Abstract
There is an urgent need to identify non-invasive biomarkers for the detection of sporadic Alzheimer’s disease (AD). We previously studied microRNAs (miRNAs) in AD autopsy brain samples and reported a connection between miR-137, -181c, -9, -29a/b and AD, through the regulation of ceramides. In this study, the potential role of these miRNAs as diagnostic markers for AD was investigated. We identified that these miRNAs were down-regulated in the blood serum of probable AD patients. The levels of these miRNAs were also reduced in the serum of AD risk factor models. Although the ability of these miRNAs to conclusively diagnose for AD is currently unknown, our findings suggest a potential use for circulating miRNAs, along with other markers, as non-invasive and relatively inexpensive biomarkers for the early diagnosis of AD, however, with further research and validation.
Keywords: Alzheimer’s disease, microRNA, blood serum
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia with a worldwide population of over 24 million, a number expected to double in the next 15 years (Ferri, et al., 2005). Early and accurate diagnosis of AD is crucial, enabling early treatments to slow or delay the progression of the disease and provide prognostic information. Current methods of neuro-imaging biomarkers include magnetic resonance imaging (MRI) structural analyses (Barber, Mungas, et al., 2002). However, the sensitivity of these imaging techniques does not afford sufficient demarcations in the medical temporal atrophy between AD and non-AD dementia to provide clear and decisive diagnosis (Scheltens, et al., 2002, Wahlund, et al., 2000). Functional neuro-imaging techniques have been developed for probable diagnosis (Ballard, et al.), but again with insufficient accuracy in discriminating between AD and control individuals (Dougall, et al., 2004). Even though positron emission tomography (PET) imaging has been able to distinguish cases with probable AD from normal and non-AD cases, wide variations in sensitivity and specificity have been reported (Patwardhan, et al., 2004). Amyloid beta (Aβ) (Sjogren, et al., 2002) and hyperphosphorylated tau (de Souza, et al.) levels in the cerebrospinal fluid (CSF) have also been suggested as diagnostic markers for AD and show great promise. However, the ability to identify biomarkers through less invasive procedures, such as in a blood test, would be significant. Attempts to measure Aβ levels in blood, thus far, have led to inconsistent results (Ballard, et al., Blennow, et al., Hampel, et al., Hansson, et al., Irizarry, 2004).
In addition to proteins, blood serum contains circulating miRNAs, endogenous small RNAs of 21–25-nucleotides that post-transcriptionally regulate gene expressions (Lagos-Quintana, et al., 2001). MiRNAs have been reported to be transported in blood in liposomes (Kosaka, et al.), high density lipoproteins (Vickers, et al.), Argonaute2 (Arroyo, et al., Turchinovich, et al.), and other proteins (Wang, et al.), protecting them from being degraded. Recently, circulating miRNA levels have been proposed as potential diagnostic tools for a number of diseases (Gilad, et al., 2008, Wang, et al., Zeng, et al.). MiRNAs have been shown to be differentially expressed in AD patients (Cogswell, et al., 2008, Hebert, et al., 2008, Lukiw, 2007, Wang, et al., 2008) and altered in response to Aβ (Schonrock, et al.). In a recent study we showed that miR-137, -181c, -9 and -29a/b are involved in AD by modulating the ceramide levels (Geekiyanage and Chan). We showed that ceramides, a sphingolipid, are increased in the brain cortices of a subgroup of sporadic AD patients along with serine palmitoyletransferase (SPT) levels, the rate limiting enzyme in the de novo synthesis of ceramide. We demonstrated that SPT long chain 1 (SPTLC1) and SPT long chain 2 (SPTLC2), two subunits of SPT, are post-transcriptionally regulated by miR-137/-181c; and miR-9-29a/b, respectively. We observed significant correlations between SPT, their corresponding miRNAs (miR-137, -181c, -9 and -29a/b), and Aβ in the autopsy AD brain samples, as well as a direct involvement of SPT and miR-137/-181c in Aβ production through transfection studies. Hebert et al. (Hebert, et al., 2008) had previously demonstrated the involvement of miR-29a/b in Aβ production. In addition, we showed negative relationships between SPT and their respective miRNAs in AD risk factor models, where miRNA levels were observed to decrease in mice fed a high fat diet and in female mice, thereby suggesting a potential therapeutic value (Geekiyanage and Chan). Here, we investigated the expression levels of these miRNAs in the blood sera of a subgroup of mild and severe sporadic AD patients and mouse risk factor models to assess the potential of using these miRNAs as early diagnostic markers.
