deficits.
Keywords: extinction, hemispheric imbalance, saccadic decision, recovery
Visual neglect is a debilitating neuropsychological disorder that occurs frequently as a consequence of right parietal and parietotemporal lesions in humans (1, 2). Neglect is characterized by an impaired or lost ability to explore and respond to events in the space contralateral to the lesion that cannot be explained by primary sensory or motor disorders. A related but distinct deficit, spatial extinction, entails the inability to perceive contralesional stimuli, but only when a simultaneous ipsilesional stimulus is also present (3). There is an ongoing debate whether extinction represents a less severe form of neglect or should be considered separately, in particular because both phenomena may differ in respect to the underlying neural substrates (4–6). One influential theory about the mechanisms of spatial neglect and extinction proposed that each brain hemisphere contains an orienting vector toward the contralateral hemispace and that the two hemispheres establish a dynamic balance by inhibiting each other (7). According to this interhemispheric competition theory (IHC), unilateral lesions result in contralesional spatial deficits because the activity in the lesioned hemisphere is reduced and the opposite hemisphere becomes hyperexcitable due to reduced inhibition from the lesioned hemisphere.
Previous studies have found evidence for interhemispheric competition in the somatosensory and motor systems: (i) functional MRI (fMRI) studies in humans revealed that unilateral finger stimulation is accompanied by increased blood oxygen level-dependent (BOLD) activity in the contralateral somatosensory cortex, as well as deactivation of the corresponding ipsilateral cortex (8, 9); (ii) excitatory transcranial magnetic stimulation (TMS) of the motor cortex in one hemisphere before stimulation of the other hemisphere caused a reduction of motor-evoked responses to the latter stimulation (10, 11). Conversely, inhibitory TMS pulses applied to one hemisphere resulted in an increase of excitability in the opposite hemisphere (12, 13).
In the visuospatial domain, support for the interhemispheric rivalry theory is mainly derived from the observation that an additional lesion in the intact hemisphere can ameliorate neglect symptoms in cats (14, 15) and humans (16), and from human TMS studies showing that “virtual lesions” in one hemisphere lead to functional improvement of the opposite hemisphere (17–19). An fMRI study in neglect patients reported that during the chronic stage of recovery, the damaged hemisphere became reactivated and dorsal parietal activity in the nonlesioned hemisphere became relatively lower in comparison with the acute stage (20), consistent with an interhemispheric push-pull pattern.
At the same time, a large body of research postulates cooperative, integrative interactions between hemispheres (21, 22), and many clinical studies suggest compensatory recruitment of the intact hemisphere following lesions (23–28). Although dynamic competitive and cooperative interhemispheric interactions may coexist, the contribution of these interactions to spatial disorders and postlesion recovery remains unclear. Therefore, animal models of spatial disorders are needed because, unlike in humans, extent and location of the lesions can be systematically varied; in addition, brain activity can be measured before long-term neural and behavioral compensations can occur (1, 29).
To investigate brain-wide functional changes underlying the spatial deficits caused by parietal lesions, we used concurrent reversible pharmacological inactivation and event-related BOLD fMRI in monkeys performing an oculomotor memory and choice task. This unique approach enabled the examination of fast lesion-induced behavioral and neural effects along with robust control over lesion location and extent.
Results
We inactivated portions of the lateral intraparietal area (LIP) by locally injecting the GABA-A agonist muscimol while monkeys performed oculomotor tasks in a vertical bore 4.7T scanner. We used a time-resolved event-related design that enabled us to separate activity from different trial types and intervals (30). Two monkeys were repeatedly tested and fMRI activity patterns were compared between interleaved control and inactivation sessions. We completed eight inactivation sessions in monkey F, of which six sessions were included in the fMRI analysis, five inactivation sessions in monkey R, and a corresponding number of control sessions without inactivation (SI Appendix, SI Methods). Throughout the article, the terms “ipsilesional” and “contralesional” refer to the visual hemifield with respect to the inactivated hemisphere (e.g., after an injection into the right hemisphere, the left visual hemifield is contralesional). Please note that when comparing control and inactivation sessions, those terms are also applied to control sessions, for consistency. “Ipsilateral” and “contralateral” refer to the visual hemifield in relation to the discussed brain hemisphere.
Delayed Memory Saccade Task-Related Activity.
To resolve brain activity changes in different trial epochs using fMRI, we used a memory saccade task with long delays and two randomly interleaved conditions (Fig. 1A). In “instructed” trials, monkeys were required to remember the location of a single unilateral target and to saccade to it after a delay; in “choice” trials monkeys were free to choose either of the two targets presented in opposite hemifields. Instructed trials were used to assess inactivation effects on sensory, memory, and oculomotor components of the task; the choice trials introduced additional target competition and selection components. Consistent with the previous study (30), visual (cue) and visuomotor (saccade) task events activated an extensive network with strong peaks in the arcuate sulcus (as), intraparietal sulcus (ips), and in the superior temporal sulcus (sts) (Fig. 1B).
Fig. 1.
