Abstract
It has been argued that bacteria communicate using small diffusible signal molecules to coordinate, among other things, the production of factors that are secreted outside of the cells in a process known as quorum sensing (QS). The underlying assumption made to explain QS is that the secretion of these extracellular factors is more beneficial at higher cell densities. However, this fundamental assumption has never been tested experimentally. Here, we directly test this by independently manipulating population density and the induction and response to the QS signal, using the opportunistic pathogen Pseudomonas aeruginosa as a model organism. We found that the benefit of QS was relatively greater at higher population densities, and that this was because of more efficient use of QS-dependent extracellular “public goods.” In contrast, the benefit of producing “private goods,” which are retained within the cell, does not vary with cell density. Overall, these results support the idea that QS is used to coordinate the switching on of social behaviors at high densities when such behaviors are more efficient and will provide the greatest benefit.
Keywords: social evolution, sociomicrobiology
A rapidly expanding body of research assumes that bacterial cells communicate via quorum sensing (QS) to coordinate behaviors at the population level (1–7). Cells produce and release QS signaling molecules, which then have two consequences. First, they regulate the production of a range of extracellular factors, which are released from cells and have various uses, including scavenging for nutrients, immune suppression, providing scaffolding for biofilms to grow, and aiding motility. These factors provide a benefit to the local population of cells and so are equivalent to what economists call “public goods.” In parasitic species, these factors often play key roles in bacterial growth, virulence, and ultimately the eventual damage caused to hosts. Second, the uptake of signal molecule also leads to an increase in the production of the signal molecule itself, in a process often termed “autoinduction.” This process leads to a positive-feedback mechanism at high cell densities, which results in a considerable increase in the production of signal and QS-controlled factors (Fig. 1).
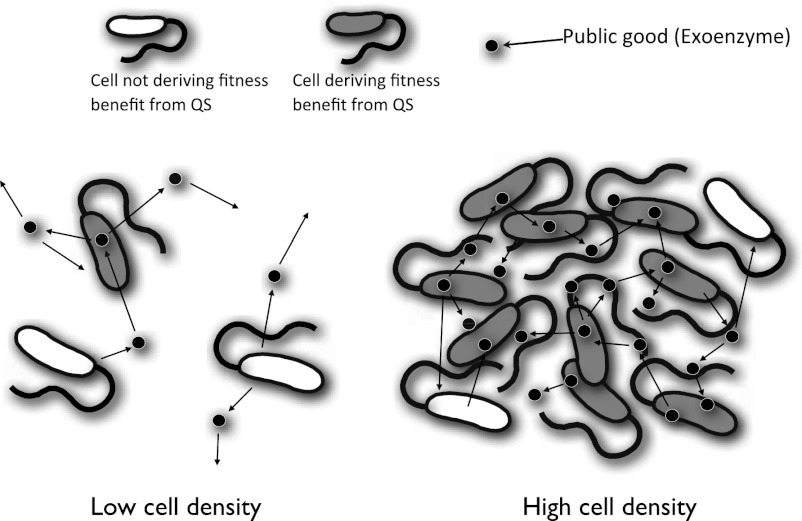
Fig. 1.
The hypothesized function of QS. At low cell densities, a large proportion of the extracellular factors (public goods or exoenzymes) disperse before they can be used (by either the cell that produced it or others), and so their production provides little direct or indirect fitness benefit. At high cell densities, a much greater proportion of the extracellular public goods (or the products they produce) can be used by the cell that produced the extracellular factors and their neighbors. Consequently, from the inclusive fitness perspective of an individual cell (33), the production of extracellular public goods is more efficient and beneficial at higher population densities.
The fundamental assumption made to explain QS is that the purpose of QS is to stimulate the production of extracellular public goods at high cell densities, when their production is most beneficial (1–8). The idea here is that at low densities, the action of extracellular factors would be relatively inefficient because they would disperse away before they could be used (Fig. 1). In contrast, at higher cell densities, a greater proportion of the products produced by extracellular factors could be used (Fig. 1), which would lead to the relative benefit of producing extracellular factors increasing with population density as their benefits are harnessed more efficiently. However, this key assumption, upon which the field of QS research is based, has never been empirically tested. Consequently, it has been argued that “this quorum-sensing hypothesis rests on very weak foundations,” and that alternative nonsocial explanations are possible (see Discussion) (8).