Material and Methods
Patient information
AD (n=7) and control (n=7) blood serum samples were from the University of Kentucky (UK) Alzheimer's disease center tissue bank (ADC). The samples have been clinically diagnosed by neurologists, neuropsychologists, and other staff members in the ADC clinic. A complete description of the samples including age of the patient, gender, MMSE scores and the clinical diagnoses is provided in Table 1. The standard MMSE test in the UDS battery was used with no correction factors. The discrimination of AD, MCI and control cases were performed using current consensus-based methodologies which are previously well- described in a clinical-pathological consensus conference (Jicha, et al.). Based on this study, we placed individuals with MMSE scores of 29 and 30 (n=7) in the “control” group and individuals with MMSE scores of 10–20 (n=6) and 1 subject with MMSE score of 8 in the “probable AD” group. Finally, based on Jicha, et al. we placed subjects with MMSE scores of 23–28 in the “MCI/probable Early AD” group. The MCI subjects included in this study only contains patients exhibiting amnestic MCI (indicative of prodromal AD) and not indicative of other type of MCI (e.g., vascular changes on MRI, or Parkinsonism). The patient dietary information is not available. Blood samples were obtained from living research subjects with appropriate IRB approval. Blood sera were separated by centrifugation at 3000 rpm for 5 mins.
Table 1. Patient information.
Patient information includes MMSE scores, clinical diagnosis, age and gender. This information was provided by the University of Kentucky (UK) Alzheimer's disease center tissue bank (ADC). The discrimination of AD and control cases were performed using current consensus-based methodologies which are previously well-described in a clinical-pathological consensus conference (Jicha, et al.).
| MMSE | Clinical diagnosis based on Jicha et al. 2011 | Group | Age | Gender |
|---|---|---|---|---|
| 30 | Normal | Normal | 90 | Female |
| 30 | Normal | Normal | 91 | Male |
| 29 | Normal | Normal | 84 | Male |
| 29 | Normal | Normal | 88 | Male |
| 30 | Normal | Normal | 82 | Female |
| 29 | Normal | Normal | 88 | Female |
| 30 | Normal | Normal | 85 | Male |
| 25 | Early AD | Amnestic MCI/Probable Early AD | 94 | Female |
| 25 | MCI | Amnestic MCI/Probable Early AD | 85 | Female |
| 28 | MCI | Amnestic MCI/Probable Early AD | 87 | Male |
| 27 | MCI | Amnestic MCI/Probable Early AD | 86 | Female |
| 23 | Early AD | Amnestic MCI/Probable Early AD | 87 | Female |
| 24 | Early AD | Amnestic MCI/Probable Early AD | 88 | Female |
| 25 | Early MCI | Amnestic MCI/Probable Early AD | 89 | Female |
| 17 | AD | Probable AD | 89 | Female |
| 16 | AD | Probable AD | 84 | Female |
| 19 | AD | Probable AD | 96 | Female |
| 17 | AD | Probable AD | 90 | Male |
| 8 | AD | Probable AD | 86 | Female |
| 15 | AD | Probable AD | 92 | Female |
| 13 | AD | Probable AD | 80 | Male |
Mice blood serum collection
Wild type C57/BL on a hybrid background, C3H/He (Charles River) x C57BL/6 (Jackson laboratories) were used in the diet and gender specific studies. Blood was collected from mice under anesthesia through puncturing of the aorta into venous collection tubes coated with clot activator and silicone. Blood serum was separated by centrifugation at 1600 g for 15 mins following 30 mins of clotting at room temperature. All procedures conducted were approved by the Institutional Animal Care and Use Committee at Michigan State University.
Quantitative RT-PCR (qRT-PCR)
Total miRNAs were extracted using miRNeasy Mini Kit (Qiagen) and RNeasy MinElute Cleanup Kit (Qiagen) and total RNA was quantified using ND-1000 nanodrop spectrophotometer. Quality control was performed by assessing the OD ration of 260/280 nm. In addition the PCR products were run on agarose gels. qRT-PCR was conducted using miScript SYBR Green PCR Kit (Qiagen) and MyiQ real time PCR detection system following reverse transcription using miScript Reverse Transcription Kit (Qiagen) according to manufacturer’s instructions. All miRNA primers were purchased from Qiagen and the relative expressions were calculated using the comparative CT method with spiked cel-miR-39 (Kroh, et al.), internal miR-22, internal miR-191 and internal miR-126 as the normalizing controls for human sera and internal miR-22 as the normalizing control for mouse sera.