The task and corresponding task-related BOLD activity. (A) Delayed memory-guided saccade task. In instructed trials, one target was presented in either the left or right hemifield at one of 18 possible positions. In the choice trials two targets appeared simultaneously, on the right and on the left equidistantly from the central fixation point. (B) Cortical areas activated in control sessions by +cue (yellow-red) and +saccade (purple-blue) contrasts, shown on the inflated brain surface of each monkey. (C) Typical ERA BOLD trial time-courses from the right LIP for the four trial conditions. The gray box denotes the time interval used for estimating cue/delay mean response amplitude, short black line under the curves represents the last two samples of initial fixation used as a baseline for estimating percent BOLD signal change. Shaded bands denote SEM across trials.
Representative event-related average (ERA) BOLD signal time-courses from the dorsal LIP (LIPd) area in the right hemisphere are depicted in Fig. 1C. Instructed (single-target) time-courses show higher cue- and delay-period responses in contralateral (left) compared with ipsilateral (right) trials (P < 0.001). Choice (two-target) time-courses demonstrate: (i) weaker contralateral tuning reflecting the upcoming choice (P < 0.001) and (ii) reduced response amplitude in comparison with contralateral instructed trials, reflecting competition between two targets (P = 0.01). Because the current study seeks to understand the neural changes that underlie the spatial selection bias associated with parietal lesions, we focus mainly on the cue/delay activity in the 5-s delay period following 200-ms cue presentation and preceding the initiation of the saccade (Fig. 1C). The mean percent BOLD signal change during the 3-s cue/delay peak-response epoch in each condition was used for quantitative comparisons in region-of-interest (ROI) analyses. This activity represents visual and cognitive components related to spatial memory, response selection, and planning.
Injection Locations and Behavioral Effects.
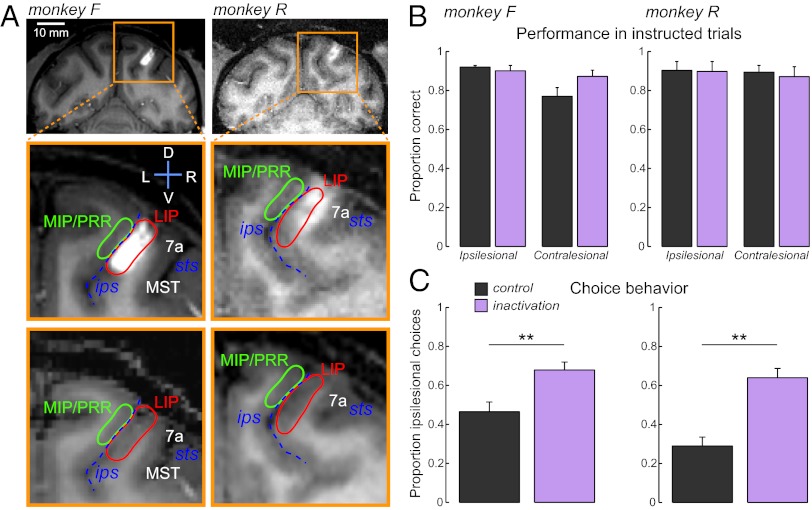
To investigate the consequences of disrupting LIP activity, we performed local injections of muscimol. Injection sites were targeted by presurgical MRI and repeatedly verified by imaging the cannula and the spread of MR contrast agent gadolinium intermixed with muscimol (SI Appendix, SI Methods). Gadolinium labeling, which corresponds closely to the spread of muscimol (31), indicated that inactivations were primarily in the LIPd but extended into the ventral part (LIPv) as well (Fig. 2A). Neighboring structures, such as area MIP in the medial bank of ips, and parieto-temporal areas MT/middle superior temporal area (MST) in caudal sts, were not labeled. Because guide cannulae were chronically preimplanted, inactivation sites were highly reproducible across sessions.
Fig. 2.
Inactivation sites and behavioral choice bias. (A, Top) Coronal T1-weighted MR sections visualizing right hemisphere injection sites with gadolinium MR contrast agent (white). (Middle and Bottom) Magnified view of inactivation area, with and without injection. The injection images were acquired 15–30 min after the 4-μL infusion. The injection spreads along the lateral bank of the intraparietal sulcus. Abbreviations: ips, intraparietal sulcus; sts, superior temporal sulcus; LIP, lateral intraparietal area (target); MIP, medial intraparietal area; MST, middle superior temporal area; PRR, parietal reach region. (B) Proportion of correct saccades to targets in the ipsi- or contralesional hemifield during control and inactivation sessions. (C) Proportion of choices toward the ipsilesional hemifield. Note the increase of choices toward ipsilesional targets in inactivation sessions. Number of sessions and single session data can be found in SI Appendix, Table S1. Error bars indicate SEM across sessions, **P < 0.01.
Fig. 2B demonstrates that muscimol inactivation did not impair the ability to perform memory saccades to instructed single targets in either hemifield (two-way ANOVA, hemifield × inactivation, P > 0.05). Consistent with previous reports (32, 33), monkeys exhibited a modest increase of saccade latencies toward contralesional targets (9–16 ms, two-tailed t test, P < 0.01). Saccade latencies toward ipsilesional space remained unaffected (P > 0.6).
