Abstract
Background
Obesity and excessive weight gain during pregnancy are associated with adverse pregnancy outcomes. Observational studies suggest that minimal or no gestational weight gain (GWG) may minimize the risk of adverse pregnancy outcomes for obese women.
Objective
This report describes the design of Healthy Moms, a randomized trial testing a weekly, group-based, weight management intervention designed to help limit GWG to 3% of weight (measured at the time of randomization) among obese pregnant women (BMI ≥30 kg/m2). Participants are randomized at 10–20 weeks gestation to either the intervention or a single dietary advice control condition.
Primary Outcomes
The study is powered for the primary outcome of total GWG, yielding a target sample size of 160 women. Additional secondary outcomes include weight change between randomization and one-year postpartum and proportion of infants with birth weight > 90th percentile for gestational age. Statistical analyses will be based on intention-to-treat.
Methods
Following randomization, all participants receive a 45-minute dietary consultation. They are encouraged to follow the Dietary Approaches to Stop Hypertension diet without sodium restriction. Intervention group participants receive an individualized calorie intake goal, a second individual counseling session and attend weekly group meetings until they give birth. Research staff assess all participants at 34-weeks gestation and at 2-weeks and one-year postpartum with their infants.
Summary
The Healthy Moms study is testing weight management techniques that have been used with non-pregnant adults. We aim to help obese women limit GWG to improve their long-term health and the health of their offspring.
Keywords: weight gain, pregnancy, diet
1. Introduction
Thirty percent of women of reproductive age are obese and an additional 25% are overweight [1;2]. Pregnancy Risk Assessment Monitoring System data from nine US states show a 70% increase over the past decade in the proportion of women who are obese at the beginning of pregnancy [3]. This is especially concerning given obese women are more likely than normal-weight women to begin pregnancy with co-morbid medical conditions and experience adverse conditions such as gestational diabetes, gestational hypertension, preeclampsia, fetal growth abnormalities, and preterm birth [4;5].
Almost 50% of obese women in the U.S gain more weight during pregnancy than recommended by the Institute of Medicine (IOM) [1]. Excessive weight gain during pregnancy is associated with adverse pregnancy outcomes including macrosomia (birth weight > 4000 grams) [6;7], preterm delivery (<37 weeks gestation) [5], and neonatal hypoglycemia and hyperbilirubinemia [8]. Excessive weight gain during pregnancy also increases the risk of both short- and long-term maternal weight retention and offspring obesity [5;9].
The IOM recently updated their guidelines for weight gain during pregnancy and noted that determining the ideal weight gain for obese women was hampered by a lack of research [1]. Currently available data are also limited primarily to observational studies. While several of these studies suggested weight loss or limited weight gain may be ideal for obese pregnant women [10–13], and that weight gain over 20 pounds may be detrimental [7], prospective randomized trials are needed to confirm this effect. The IOM Working group has also identified a need for further research to determine how to help women stay within weight-gain recommendations [1].
Whether dietary and/or physical activity interventions during pregnancy can successfully control weight gain during pregnancy and improve pregnancy outcomes is uncertain. Among seven randomized trials of dietary and lifestyle interventions that reported results specifically for obese, nondiabetic pregnant women [14–20], only three [14;15;20] were successful in limiting gestational weight gain among obese women. The three successful interventions were the most intensive. They included ten one-hour dietary consultations[14], dietary education with daily food records reviewed at prenatal visits [15], and four dietary counseling sessions coupled with a free 6-month membership to a fitness center, individual physical activity training classes for 1 hour each week with a physiotherapist, and 4–6 group physical activity training sessions[20]. The results of the seven randomized trials suggest that less-intensive interventions are not effective for limiting weight gain among obese pregnant women [18;19]. This is consistent with the weight-loss and weight-maintenance literature in non-pregnant adults, which suggests that the best results are obtained with a long series of weekly group sessions [21].