Statistical analysis
Statistical significances were determined using both 2 tailed t tests and Mann-Whitney tests for the human sera samples and 2 tailed t tests were used on mice sera samples.
Results
MiRNAs are down-regulated in blood serum of probable AD patients
The expression levels of the miRNAs, that were previously shown to regulate SPT and Aβ, and were down-regulated in the brain cortices of a subgroup of sporadic AD patients (Geekiyanage and Chan), were quantified in blood sera of 7 control (MMSE scores 29 and 30), 7 amnestic MCI/ probable early AD (MMSE scores 23–28) and 7 probable sporadic AD (MMSE scores 8–19) subjects (see Table 1 for patient information). Currently there is no generally agreed upon normalizing RNA with respect to blood serum or plasma. The generally used normalizing ribosomal RNA (RNU6B etc.) in miRNA analysis is typically not present in the blood. Therefore, the human blood sera from patients were spiked with C-elegance miRNA-39, cel-miR-39 (Kroh, et al.), prior to miRNA extraction. Cel-miR-39 was selected as it demonstrates no sequence homology to any known human, mouse, or rat miRNA. In addition miR-22 and miR-191 are abundantly expressed in the blood serum (Qiagen, 2011) and have not shown to be differentially expressed in the literature with respect to AD and therefore were also used for normalization. Further, we have observed that miR-126 expression levels remained unchanged in the brains of AD patients. Therefore, the expressions of the respective miRNAs in the blood serum were also normalized to miR-126. The expression levels of miR-137, miR-181c, miR-9, miR-29a and miR-29b (Figure 1) were significantly (both P<0.05, student’s t test and Mann-Whitney test) down-regulated in the blood serum of probable AD patients when normalized to spiked cel-miR-39 (Figure 1A), internal miR-22 (Figure 1B), internal miR-191(Figure 1C) or internal mir-126 (Figure 1D). The expression levels of the respective miRNAs (Figure 1) were significantly (both P<0.05, student’s t test and Mann-Whitney test) down-regulated in the blood serum of amnestic MCI/ probable early AD patients when normalized to spiked cel-miR-39 (Figure 1A), internal miR-22 (Figure 1B), internal miR-191(Figure 1C) or internal mir-126 (Figure 1D) with the exception of miR-137, where the down regulation was not statistically significant when normalized to spiked cel-miR-39 and internal miR-191. However, a statistical significance may be achieved with the exclusion of the case exhibiting an MMSE score of 28, from the amnestic MCI/ probable early AD group.
Figure 1. Down-regulated miRNA expression levels in probable AD patients.
The expression levels of miR-137, -181c, -9, -29a and -29b in blood serum of probable AD (n=7), amnestic MCI/Probable Early AD (n=7) and control (n=7) patients measured by qRT-PCR. Relative expressions shown are normalized to spiked cel-miR-39 (A), internal miR-22 (B), internal miR-191 (C) and internal miR-126 (D) and average control patient expressions. The statistical significance between control and AD sera were determined by 2-tailed student t tests and Mann- Whitney tests (*, P<0.05).
Decreased miRNA expression levels in the blood serum of high fat diet fed mice
High dietary fat intake is identified as a potential risk factor for AD (Baker, et al., Bayer-Carter, et al., Julien, et al., Oksman, et al., 2006, Refolo, et al., 2000). We previously showed that the expression levels of miR-137, miR-181c and miR-9 were down-regulated in the brain cortices of high fat diet fed mice (Geekiyanage and Chan). The miRNA expression levels were measured in the blood serum of male wild-type mice fed a 60% kcal high fat diet for a period of 5 months (starting at 4 months of age). In accordance with the expression levels in the brain, the expression levels of miR-137 (P=0.045, student’s t test), miR-181c (P=0.046) and miR-9 (P=0.03) (Figure 2) expression levels were down-regulated in the blood serum of mice fed a high diet. As the expression levels of miR-22 were stable across the human sera samples, miR-22 was used for normalization of miRNA expressions in the sera of mice fed a high fat diet.
Figure 2. Reduced miRNA expression in high fat diet fed mice.