In contrast to the modest effect on instructed trials, LIP inactivation resulted in a profound choice bias toward ipsilesional targets (two-tailed t test, P < 0.01) (Fig. 2C and SI Appendix, Table S1). Following inactivation of the right LIP, monkey F choose the ipsilesional (right) target in 67.9% of the trials (46.3% in control sessions) and monkey R in 63.9% of the trials (28% in control sessions). Thus, although sensory and oculomotor capabilities remained largely intact after LIP inactivation, monkeys exhibited a spatial choice bias reminiscent of extinction symptoms observed in human patients with parietal damage.
Effects of LIP Inactivation on BOLD Activity.
Instructed trials.
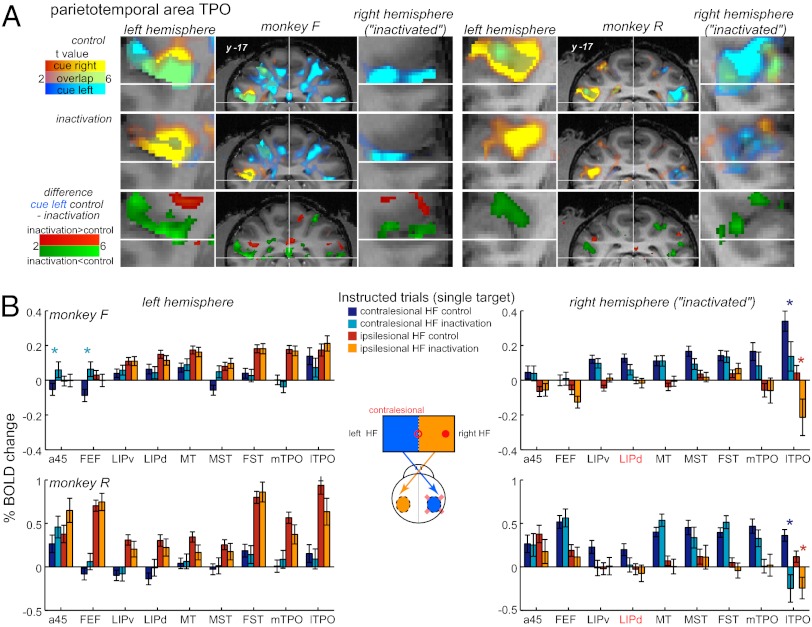
To understand the neural activity changes that led to the behavioral effects described above, we first analyzed the effects of LIP inactivation on brain activity in instructed single-target trials. We focused on several areas that were significantly activated during the cue/delay period in the contralateral instructed trials in control sessions. Frontal (FEF, area 45), parietal (LIP), and parieto-temporal areas in the superior temporal sulcus [sts: MT, MST, temporal parietal occipital (TPO), fundus superior temporal (FST)] showed robust and predominantly contralateral cue responses, although ipsilateral activation was also present (Fig. S1A).
Following right LIP inactivation, we observed an activity reduction for contralesional (left) cues (cyan-blue voxels; Fig. 3A). As expected from the injection location, pronounced activity reduction occurred in the inactivated (right) hemisphere, locally in the LIPd and adjacent LIPv (SI Appendix, Fig. S1A), partially attributable to gadolinium-induced magnetic susceptibility (Table S2). Beside the expected local decrease, in both monkeys the activity for contralesional (left) cues was most notably reduced in the upper bank of the sts, in an area referred to as “superior temporal polysensory area” (STP) or TPO (Fig. 3A and SI Appendix, SI Methods). In the inactivated hemisphere, both ipsi- and contralesional cue activity was reduced, and mostly contralesional cue activity was affected in the intact hemisphere (Fig. 3A). Beyond parieto-temporal areas, similar consequences were observed in frontal regions (SI Appendix, Fig. S2) and in the medial aspect of both hemispheres (SI Appendix, Fig. S3).
Fig. 3.
Inactivation effects in instructed trials. (A) Superimposed activity maps for +cue left and +cue right contrast (first two rows in each panel), and contralesional cue left “control minus inactivation” difference (third row) in example coronal sections through sts, with enlarged maps around area TPO. Inactivation effects on contralesional cue activity can be seen as diminished cyan-blue clusters and as green clusters in difference maps in both hemispheres (see Fig. S1B for more sections). Coordinates are in AC-PC plane. (B) ROI analysis showing cue/delay activity in activated areas, as a function of visual hemifield, hemisphere, and session type. Error bars indicate SEM across trials. The asterisk represents significant difference for the control vs. inactivation comparison for the same hemifield (P < 0.05, t test, star color signifies the direction of the change). De-emphasized bars for the right LIPd/v signify a mixture of local inactivation and gadolinium effects. Trial numbers for each monkey and condition are listed in SI Appendix, Table S4 and statistics are presented in SI Appendix, Table S5. HF, hemifield.
The effects of inactivation on instructed trials are further quantified in Fig. 3B, which plots average cue/delay period activity extracted from the ERAs in ipsi- and contralesional trials in control and inactivation sessions. Each ROI was defined using “+ contralateral cue” contrast in the control condition and identical ROIs were used for the control and inactivation datasets (see SI Appendix, SI Methods for ROI definition; SI Appendix, Table S3 for coordinates; and SI Appendix, Table S4 for number of trials in each condition). In the inactivated hemisphere, apart from the local decrease in the LIP and significant activity reduction in the lTPO, both monkeys showed a similar reduction trend in neighboring areas mTPO and MST (Fig. 3B and SI Appendix, Table S5).