Here, we test whether the fitness benefits of responding to QS are density-dependent, with a greater benefit occurring at higher cell densities (1–7). We examine this using Pseudomonas aeruginosa, a Gram-negative opportunistic pathogen, which causes disease in plants and animals, including humans. This organism uses a complex, hierarchical QS system to regulate numerous processes, including virulence factor production (9, 10). To test whether the fitness benefits of QS are density-dependent, it is necessary to be able to independently manipulate density and to control when the QS system in P. aeruginosa is induced. We do this by manipulating the growth medium, and by using artificial signal to control the behavior of a mutant that does not produce signal. We carry out three controls, one which removes the need for a cooperative QS response, a second which requires a QS response to generate a nonsocial, intracellular “private good,” and a third that manipulates the response to QS by altering the concentration of QS signals.
Results
Manipulating Density and QS.
We independently manipulated both density and when the QS system of P. aeruginosa was induced. We manipulated density by varying the concentration of casamino acids (CAA) in a minimal growth medium where CAA was the only carbon source available for growth. We found that as we increased the percentage of CAA in the growth environment, this led to an increase in the final population density (Fig. 2A) (P < 0.0001).
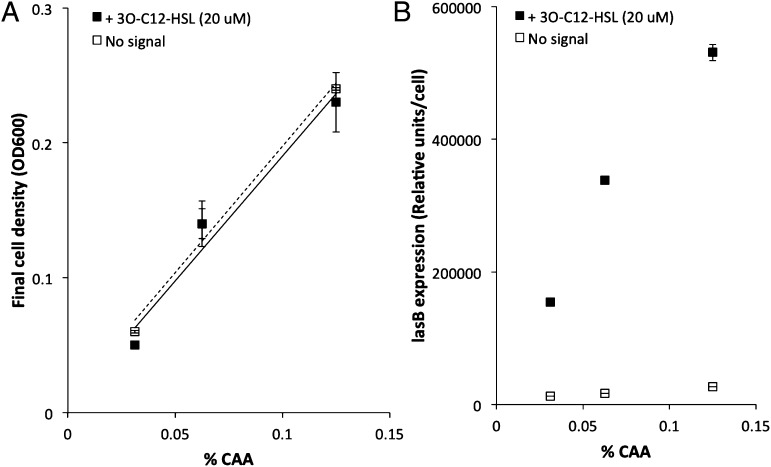
Fig. 2.
Manipulating cell density with CAA. (A) The PA01 lasI::GmR (signal-negative) mutant grew to a higher cell density when provided with more CAA, both in the presence (■) and absence (□) of the signal 3O-C12-HSL (20 μM). This process demonstrates that cell density and the size of the bacterial population can be tightly controlled in this media. (B) The lasB expression per cell (in relative light units) of the PA01 lasI::GmR (signal-negative) mutant was increased by the addition of the signal 3O-C12-HSL (20 μM). All results are shown as means (± SD), eight replicates per treatment.
We controlled the induction of QS by using a lasI (signal-negative) QS mutant, that doesn’t produce signal, but does respond to signal. We added 20 μM of chemically synthesized N-(3-oxododecanoyl)-l-homoserine lactone (3O-C12-HSL), to induce the expression of QS-dependent genes in this mutant, which includes the lasB gene, which codes for elastase. We found that when we added synthesized signal this led to QS induction, measured by expression of the lasB gene, at both low and high densities (Fig. 2B) [F(1,4) = 23.16, P = 0.04]. Our finding that QS can be induced at low population densities is in agreement with previous work performed on the QS system of P. aeruginosa (11, 12).
Fitness Consequences of QS.
We then tested the idea that the addition of signal, and therefore induction of QS-dependent genes, provides a greater benefit at higher cell densities. To do this, we used a medium containing two carbon sources, CAA and BSA. BSA can only be used as a nutrient source by cells when it is broken down by the action of QS-dependent proteases, such as LasB (13). Consequently, we could vary the amount of CAA to vary population density and then add artificial signal to test the fitness benefit of inducing QS to break down BSA. Our hypothesis was that the fitness consequences of adding signal (hence, inducing QS), as measured by dividing the final population density in the presence of signal by the final population density in the absence of signal, will be greater with increasing density.
