Abstract
Hematopoietic stem cells (HSCs) repopulate the immune system during normal replenishment as well as under the burden of pathogen stress, but the respective outcomes of differentiation are not the same. Under homeostatic conditions such as those which accompany turnover of immune cell subsets, HSCs appear to co-equally prime genes associated with the major downstream lineages: lymphoid, myeloid, and megakaryocyte/erythroid. Recent studies reveal, however, that during pathogen exposure, hematopoiesis may yield progeny in proportions different than those produced under homeostasis. At least some of these effects may be due to pathogen engagement of Toll-like receptors (TLRs) expressed on HSCs. HSCs are also responsive to inflammatory cytokines that are produced in response to pathogen burden and are present in the bone marrow microenvironment. Thus, hematopoiesis is not a formulaic process that produces the same, predictable outcome regardless of the specific environmental context. Rather, hematopoiesis represents a dynamic biological system that can be appreciably responsive to environmental factors, an influence that extends to the level of the HSC itself. Knowledge of functional consequences of TLR ligation on HSCs may be therapeutically exploited and applied to treatment of hematopoietic insufficiency in the setting of infection and disease.
Keywords: Toll-like receptors, Hematopoietic stem cell (HSC), Interferon (IFN), Tumor necrosis factor (TNF)
1. Introduction
The white blood cells of the immune system are terminally differentiated and must be continually replenished from self-renewing hematopoietic stem cells (HSCs). One view of hematopoiesis is that of a process that responds in a canalized manner to normal cellular turnover as well as to dramatic immune cell depletion. This concept of canalization, or robustness, refers to developmental stability regardless of genotypic or environmental variability [1]. While hematopoiesis must be a robust process in order to sustain the organism, it need not be inflexible.
It is increasingly clear that not all routes of HSC activation are equivalent. Rather, HSC differentiation and maturation are sensitive to the physiologic context of blood cell depletion. Exposure of mice to chemotherapeutics or radiation activates HSC cell cycling as assessed in functional assays of stem cell activity [2,3]. Repeated sterile bleeding, a murine model of hemorrhage, also stimulates HSC proliferation in bone marrow (BM) and spleen [4]. In this case, HSC activation is suppressed when red blood cells but not white cells or fluid volumes are restored, suggesting density-dependent feedback. Similar density-dependent effects are also observed in a murine model of neutropenia induced by the sterile adjuvant alum. Alum exposure depletes BM neutrophils and rapidly activates IL-1RI-mediated granulocyte colony stimulating factor (G-CSF) production, leading to HSC cycling [5]. However, neutrophil depletion can trigger a mechanistically distinct process of granulopoiesis depending on the presence or absence of an inflammatory cytokine environment. Homeostatic granulopoiesis uses a c/EBPα-dependent pathway whereas inflammatory granulopoiesis uses a c/EBPβ-dependent pathway [6]. Thus, HSC activation is context-dependent, and distinct developmental routes may be available to HSCs depending on whether repopulation occurs under circumstances of homeostasis, sterile wounding, or inflammation.
Recent studies reveal that not only do HSCs respond to inflammatory cytokine signals but that these multipotent progenitors can also directly sense pathogens. HSCs express Toll-like receptors (TLRs) and respond to receptor engagement. The realization that pathogens and their products directly influence HSCs raises questions about whether microbes bias differentiation of blood and immune cells. Although most hematopoietic progeny express TLRs and can respond to receptor ligation, TLR engagement at the primitive level of the HSC may have the greatest impact to hematopoiesis. Alterations in hematopoiesis may reflect a targeted redirection of the immune response to confront invading pathogens or, conversely, a mechanism by which pathogens subvert the immune system.
Here, we review recent discoveries that suggest a new role for HSCs as direct sensors of pathogens and associated inflammatory signals, rather than merely as indirect sensors of generic hematopoietic depletion. The recognition that HSC potential is directly shaped by pathogens has broad biological implications for our understanding of hematopoiesis not only under acute infection stress but also during chronic infections in which pathogen products may constantly stimulate HSCs.
2. Overview of hematopoiesis in mouse and human
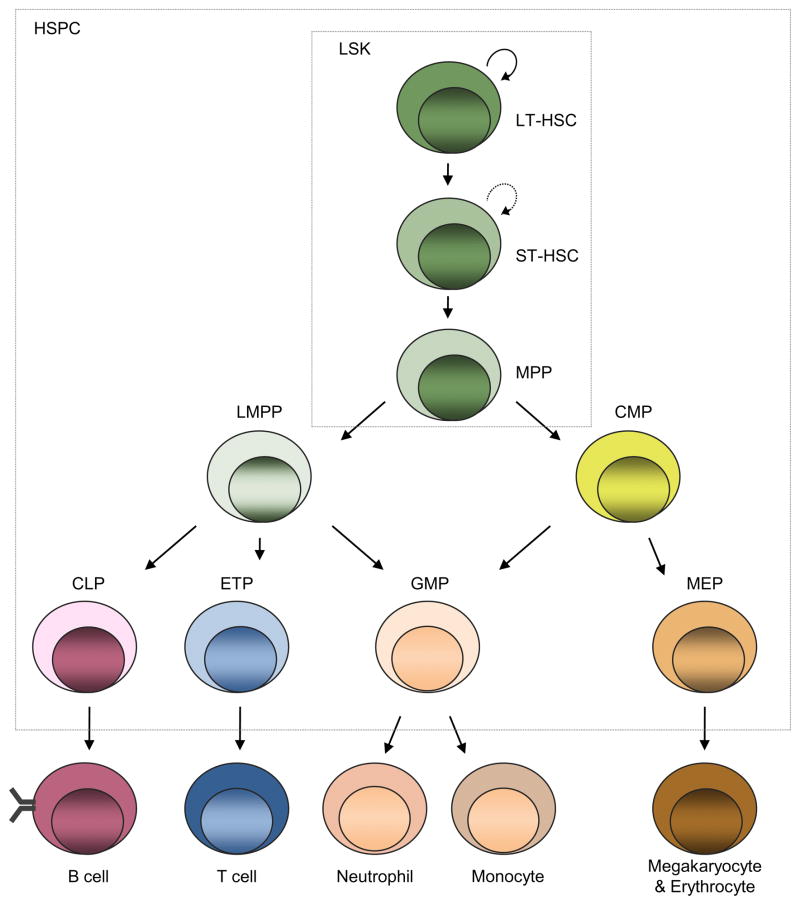