The expression levels of blood serum miR-137 (*, P<0.05), -181c (*, P<0.05), -9, (*, P<0.04) -29a and -29b in mice fed a high fat diet were measured by qRT-PCR. The expression of miR-126 is shown as a control. Relative expressions shown are normalized to miR-22 and average chow control diet expressions. The statistical significance between control and AD sera were determined by 2-tailed student t tests.
miRNA are differentially expressed in blood serum according to gender
Research suggests that AD pathology may be more prevalent in females than in males (Alberca, et al., 2002, Bachman, et al., 1992, Brookmeyer, et al., 1998, Burns and Zaudig, 2002, Henderson and Buckwalter, 1994, McPherson, et al., 1999, Ripich, et al., 1995). We previously reported that the miR-137, miR-181c and miR-29a/b-1 expression levels are down-regulated in the cerebral cortices of female wild-type mice compared to males (Geekiyanage and Chan). Here we demonstrate that the expression levels of miR-137 (P=0.01), miR-181c (P=0.02) and miR-29b-1 (P=0.046) expression levels are down-regulated (Figure 3) in the blood serum of female mice (9 months of age) vs. male mice. However, the expression levels of miR-29a did not differ between the groups. As the expression levels of miR-22 were stable across the human sera samples, miR-22 was used for normalization of miRNA expressions in female vs. male mice sera.
Figure 3. Gender specific down-regulation of miRNA.
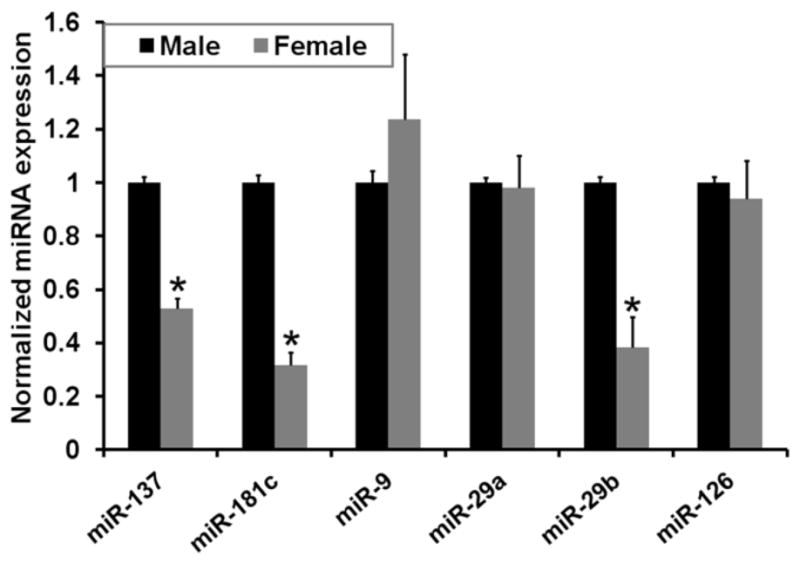
The expression levels of blood serum miR-137 (*, P<0.02), -181c (*, P<0.02), -29a, -29b (*, P<0.05), and -9 in male and female mice (n=6) were measured by qRT-PCR. The expression of miR-126 is shown as a control. Relative expressions shown are normalized to miR-22 and average male (n=6) expressions. The statistical significance between control and AD sera were determined by 2-tailed student t tests.
Discussion
MiRNA levels were down-regulated in the sera of patients with probable AD (MMSE scores 8–19) and amnestic MCI/probable early AD (MMSE score 23–28) compared to normal patients (MMSE scores 29 and 30). This suggests a potential role for these miRNAs as early diagnostic markers. Moreover, screening for miRNAs in the sera as biomarkers, that i) directly affect a fundamental feature of AD neuropathology, ii) are diagnostically sensitive enough to detect, iii) can detect AD early in the course of the disease progression, and iv) are non-invasive, simple to perform and inexpensive, make them potentially good diagnostic biomarkers in accordance with the criteria described by the National Institute on Aging (1998).