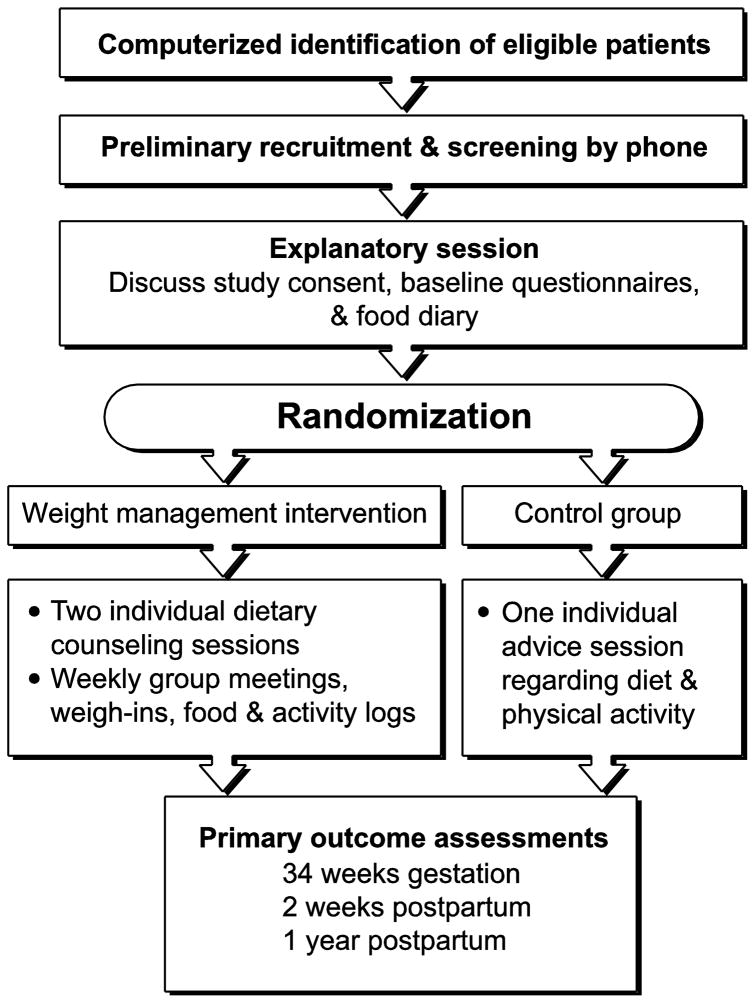
This report describes the development and methodology for the Healthy Moms study (Figure 1) (Clinicaltrials.gov # NCT00950235), a randomized clinical trial among obese pregnant women designed to test the following: 1) the primary feasibility and efficacy of a group-based weight management intervention for obese pregnant women for the primary outcome of gestational weight gain, and secondary outcomes of postpartum weight retention and newborn size; and 2) the feasibility and safety of setting a weight-maintenance goal for obese pregnant women during pregnancy.
Fig.1.
2. Study Design
2.1 Overview
Healthy Moms is randomizing pregnant women who were obese at the start of pregnancy (BMI ≥30 kg/m2) and aged 18 years or older. Women are being randomized between 10–20 weeks gestation to either a weekly, group-based, diet and lifestyle intervention, or to an information-only control condition consisting of a single dietary advice session. The Kaiser Permanente Northwest’s Institutional Review Board approved the study protocol and consent procedures. All study procedures are carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) [22].
2.2 Study Aims
The Healthy Moms study’s overarching hypothesis is that obese women who maintain a constant weight during pregnancy will have superior maternal and neonatal outcomes when compared to women who gain >15 lbs (the amount of weight gain recommended for obese women by the Institute of Medicine at the time our study was designed [23]). The goal for women in the intervention group is to maintain their weight during pregnancy to within 3% of their weight at randomization. The study’s specific aims are to test the intervention’s efficacy in reducing: 1) total gestational weight gain (mean weight gain and proportion with excessive weight gain [>15 lbs]), 2) postpartum weight retention at one year (mean difference between postpartum and baseline weight), and 3) proportion of large-for-gestational-age neonates. Secondary outcomes include measures of feasibility and acceptability of the intervention; maternal dietary intake and physical activity; and infant birth weight, feeding patterns, and growth during the first year of life.
2.3 Study Population
Kaiser Permanente Northwest, (KPNW) is a not-for-profit health maintenance organization serving ~475,000 members. Most pregnant women in KPNW receive prenatal care at one of eight obstetrics and gynecology clinics across the region. Eligibility for Healthy Moms is limited to KPNW members with a singleton gestation and a body mass index (BMI) of ≥ 30 kg/m2 at randomization. Exclusion criteria are a gestational age of greater than 20 weeks at randomization, multifetal pregnancy (twins or more), anticipated disenrollment from KPNW prior to delivery, non-English speaking, plans to move out of the area within one year of delivery, type 1 and type 2 diabetes mellitus (or a diagnosis of gestational diabetes prior to randomization), and other medical conditions that require specialized nutritional care (e.g., history of bariatric surgery) or conditions that may affect weight gain (e.g., severe hyperemesis gravidarum).