Pathogens impact multipotent hematopoietic subsets including the lineage marker-negative, Sca-1+ c-kit+ (LSK) population. For example, infection with microbes representing distinct pathogen classes elicits a significant expansion of murine LSKs in vitro or in vivo, as well as peripheral mobilization and maturation [7–9]. Historically, LSKs were equated with HSCs, since the LSK subset contains all cells capable of reconstituting the hematopoietic system. However, current resolution schemes demonstrate that total LSKs are a heterogeneous population that contains long-term (LT-) HSCs that retain full self-renewal potential and long-term reconstitution activity, short-term (ST-) HSCs that have begun to lose self-renewal capacity, and multipotent progenitors that have little to no detectable renewal activity (Fig. 1). By current estimates, LT-HSCs represent only ~1% of total LSKs, and this percentage is likely to decrease in the future as detection methodologies improve to allow the sensitive and reproducible isolation of pure stem cells. Moreover, the Sca-1 antigen used to identify LSKs is influenced by inflammatory cytokines, as type I IFN, type II IFN and TNFα increase the per-cell density of this surface antigen [10,11]. Therefore, it is important to complement phenotypic resolution of putative HSC subsets with functional studies that directly measure in vivo reconstitution competence. Current schemes of phenotypic resolution now afford the opportunity to interrogate the effects of pathogen on shaping the lineage-potential, durability, and functional competence of true LT-HSCs.
Fig. 1.
Hematopoiesis. All blood cells arise from self-renewing hematopoietic stem cells that possess long-term reconstituting capabilities (LT-HSCs). During differentiation, LT-HSCs give rise to short-term reconstituting stem cells (ST-HSC) with limited self-renewal capabilities which, in turn, produce multipotent progenitors (MPPs) that retain multi-lineage differentiation capabilities but have little or no renewal activity. MPPs then become increasingly specified to the megakaryocyte/erythroid, myeloid, or lymphoid lineages. Lymphoid-myleoid primed multipotent progenitors (LMPPs) give rise to common lymphoid progenitor (CLPs) and early thymic progenitors (ETPs), precursors to the B and T lineages, as well as granulocyte–macrophage progenitors (GMPs). Common myeloid progenitors (CMPs) give rise to megakaryocyte/erythroid precursors (MEPs) as well as GMPs. LSKs (lineage−Sca1+c-kit+ cells) contain LT-HSC, ST-HSC and MPP subsets, while hematopoietic stem and progenitor cells (HSPCs) additionally include their immediate downstream progeny. This schematic represents hematopoietic development under homeostasis, and not all pathways may be obligate. For example, TLR4 ligation fosters rapid myeloid development independent of the usual requirement for the myeloid colony stimulating factors [35].
True HSCs are functionally defined by the capabilities of self-renewal and multi-lineage differentiation [12]. Maturation of HSCs into terminally differentiated progeny involves the loss of self-renewal potential followed by progression through downstream subsets of increasingly restricted lineage potential. Characterization of HSC responses to pathogen and inflammation requires careful attention to the identity of the uncommitted hematopoietic progenitor subset being interrogated. Within total murine LSKs, HSCs can be appreciably purified as side-population (SP) cells based on Hoechst dye efflux or as CD150+CD48− cells based on the signaling lymphocyte activation molecule (SLAM) family markers [13,14] (Table 1). In limiting dilution analyses, approximately 1 in 3–5 progenitors purified using either method is sufficient to reconstitute immune-deficient recipients for at least 16 weeks, one standard measure of long-term reconstituting activity [12].
Table 1.
Functional repopulation potential of phenotypically defined HSC subsets.
| Population | Phenotype | Functional repopulation activity* | Comments | Citation |
|---|---|---|---|---|
| Mouse | ||||
| HSPC | lin−Sca-1+c-kit+ (LSK) | ~1 in 100 | Heterogeneous, contains self-renewing and non-renewing subsets | [64,65] |
| HSC | flk2/flt3− LSK | ~1 in 50 | Can be further enriched for HSC activity using VCAM-1 | [66–68] |
| HSC | CD27− LSK | ~1 in 50 | Does not capture all HSCs as some self-renewal activity is detectable within the CD27+ subset | [69] |
| HSC | CD150+CD48− LSK | ~1 in 2–5 | [13] | |
| HSC | SP (side population) LSK | ~1 in 2–5 | [70,71] | |
| HSC | Thy-1.1loSca-1+ LSK | 1 in 5–22 | Thy-1 and Sca-1 not conserved across mouse strains | [13,72–74] |
| Human | ||||
| HSC | CD34+CD38− | 1 in 617 | [15] | |
| HSC | CD133+ALDHhilin− | 1 in 691 | [16] | |
| HSC | CD34+CD38−CD45RA−Thy1+CD49f+ | 1 in 10.5 | [18] | |
As measured by the ability to sustain multi-lineage hematopoiesis long-term, usually 16 weeks, after transplantation to an immune deficient recipient.
In humans, self-renewing HSCs are contained within a lineage marker-negative subset that expresses CD34 and CD133. CD34 is a traditional marker used to phenotypically identify human HSCs; mice differ in that their LT-HSCs are CD34− and this marker is acquired as cells begin to lose self-renewal potential. However, in functional assays, only 1 in 617 human CD34+ cells has long-term reconstituting activity [15], suggesting that the CD34+ population is heterogeneous for stem cell activity. Moreover, the CD34+ subset may not contain all LT- and ST-HSCs. Alternative markers used to resolve HSCs include aldehyde dehydrogenase (ALDH), an enzyme involved in retinoid metabolism; CD133, a tetraspan protein possibly involved in HSC migration; and the integrin CD49f. Reconstitution activity of cord blood progenitors is enriched to 1 in 691 ALDH+CD133+lin− cells [16,17] and to 1 in 10.5 CD34+CD38−CD45RA−Thy1+CD49f+ cells [18] (Table 1).
