Abstract
A platelet-aggregating activity was found in many snake venoms, predominantly those of the genus Bothrops, that is apparent only in the presence of the platelet-aggregating von Willebrand factor of plasma. It is designated "venom coagglutinin." The coagglutinin can be largely separated from the thrombin-like enzyme of the venoms by ion-exchange chromatography. The venom factor acts on formaldehyde-fixed platelets and is effective with decalcified, heparinized, and afibrinogenemic plasmas but not with severe von Willebrand disease plasmas or with normal plasmas in which the von Willebrand factor has been neutralized by specific antibodies. Use of this coagglutinin permits the assay of von Willebrand factor without the many disadvantages of the ristocetin test. The coagglutinin is active with human, dog, pig, and bovine plasmas and with platelets of any one of these species. This broad-spectrum activity without regard to species contrasts with the ristocetin-resistance of many combinations of plasma and platelets from various species. The assay provides a procedure for studying human, porcine, and canine von Willebrand disease. The lack of species specificity of the coagglutinin suggests that it may be a universal activator of the von Willebrand factor-platelet reaction.
Full text
PDF
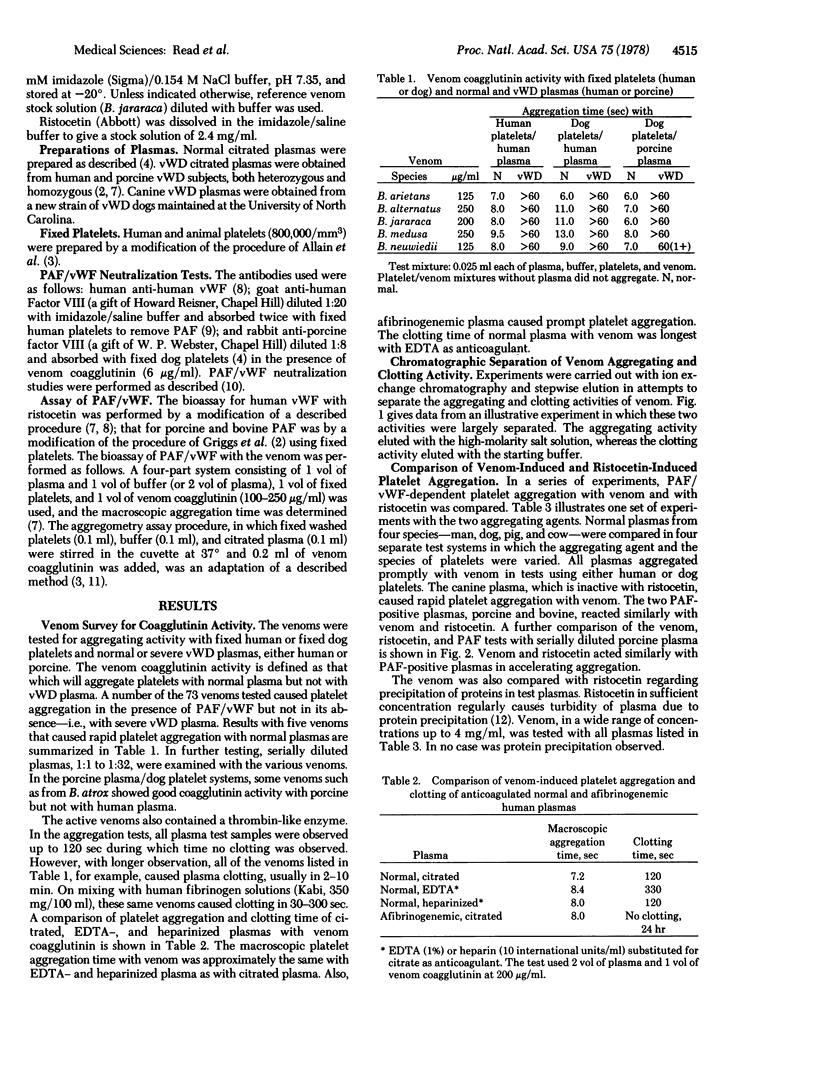
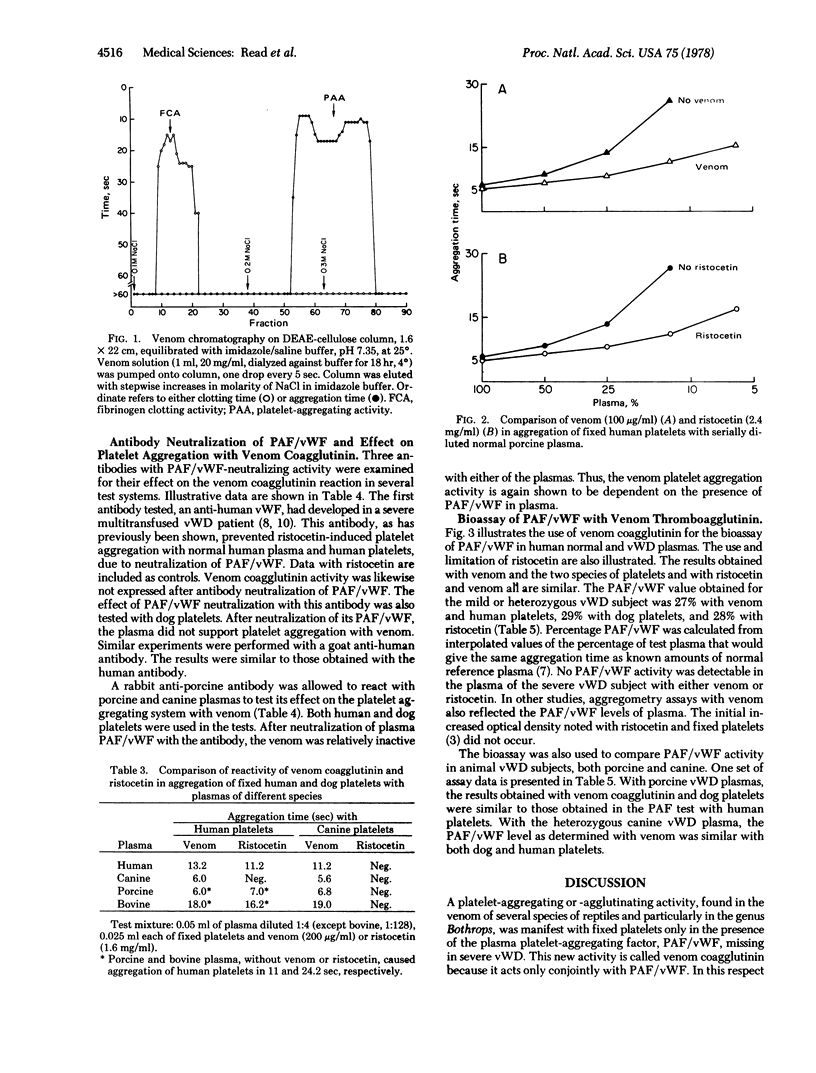
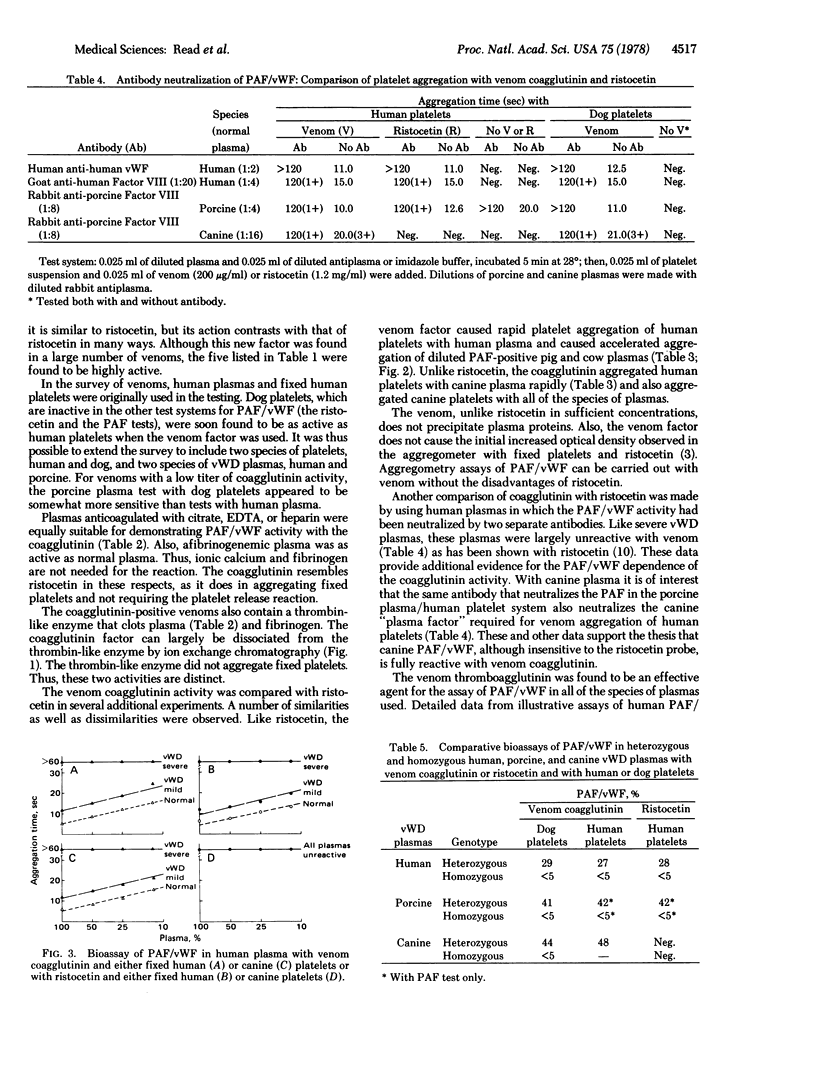