2.4 Recruitment and Randomization
KPNW patients typically undergo their first prenatal visit at approximately eight weeks gestation. To identify potential participants, we use an electronic algorithm to search the KPNW appointment system and the electronic medical record (EMR) weekly. Center for Health Research (CHR) recruitment staff mail a recruitment letter to patients who appear to meet eligibility criteria based on BMI, maternal age, and gestational age. Because approximately 20% of women do not have height recorded in the EMR, letters are also sent to women without a recorded height if they weigh >180 pounds. A project recruiter calls women who do not decline participation. Recruiters make up to eight contact attempts for each potentially eligible patient. If a potential participant is not reached after eight attempts, no further calls are made. When the recruiter reaches a potential participant, she describes the study, answers any study-related questions, and conducts a preliminary eligibility interview. Eligible participants are then scheduled to attend an explanatory meeting. A project investigator conducts weekly explanatory meetings to describe the study and encourage potential participants to ask questions. Interested women are then scheduled to return for a randomization visit in approximately one week. Potential participants receive a set of baseline questionnaires and are asked to keep a food diary for at least five days for the randomization visit. The study dietitian uses the food diary to create a personalized eating plan. At the randomization visit, clinic staff meet with the participant to confirm that all study questionnaires and food diaries are complete, obtain written informed consent, and measure the participant’s height and weight. The participant is then referred to the study dietician where eligibility criteria are reviewed. Randomization then occurs using a computerized algorithm to generate the random assignments in blocks of four. Random assignment is stratified on age (<30, 30 and older) and BMI (30 to 34.9 kg/m2, 35 to 39.9 kg/m2, and 40 or greater kg/m2).
Although KPNW patients typically enter prenatal care in the first trimester, we chose to allow randomization up to 20 weeks gestation to maximize our sample size and ability to recruit. While allowing randomization up to 20 weeks gestation may seem generous, women’s baseline dietary behaviors are set well before pregnancy and, although entering the study at 20 weeks rather than 10 means we have less cumulative time to intervene, there are still potentially 20 weeks left of pregnancy to have an impact. Our preliminary analysis of baseline data collected to date has demonstrated the average gestational age at randomization is 15 weeks.
2.5 Intervention Goals, Approach and Rationale
2.5.1. Intervention goals
Weight Goal
The intervention’s goal is to help participants maintain their weight during pregnancy to within 3% of their weight at enrollment (randomization). This differs from current recommendations for obese women for a gestational weight gain of 11–20 pounds [1]. We chose a maintenance goal based on observational studies suggesting that limited weight gain, weight maintenance, or even weight loss may result in better pregnancy outcomes for obese women. There were no prior to studies to help us determine how the weights of obese women would fluctuate on a week to week basis during pregnancy so we chose to define maintenance as +/− 3% of baseline weight. There may be other ways to define weight maintenance and track weight change on weekly basis but, in designing this study, there were several reasons we chose +/− 3%. First, it had been suggested as a reasonable way to define weight maintenance [24], even though not empirically tested. Second, we believe this percentage is large enough to allow for fluctuations in weight gain from week to week based on changes in fluid retention. Third, we believe it will provide a standardized way (percent change) to track weight week to week during the intervention period and give us a range to act upon in the instance of weight loss or weight gain and potential need for revision in caloric intake. A 3% weight gain over the course of pregnancy for a 300 lb woman is equivalent to 9 lbs, which is below the current IOM guidelines of 11–20 pounds and the old IOM guidelines of at least 15 pounds.
It is not possible to track energy consumption with precision in free-living participants. Thus, even though participants are given daily calorie targets, actual consumption is only a crude estimate that is based on food records. To help a participant stay within her weight goals, intervention staff monitor participants’ weights and food records weekly to adjust calorie targets, as needed. Rapid weight gain is defined as a weight increase of 3% or more from the preceding week. If rapid weight gain is felt by the participant or study staff to be attributable to edema, the intervention staff notifies the study’s medical director (Dr. Vesco), who determines if further evaluation is needed by the patient’s obstetrical care provider. Similarly, if a participant’s weight drops below 3 percent, then her food records are reviewed by the interventionist and study dietician. They consider the caloric intake recommendation originally made for the participant and an estimate of her current caloric intake in making a recommendation for how to increase caloric intake (and how much to increase) to avoid further weight loss.