3. Hematopoiesis under homeostasis versus pathogen burden
While HSCs normally reside in specialized niches in the bone marrow, they also circulate [19,20]. Pathogens may perturb hematopoiesis through direct effects on HSCs, either by infection or through exposure to microbial products, or through indirect effects on the BM microenvironment that supports stem cells [21]. Given that HSCs transiently traverse the peripheral circulation, it is possible that encounters with localized infections directly regulate HSC biology [22].
Progress over the last 10 years defines the molecular mechanisms that control HSC maintenance in homeostasis, a process in which relative levels of blood cell production are maintained, and following adoptive transfer to immunocompromised recipients, a process that entails rapid repopulation stress. The most recent work identifies the regulatory networks that orchestrate the interplay of key transcription factors (FOXO, PTEN, Gfi1, E47, Notch), survival genes (Bcl2, Mcl1, Bcl-XL), and cell cycle regulators (p16, p18, p21) [23–28]. What is of potential impact is how immune system duress or direct exposure to pathogens influences these signaling networks to redirect the lineage-specific differentiation of HSCs. Alterations in numbers and proportions of uncommitted progenitors may occur rapidly following acute microbial infection or more gradually in situations of chronic infection or inflammation. Acute microbial infection elicits profound changes in hematopoiesis. Sepsis, for example, is marked by immune system hyperactivity including excessive production of pro-inflammatory cytokines and chemokines, followed by hyporeactivity and neutropenia [29,30]. Likewise, infection with Erlichia muris or Anaplasma phagocytophilum, both clinically relevant models of tick-borne disease, decreases bone marrow cellularity accompanied by increased extramedullary hematopoiesis in the spleen [31,32]. Acute versus chronic exposure to pathogens differentially impacts hematopoiesis, and the underlying mechanisms are beginning to be resolved. Here, we focus on the direct effects of pathogen products and inflammatory mediators on HSCs and their immediate downstream progeny.
4. TLR-dependent effects on hematopoiesis
Stimulation of HSCs by microbial ligands may affect the timing and manner in which HSCs differentiate, as well as the preferred progeny into which they develop. Pattern recognition receptors (PRRs) which may regulate HSC differentiation include TLRs, C-type lectin, RIG-I-like, and Nod-like receptors that recognize conserved moieties of viruses, bacteria, and fungi. Recent studies reveal that TLR ligands can exert a direct effect on HSC activation and hematopoietic potential.
First identified as components of the innate immune system, TLRs are transmembrane proteins which recognize conserved pathogen-associated molecular patterns (PAMPs). There is considerable cross-talk between the innate and adaptive branches of the immune system, and TLRs enable priming of adaptive responses to pathogens, chiefly through abundant expression on antigen presenting cells which phagocytose pathogens and present peptides to naive lymphocytes. TLRs recognize dsRNA (TLR-3 and -9), LPS (TLR-4), flagellin (TLR-5), and CpG motifs (TLR-9), among other PAMPs [33]. The intracellular Toll/IL-1 receptor (TIR) homologous domain is required for signal transduction and activation of several transcription factors, including NF-κB and several IFN-regulatory factors (IRFs), to produce inflammatory cytokines such as TNFα, IL-6, and type I IFNs [33,34]. All TLRs except TLR-3 utilize myeloid differentiation primary response protein 88 (MyD88), which carries the TIR domain and associates with IL-1 receptor-associated kinase (IRAK) family members to effect downstream activation of IRFs and NF-κB. TLR-3 and TLR-4 can utilize a MyD88-independent activation mechanism whereby it associates with TIR-domain-containing adapter-inducing interferon-β (TRIF) to activate NF-κB.
Knowing that splenic B cells respond efficiently to TLR ligands, Kincade and colleagues interrogated upstream subsets to establish when lymphocytes acquire ligand sensitivity [35]. Strikingly, the authors found that murine HSCs (flk2/flt3− LSK or IL7Rα− LSK) express TLRs and functionally respond to receptor stimulation. HSCs and progenitor cells express TLR-2 and -4, and respond to receptor-specific agonists [35]. Low levels of TLR-9 transcript are also detectable in HSCs [36]. The TLR-4 ligand LPS triggers HSC cell cycling and fosters myeloid lineage outgrowth at the expense of lymphoid lineage outgrowth, possibly via E2A degradation [35,37] (Fig. 2A and B). In a model of chronic infection, even very low-dose LPS treatment of mice stimulates HSC (CD150+CD48− LSK) proliferation, myeloid skewing, and diminished reconstitution activity. In LSKs, exposure to Candida albicans provokes differentiation into lineage-positive cells through a MyD88-dependent pathway, which is followed by differentiation of common myeloid progenitors (CMPs) into macrophages and neutrophils [38].
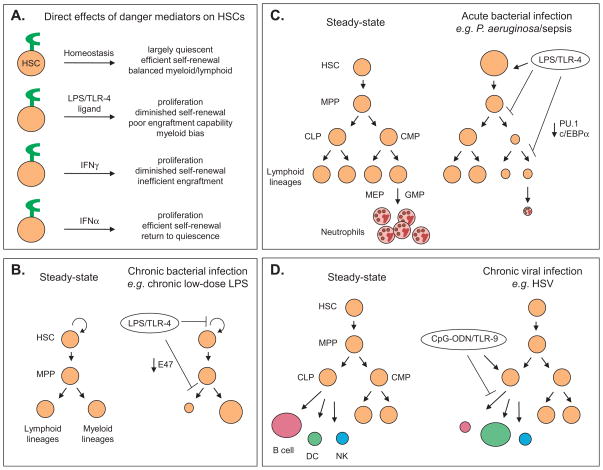
Fig. 2.