In a previous study (Geekiyanage and Chan) we identified that a subgroup of AD patients display increased levels of ceramide along with increased SPTLC1/2 protein levels in neocortices. However, the SPTLC1/2 mRNA levels did not differ from their control counterparts. We observed negative correlations between the expression levels of miR-137/-181c and SPTLC1, and between miR-9/-29a/b and SPTLC2 protein expressions, in these autopsy brain samples. These results in combination with cell culture studies suggested that SPTLC1/2 are post-transcriptionally regulated by their respective miRNAs. Similar negative relationships were identified between SPTLC1/2 and the corresponding miRNAs in AD risk factor models, i.e. high fat diet and gender specific. In addition, positive correlations between SPTLC1/2 and Aβ, and negative correlations between the respective miRNAs and Aβ were observed in these brain samples. Cell culture studies with “target protectors” and over-expression assays showed a direct effect of miRNAs on SPT and in turn on Aβ protein expression. These results together suggested that these miRNAs and SPT are involved in AD and represents a potential therapeutic target. In this current study, we suggest a prospective use for the circulating miRNAs as diagnostic markers.
We also observed that the same miRNAs were down-regulated in the blood serum of high fat diet and gender specific models. Expression of miR-137 is negatively regulated, epigenetically and transcriptionally by MeCP2 and Sox2 (Szulwach, et al.). Additionally, Sox2 genetic polymorphisms are associated with diabetic neuropathy in female patients but not in males (Gu, et al., 2009), providing a possible explanation for the dysregulation of miR-137 in female mice. Research performed on the treatment for Rett syndrome, a disease caused by mutations in MeCP2, suggests an increase in MeCP2 levels with the consumption of a high fat diet (Haas, et al., 1986, Liebhaber, et al., 2003). This may provide a potential explanation for the down-regulated miR-137 levels observed in mice fed a high fat diet. Further, miR-181c is positively and transcriptionally regulated by Akt1 (Androulidaki, et al., 2009). Akt activity has been observed to be decreased in response to high fat diet (Tremblay, et al., 2001), providing a possible explanation for the reduced miR-181c levels observed in mice fed a high fat diet. Interestingly, Akt1 expression levels have been shown to decrease considerably with age in the myocardial tissue of women (Camper-Kirby, et al., 2001) while MeCP2 expression levels increases with age in frontal cortices of males and females (Samaco, et al., 2004). The reduction in Akt expression in these elderly women may provide an explanation for the gender specific reduction of miR-181c observed with the mice in this study. However, further analysis is needed including age matched male samples to conclude the gender specific regulation of miR-181c by Akt. MiR-9 is negatively regulated by RE1-silensing transcription factor (REST) while it is positively regulated by cAMP-response element binding protein (CREB) (Laneve, et al.). In contrast, NF B, c-Myc and hedgehog signaling transcriptionally repress miR-29a/b (Mott, et al.). High dietary fat intake has been shown to activate cortical NF B in rats (Zhang, et al., 2005); however we did not observe miR-29a/b-1 to be down-regulated in mice fed a high fat diet. Differential expression patterns of hepatic miR-29 family have been observed between different mouse strains fed with methyl-deficient diets (Pogribny, et al.), suggesting species and strain variations may contribute to the unchanged miR-29a/b expression level observed in this study. In support of the gender difference observed in the miRNA expressions in this study, estrogen is known to protect neurons against inflammation by suppressing the activation of NF B (Wen, et al., 2004). However, this protection may be reduced in post-menopausal elderly women making them more vulnerable to NF B mediated suppression of miR-29a/b.
It must be noted that the blood serum samples used in this study were only clinically diagnosed and further studies are necessary to assess their ability to discriminate them from other forms of dementia. In addition, independent validation of these miRNAs as biomarkers will also be required. We note that the circulating miR-137, -181c, -9 and 29a/b levels are low in the blood serum. Nevertheless, the qRT-PCR are able to distinguish specifically between normal and AD patients.
The discrimination of the AD patients was performed using a current consensus-based methodology described in (Jicha, et al.). Nevertheless, it is acknowledged that comorbid pathologies can contribute to the development of cognitive impairment, and thereby confound the application of the Preclinical Alzheimer's disease Workgroup recommendations (Jicha, et al.). Therefore, we recognize the possibility that some subjects included in this study may contain non-AD pathologies. Consequently, all clinical diagnosis may not necessarily coincide directly with AD neuropathological features, i.e. some amnestic MCI/probable early AD and probable AD subjects included in this study may not develop AD neuropathologies and some normal subjects may develop AD. Finally, as with any biomarker, other diseases with similar risk factors, cerebovascular disease, cardiovascular disease and diabetes (Beeri, et al., 2009, Ewers, et al., McClean, et al., Slevin and Krupinski, 2009), may have similar blood miRNA profiles. Although, these diseases play a significant role in AD as major risk factors (Carlsson, Caselli, et al., Ettorre, et al.) and thus would provide insight into the possible risk of developing AD, further studies are needed to determine whether these miRNAs can be used to distinguish AD from these other diseases. Therefore, whether these miRNA profiles could provide conclusive diagnosis is currently unknown, nonetheless, miRNA profiles along with other biomarkers and cognitive tests could potentially provide a more comprehensive and early diagnosis and prognosis of AD.