Physical Activity Goal
Women in the intervention arm are encouraged to accumulate at least 30 minutes of moderate physical activity per day unless restrictions are advised by their primary obstetrical care provider (Table 1). This goal is consistent with the recommendations of the Centers for Disease Control and Prevention (CDC), the American College of Sports Medicine, and the American College of Obstetricians and Gynecologists (ACOG) [25]. Medical conditions, such as placenta previa and preterm labor, could arise during the course of pregnancy and lead a participant’s health care provider to restrict her physical activity. If a participant’s health care provider recommends she limit her activity or go on bedrest and the participant is unable to travel, our interventionists talk with the participant weekly by phone and a home visit is performed to obtain the primary outcome data (e.g. patient’s weight) if needed.
Table 1.
Healthy Moms Study Intervention goals
Nutrition goals
|
Physical activity goals
|
Women in the intervention group are provided pedometers to encourage physical activity. The pedometer also provides participants a means to track their physical activity level. Participants are asked to record their daily step totals or minutes of physical activity and report on their physical activity at each weekly group-session. Because the pedometer is viewed as an intervention in and of itself, pedometers are not provided to the control group until they have completed the one-year follow-up visit.
2.5.2. Intervention Diet
There are no empirically tested recommendations of the most appropriate diet for weight control during pregnancy. An ideal pregnancy diet should provide adequate nutrition for the mother and fetus, prevent ketonemia, and stabilize glucose levels, while allowing normal fetal growth. When considering alternative diet patterns, we examined guidelines provided by ACOG [26], the United States Department of Agriculture (USDA) MyPyramid for Moms [27], and the American Dietetic Association (ADA) nutrition recommendation for the prevention and treatment of diabetes including gestational diabetes mellitus (GDM) [28]. All three agencies recommend a diet that is nutrient dense, varied, and balanced [26–28]. We chose the Dietary Approaches to Stop Hypertension (DASH) dietary pattern [29;30] because it meets these goals and is cited as an example of a dietary pattern consistent with the most current USDA guidelines for a healthy diet [31].
Briefly, the DASH combination diet emphasizes consumption of fruits, vegetables, and low-fat dairy products. It includes whole grains, poultry, fish, and nuts, and limits fats, red meat, sweets, and sugar-containing beverages. The DASH diet is high in the nutrients found in fruits, vegetables, and low fat dairy products—particularly calcium, magnesium, potassium, and fiber. Compared to the typical diets Americans eat, the DASH diet contains lower amounts of total fat (most notably saturated fat and cholesterol), and is higher in protein (18% versus 15%). The DASH diet has a lower energy density and has been shown to lower blood pressure, lipids, and fasting glucose [30;32]. While the DASH diet has not been directly tested in pregnant women, we have no reason to believe it would not have the same metabolic benefits in overweight and obese pregnant women, who are at risk for metabolic complications such gestational hypertension and diabetes, as it has in non-pregnant individuals. Further, the DASH diet has been successfully used in combination with intensive behavioral interventions to promote weight loss [33]. The DASH diet (as originally designed) does not restrict sodium intake. Therefore, participants are counseled not to restrict sodium intake, since a sodium-restricted diet could potentially prevent adequate expansion of the circulating blood volume required for pregnancy. Because pregnancy is a state of mild insulin resistance, participants are given tools to facilitate the consumption of 45–60 grams of carbohydrates (primarily whole grain carbohydrates) per meal and 15–30 grams during snacks to prevent extreme fluctuation in postprandial glycemia. Table 1 depicts the nutrition goals for the intervention group and Table 2 shows two sample meal plans (1500 and 2000 kilocalories) that represents the meal pattern that we recommended to achieve a steady level of postprandial glycemia while meeting the goals of the DASH diet.
Table 2.