Differential responses of HSCs to inflammatory mediators. (A) Direct effects of danger mediators. HSCs express surface receptors for pathogen products as well as receptors for inflammatory cytokines, and this is depicted schematically by a green receptor on HSCs under each environmental condition. Under homeostatic replenishment such as that which occurs in the absence of microbial or inflammatory ligand, HSCs cycle infrequently and co-equally prime lymphoid, myeloid and megakaryocyte/erthyroid lineages; see text for details. Exposure to the TLR-4 ligand LPS or the inflammatory cytokines IFNγ or IFNα differentially activates the appropriate cognate receptor. LPS drives HSC proliferation accompanied by diminished long-term repopulation activates and a strong myeloid development at the expense of lymphoid production [35,37]. IFNγ and IFNα also elicit proliferation, but the former is selectively associated with poor HSC self-renewal [11,14]. No observations of lineage-skewing have been reported. (B) Experimental model of chronic bacterial infection. HSCs from mice treated with 0.6 μg of LPS/day for 4–6 weeks exhibit hyperproliferation and a strong myeloid bias associated with loss of the lymphoid-lineage transcription factor E47 [37]. (C) Model of acute bacterial infection. Mice infected with Pseudomonas aeruginosa or exposed to LPS from this bacterium develop severe neutropenia accompanied by an accumulation of the LSK subset that contains HSCs. Neutropenia is accompanied by a loss of the key myeloid transcription factors PU.1 and c/EBPα in common myeloid progenitors (CMP) [58]. (D) Model of viral infection. Exposure to the TLR-9 ligand CpG-ODN redirects CLP activity. CLPs normally have strong B cell potential with lesser activity for dendritic cell (DC) and natural killer (NK) lineages. However, in vivo exposure to CpG-ODN drives CLPs to make DCs over B cells, an effect observed at the single cell level [36].
Loss of G0 quiescence and the induction of hyperproliferation following LPS stimulation of TLRs may functionally exhaust HSCs, leading to senescence. Some aspects of HSC dysfunction including hyperproliferation, exhaustion, and lymphopenia are reminiscent of HSC activity in mice deficient in the transcription factor E47 [27,39]. Indeed, HSCs from LPS-treated animals have reduced levels of E47 [37]. Moreover, the diminished reconstitution potential of LPS-exposed HSCs persists through two rounds of serial adoptive transfer through untreated hosts. Thus, changes to HSC activity are durable and persist weeks after the initial exposure to TLR ligand.
LPS can influence hematopoietic dynamics through direct effects on hematopoietic stem and progenitor cells (HSPCs; Fig. 1) as well as through indirect effects on BM resident cells. For example, Kincade’s group used defined, serum-free, stromal cell-free cultures to show that LPS (TLR-4 ligand) or Pam3CSK4 (TLR-2 ligand) directly induces cell cycling in flk2/flt3− LSKs and total LSKs [35]. In parallel cultures, purified LKS− or granulocyte macrophage progenitor (GMP) subsets are driven to rapid myeloid differentiation under these same stromal cell-free conditions. Likewise, common lymphoid progenitors (CLPs) stimulated with CpG (TLR-9 ligand) are driven to dendritic cell (DC) lineage fate at the expense of the B lineage; this outcome was confirmed at the single cell level using in vitro clonal assays devoid of stroma [36]. In vivo work from the von Andrian lab showed that that HSPCs pre-incubated with LPS for 12 h prior to implantation in the kidney capsule of recipients exhibited a level of robust myeloid development twofold greater than HSPCs that were mixed with LPS at the time of implantation [22]. Together, these studies point to an important role for TLRs on HSPCs. Recent findings from the Pamer lab add a new layer of biological complexity, namely that LPS can also influence hematopoietic dynamics through indirect effects via TLRs on non-hematopoietic BM cells. BM mesenchymal stem cells with osteoblast potential express TLR-4, and in vivo exposure to LPS elicits chemokine-mediated monocyte emigration into the bloodstream [21]. While the relative sensitivity of HSPCs and BM stroma to microbial ligands remains to be carefully examined, it is clear that HSPC subsets differ in both the array of TLRs expressed and in the density of surface expression. GMPs have higher levels of TLR-4 than CLPs, and LSKs have intermediate expression [35]. Moreover, LPS dose responsiveness of HSPCs is extraordinarily sensitive to the presence of the CD14 co-receptor. Soluble CD14 is normally present in serum, and addition of exogenous CD14 to defined, serum-free cultures enhances LPS sensitivity ~1000-fold. Thus, the ability of HSPCs and stromal cells to respond to low-dose LPS in vivo may be influenced by the presence of CD14 in the local microenvironment.
One major implication of these findings is that chronic exposure to systemic TLR ligands may alter HSC biology. For example, HIV infection is associated with leakage of microbes from the gut, persistent inflammation, and immune exhaustion. HIV-infected patients have detectable levels of circulating LPS as well as bacterial 16S rDNA [40,41]. With the success of highly active antiretroviral therapies (HAART), HIV is now considered a chronic condition, and the maintenance of robust HSC integrity in the presence of this chronic infection is increasingly important for basic health as well as for vaccine responsiveness. These observations of durable changes to HSC function following persistent TLR stimulation are accordingly pertinent to other chronic infections including periodontal disease and endocarditis [42]. Indeed, chronic inflammation is also a hallmark characteristic of metabolic diseases including obesity and type II diabetes. Notably, circulating fatty acids associated with such metabolic syndromes can activate TLRs [43], and this chronic stimulation of HSCs may alter global patterns of gene expression, thereby skewing hematopoietic potential [37] and further perpetuating inflammation. Not only do the direct versus indirect roles of these PRRs on HSCs remain to be fully established but so do their dose dependencies. While chronic administration of low dose Escherichia coli LPS (0.6 μg/day for 4–6 weeks) elicits HSC exhaustion and loss of self-renewal activity following adoptive transfer, higher dose E. coli LPS (35 μg administered four times with a 2 day interval) enhances self-renewal capabilities [37,44].