We found that the fitness benefits of QS were greater at higher population densities (Fig. 3) (P < 0.0001). At low cell densities, the addition of 20 μM 3O-C12-HSL signal led to a relatively small increase in population growth, suggesting that despite the induction of a QS response (Fig. 2B), the production of QS-regulated extracellular factors is relatively inefficient. In contrast, at high cell densities, the addition of signal led to significant increase in population growth, suggesting considerable benefit from the production of extracellular factors at such densities. We carried out three control experiments to test the validity of this result.
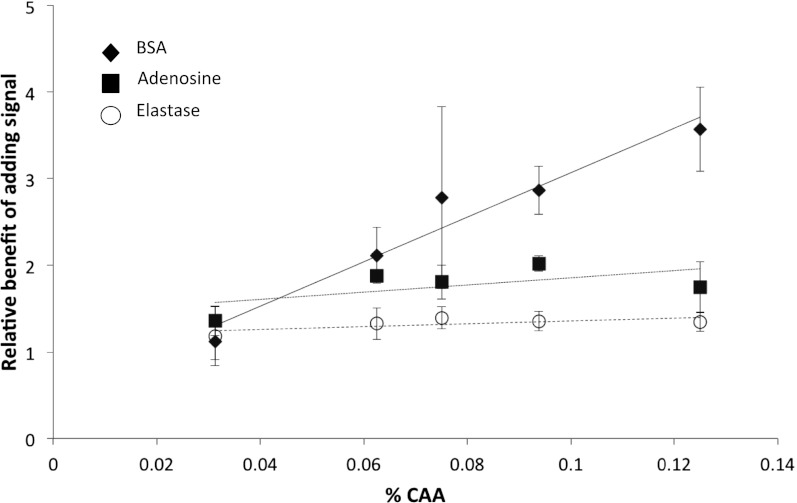
Fig. 3.
The fitness benefit of responding to QS. The fitness benefit of adding signal (relative final growth), and therefore inducing QS, was greater when CAA was provided at a higher concentration, and hence at higher population densities (◆). Two control treatments showed that when the social aspect of QS was removed, this also removed the effect of population density. Specifically, through: (i) the addition of elastase, which digests the BSA carbon source directly, without the need for QS (○); and (ii) the replacement of the BSA carbon source with adenosine, which is digested intracellularly (■). All results are shown as means (± SD), eight replicates per treatment.
Control I: Public goods.
To test that the effect of density was mediated through the production of extracellular factors, we experimentally added purified elastase (porcine elastase) directly to the dual carbon-source medium. This process breaks down the BSA carbon source and, hence, removes the benefit to the bacteria of producing extracellular proteolytic factors. In this case, we predict that density would have no influence on the relative growth consequences of adding QS signal. We found no significant relationship between relative growth and cell density when elastase had been added (Fig. 3) (P = 0.287).
Control II: Private goods.
We tested whether the effect of density was removed when examining a QS-regulated factor that operates within the cell. Our predicted positive relationship between cell density and the benefit of QS arises because the factors are released out of the cell, providing a benefit to the local population of cells (public goods). In contrast, some benefits produced by the action of QS are not released and instead act intracellularly. We would predict that the fitness benefit of such private goods should not vary with cell density because their benefit is only to the individual cell that produced them, and not the local population.
We chose adenosine as a carbon source to examine this. In P. aeruginosa, adenosine is taken up by cells and degraded via inosine, hypoxanthine, xanthine, and urate to glyoxylate and urea, providing direct fitness benefits to individual cells proficient in this pathway. P. aeruginosa lasR QS mutants have previously been shown to be unable to grow on adenosine as a sole carbon source because the nucleoside hydrolase Nuh, which degrades inosine to hypoxanthine, is under positive QS-control (14). We found that our lasI mutant was unable to grow using adenosine as a carbon source. We also found that when the lasI (signal-negative) mutant was grown in a dual carbon-source medium containing CAA and adenosine, the addition of signal leads to an increase in growth, but this increase and the relative fitness benefit does not vary with cell density (P = 0.598) (Fig. 3).
Control III: QS response.
We tested whether our results could be explained by a lower response to QS signal at lower densities. Although the addition of signal induced QS at all cell densities, there was a reduced expression of lasB per cell at lower densities compared with high densities (Fig. 2B). Consequently, it is possible that the reduced benefit of QS at lower cell densities (Fig. 3) may be because of density influencing the production of extracellular factors, rather than the efficiency with which those factors are used. We tested this theory by increasing the expression of lasB in the low cell-density cultures in the dual carbon-source medium by varying the amount of signal added.