Healthy Moms Study sample meal plans
| 1500 Kcal | 2000 Kcal | |
|---|---|---|
| Meal | (45 g carb/meal) | (60 g carb/meal) |
| Breakfast | 1 whole grain | 2 whole grains |
| 1 dairy | 1 dairy | |
| 1 fruit | 1 fruit | |
| 1 fat | 1 fat | |
| AM Snack | 1 vegetable | 1 fruit |
| 1 grain | ||
| 1 fat | ||
| Lunch | 2 whole grains | 2 whole grains |
| 1 fruit | 1 fruit | |
| 2 vegetables | 2 vegetables | |
| 2 proteins | 3 proteins | |
| 1 fat | 1 fat | |
| PM Snack | 1 dairy | 1 dairy |
| 1grain | ||
| Dinner | 2 whole grains | 2 whole grains |
| 1 fruit | 1 fruit | |
| 3 vegetables | 3 vegetables | |
| 2 proteins | 3 proteins | |
| 1 fat | 2 fats | |
| PM Snack | 1 whole grain | 1 whole grain |
| 1 dairy | 1 dairy | |
| 1 fat | 1 fat | |
2.5.3 Energy (Caloric) Intake Management and Rationale
Women randomized to the intervention arm are assigned a daily caloric intake goal that creates sufficient caloric deficit to maintain weight using the following formula: . We created this formula since there were no guidelines on how best to limit energy during pregnancy. The formula first calculates energy needs based on pre-pregnancy weight using the ACOG factor of 30 kcal/day, reduces that number by 30%, and then adds 10 Kcal per week of gestation to accommodate rising basal metabolic rate (BMR) as gestation progresses. This approach allows us to tailor energy need to stage of pregnancy. A woman with a pre-pregnancy weight of 91 kg who is eight weeks pregnant, for example, would be assigned a caloric intake of 1991 Kcal/day. The same woman would need 2091 Kcal/day at 18 weeks.
We chose to reduce calorie consumption by 30% from the calculated energy needs at the pre-pregnancy weight as there is not extensive information on the best approach to limit energy intake during pregnancy. The rationale for our choice is supported by the ADA’s suggestion that women with a BMI >30 may do well with moderate caloric restriction (30–33%), particularly if they have gestational diabetes mellitus (GDM) [34]. In perhaps the only study to restrict caloric intake by 30% in obese women with GDM, pregnancy outcomes were similar to those of a group of matched controls without GDM [34]. Our approach allows us to use one protocol that appropriately meets the energy needs of women with and without GDM.
2.5.4. Intervention format and content
Participants randomized to the intervention arm attend two individual counseling sessions designed to tailor the intervention to the participant and prepare them for the group sessions. The first counseling session is conducted immediately after randomization and the second session is conducted a week later. Participants are introduced to the Healthy Moms’ diet and physical activity goals and receive their calorie assignment at the first individual counseling session. The interventionist helps the participant analyze her food diaries to determine how many calories she is eating and whether she is meeting the DASH dietary goals. The interventionist uses motivational interviewing techniques to help the participant set personal goals and action plans to align their eating patterns to the DASH pattern and follow the calorie recommendations. Where appropriate, the participant receives two weeks’ worth of sample meal plans adapted to their calorie level.
Tailoring the intervention to the participant’s lifestyle occurs during the second individual counseling session. The interventionist and participant review the previous week’s goals, discuss challenges the participant may have encountered, and streamline personal action plans to meet intervention goals. Participants also receive a pedometer and instruction on how to use it at this session.
Participants begin weekly group sessions after their second individual counseling session. Participants are expected to attend for the duration of their pregnancy. The group meeting is conducted one night per week at the CHR, which is centrally located in the KPNW region. Group counseling is delivered in a series of 16 sessions that form the core. The curriculum is delivered in a cyclical manner such that session one starts the week after session 16 ends, in a repeating fashion. Similarly, participants also join group sessions in a cyclical manner when they are assigned to the intervention condition. A participant joining during session eight is expected to stay thorough session 16 and the repeat of session 1–7. This group approach has been proven to be effective in many previous weight loss studies [21] and is used by some popular commercial weight-loss programs. We have successfully used a similar model in another weight loss program within KPNW [35].