In the framework of the IHC theory, the observed decrease of activity in the inactivated hemisphere might lead to a hyperactivation of homotopic areas in the intact hemisphere, leading to an overrepresentation of the ipsilesional space (7, 34). However, we did not observe any activity enhancement for ipsilesional right cues in the intact hemisphere during inactivation sessions, neither in the maps (Fig. 3A and SI Appendix, Figs. S1–S3), nor in the ROI analysis (Fig. 3B).
Choice trials.
Next, we investigated how LIP inactivation altered BOLD responses in trials when monkeys had to choose between two targets in opposite hemifields. For each pair of bilateral targets, left and right trials were visually identical before the saccade event, and were sorted according to the monkey choice. Monkeys received the same reward for choosing either target as long as they successfully completed the trial.
Our main question was how the cue/delay activity preceding the oculomotor choice is altered by the LIP inactivation. In control sessions, the LIPd showed modest contralateral choice tuning of delay activity in both hemispheres (Fig. 4A and SI Appendix, Fig. S5, Top row). After inactivation, LIP activity in the intact hemisphere was enhanced in those infrequent trials in which the monkeys chose the contralesional (“affected”) left target, and it was decreased for the ipsilesional rightward choices. Thereby, following inactivation, the intact LIP exhibited stronger activity for contralesional (left) compared with ipsilesional (right) choices, effectively reversing its spatial tuning (Fig. 4B, Left Inset). Similar effects were found in the lTPO area: after LIP inactivation, left and right lTPO in both monkeys exhibited elevated activity for contralesional choices, relative to ipsilesional choices (Fig. 4B, Right Inset, and SI Appendix, Fig. S5).
Fig. 4.
Inactivation effects in choice trials. (A) ROI analysis showing average cue/delay activity in choice trials in control and inactivation sessions. Error bars indicate SEM across trials, *P < 0.05, star color corresponds to the larger condition (statistics presented in SI Appendix, Table S6). (Inset) Predictions of the IHC model. (B) Relative activity difference between ipsi- and contralesional choice trials. Bar amplitudes represent average cue/delay activity difference across sessions (left contralesional choices minus right ipsilesional choices; positive values: left > right; negative values: left < right). Note the increased positive values in several areas, in both hemispheres, following the inactivation (purple > gray), indicating differential enhancement in trials when monkeys choose the contralesional hemifield. Error bars indicate SEM across sessions, *P < 0.05 (statistics presented in SI Appendix, Table S7). (Left and Right Insets) ERA BOLD time-courses from left LIP (Left) and right lTPO (Right) before and after inactivation (see SI Appendix, Fig. S5 for other ROIs). Shaded error bands indicate SEM across trials; blue/orange stars, significant difference between left and right trials in at least one time sample (P < 0.05, sample-wise t test). (Center Inset) Predictions of the IHC model.
Hypothetically, inactivation effects can be expressed in two ways: (i) as an “absolute” activity increase or decrease in inactivation sessions, compared with control sessions, within the same trial type; and (ii) as a “relative” activity difference between contralesional and ipsilesional choice trials (regardless of the absolute activity level). We observed both effects; however, the relative effects were more pronounced.
Considering the absolute activity changes, the IHC model makes different predictions for ipsi- and contralesional choices in the two hemispheres after inactivation. Generally, the IHC predicts the following outcome for two competing bilateral stimuli: the cue in the ipsilesional hemifield activates the intact hemisphere, which in turn further suppresses the inactivated hemisphere because of interhemispheric inhibition. As a result, subjects tend to choose the ipsilesional target. On the behavioral level, this prediction proved correct: monkeys chose the ipsilesional target more frequently after the inactivation.
With respect to neural activity, for ipsilesional choices the model predicts elevated activity in the intact hemisphere as well as reduced activity in the lesioned hemisphere (Fig. 4A, Inset). Contrary to the prediction, none of the areas in the intact hemisphere showed elevated activity for ipsilesional choices after inactivation (Fig. 4A, and SI Appendix, Table S6). For the infrequent contralesional choices (“affected hemifield” after inactivation), the model postulates that the hemispheric balance should be restored. This process could be achieved by an activity decrease in the intact hemisphere, or by a hyperactivity in structurally intact areas within the lesioned hemisphere. Again, this is not what we found for the intact hemisphere: instead, we observed a cue/delay activity increase in both hemispheres when monkeys chose targets in the contralesional, “affected” hemifield (Fig. 4A). At the same time and consistent with the prediction, activity for contralesional choices in the lesioned hemisphere (except in LIP and lTPO) tended to be higher.
Another way to look at absolute activity changes is to compare activity in the left vs. right hemisphere in control and inactivation sessions. According to the IHC formulation, after inactivation we expected more activity in the intact left hemisphere and less activity in the right hemisphere, and thus increased left minus right hemisphere difference for the ipsilesional choices, and decreased difference for contralesional choices. However, none of the bilateral ROI pairs exhibited this pattern (SI Appendix, Fig. S6), failing to concur with the IHC model.