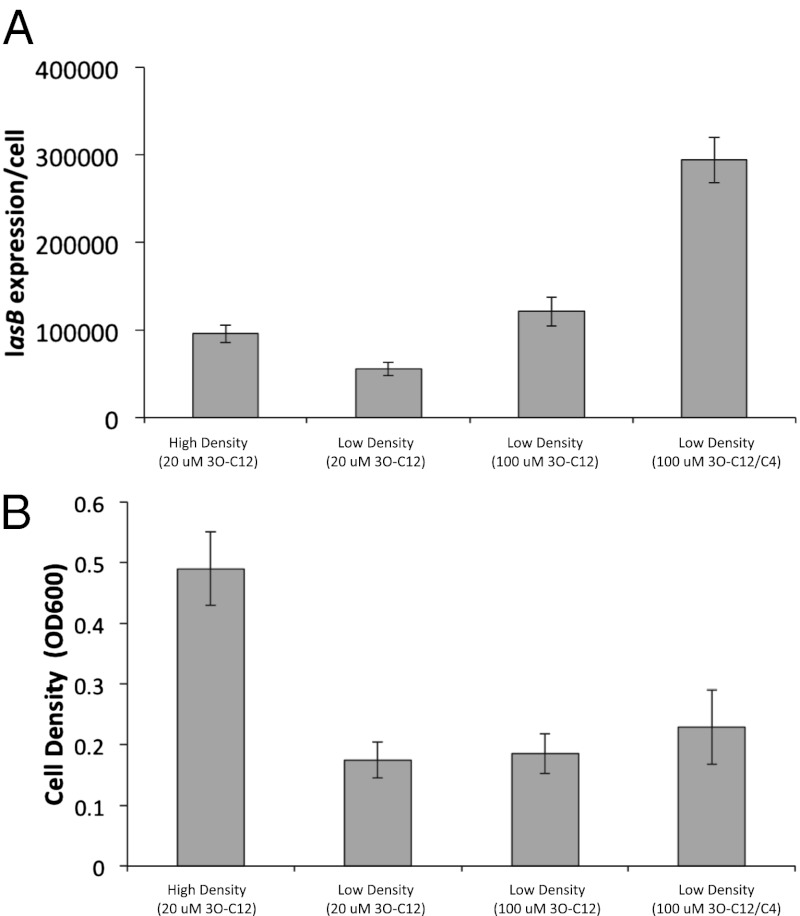
Compared with high cell-density cultures, low cell-density cultures containing 20 μM of added 3O-C12-HSL showed significantly reduced lasB expression (Fig. 4A) (P < 0.0001) and a reduced fitness as measured by growth (Fig. 4B) (P < 0.0001). When we increased the concentration of 3O-C12-HSL in the low cell-density culture to 100 μM, this resulted in an increase in lasB expression (Fig. 4A) (P < 0.0001) but no effect on cell growth (Fig. 4B) (P = 0.59). It has previously been shown that a combination of 3O-C12-HSL and C4-HSL leads to maximal expression of the lasB gene (15), so when we added 100 μM of both 3O-C12-HSL and C4-HSL together to low cell-density cultures, this resulted in a further significant increase in lasB expression compared with the high cell-density culture containing 20 μM of 3O-C12-HSL alone (Fig. 4A) (P < 0.0001), but no increase in cell growth (Fig. 4B) (P = 0.1538). We repeated this control using a lasI/rhlI double-mutant and found the same effects (Fig. S1).
Fig. 4.
Response to signal and cell density. (A) lasB expression per cell is highly induced by 3O-C12-HSL at high density (0.125% CAA) but less so at low density (0.03125% CAA) cultures. A combination of 3O-C12-HSL and C4-HSL induces lasB to a high level at low cell density. (B) Increased fitness (growth) of the population occurs under conditions of high cell density but not low cell density, even with the addition of both signals. All results are shown as means (± SD), six replicates per treatment.
Discussion
Here we provide clear support for a fundamental assumption of the QS literature, that the production of extracellular factors is more beneficial at higher cell densities. Specifically, we found that: (i) the fitness benefit provided by turning on the QS response was greater at higher cell densities (Fig. 3); (ii) the effect of density was removed if extracellular factors (elastase) were added directly to the medium, or if growth was dependent upon intracellular consumption of private goods (Fig. 3); (iii) our results cannot be explained by a reduced response to QS at lower cell densities.