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain J. P., Cooper H. A., Wagner R. H., Brinkhous K. M. Platelets fixed with paraformaldehyde: a new reagent for assay of von Willebrand factor and platelet aggregating factor. J Lab Clin Med. 1975 Feb;85(2):318–328. [PubMed] [Google Scholar]
- Blatt P. M., Brinkhous K. M., Culp H. R., Krauss J. S., Roberts H. R. Antihemophilic factor concentrate therapy in von Willebrand disease. Dissociation of bleeding-time factor and ristocetin-cofactor activities. JAMA. 1976 Dec 13;236(24):2770–2772. [PubMed] [Google Scholar]
- Brinkhous K. M., Graham J. E., Cooper H. A., Allain J. P., Wagner R. H. Assay of von Willebrand factor in von Willebrand's disease and hemophilia: use of a macroscopic platelet aggregation test. Thromb Res. 1975 Mar;6(3):267–272. doi: 10.1016/0049-3848(75)90074-2. [DOI] [PubMed] [Google Scholar]
- Brinkhous K. M., Thomas B. D., Ibrahim S. A., Read M. S. Plasma levels of platelet aggregating factor/vom Willebrand factor in various species. Thromb Res. 1977 Sep;11(3):345–355. doi: 10.1016/0049-3848(77)90186-4. [DOI] [PubMed] [Google Scholar]
- Cooper H. A., Mason R. G., Brinkhous K. M. The platelet: membrane and surface reactions. Annu Rev Physiol. 1976;38:501–535. doi: 10.1146/annurev.ph.38.030176.002441. [DOI] [PubMed] [Google Scholar]
- Cooper H. A., Wilkins K. W., Jr, Johnson P. R., Jr, Wagner R. H. Platelet-aggregating factor and the aggregation of fixed washed platelets. J Lab Clin Med. 1977 Sep;90(3):512–521. [PubMed] [Google Scholar]