Apart from the absolute activity level changes described above, inactivation enhanced and in some cases even reversed the relative difference between contralesional and ipsilesional choice trials. To quantify those activity changes, we computed the relative contralesional minus ipsilesional choice difference for each ROI separately for control and inactivation sessions (Fig. 4B). Because this procedure involved subtracting means of different trial types, we performed this analysis on a session-by-session basis. Following the IHC model, the difference between contralesional and ipsilesional activity may increase in the intact areas of the inactivated hemisphere and decrease in the opposite hemisphere (Fig. 4B, Center Inset). However, the analysis revealed activity enhancement for contralesional choices in specific ROIs in both hemispheres. In the inactivated (right) hemisphere, lTPO (significant in both monkeys) and frontal ROIs (FEF/a45, significant in monkey R) showed the effect. In the intact hemisphere, relative activity enhancement was found in the LIPd (significant in monkey R) and in frontal areas, which reversed its contralateral tuning, showing more activity for contralesional choices in inactivation sessions. Although apart from the right lTPO, areas with statistically significant relative enhancement differed between the two monkeys, the overall trend for bihemispheric activity increase was similar. To summarize, the selection of targets in the “affected” contralesional space was associated with activity enhancement in both hemispheres, as opposed to a push-pull pattern predicted by the IHC model.
Discussion
In the present study we investigated in which brain areas and how unilateral LIP inactivation alters visuomotor and choice-related neural activity. Using a combination of reversible inactivation and time-resolved fMRI, we found that LIP lesions led to both anatomically and functionally specific changes: reduction of cue/delay responses to single contralesional targets in structurally intact parieto-temporal areas, especially in the lesioned hemisphere, and putatively compensatory bihemispheric activity enhancement accompanying the selection of targets in the contralesional (“affected”) hemifield in bilateral choice trials.
Reduction of Activity in Distant Parieto-Temporal Areas.
LIP inactivation modestly increased saccade latencies in the contralesional hemifield. This increase was accompanied by reduced contralesional cue/delay BOLD activity in parieto-temporal areas, most notably in area lTPO in the upper bank of the sts. The apparent functional relationship between LIP and lTPO is consistent with anatomical studies indicating that the dorsal bank of the sts receives direct input from LIP (35, 36) and agrees with physiological, fMRI and ablation studies showing that STP/TPO is involved in the control of eye movements and visuospatial attention (30, 37–42). Putatively corresponding regions in human inferior parietal lobe and temporal parietal junction are critically involved in spatial awareness, and damage to these areas results in spatial deficits, although the involvement of human sts in neglect vs. extinction is under debate (20, 43, 44), and the monkey-human homologies in the IPL and sts are not fully established. Together with the relative activity enhancement in lTPO for contralesional choices (see below), these observations emphasize the importance of monkey TPO in goal-directed visuospatial functions, and call for further investigations of functional analogies and differences between parieto-temporal areas in monkeys and humans.
More generally, the temporary deactivation of intact, remote brain regions connected to the primary lesion site is a well-known consequence of brain injury, and has been measured as a reduction in electric activity, blood flow, and metabolism (diaschisis) (45). In the context of parietal lesions, reduced activity in connected areas after local inactivation is consistent with several studies in acute neglect patients, demonstrating hypometabolism within the lesioned hemisphere (20, 46–48). However, the results of the current inactivation study are easier to interpret functionally than those patient studies because muscimol does not affect fibers of passage (49) and the neurovascular coupling in distant areas is likely not compromised by the local muscimol injection, contrary to lesions with vascular etiology (50).
Compensatory Activity Enhancement in both Hemispheres.
The main behavioral consequence of LIP inactivation was a choice bias toward the ipsilesional hemifield, agreeing with previous studies showing that in monkeys, parietal lesions or inactivation rarely lead to full-blown neglect but result in contralesional extinction (51–55). This finding for delayed memory saccades extends previous studies that used visually guided saccades (51, 54). Although best described as extinction, the choice bias can also be viewed as diminished contralesional exploration (56). Nonetheless, monkeys occasionally chose targets in the contralesional hemifield. We found that those contralesional choices were preceded by cue/delay activity enhancement in both hemispheres.
This choice-specific bihemispheric activity increase does not fully concur with predictions of the interhemispheric push-pull models of neglect (7, 19, 20) and the contralateral organization of visuomotor and attentional activity in monkeys (30, 57–59). Although the compensatory increase for contralesional choices in structurally intact areas of the lesioned hemisphere was expected, we also expected the intact hemisphere, which encodes predominantly the ipsilesional hemifield under normal conditions, to be either suppressed or unaffected in those trials. Although the recruitment of the intact hemisphere may be surprising in the context of spatial choices, such recruitment for the control of contralesional limbs is a frequently reported consequence of brain lesions with different etiologies, such as stroke (24), tumor removal (25), and “virtual lesions” with TMS (26). The degree to which such activity increase in the intact hemisphere represents a true correlate of functional compensation/recovery, an epiphenomenon, or a “maladaptive strategy” remains a topic of intense debate and may depend on the time after injury (50, 60).