Human HSCs also bear functional TLRs. Adult CD34+ BM cells express TLR-4, -7 and -8 transcripts, and activation of TLR-7, -8 with resiquimod elicits differentiation to the myeloid and DC lineages [45]. The latter but not former response is inhibited by anti-TNFα neutralizing antibodies, suggesting two different routes of cellular activation, one of which is independent of this inflammatory cytokine [46]. TLR-2 stimulation of lineage marker-negative CD34+ HSCs promotes myeloid differentiation at the expense of lymphoid development [47]. Stimulation with the specific ligand Pam3CSK4, which activates TLR-1, promotes commitment to the myelomonocytic lineage. CD34+ cord blood HSCs express TLRs 1– 9 at the RNA level; however, protein expression with appropriate sub-cellular localization could not be confirmed [48]. Characterization of the TLR protein expression profile on HSCs in both humans and mice remains difficult, as few commercial antibodies are presently available.
Although HSPCs (here defined as IL7R− LSKs) primarily reside in the BM, they also recirculate, raising the possibility that uncommitted progenitors encounter pathogen and undergo TLR activation outside of the marrow [22]. Approximately 100–400 HSPCs are present in the periphery at any given time, and that residence time in the circulation typically lasts for fewer than 6 s [49]. Indeed, a migratory pool of HSPCs appears to constitutively survey peripheral tissue, proliferate in response to microbial danger signals such as LPS, and boost the local supply of innate effector cells [22]. The ability of HSPCs to directly respond to TLR signals suggests one means by which the immune system can rapidly and effectively respond to both systemic and local infection. Specifically, in the case of local inflammation, migratory HSPCs that sense microbial danger signals in peripheral tissues can proliferate within that pathogen-challenged location and contribute to the supply of effector cells [50]. Therefore, TLR ligation directly activates HSCs and immediate downstream progeny, suggesting a mechanism by which pathogens may sculpt hematopoiesis in real time.
5. Cytokine effects on hematopoiesis
In addition to being activated by pathogen products, HSCs can directly respond to inflammatory cytokines that are elicited by TLRs as well as by other signaling pathways in the BM microenvironment. New findings demonstrate the ability of HSCs to directly sense danger-associated inflammatory cytokines (recently reviewed in [51]). Such factors may be produced upon TLR agonist exposure by TLR-expressing hematopoietic cells or by non-hematopoietic BM resident populations such as mesenchymal stem cells and reticular cells [21]. As well as responding to insult via depletion of marrow subsets following cytokine-mediated mobilization, HSCs are directly responsive to both interferons (IFN) and tumor necrosis factor (TNF) (Fig. 2A). IFNs, in particular, are emerging as major regulators of HSC activity in homeostasis as well as in response to pathogens. Most cells synthesize type I IFNs, IFNα and IFNβ, following viral infection. Type II IFN, namely IFNγ, is produced by immune effector populations activated by antigen or mitogen. In contrast to initial in vitro findings that demonstrated an inhibitory effect of IFNa on hematopoietic progenitors, HSCs (CD150+ LSKs) are now known to be activated by this cytokine in vivo [11]. Injection of mice with recombinant IFNα or the poly(I:C) inducer of IFNα coaxes HSCs from G0 into the active stages of cycling. These activated HSCs subsequently return to dormancy and retain normal reconstitution activity. IFNγ likewise triggers HSC (SP LSK) cycling in vitro and in vivo; however, this activation has functional consequences for HSC competence [14]. Chronic cycling can lead to HSC exhaustion and, in fact, HSCs from IFNγ-deficient mice are more quiescent and can sustain better long-term engraftment relative to their wild-type counterparts following competitive adoptive transfer to lethally irradiated recipients. The cellular sources of IFN produced within the BM compartment remain to be defined.
As with IFN, TNFα was originally described as an inhibitor of hematopoietic stem cell and progenitor activities [51]. However, mice deficient in the p55 form of the TNF receptor exhibit an age-associated increase in BM cellularity, accompanied by decreased stem cell function as assessed by both competitive repopulation and serial adoptive transfer studies [52]. These data raise the possibility of positive regulatory effects of TNF on HSCs. However, a recent study examining mice singly deficient in the p55 or p75 forms of the TNF receptor as well as double-deficient animals had appreciably different findings. All three strains deficient in TNF receptors exhibited increased hematopoietic activity following competitive adoptive transfer, and this advantage was sustained followed secondary adoptive transfer [53]. Consistent with historical findings, these data suggest that TNF suppresses HSC function. The nature of the discordance in the findings between these two studies remains perplexing but may be resolved with further information about the background strain of mice bearing the mutated alleles [53]. Other cytokines including G-CSF also activate HSCs; however this effect appears to be indirect via the induction of proteolytic enzymes secreted by BM neutrophils which, in turn, liberate HSCs from their niche. The observations that danger signals delivered by TLR ligands and inflammatory cytokines directly affect HSC activity raise questions about the relative contribution of each mechanism following infection with pathogen.