Our results are expected if QS is social, with individual cells being influenced by the signal and extracellular factors produced by other cells (13, 16–18). In contrast, if QS was not social, such that the benefits of producing extracellular factors only pass to the cell that produces them, then the fitness benefit of responding to QS would not have varied with cell density (19). However, it is important to not overextrapolate from these results in two ways. First, just because QS can have social fitness consequences, this does not mean that all traits controlled by QS are social. In cases where QS induces intracellular factors, such as those required for the metabolic breakdown of adenosine, then the benefits of such private goods are not social, and only accrue directly to the cell producing them (11).
Second, our results do not refute the idea that QS may also act to sense properties of the environment, such as the rate at which signals and QS-dependent factors diffuse away from cells. Redfield suggested that an alternative explanation for QS was that it was a nonsocial trait, the role of which was to measure the diffusion rate, termed diffusion sensing (8, 20). However, it is wrong to contrast QS and diffusion sensing as competing hypotheses because the rate of diffusion will also alter the social costs and benefits of QS, by altering the extent to which benefits flow to relatives (6). Consequently, it is more useful to ask the separate questions of whether the trait is social, whether density matters and whether diffusion matters, and how this varies across different factors produced. Indeed, a comparison of our main result with our adenosine control shows how autoinducers can control both social (public goods) and nonsocial (private goods) traits, and that one does not exclude the other. Analogously, the result that QS can be induced at low cell densities does not eliminate the potential for social fitness consequences (11, 12, 21, 22).
Our results should be seen as an empirical first step, demonstrating the fitness consequences of varying cell density. A limitation of our work is that it was carried out in the relatively unnatural environment of a liquid culture in a test tube; we did this because it allowed us to independently control both density and the induction and response to QS. A key task for the future is to develop methods that allow this experiment to be replicated in increasingly realistic environments, including in vivo. Although, it should be noted that our previous test tube results with P. aeruginosa (13) have since been replicated in other laboratory conditions, with acute and chronic infections of mice, and within human intubated infections (13, 18, 23–25). These previous studies demonstrated the social nature of QS, and in particular how QS could be exploited by free-loaders that did not either produce or respond to signal. A laboratory study of Myxococcus xanthus grown on casein elegantly showed that growth rates were greater at higher cell densities, and could be raised by the addition of prehydrolyzed casein (26). However, although this result is consistent with our results, it was not on a trait controlled by QS and did not involve the relevant controls that we carried out.
More generally, by demonstrating that signaling is favored to coordinate behavior at the population level, our results show that QS shares conceptual links with other forms of signaling, such as alarm or food calls in birds and mammals (27–29). Consequently, QS could be exploited as an easily manipulated model system for testing very general theory that was developed to explain animal signals. Furthermore, this social nature of QS is not only of interest from a pure science perspective, because it can also be exploited as a novel medical intervention strategy (30). Specifically, the fact that QS can be exploited by free-loaders, who do not either produce or respond to the QS signaling molecules (13, 18, 23–25), could be used to reduce population size and virulence, or even introduce medically beneficial alleles into infective populations (30).
Materials and Methods
Bacterial Strains, Growth Media, and Culture Conditions.
The bacterial strains we used were P. aeruginosa PA01 lasI::GmR (31) (PA01 carrying an insertion in the lasI gene) and PA01 lasI::GmR (31), containing a chromosomally integrated lasB::luxCDABE fusion constructed using the mini-CTXlux system (32). For growth of all overnight cultures we used Luria-Bertani (LB) broth and we incubated cultures with shaking at 37 °C. For all growth-curve experiments we used a minimal growth medium, QS medium (QSM) (13). This medium was composed of an autoclaved basal salts solution (Na2HPO4, 6.8 gL−1; KH2PO4, 3.0 gL−1; NaCl, 0.5 gL−1) and filter-sterilized 1 M supplement stock solutions of NH4Cl, CaCl2, and MgSO47H2 . Volumes were constructed and filter-sterilized (150 mL basal salts, 1,500 μL NH4Cl, 15 μL CaCl2, 150 μL MgSO4). For a carbon source, we either added CAA (at different concentrations to vary density) or BSA (Sigma) at a concentration of 1% or adenosine (Sigma) at 0.1%.
Manipulating Density and QS.