The meeting format and many aspects of the behavior change intervention techniques are based on the weight loss interventions employed in the PREMIER and Weight Loss Maintenance (WLM) trials. The PREMIER and WLM weight loss interventions have been effective in a broad range of participants [33;36–38], and the basic approach was adapted from the TOHP I and TOHP II hypertension-prevention trials [36;37;39;40] and other weight loss studies [41]. Using this model, each 90-minute group session comprises four components: a check-in, a nutrition topic and/or exercise topic, a behavior change topic, and a goal-setting segment for the next week with a plan for how to meet the goals [42]. The meetings begin with the check-in component, during which participants report on their experiences during the past week. Participants are encouraged to talk about the behavior change techniques they found helpful. Next, the leaders present a nutrition and/or exercise information module relevant to the intervention goals (e.g., managing calories by controlling portion size). Questions and group discussions are encouraged, as is group problem solving around potential barriers to behavior change. The content of the sessions is behavior change oriented, rather than simply presenting information. The third module in each session is devoted to a behavior change technique, such as building social support for physical activity, getting family members to help manage the household food supply, developing personalized record keeping techniques. Group leaders present examples for how the behavior change technique can be directly applied to that session’s nutrition and physical activity topics. In the final part of the group session, participants are asked to develop specific behavior change goals and action plans for the next week and share them with the group. This social disclosure of goals and plans has been shown to be a powerful intervention technique [43].
Participants in the intervention group are also asked to keep weekly food and activity records and bring them to each group session. The interventionists and study dietician review these records and provide written comments to encourage participants to keep the food diaries, provide positive comments on nutritional choices, and answer any questions raised by the participants. If a participant does not appear to be receiving adequate nutrition, the study dietician will either meet with the participant after the weekly group session or schedule a follow-up individual counseling session either in person or by telephone.
2.6 Control Group Strategy
Immediately following randomization, women assigned to the control group receive onetime feedback about their food diaries, a structured dietary plan (Table 2), and advice to follow the ACOG guidelines for physical activity during pregnancy [25]. We also provide control participants with information on a considerable array of pregnancy support services available to them within the KPNW health care program and the community. We chose to offer a single dietary advice session to women in the control group because this is available through their prenatal care with KPNW and this allowed us to standardize usual care related to nutrition. This approach also promotes interest in the study among patients and their clinicians. We expect that women who participate in the Healthy Moms study will have a high degree of motivation and, therefore, weight gain in the control group will likely be less than among similarly eligible KPNW members who decline to participate. Since weight loss and weight maintenance studies in non-pregnant adults have shown that accountability is important for short- and long-term success in achieving weight goals [21], we anticipate that a single dietary advice session will not yield the same effect on weight gain or dietary change as weekly, group sessions. We do not make weight gain recommendations for the control group. They are asked to follow the advice of their obstetrical care provider. We recognize the recommendations may vary by provider and we ask all participants prior to randomization what weight gain advice they have received.
2.7 Study Retention
A number of incentives to keep participants engaged and improve retention of both the intervention and control are provided on an intermittent schedule. Incentives include gift cards, birthday and birth congratulations cards. For women in the intervention group, intervention counselors conduct follow-up telephone calls with participants who miss group sessions. Incentives for attending intervention sessions include drawings for door prizes given at group meetings and services provided postpartum (e.g., gift certificates for food/cleaning services for attendance at a preset proportion of group sessions).
3. Outcome Measures
The data collection schedule is shown in Table 3. CHR clinic staff members (who are blinded to the participants’ assignment) perform the outcomes measures. Assessments are made at 34 weeks of gestation and again at 2 weeks, 6 and 12 months postpartum. We chose to conduct an assessment for all participants at 34 weeks of gestation to capture potential changes implemented by women during pregnancy and to ensure that we have an adequate window of time to bring all women in for the 34-week assessment. Although a later gestational age (such as 36 to 37 weeks) may be more desirable for the late third trimester assessment of maternal secondary outcomes, obese women are at high risk for a number of pregnancy complications, including hypertension and gestational diabetes, which can result in preterm (<37 weeks gestation), early term delivery (37 to 38 weeks gestation) or need for bed rest during the last several weeks of pregnancy. We conduct home visits for women in the study who are unable to travel to a CHR clinic visit (for example, women on clinician-prescribed bed rest).
Table 3.