The availability of mice selectively deficient in TLRs, TLR signaling adapters, cytokines, and cytokine receptors enables separation of function studies to establish the individual contributions of TLR signals and cytokines to HSC integrity. In the absence of pathogens, MyD88-dependent signals as well as IFNα-dependent signals appear to be important for HSC functioning. BM from mice deficient in TLR-4, -9 or the adapter MyD88 exhibits enhanced reconstitution activity relative to wild-type mice in competitive adoptive transfers [54]. Likewise, HSCs from mice deficient in IFNγ have decreased tonic proliferation and improved engraftment compared to their wild-type counterparts [14]. No reconstitution advantage is detectable in BM from TLR-2 deficient mice [54]. Interestingly, TLR signaling also influences lineage fate decisions of downstream multipotent subsets. CLPs are a subset of cells which are downstream of HSCs and have lost self-renewal activity as well as megakaryocyte/erythroid potential. CLPs from unmanipulated mice retain strong B cell potential with lesser activity for DC, T cell, or myeloid lineages [55,56]. Infection with HSV-1 or exposure to the TLR-9 ligand CpG redirects CLP lineage fate from B cell to DC, including at the single cell level [36] (Fig. 2D). This lineage redirection depends on the presence of the TLR-9 receptor and the MyD88 signaling adapter but is independent of the IFNα/β receptor, TNFα or IFNγ. Whether these effects extend to the HSC remains to be examined. Conversely, HSC (CD150+flk2/flk3− LSK) differentiation in response to Staphylococcus aureus infection or polymicrobial sepsis following cecal ligation is independent of MyD88, TRIF, and IFNα, suggesting the importance of other regulatory pathways [9]. Ablation of BM neutrophils with antibodies to Gr-1, a depletion strategy thought to occur without inflammation [5], recapitulates the observed expansion in HSCs. Together, these data suggest that HSC responses to infections rely on a combination of inflammatory pathways, including TLR stimulation.
6. Translational implications of TLR ligation on HSCs
Research on the effects of TLR ligation on mouse HSCs prompts translational questions concerning human health and disease. In vitro and in vivo studies reveal a shift toward myeloid differentiation following TLR ligation of murine or human HSCs [35,37,45]. It is plausible that chronic infection continually stimulates TLRs, biasing HSCs toward myeloid differentiation for extended periods. Such lineage bias may adversely impact the ability to mount effective adaptive immune responses. Of note, in a biologically complex system, multiple TLRs may be ligated simultaneously not only on HSCs but also on downstream progeny as well as on TLR-expressing non-hematopoietic tissue. The biological outcome will likely reflect the concerted effects of developmental stage-specific activation as well as the precise array of receptors ligated. The relative importance of TLR ligation on HSCs versus downstream progeny on short-term and long-term hematopoiesis remains to be established.
TLR expression and ligation may contribute to neutropenia in septic patients, making them more vulnerable to fungal infections such as invasive aspergillosis and exacerbating their morbidity [57]. Recent observations in a mouse burn model of acute sepsis demonstrate the effects of TLR-4 ligand on intermediate populations between HSCs and mature neutrophils [58]. During myeloid lineage development, HSCs progress through the sequential stages of CMP followed by GMPs which, in turn, give rise to Gr-1+Mac1+ neutrophils (Fig. 1). Megakaryocyte/erythroid potential (MEP) is lost prior to the GMP stage. Following exposure to LPS from Pseudomonas aeruginosa, mice exhibit neutropenia concomitant with an increase in the frequency of CLPs, suggesting a selective, mechanistic block during CMP differentiation to GMP (Fig. 2C). Studies in C3H/HeJ mice, which lack TLR-4 functionality, demonstrate that TLR-4 is necessary and sufficient for these effects [58]. Therefore, while low-dose LPS stimulation appears to activate the myeloid potential of HSCs [37], acute high-dose LPS mechanistically inhibits the neutrophil potential of CMP and GMP via suppression of the myeloid transcription factors PU.1 and c/EBPα [58].
Regulation of TLRs on HSCs may correlate with human pathology. TLR-4 is upregulated on CD34+ cells in patients with myelodysplastic syndrome (MDS) [59]. MDS is a group of hematologic disorders characterized by insufficient myeloid differentiation, frequently manifesting with anemia, thrombocytopenia, and neutropenia; one-third of patients progress to acute myeloid leukemia due to accumulation of mutations [60]. Additionally, stimulation with the agonist LPS appears to contribute to the hyper-apoptotic phenotype of CD34+ cells and BM mononuclear cells observed in MDS. TLR pathways converge on NF-κB, which regulates apoptosis through TNF signaling, and it is still unclear whether TLR-4 directly mediates MDS effects or whether additional factors modulate pathology. Notably, increased TNFα and IFN signaling are associated with MDS. Thus, TLR-4 stimulation may constitute only one means by which inflammatory cytokines provoke the MDS phenotype. Future studies may examine whether chronic infection accelerates MDS progression and whether ligation of other TLRs exacerbates apoptotic tendencies.
Recent therapeutic interest in TLR stimulation on hematopoietic progenitors has spurred research on protection from apoptosis during acute radiation sickness. High radiation levels damage rapidly dividing progenitor cells and can endanger the functional integrity of HSCs, proving lethal [61]. Burdelya et al. found that the flagellin derivative CBLB502 (TLR-5 ligand) protects mice when administered 24 h before lethal total body irradiation and 1 h post-irradiation [62]. These effects were not seen in mice which lack a functional TLR-5 response due to a single nucleotide polymorphism. It is proposed that TLR-5 ligation prevents apoptosis through NF-κB activation, thus preserving radiation-sensitive tissues, significantly HSCs. Of note, CBLB502 injection in non-irradiated mice increased superoxide dismutase as well as circulating G-CSF, IL-6, and TNFα levels. Thereby, TLR agonists may promote systemic contributions to hematopoiesis. An open question is whether these cytokines ultimately facilitate normal hematopoiesis, as TNF is known to suppress HSC function [53]. Importantly, if TLR agonists are to be used therapeutically, it will be important to determine the long-term consequences of TLR stimulation to HSC function including self-renewal [37].