We manipulated density by growing strains in a minimal QSM growth medium, with varying concentrations of CAA as the sole carbon and energy source. We generated a CAA concentration range between 0.125% and 0.03125% by serial dilution. We then grew overnight cultures in LB and centrifuged them at 8,900 × g for 5 min, and the cells were washed twice and resuspended in QSM containing no carbon source. We adjusted cultures to OD600 0.03 ± 0.01. Aliquots of QSM containing CAA were inoculated with the appropriate strain at a concentration of 10 μL⋅mL−1. Next, 200 μL of each sample was added to a 96-well microtiter plate (Corning) and blank QSM was used as a negative control. Growth curves are shown in Fig. S2A. We controlled the induction of QS by using a lasI (signal-negative) QS mutant that does not produce the primary signal of the P. aeruginosa QS system, 3O-C12-HSL, but still produces extracellular factors in response to the presence of this signal. We were then able to turn on QS experimentally, by adding chemically synthesized signal, to induce the expression of QS-dependent genes and subsequently the production of extracellular factors. We verified that this leads to QS induction using a lasB::lux fusion where the expression of the QS-regulated lasB gene leads to light output. We measured lasB expression, as shown in Fig. 2B, after 7 h of growth; a full-expression profile is shown in Fig. S2B. We analyzed all growth-curve experiments using a Tecan Infinite 200 microplate reader, with absorbance (600 nm) and bioluminescence readings taken at 30-min intervals for a period of 24 h. Where required, we added synthetically prepared 3O-C12-HSL at appropriate concentrations.
Analyzing the Fitness Consequences of QS.
We performed growth-curve experiments as previously described, using PAO1 lasI::GmR grown in a dual carbon source QSM in both the presence and absence of QS signal. For one carbon source, we added CAA at varying concentrations, ranging from 0.0325% to 0.125%, to produce a range of population densities from relatively low to relatively high. For the second carbon source, we added 1% BSA, which can only be used when it is broken down by the action of QS-dependent proteases, such as LasB (12). For control experiments, we used a CAA dilution range of 0.125–0.03125% with either a 1% constant source of BSA, 0.1% of adenosine (Sigma), or 1 unit of elastase (Sigma). We calculated, using an Elastin Congo red assay, that the concentration of elastase we added (0.0067 units/mL) is similar to the level produced in the supernatant by PAO1 (0.0042 units/mL) and the lasI mutant (+ 20 μM 3O-C12-HSL; 0.0059 units/mL) when we grew the strains in QSM for 18 h. We analyzed growth using a Tecan Infinite 200 microplate reader, with absorbance (600 nm) and bioluminescence readings taken at 30-min intervals for a period of 24 h. Where required, we added synthetically prepared 3O-C12-HSL and C4-HSL at appropriate concentrations. We calculated the relative benefit of QS by dividing the final population density in the presence of signal by the final population density in the absence of signal.
Analyses.
We analyzed our data using the statistics package GraphPad Prism v.5. In all analyses we used either standard two-tailed t tests or linear regressions.
Supplementary Material
Acknowledgments
We thank Paul Williams, Karima Rhigetti, and James Gurney for the strains used in the study; and Sam Brown, Roman Popat, and Andrew Fogarty for discussion and comments. This study was supported in part by the European Research Council, the Medical Research Council, the Leverhulme Trust, and the Royal Society.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118131109/-/DCSupplemental.