Healthy Moms Study data collection schedule
| Baseline 10 to 20 weeks gestation | 34-weeks gestation | 2-weeks postpartum | 4 months postpartum | 6 months postpartum | 9 months postpartum | 1-year postpartum | |
|---|---|---|---|---|---|---|---|
| Maternal | |||||||
| Informed consent | X | ||||||
| Randomization | X | ||||||
| Weight | X | X | X | X | |||
| Height | X | ||||||
| Demographics | X | ||||||
| Dietary intake (Block FFQa) | X | X | X | X | |||
| Food diaries | X | ||||||
| IPAQb | X | X | X | X | |||
| EDSc | X | X | X | X | |||
| SF-12d | X | X | X | x | |||
| Satisfaction questionnaire | X | X | X | ||||
| Neonatal/infant | |||||||
| Skinfolds, weight, length; arm and head circumference | X | X | |||||
| Feeding practices | X | X | X | X | X | ||
FFQ – food frequency questionnaire
IPAQ – International Physical Activity Questionnaire
EDS – Edinburgh Depression Survey
SF-12 – Short Form 12
The six-month and 1-year postpartum assessments will allow us to determine if there is a lasting effect of the intervention on weight and secondary measures such as diet, physical activity, and child growth.
3.1 Primary Outcomes, Assessment, and Rationale
Maternal Outcomes
This trial’s primary maternal outcomes are mean total gestational weight gain and proportion of participants with gestational weight gain >15 lbs. We chose gestational weight gain of >15 lbs because the 2009 IOM guidelines [1] (recommending 11–20 pounds pregnancy weight gain for obese women) were not available at the time the study began and previous IOM guidelines [44] did not provide an upper weight gain limit for obese women, they just stated that obese women should gain at least 15 pounds. Gestational weight gain will be based on the difference between the participant’s weight at randomization and 2 weeks postpartum. The weight at randomization is the baseline weight for our study and is measured at the CHR clinic. We chose the weight at 2 weeks postpartum, rather than at an end point during pregnancy, to avoid the contribution of products of conception, maternal edema, and increased maternal blood volume to the weight gain. We will also calculate weight gain as the difference between weight at 34 weeks gestation and baseline, adjusted for baseline gestational age. Further, using data from the electronic medical record, we will calculate total pregnancy weight gain as last prenatal weight minus first prenatal weight. These additional calculations will make the study results directly comparable to other intervention studies focused on limiting gestational weight gain. As a secondary maternal outcome we will evaluate postpartum weight retention, which will be determined as weight at one year postpartum minus weight at baseline.
Offspring Outcomes
As secondary outcomes, we will evaluate newborn size as a continuous measure (birth weight in grams) and as weight-for-gestational age. Large-for-gestational-age (LGA) and small-for-gestational age (SGA) will be determined based on gender and race/ethnicity specific growth curves. LGA will be defined as weight greater than the 90th percentile for gestational age and SGA will be defined as weight less than the 10th percentile for gestational age at birth. Additional analyses will also be performed to evaluate differences between the two groups in prevalence of macrosomia (birth weight > 4000 grams). We don’t anticipate having adequate power to detect a statistically significant difference between the intervention and control groups in the proportion of LGA or SGA newborns.
3.2 Secondary Outcomes
Maternal Outcomes
Secondary outcomes include dietary intake, physical activity, assessment of depressive symptoms, and mother’s health-related quality of life (Table 3). We will also obtain data from the EMR on pregnancy outcomes, such as mode of delivery (cesarean section versus vaginal), hypertensive disorders of pregnancy, gestational diabetes, delivery complications, and neonatal hypoglycemia and hyperbilirubinemia.
The Block Food Frequency Questionnaire (FFQ) [45] is being used to assess dietary intake at baseline, 34 weeks of gestation, and at 6 and 12 months postpartum. The Block FFQ has been validated [46], is self-administered, and requires about 20 minutes to complete. Data from the FFQ will be summarized to include daily energy, macro- and micronutrients, food group servings, and nutrients that reflect the DASH eating plan (e.g., total fat, saturated fat, fruits and vegetables, low-fat dairy) [45].
We chose to assess physical activity using the International Physical Activity Questionnaire (IPAQ), because it has been validated against accelerometry and has good test-retest metrics [47]. While measures such as accelerometers or some of the newer motion detection technologies might be more objective, we were constrained by the costs of obtaining instrumentation, creating visits to load and unload the instruments, and resources to analyze the large volume of data produced by these instruments.
We are using the Edinburgh Depression Scale (EDS) [48;49] to assess depressive symptoms, and the SF-12 [50] to assess health-related quality of life. These tools have been used elsewhere to study health status in pregnant populations [51–55]. These short surveys are self-administered and can be completed within a few minutes.