Different interpretations other than adaptive plasticity are also possible, such that the activity increase in the hemisphere opposite to the lesion reflects a reduced interhemispheric inhibition from the lesioned hemisphere (7). Evidence for this proposal is derived from TMS studies in neglect patients showing that spatial extinction can be transiently alleviated by deactivating the hemisphere opposite to the lesion (19, 61, 62) and by demonstrating hyperexcitability of parietal-motor pathways in the intact hemisphere (18). Similarly, studies in cats have shown a restoration of spatial functions as a consequence of deactivation of homolog areas in the hemisphere opposite to the lesion (14, 29, 63).
With respect to the current study, functionally pertinent compensation is supported by the observation that enhancement in the intact hemisphere was specific for contralesional choices and was neither observed in instructed trials nor in choice trials in which the ipsilesional target was chosen (as one would expect from a release of interhemispheric inhibition). Furthermore, most areas did not show hyperactivity per se but a relative higher activity for contralesional compared with ipsilesional choices, arguing against a general release from inhibition as a sole source of enhancement in the intact hemisphere. One conceivable explanation is that the bihemispheric activity pattern represents additional recruitment of contralesionally tuned neuronal populations because of increased effort.
Finally, we have to ask whether the increase of BOLD signal, especially in the intact hemisphere, indeed reflects an increase of neuronal activity. Although there is generally a good correspondence between increases and decreases in neuronal and BOLD activation (64), the latter is a global signal that pools over inhibitory and excitatory, contra- and ipsilaterally tuned neuronal populations. Thus, current fMRI results (and likewise, human neuroimaging studies) need to be interpreted with caution and specific excitatory vs. inhibitory effects can only be disambiguated by electrophysiological recordings.
Because of differences in lesion etiology and different delays between lesion and measurement (days or months after lesion vs. hours in the present study), the direct comparison between clinical work and our study is limited. Further limitations are anatomical and functional differences in visuomotor organization of monkeys and humans, in particular profound hemispheric asymmetries found in humans but not in monkeys (2, 30, 53). Nonetheless, the combined reversible lesions and fMRI approach enables a more precise experimental control for investigations of functional deficits and compensation, thus complementing clinical studies. It is an attractive possibility that the bihemispheric activity pattern observed in the present study represents a precursor of adaptive reorganization observed in recovering patients.
In summary, our results demonstrate that a local LIP lesion engenders fast anatomically and functionally specific changes in both hemispheres. With respect to the interhemispheric spatial processing, the absence of overactivation in the intact hemisphere for upcoming ipsilesional saccades and bihemispheric enhancement preceding contralesional choices deviate from the current models of interhemispheric competition and suggests additional compensatory cooperative interactions between hemispheric representations.
Methods
All surgical and animal care procedures were done in accordance with National Institutes of Health guidelines and were approved by the California Institute of Technology Animal Care and Use Committee. Two male rhesus macaques (Macaca mulatta) were chronically implanted with a 22 gauge guide PEEK cannula (Plastics One) penetrating the dura and targeting the lateral bank of the ips (LIP) in the right hemisphere. This chronically implanted outer cannula served as a guide for inserting a 28-gauge internal PEEK cannula for the microinfusions of the GABA-A agonist muscimol (Tocris Bioscience). Monkeys were scanned in a Bruker Biospec 4.7T/60 cm vertical bore scanner (30). Detailed description of experimental procedures, data acquisition, and data analysis are available in SI Appendix, SI Methods.
Supplementary Material
Acknowledgments
We thank Dr. D. Procissi for help with scanning; Dr. F. Ye for providing the source code for the phase labeling for additional coordinate encoding echo-planar imaging sequence; K. Pejsa and N. Sammons for animal care; Dr. V. Shcherbatyuk for computer support; and Drs. W. Vanduffel, A. Gerits, and J. Bonaiuto for comments on the manuscript. This work was supported by the Moore Foundation, the National Eye Institute, the National Science Foundation, the Defense Advanced Research Projects Agency, and the Boswell Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204789109/-/DCSupplemental.