References
- 1.Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diggle SP, Gardner A, West SA, Griffin AS. Evolutionary theory of bacterial quorum sensing: When is a signal not a signal? Philos Trans R Soc Lond B Biol Sci. 2007;362:1241–1249. doi: 10.1098/rstb.2007.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams P, Winzer K, Chan WC, Cámara M. Look who’s talking: Communication and quorum sensing in the bacterial world. Philos Trans R Soc Lond B Biol Sci. 2007;362:1119–1134. doi: 10.1098/rstb.2007.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng W-L, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller L, Surette MG. Communication in bacteria: An ecological and evolutionary perspective. Nat Rev Microbiol. 2006;4:249–258. doi: 10.1038/nrmicro1383. [DOI] [PubMed] [Google Scholar]
- 6.Brown SP, Johnstone RA. Cooperation in the dark: Signalling and collective action in quorum-sensing bacteria. Proc Biol Sci. 2001;268:961–965. doi: 10.1098/rspb.2001.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nat Rev Microbiol. 2006;4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 8.Redfield RJ. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 2002;10:365–370. doi: 10.1016/s0966-842x(02)02400-9. [DOI] [PubMed] [Google Scholar]
- 9.Schuster M, Lostroh CP, Ogi T, Greenberg EP. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: A transcriptome analysis. J Bacteriol. 2003;185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams P, Cámara M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: A tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol. 2009;12:182–191. doi: 10.1016/j.mib.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Boedicker J, Vincent M, Ismagilov R. Microfluidic confinement of single cells of bacteria in small volumes initiates high-density behavior of quorum sensing and growth and reveals its variability. Angew Chem Int Ed Engl. 2009;48:5908–5911. doi: 10.1002/anie.200901550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connell JL, et al. Probing prokaryotic social behaviors with bacterial “lobster traps.”. MBio. 2010;1:e00202–e00210. doi: 10.1128/mBio.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:411–414. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 14.Heurlier K, et al. Quorum-sensing-negative (lasR) mutants of Pseudomonas aeruginosa avoid cell lysis and death. J Bacteriol. 2005;187:4875–4883. doi: 10.1128/JB.187.14.4875-4883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiteley M, Lee KM, Greenberg EP. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1999;96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank SA. Foundations of Social Evolution. Princeton, NJ: Princeton Univ Press; 1998. [Google Scholar]
- 17.West SA, Griffin AS, Gardner A. Social semantics: Altruism, cooperation, mutualism, strong reciprocity and group selection. J Evol Biol. 2007;20:415–432. doi: 10.1111/j.1420-9101.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 18.Sandoz KM, Mitzimberg SM, Schuster M. Social cheating in Pseudomonas aeruginosa quorum sensing. Proc Natl Acad Sci USA. 2007;104:15876–15881. doi: 10.1073/pnas.0705653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross-Gillespie A, Gardner A, Buckling A, West SA, Griffin AS. Density dependence and cooperation: Theory and a test with bacteria. Evolution. 2009;63:2315–2325. doi: 10.1111/j.1558-5646.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- 20.Hense BA, et al. Does efficiency sensing unify diffusion and quorum sensing? Nat Rev Microbiol. 2007;5:230–239. doi: 10.1038/nrmicro1600. [DOI] [PubMed] [Google Scholar]
- 21.Dulla G, Lindow S. Quorum size of Pseudomonas syringae is small and dictated by water availability on the leaf surface. Proc Natl Acad Sci USA. 2008;105:3082–3087. doi: 10.1073/pnas.0711723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carnes EC, et al. Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nat Chem Biol. 2010;6:41–45. doi: 10.1038/nchembio.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rumbaugh KP, et al. Quorum sensing and the social evolution of bacterial virulence. Curr Biol. 2009;19:341–345. doi: 10.1016/j.cub.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 24.Köhler T, Buckling A, van Delden C. Cooperation and virulence of clinical Pseudomonas aeruginosa populations. Proc Natl Acad Sci USA. 2009;106:6339–6344. doi: 10.1073/pnas.0811741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilder CN, Diggle SP, Schuster M. Cooperation and cheating in Pseudomonas aeruginosa: The roles of the las, rhl and pqs quorum-sensing systems. ISME J. 2011;5:1332–1343. doi: 10.1038/ismej.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg E, Keller KH, Dworkin M. Cell density-dependent growth of Myxococcus xanthus on casein. J Bacteriol. 1977;129:770–777. doi: 10.1128/jb.129.2.770-777.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Searcy W, Nowicki S. The Evolution of Animal Communication. Princeton, Oxford: Princeton Univ Press; 2005. [Google Scholar]
- 28.Bradbury J, Vehrencamp S. Principles of Animal Communication. 2nd Ed. Sunderland, MA: Sinauer Associates; 2011. [Google Scholar]
- 29.Maynard-Smith J, Harper D. Animal Signals. Oxford: Oxford Univ Press; 2003. [Google Scholar]
- 30.Brown SP, West SA, Diggle SP, Griffin AS. Social evolution in micro-organisms and a Trojan horse approach to medical intervention strategies. Philos Trans R Soc Lond B Biol Sci. 2009;364:3157–3168. doi: 10.1098/rstb.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jadhav GP, et al. Immunosuppressive but non-LasR-inducing analogues of the Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone. J Med Chem. 2011;54:3348–3359. doi: 10.1021/jm2001019. [DOI] [PubMed] [Google Scholar]
- 32.Becher A, Schweizer HP. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. Biotechniques. 2000;29:948–950. doi: 10.2144/00295bm04. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton WD. The genetical evolution of social behaviour I & II. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.