Acknowledgments
We thank the UK ADC NIA P30-AG0-28383 for providing the human blood serum samples. This work was supported in part by the National Institute of Health (R01GM079688, R01GM089866 and R21RR024439), the National Science Foundation (CBET 0941055 and CBET 1049127) and the MSU Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Consensus report of the Working Group on: "Molecular and Biochemical Markers of Alzheimer's Disease". The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and the National Institute on Aging Working Group. Neurobiol Aging. 1998;19:109–116. [PubMed] [Google Scholar]
- 2.Alberca R, Montes-Latorre E, Gil-Neciga E, Mir-Rivera P, Lozano-San Martin P. Alzheimer's disease and women. Rev Neurol. 2002;35:571–579. [PubMed] [Google Scholar]
- 3.Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, Margioris AN, Tsichlis PN, Tsatsanis C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220–231. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachman DL, Wolf PA, Linn R, Knoefel JE, Cobb J, Belanger A, D'Agostino RB, White LR. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42:115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- 6.Baker LD, Bayer-Carter JL, Skinner J, Montine TJ, Cholerton BA, Callaghan M, Leverenz J, Walter BK, Tsai E, Postupna N, Lampe J, Craft S. High-Intensity Physical Activity Modulates Diet Effects on Cerebrospinal Amyloid-beta Levels in Normal Aging and Mild Cognitive Impairment. J Alzheimers Dis. doi: 10.3233/JAD-2011-111076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 8.Barber RC. Biomarkers for early detection of Alzheimer disease. J Am Osteopath Assoc. 110:S10–15. [PubMed] [Google Scholar]
- 9.Bayer-Carter JL, Green PS, Montine TJ, VanFossen B, Baker LD, Watson GS, Bonner LM, Callaghan M, Leverenz JB, Walter BK, Tsai E, Plymate SR, Postupna N, Wilkinson CW, Zhang J, Lampe J, Kahn SE, Craft S. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol. 68:743–752. doi: 10.1001/archneurol.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beeri MS, Ravona-Springer R, Silverman JM, Haroutunian V. The effects of cardiovascular risk factors on cognitive compromise. Dialogues Clin Neurosci. 2009;11:201–212. doi: 10.31887/DCNS.2009.11.2/msbeeri. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 12.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns A, Zaudig M. Mild cognitive impairment in older people. Lancet. 2002;360:1963–1965. doi: 10.1016/S0140-6736(02)11920-9. [DOI] [PubMed] [Google Scholar]
- 14.Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res. 2001;88:1020–1027. doi: 10.1161/hh1001.090858. [DOI] [PubMed] [Google Scholar]
- 15.Carlsson CM. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer's disease. J Alzheimers Dis. 20:711–722. doi: 10.3233/JAD-2010-100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caselli RJ, Dueck AC, Locke DE, Sabbagh MN, Ahern GL, Rapcsak SZ, Baxter LC, Yaari R, Woodruff BK, Hoffman-Snyder C, Rademakers R, Findley S, Reiman EM. Cerebrovascular risk factors and preclinical memory decline in healthy APOE epsilon4 homozygotes. Neurology. 76:1078–1084. doi: 10.1212/WNL.0b013e318211c3ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, Kelnar K, Kemppainen J, Brown D, Chen C, Prinjha RK, Richardson JC, Saunders AM, Roses AD, Richards CA. Identification of miRNA changes in Alzheimer's disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimers Dis. 2008;14:27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- 18.de Souza LC, Chupin M, Lamari F, Jardel C, Leclercq D, Colliot O, Lehericy S, Dubois B, Sarazin M. CSF tau markers are correlated with hippocampal volume in Alzheimer's disease. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Dougall NJ, Bruggink S, Ebmeier KP. Systematic review of the diagnostic accuracy of 99mTc-HMPAO-SPECT in dementia. Am J Geriatr Psychiatry. 2004;12:554–570. doi: 10.1176/appi.ajgp.12.6.554. [DOI] [PubMed] [Google Scholar]