References
- 1.Kerkhoff G. Spatial hemineglect in humans. Prog Neurobiol. 2001;63:1–27. doi: 10.1016/s0301-0082(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 2.Mesulam MM. Spatial attention and neglect: Parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vuilleumier PO, Rafal RD. A systematic study of visual extinction. Between- and within-field deficits of attention in hemispatial neglect. Brain. 2000;123:1263–1279. doi: 10.1093/brain/123.6.1263. [DOI] [PubMed] [Google Scholar]
- 4.Bisiach E. Extinction and neglect: Same or different? In: Paillard J, editor. Brain and Space. Oxford: Oxford Univ Press; 1991. pp. 251–257. [Google Scholar]
- 5.Karnath HO, Himmelbach M, Küker W. The cortical substrate of visual extinction. Neuroreport. 2003;14:437–442. doi: 10.1097/01.wnr.0000059778.23521.88. [DOI] [PubMed] [Google Scholar]
- 6.Karnath HO, Rennig J, Johannsen L, Rorden C. The anatomy underlying acute versus chronic spatial neglect: A longitudinal study. Brain. 2011;134:903–912. doi: 10.1093/brain/awq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinsbourne M. Hemi-neglect and hemisphere rivalry. Adv Neurol. 1977;18:41–49. [PubMed] [Google Scholar]
- 8.Hlushchuk Y, Hari R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J Neurosci. 2006;26:5819–5824. doi: 10.1523/JNEUROSCI.5536-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastrup A, et al. Behavioral correlates of negative BOLD signal changes in the primary somatosensory cortex. Neuroimage. 2008;41:1364–1371. doi: 10.1016/j.neuroimage.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 10.Ferbert A, et al. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schambra HM, Sawaki L, Cohen LG. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol. 2003;114:130–133. doi: 10.1016/s1388-2457(02)00342-5. [DOI] [PubMed] [Google Scholar]
- 13.Plewnia C, Lotze M, Gerloff C. Disinhibition of the contralateral motor cortex by low-frequency rTMS. Neuroreport. 2003;14:609–612. doi: 10.1097/00001756-200303240-00017. [DOI] [PubMed] [Google Scholar]
- 14.Lomber SG, Payne BR. Removal of two halves restores the whole: Reversal of visual hemineglect during bilateral cortical or collicular inactivation in the cat. Vis Neurosci. 1996;13:1143–1156. doi: 10.1017/s0952523800007781. [DOI] [PubMed] [Google Scholar]
- 15.Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science. 1966;153:1544–1547. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- 16.Weddell RA. Subcortical modulation of spatial attention including evidence that the Sprague effect extends to man. Brain Cogn. 2004;55:497–506. doi: 10.1016/j.bandc.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 17.Hilgetag CC, Théoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions’ of human parietal cortex. Nat Neurosci. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- 18.Koch G, et al. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain. 2008;131:3147–3155. doi: 10.1093/brain/awn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveri M, et al. rTMS of the unaffected hemisphere transiently reduces contralesional visuospatial hemineglect. Neurology. 2001;57:1338–1340. doi: 10.1212/wnl.57.7.1338. [DOI] [PubMed] [Google Scholar]
- 20.Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- 21.Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? Neuropsychol Rev. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- 22.Gazzaniga MS. Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 23.Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: A review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- 24.Cramer SC, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 25.Krainik A, et al. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology. 2004;62:1323–1332. doi: 10.1212/01.wnl.0000120547.83482.b1. [DOI] [PubMed] [Google Scholar]
- 26.O’Shea J, Johansen-Berg H, Trief D, Göbel S, Rushworth MF. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54:479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Nyffeler T, Müri R, Pflugshaupt T, Wartburg R, Hess CW. Cortical reorganization after brain damage: The oculomotor model. Eur J Neurosci. 2006;23:1397–1402. doi: 10.1111/j.1460-9568.2006.04648.x. [DOI] [PubMed] [Google Scholar]
- 28.Thimm M, Fink GR, Sturm W. Neural correlates of recovery from acute hemispatial neglect. Restor Neurol Neurosci. 2008;26:481–492. [PubMed] [Google Scholar]
- 29.Payne BR, Rushmore RJ. Animal models of cerebral neglect and its cancellation. Neuroscientist. 2003;9:446–454. doi: 10.1177/1073858403256689. [DOI] [PubMed] [Google Scholar]
- 30.Kagan I, Iyer A, Lindner A, Andersen RA. Space representation for eye movements is more contralateral in monkeys than in humans. Proc Natl Acad Sci USA. 2010;107:7933–7938. doi: 10.1073/pnas.1002825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heiss JD, Walbridge S, Asthagiri AR, Lonser RR. Image-guided convection-enhanced delivery of muscimol to the primate brain. J Neurosurg. 2010;112:790–795. doi: 10.3171/2009.7.JNS09652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li CS, Mazzoni P, Andersen RA. Effect of reversible inactivation of macaque lateral intraparietal area on visual and memory saccades. J Neurophysiol. 1999;81:1827–1838. doi: 10.1152/jn.1999.81.4.1827. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Yttri EA, Snyder LH. Intention and attention: Different functional roles for LIPd and LIPv. Nat Neurosci. 2010;13:495–500. doi: 10.1038/nn.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seyal M, Ro T, Rafal R. Increased sensitivity to ipsilateral cutaneous stimuli following transcranial magnetic stimulation of the parietal lobe. Ann Neurol. 1995;38:264–267. doi: 10.1002/ana.410380221. [DOI] [PubMed] [Google Scholar]