7. Summary and future studies
The recognition that TLRs are expressed on early hematopoietic progenitors, including the HSC, furthers our mechanistic understanding of how the immune system responds to pathogen burden and, in turn, how pathogens manipulate hematopoiesis. Significant progress about the role of TLRs in activating differentiated immune cells provides a platform to understand how specific classes of microbes elicit alterations in self-renewing HSCs, including lineage-skewing. A major unanswered question is whether the two major signaling pathways, MyD88 (exploited by all TLRs except TLR-3) or TRIF (TLR-3 and -4-driven), elicit distinct responses in HSCs upon TLR stimulation. Moreover, do HSCs differentially exploit signals from the distinct TLR and non-TLR PRRs that sense bacteria versus fungi versus viruses (Table 2)? The recruitment of distinct co-adapters confers signaling specificity to individual TLRs, and HSCs may exploit these cues to mediate distinct pathways of hematopoiesis [34,63]. TLR ligands may also be useful for therapeutic applications in which it is desirable to restore or expand a select hematopoietic lineage. For example, inefficient expansion of donor-derived neutrophils following allogeneic BM transfer is associated with poor outcomes for recipients; expanding the GMP compartment could improve survival. Finally, non-hematopoietic BM resident cells including mesenchymal stem cells express TLRs and have recently been shown to regulate monocyte efflux into the bloodstream [21]. Mesenchymal stem cells are precursors to osteoblasts, a major cell type associated with stem cell niches. The impact of TLR ligation of mesenchymal stem cells or their reticular cell progeny to HSC retention or differentiation potential remains to be examined.
Table 2.
Major unanswered questions.
| Do HSCs differentially respond to signals transmitted through MyD88 versus TRIF, or even through the individual TLRs? |
| Memory T cells and memory B cells share with HSCs the properties of quiescence, periodic reactivation, and a return to quiescence. Do TLRs signals or inflammatory signals similarly influence integrity of these long-lived memory compartments, including proliferation and exhaustion? |
| What are the implications of concerted stimulation of TLRs on the HSCs and their downstream progeny? |
| Do therapeutic TLR agonists affect long-term HSC potential including self-renewal and multi-lineage differentiation? |
To summarize, HSCs not only sustain blood cell formation following bleeding, chemotherapeutic ablation, or BM damage, but HSCs respond directly to microbial products as well as to inflammatory cytokines, permitting real-time changes in the direction of hematopoiesis in response to pathogen exposure burden.
Acknowledgments
We would like to thank members of the Borghesi and Milcarek labs as well as the University of Pittsburgh Cancer Institute writing group for critical input. This work is supported by NIH AR054529 and AI079047.
References
- 1.Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563–5. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- 2.Harrison DE, Lerner CP. Most primitive hematopoietic stem cells are stimulated to cycle rapidly after treatment with 5-fluorouracil. Blood. 1991;78(5):1237–40. [PubMed] [Google Scholar]
- 3.Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA. 1997;94(5):1908–13. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheshier SH, Prohaska SS, Weissman IL. The effect of bleeding on hematopoietic stem cell cycling and self-renewal. Stem Cells Dev. 2007;16(5):707–17. doi: 10.1089/scd.2007.0017. [DOI] [PubMed] [Google Scholar]
- 5.Cain DW, et al. Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PLoS One. 2011;6(5):e19957. doi: 10.1371/journal.pone.0019957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirai H, et al. C/EBPbeta is required for ‘emergency’ granulopoiesis. Nat Immunol. 2006;7(7):732–9. doi: 10.1038/ni1354. [DOI] [PubMed] [Google Scholar]
- 7.Zhang P, et al. The lineage-c-Kit+Sca-1+ cell response to Escherichia coli bacteremia in Balb/c mice. Stem Cells. 2008;26(7):1778–86. doi: 10.1634/stemcells.2007-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanez A, et al. Candida albicans triggers proliferation and differentiation of hematopoietic stem and progenitor cells by a MyD88-dependent signaling. Microbes Infect. 2009;11(4):531–5. doi: 10.1016/j.micinf.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Scumpia PO, et al. Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. J Immunol. 2010;184(5):2247–51. doi: 10.4049/jimmunol.0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malek TR, Danis KM, Codias EK. Tumor necrosis factor synergistically acts with IFN-gamma to regulate Ly-6A/E expression in T lymphocytes, thymocytes and bone marrow cells. J Immunol. 1989;142(6):1929–36. [PubMed] [Google Scholar]
- 11.Essers MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458(7240):904–8. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 12.Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1(3):263–70. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Baldridge MT, et al. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465(7299):793–7. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia M, et al. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci USA. 1997;94(10):5320–5. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess DA, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107(5):2162–9. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake AC, et al. Human CD34+ CD133+ hematopoietic stem cells cultured with growth factors including Angptl5 efficiently engraft adult NOD-SCID Il2rgamma−/− (NSG) mice. PLoS One. 2011;6(4):e18382. doi: 10.1371/journal.pone.0018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Notta F, et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218–21. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 19.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 20.Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117(20):5281–8. doi: 10.1182/blood-2011-01-315069. [DOI] [PubMed] [Google Scholar]
- 21.Shi C, et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34(4):590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massberg S, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131(5):994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9(2):115–28. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 24.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631–44. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson NK, et al. The transcriptional program controlled by the stem cell leukemia gene Scl/Tal1 during early embryonic hematopoietic development. Blood. 2009;113(22):5456–65. doi: 10.1182/blood-2009-01-200048. [DOI] [PubMed] [Google Scholar]
- 26.Santos PM, Borghesi L. Molecular resolution of the B cell landscape. Curr Opin Immunol. 2011;23(2):163–70. doi: 10.1016/j.coi.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Q, Esplin B, Borghesi L. E47 regulates hematopoietic stem cell proliferation and energetics but not myeloid lineage restriction. Blood. 2011;117(13):3529–38. doi: 10.1182/blood-2010-07-297689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu S, et al. GABP controls a critical transcription regulatory module that is essential for maintenance and differentiation of hematopoietic stem/progenitor cells. Blood. 2011;117(7):2166–78. doi: 10.1182/blood-2010-09-306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barthlen W, et al. Impact of experimental peritonitis on bone marrow cell function. Surgery. 1999;126(1):41–7. doi: 10.1067/msy.1999.99060. [DOI] [PubMed] [Google Scholar]