- 20.Ettorre E, Cerra E, Marigliano B, Vigliotta M, Vulcano A, Fossati C, De Benedetto G, Servello A, Andreozzi P, Marigliano V. Role of cardiovascular risk factors (CRF) in the patients with mild cognitive impairment (MCI) Arch Gerontol Geriatr. doi: 10.1016/j.archger.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Ewers M, Mielke MM, Hampel H. Blood-based biomarkers of microvascular pathology in Alzheimer's disease. Exp Gerontol. 45:75–79. doi: 10.1016/j.exger.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geekiyanage H, Chan C. MicroRNA-137/181c Regulates Serine Palmitoyltransferase and In Turn Amyloid {beta}, Novel Targets in Sporadic Alzheimer's Disease. J Neurosci. 31:14820–14830. doi: 10.1523/JNEUROSCI.3883-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed N, Bentwich Z, Hod M, Goren Y, Chajut A. Serum microRNAs are promising novel biomarkers. PLoS One. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu HF, Alvarsson A, Efendic S, Brismar K. SOX2 has gender-specific genetic effects on diabetic nephropathy in samples from patients with type 1 diabetes mellitus in the GoKinD study. Gend Med. 2009;6:555–564. doi: 10.1016/j.genm.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Haas RH, Rice MA, Trauner DA, Merritt TA. Therapeutic effects of a ketogenic diet in Rett syndrome. Am J Med Genet Suppl. 1986;1:225–246. doi: 10.1002/ajmg.1320250525. [DOI] [PubMed] [Google Scholar]
- 27.Hampel H, Shen Y, Walsh DM, Aisen P, Shaw LM, Zetterberg H, Trojanowski JQ, Blennow K. Biological markers of amyloid beta-related mechanisms in Alzheimer's disease. Exp Neurol. 223:334–346. doi: 10.1016/j.expneurol.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansson O, Stomrud E, Vanmechelen E, Ostling S, Gustafson DR, Zetterberg H, Blennow K, Skoog I. Evaluation of Plasma Abeta as Predictor of Alzheimer's Disease in Older Individuals Without Dementia: A Population-Based Study. J Alzheimers Dis. doi: 10.3233/JAD-2011-111418. [DOI] [PubMed] [Google Scholar]
- 29.Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson VW, Buckwalter JG. Cognitive deficits of men and women with Alzheimer's disease. Neurology. 1994;44:90–96. doi: 10.1212/wnl.44.1.90. [DOI] [PubMed] [Google Scholar]
- 31.Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1:226–234. doi: 10.1602/neurorx.1.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jicha GA, Abner EL, Schmitt FA, Kryscio RJ, Riley KP, Cooper GE, Stiles N, Mendiondo MS, Smith CD, Van Eldik LJ, Nelson PT. Preclinical AD Workgroup staging: pathological correlates and potential challenges. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, Calon F. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol Aging. 31:1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR) Methods. 50:298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 37.Laneve P, Gioia U, Andriotto A, Moretti F, Bozzoni I, Caffarelli E. A minicircuitry involving REST and CREB controls miR-9-2 expression during human neuronal differentiation. Nucleic Acids Res. 38:6895–6905. doi: 10.1093/nar/gkq604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liebhaber GM, Riemann E, Baumeister FA. Ketogenic diet in Rett syndrome. J Child Neurol. 2003;18:74–75. doi: 10.1177/08830738030180011801. [DOI] [PubMed] [Google Scholar]
- 39.Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer's disease hippocampus. Neuroreport. 2007;18:297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- 40.McClean PL, Parthsarathy V, Faivre E, Holscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci. 31:6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McPherson S, Back C, Buckwalter JG, Cummings JL. Gender-related cognitive deficits in Alzheimer's disease. Int Psychogeriatr. 1999;11:117–122. doi: 10.1017/s1041610299005670. [DOI] [PubMed] [Google Scholar]
- 42.Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem. 110:1155–1164. doi: 10.1002/jcb.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mungas D, Reed BR, Jagust WJ, DeCarli C, Mack WJ, Kramer JH, Weiner MW, Schuff N, Chui HC. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology. 2002;59:867–873. doi: 10.1212/wnl.59.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oksman M, Iivonen H, Hogyes E, Amtul Z, Penke B, Leenders I, Broersen L, Lutjohann D, Hartmann T, Tanila H. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol Dis. 2006;23:563–572. doi: 10.1016/j.nbd.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Patwardhan MB, McCrory DC, Matchar DB, Samsa GP, Rutschmann OT. Alzheimer disease: operating characteristics of PET--a meta-analysis. Radiology. 2004;231:73–80. doi: 10.1148/radiol.2311021620. [DOI] [PubMed] [Google Scholar]