- 35.Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- 36.Seltzer B, Pandya DN. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: A retrograde tracer study. J Comp Neurol. 1994;343:445–463. doi: 10.1002/cne.903430308. [DOI] [PubMed] [Google Scholar]
- 37.Bruce C, Desimone R, Gross CG. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J Neurophysiol. 1981;46:369–384. doi: 10.1152/jn.1981.46.2.369. [DOI] [PubMed] [Google Scholar]
- 38.Scalaidhe SP, Albright TD, Rodman HR, Gross CG. Effects of superior temporal polysensory area lesions on eye movements in the macaque monkey. J Neurophysiol. 1995;73:1–19. doi: 10.1152/jn.1995.73.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Ungerleider LG. Functional brain imaging studies of cortical mechanisms for memory. Science. 1995;270:769–775. doi: 10.1126/science.270.5237.769. [DOI] [PubMed] [Google Scholar]
- 40.Watson RT, Valenstein E, Day A, Heilman KM. Posterior neocortical systems subserving awareness and neglect. Neglect associated with superior temporal sulcus but not area 7 lesions. Arch Neurol. 1994;51:1014–1021. doi: 10.1001/archneur.1994.00540220060015. [DOI] [PubMed] [Google Scholar]
- 41.Luh KE, Butter CM, Buchtel HA. Impairments in orienting to visual stimuli in monkeys following unilateral lesions of the superior sulcal polysensory cortex. Neuropsychologia. 1986;24:461–470. doi: 10.1016/0028-3932(86)90091-6. [DOI] [PubMed] [Google Scholar]
- 42.Kamphuis S, Dicke PW, Thier P. Neuronal substrates of gaze following in monkeys. Eur J Neurosci. 2009;29:1732–1738. doi: 10.1111/j.1460-9568.2009.06730.x. [DOI] [PubMed] [Google Scholar]
- 43.Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411:950–953. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- 44.Mort DJ, et al. The anatomy of visual neglect. Brain. 2003;126:1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- 45.Feeney DM, Baron JC. Diaschisis. Stroke. 1986;17:817–830. doi: 10.1161/01.str.17.5.817. [DOI] [PubMed] [Google Scholar]
- 46.Fiorelli M, Blin J, Bakchine S, Laplane D, Baron JC. PET studies of cortical diaschisis in patients with motor hemi-neglect. J Neurol Sci. 1991;104:135–142. doi: 10.1016/0022-510x(91)90302-n. [DOI] [PubMed] [Google Scholar]
- 47.Baron JC, et al. Effects of thalamic stroke on energy metabolism of the cerebral cortex. A positron tomography study in man. Brain. 1986;109:1243–1259. doi: 10.1093/brain/109.6.1243. [DOI] [PubMed] [Google Scholar]
- 48.Vuilleumier P, et al. Abnormal attentional modulation of retinotopic cortex in parietal patients with spatial neglect. Curr Biol. 2008;18:1525–1529. doi: 10.1016/j.cub.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majchrzak M, Di Scala G. GABA and muscimol as reversible inactivation tools in learning and memory. Neural Plast. 2000;7:19–29. doi: 10.1155/NP.2000.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johansen-Berg H. Functional imaging of stroke recovery: What have we learnt and where do we go from here? Int J Stroke. 2007;2:7–16. doi: 10.1111/j.1747-4949.2007.00093.x. [DOI] [PubMed] [Google Scholar]
- 51.Lynch JC, McLaren JW. Deficits of visual attention and saccadic eye movements after lesions of parietooccipital cortex in monkeys. J Neurophysiol. 1989;61:74–90. doi: 10.1152/jn.1989.61.1.74. [DOI] [PubMed] [Google Scholar]
- 52.Schiller PH, Tehovnik EJ. Cortical inhibitory circuits in eye-movement generation. Eur J Neurosci. 2003;18:3127–3133. doi: 10.1111/j.1460-9568.2003.03036.x. [DOI] [PubMed] [Google Scholar]
- 53.Gaffan D, Hornak J. Visual neglect in the monkey. Representation and disconnection. Brain. 1997;120:1647–1657. doi: 10.1093/brain/120.9.1647. [DOI] [PubMed] [Google Scholar]
- 54.Wardak C, Olivier E, Duhamel JR. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J Neurosci. 2002;22:9877–9884. doi: 10.1523/JNEUROSCI.22-22-09877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wardak C, Olivier E, Duhamel JR. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron. 2004;42:501–508. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]
- 56.Karnath HO, Niemeier M, Dichgans J. Space exploration in neglect. Brain. 1998;121:2357–2367. doi: 10.1093/brain/121.12.2357. [DOI] [PubMed] [Google Scholar]
- 57.Patel GH, et al. Topographic organization of macaque area LIP. Proc Natl Acad Sci USA. 2010;107:4728–4733. doi: 10.1073/pnas.0908092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thier P, Andersen RA. Electrical microstimulation suggests two different forms of representation of head-centered space in the intraparietal sulcus of rhesus monkeys. Proc Natl Acad Sci USA. 1996;93:4962–4967. doi: 10.1073/pnas.93.10.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol. 1991;66:1109–1124. doi: 10.1152/jn.1991.66.3.1109. [DOI] [PubMed] [Google Scholar]
- 60.Rossini PM, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- 61.Fierro B, Brighina F, Bisiach E. Improving neglect by TMS. Behav Neurol. 2006;17:169–176. doi: 10.1155/2006/465323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sparing R, et al. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain. 2009;132:3011–3020. doi: 10.1093/brain/awp154. [DOI] [PubMed] [Google Scholar]
- 63.Rushmore RJ, Valero-Cabre A, Lomber SG, Hilgetag CC, Payne BR. Functional circuitry underlying visual neglect. Brain. 2006;129:1803–1821. doi: 10.1093/brain/awl140. [DOI] [PubMed] [Google Scholar]
- 64.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.