- Griggs T. R., Webster W. P., Cooper H. A., Wagner R. H., Brinkhous K. M. Von Willebrand factor: gene dosage relationships and transfusion response in bleeder swine--a new bioassay. Proc Natl Acad Sci U S A. 1974 May;71(5):2087–2090. doi: 10.1073/pnas.71.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarji K. E., Stratton R. D., Wagner R. H., Brinkhous K. M. Nature of von Willebrand factor: a new assay and a specific inhibitor. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2937–2941. doi: 10.1073/pnas.71.8.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibbe J., Kirby E. P. The influence of Haemaccel, fibrinogen and albumin on ristocetin-induced platelet aggregation. Relevance to the quantitative measurement of the ristocetin cofactor. Thromb Res. 1976 Feb;8(2):151–165. doi: 10.1016/0049-3848(76)90258-9. [DOI] [PubMed] [Google Scholar]
- Stratton R. D., Wagner R. H., Webster W. P., Brinkhous K. M. Antibody nature of circulating inhibitor of plasma von Willebrand factor. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4167–4171. doi: 10.1073/pnas.72.10.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. J., Hoyer L. W., Rickles F. R., Varma A., Rogers J. Quantitative assay of a plasma factor deficient in von Willebrand's disease that is necessary for platelet aggregation. Relationship to factor VIII procoagulant activity and antigen content. J Clin Invest. 1973 Nov;52(11):2708–2716. doi: 10.1172/JCI107465. [DOI] [PMC free article] [PubMed] [Google Scholar]