- 30.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9(5):517–24. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 31.MacNamara KC, et al. Diminished hematopoietic activity associated with alterations in innate and adaptive immunity in a mouse model of human monocytic ehrlichiosis. Infect Immun. 2009;77(9):4061–9. doi: 10.1128/IAI.01550-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johns JL, et al. Infection with Anaplasma phagocytophilum induces multilineage alterations in hematopoietic progenitor cells and peripheral blood cells. Infect Immun. 2009;77(9):4070–80. doi: 10.1128/IAI.00570-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388(4):621–5. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins KA, Mansell A. TIR-containing adaptors in Toll-like receptor signalling. Cytokine. 2010;49(3):237–44. doi: 10.1016/j.cyto.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Nagai Y, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24(6):801–12. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welner RS, et al. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood. 2008;112(9):3753–61. doi: 10.1182/blood-2008-04-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esplin BL, et al. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol. 2011;186(9):5367–75. doi: 10.4049/jimmunol.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanez A, et al. Signalling through TLR2/MyD88 induces differentiation of murine bone marrow stem and progenitor cells to functional phagocytes in response to Candida albicans. Cell Microbiol. 2010;12(1):114–28. doi: 10.1111/j.1462-5822.2009.01382.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang Q, et al. E47 controls the developmental integrity and cell cycle quiescence of multipotential hematopoietic progenitors. J Immunol. 2008;181(9):5885–94. doi: 10.4049/jimmunol.181.9.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang W, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199(8):1177–85. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, et al. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13(4):547–58. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takizawa H, et al. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med. 2011;208(2):273–84. doi: 10.1084/jem.20101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sioud M, et al. Signaling through toll-like receptor 7/8 induces the differentiation of human bone marrow CD34+ progenitor cells along the myeloid lineage. J Mol Biol. 2006;364(5):945–54. doi: 10.1016/j.jmb.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 46.Sioud M, Floisand Y. TLR agonists induce the differentiation of human bone marrow CD34+ progenitors into CD11c+ CD80/86+ DC capable of inducing a Th1-type response. Eur J Immunol. 2007;37(10):2834–46. doi: 10.1002/eji.200737112. [DOI] [PubMed] [Google Scholar]
- 47.De Luca K, et al. The TLR1/2 agonist PAM(3)CSK(4) instructs commitment of human hematopoietic stem cells to a myeloid cell fate. Leukemia. 2009;23(11):2063–74. doi: 10.1038/leu.2009.155. [DOI] [PubMed] [Google Scholar]
- 48.Dorner M, et al. Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunology. 2009;128(4):573–9. doi: 10.1111/j.1365-2567.2009.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7(4):333–7. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 50.Massberg S, von Andrian UH. Novel trafficking routes for hematopoietic stem and progenitor cells. Ann NY Acad Sci. 2009;1176:87–93. doi: 10.1111/j.1749-6632.2009.04609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32(2):57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rebel VI, et al. Essential role for the p55 tumor necrosis factor receptor in regulating hematopoiesis at a stem cell level. J Exp Med. 1999;190(10):1493–504. doi: 10.1084/jem.190.10.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pronk CJ, et al. Tumor necrosis factor restricts hematopoietic stem cell activity in mice: involvement of two distinct receptors. J Exp Med. 2011 doi: 10.1084/jem.20110752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ichii M, et al. Functional diversity of stem and progenitor cells with B-lymphopoietic potential. Immunol Rev. 2010;237(1):10–21. doi: 10.1111/j.1600-065X.2010.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004;5(9):953–60. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- 56.Mansson R, et al. Single-cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 2010;115(13):2601–9. doi: 10.1182/blood-2009-08-236398. [DOI] [PubMed] [Google Scholar]
- 57.Gangneux JP, et al. Epidemiology of invasive aspergillosis and risk factors in non neutropaenic patients. Rev Mal Respir. 2010;27(8):e34–46. doi: 10.1016/j.rmr.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez S, et al. Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis. Blood. 2009;114(19):4064–76. doi: 10.1182/blood-2009-04-214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maratheftis CI, et al. Toll-like receptor-4 is up-regulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clin Cancer Res. 2007;13(4):1154–60. doi: 10.1158/1078-0432.CCR-06-2108. [DOI] [PubMed] [Google Scholar]
- 60.Kerbauy DB, Deeg HJ. Apoptosis and antiapoptotic mechanisms in the progression of myelodysplastic syndrome. Exp Hematol. 2007;35(11):1739–46. doi: 10.1016/j.exphem.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kulkarni S, et al. Hematological targets of radiation damage. Curr Drug Targets. 2010;11(11):1375–85. doi: 10.2174/1389450111009011375. [DOI] [PubMed] [Google Scholar]
- 62.Burdelya LG, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320(5873):226–30. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horng T, et al. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420(6913):329–33. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 64.Jordan CT, Lemischka IR. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990;4(2):220–32. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- 65.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA. 1992;89(4):1502–6. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adolfsson J, et al. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15(4):659–69. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 67.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98(25):14541–6. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175(8):5016–23. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- 69.Wiesmann A, et al. Expression of CD27 on murine hematopoietic stem and progenitor cells. Immunity. 2000;12(2):193–9. doi: 10.1016/s1074-7613(00)80172-7. [DOI] [PubMed] [Google Scholar]
- 70.Goodell MA, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weksberg DC, et al. CD150− side population cells represent a functionally distinct population of long-term hematopoietic stem cells. Blood. 2008;111(4):2444–51. doi: 10.1182/blood-2007-09-115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uchida N, Weissman IL. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin− Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1. 1 bone marrow. J Exp Med. 1992;175(1):175–84. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spangrude GJ, Brooks DM. Phenotypic analysis of mouse hematopoietic stem cells shows a Thy-1-negative subset. Blood. 1992;80(8):1957–64. [PubMed] [Google Scholar]
- 74.Spangrude GJ, Brooks DM. Mouse strain variability in the expression of the hematopoietic stem cell antigen Ly-6A/E by bone marrow cells. Blood. 1993;82(11):3327–32. [PubMed] [Google Scholar]