- 46.Pogribny IP, Starlard-Davenport A, Tryndyak VP, Han T, Ross SA, Rusyn I, Beland FA. Difference in expression of hepatic microRNAs miR-29c, miR-34a, miR-155, and miR-200b is associated with strain-specific susceptibility to dietary nonalcoholic steatohepatitis in mice. Lab Invest. 90:1437–1446. doi: 10.1038/labinvest.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiagen . Profiling miRNA in Serum and Plasma: Perils and Pitfalls on the Road to Biomarker Development. 2011. [Google Scholar]
- 48.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 49.Ripich DN, Petrill SA, Whitehouse PJ, Ziol EW. Gender differences in language of AD patients: a longitudinal study. Neurology. 1995;45:299–302. doi: 10.1212/wnl.45.2.299. [DOI] [PubMed] [Google Scholar]
- 50.Samaco RC, Nagarajan RP, Braunschweig D, LaSalle JM. Multiple pathways regulate MeCP2 expression in normal brain development and exhibit defects in autism-spectrum disorders. Hum Mol Genet. 2004;13:629–639. doi: 10.1093/hmg/ddh063. [DOI] [PubMed] [Google Scholar]
- 51.Scheltens P, Fox N, Barkhof F, De Carli C. Structural magnetic resonance imaging in the practical assessment of dementia: beyond exclusion. Lancet Neurol. 2002;1:13–21. doi: 10.1016/s1474-4422(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 52.Schonrock N, Ke YD, Humphreys D, Staufenbiel M, Ittner LM, Preiss T, Gotz J. Neuronal microRNA deregulation in response to Alzheimer's disease amyloid-beta. PLoS One. 5:e11070. doi: 10.1371/journal.pone.0011070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sjogren M, Davidsson P, Wallin A, Granerus AK, Grundstrom E, Askmark H, Vanmechelen E, Blennow K. Decreased CSF-beta-amyloid 42 in Alzheimer's disease and amyotrophic lateral sclerosis may reflect mismetabolism of beta-amyloid induced by disparate mechanisms. Dement Geriatr Cogn Disord. 2002;13:112–118. doi: 10.1159/000048642. [DOI] [PubMed] [Google Scholar]
- 54.Slevin M, Krupinski J. A role for monomeric C-reactive protein in regulation of angiogenesis, endothelial cell inflammation and thrombus formation in cardiovascular/cerebrovascular disease? Histol Histopathol. 2009;24:1473–1478. doi: 10.14670/HH-24.1473. [DOI] [PubMed] [Google Scholar]
- 55.Szulwach KE, Li X, Smrt RD, Li Y, Luo Y, Lin L, Santistevan NJ, Li W, Zhao X, Jin P. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol. 189:127–141. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tremblay F, Lavigne C, Jacques H, Marette A. Defective insulin-induced GLUT4 translocation in skeletal muscle of high fat-fed rats is associated with alterations in both Akt/protein kinase B and atypical protein kinase C (zeta/lambda) activities. Diabetes. 2001;50:1901–1910. doi: 10.2337/diabetes.50.8.1901. [DOI] [PubMed] [Google Scholar]
- 57.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wahlund LO, Julin P, Johansson SE, Scheltens P. Visual rating and volumetry of the medial temporal lobe on magnetic resonance imaging in dementia: a comparative study. J Neurol Neurosurg Psychiatry. 2000;69:630–635. doi: 10.1136/jnnp.69.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 61.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang WX, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, Rigoutsos I, Nelson PT. The expression of microRNA miR-107 decreases early in Alzheimer's disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28:1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, Simpkins JW. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain Res. 2004;1008:147–154. doi: 10.1016/j.brainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 64.Zeng L, Liu J, Wang Y, Wang L, Weng S, Tang Y, Zheng C, Cheng Q, Chen S, Yang GY. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front Biosci (Elite Ed) 3:1265–1272. doi: 10.2741/e330. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. 2005;191:318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]