Offspring Outcomes
Anthropometric measurements collected at age two weeks and one year include weight, length, head circumference, and triceps and subscapular skin fold thickness. A pediatrician (Dr. McEvoy, who is also blinded to study assignment) trained the clinic staff collecting these measures. A blinded observer conducts periodic observations of clinic staff for these and all other measurements to ensure adherence to the study procedures.
We will use the EMR to gather supplemental data on infant growth from routine pediatric well-child visits completed during the first year of life. For neonatal and infant growth and body composition, we are using the World Health Organization growth curves to calculate weight for length, weight for age, and length for age calculations [56], and body fat will be estimated using the sum of the triceps and subscapular skin fold measurements [57;58].
We are assessing infant feeding practices and diet at 2-weeks, 4-months, 6-months, 9-months and 12-months of age, including breastfeeding initiation, duration and exclusivity. These data might allow us to separate the effect of maternal weight gain during pregnancy on infant growth patterns, and infant growth patterns that may be attributed to feeding, or other parenting practices.
As part of evaluating the safety of the intervention, we will use the participants’ and their infants’ medical records to assess the rate of intrauterine growth restriction and other measures of fetal compromise including admissions to the special care nursery or neonatal intensive care unit, perinatal mortality and need for complementary formula feeding.
Acceptability and Feasibility of Intervention
To assess the acceptability and feasibility of conducting this group-based intervention, we are tracking study disenrollment rates and attendance at intervention sessions.
3.3 Data Analysis Plan
When final outcome data become available, we will use an intention-to-treat approach. Initial power calculations for this study estimated a final sample size of 160 women (80 in each arm) would be needed to identify a 6.6 pound difference between the groups in mean total gestational weight gain and a 20.6 percent difference in gestational weight gain >15 pounds with power of .80 and alpha = 0.05. Given limited data by which to assess the expected differences, we used historical data regarding weight gain within our KPNW obstetrical population and our desired outcomes to inform the calculations. We were unable to determine a priori how the motivation of women willing to join this type of study would influence group differences in outcomes. We suspect that even women in our control arm will gain less weight than those who do not participate in the study. This hypothesis could be explored secondarily using electronic medical record data.
Prior to conducting the main analyses, baseline characteristics such as presence of chronic hypertension, tobacco use, age, parity, and Medicaid status will be compared between the intervention and control groups to identify potential confounders. These characteristics were selected for theoretical reasons, such as the effect of tobacco on weight or access to healthy food for women with limited financial resources. To examine total weight gain, multiple regression will be used with gestational weight gain as the dependent variable. To account for regression towards the mean, baseline weight will be included in the model as a covariate along with potential confounders. Logistic regression will be used to examine differences between the groups in weight gain >15 pounds controlling for baseline weight and potential confounders. Baseline characteristics will be evaluated as potential effect modifiers. GDM is anticipated to occur in about eight percent of our study participants. As such, we will not have the power to evaluate the intervention’s impact on GDM diagnosis and treatment. Diagnosis of gestational diabetes will be included in the statistical models as a covariate if the proportion of women with GDM differs between the groups.
4. Design Limitations and Strengths
Our goal with this study was to determine whether it is feasible to recruit obese pregnant women to an intensive weight management intervention to significantly impact pregnancy weight gain, our primary outcome of interest. Our sample size will not be large enough to identify statistically significant differences in clinical outcomes such as SGA, LGA, or GDM. Much larger studies would be needed to determine the impact of a weight management intervention on these outcomes. Nevertheless, we are collecting information on important clinical outcomes such as SGA, LGA, GDM, and preeclampsia, and will plan to evaluate for between group differences.
For this preliminary trial we excluded non-English speaking populations. While it is unclear if uptake and satisfaction would be similar in non-English speaking, minority, or low-income populations, access to the intensive services provided may be appealing to women with limited resources. Budget limitations prevented us from collecting biologic measures to assess adherence to the dietary intervention or the effect of the intervention on maternal glucose and lipid levels during pregnancy, as well as lack of body composition assessments on the mother, and lack of metabolic assessments of the offspring.
Our study does not include women with BMIs of less than 30 kg/m2, but excessive gestational weight gain is also a concern for both normal and overweight women. Currently, 38% of normal and 63% of overweight pregnant women in KPNW gain more weight than is recommended by the IOM [1]. Whether women of lower BMIs would be receptive to a high-intensity intervention with the goal of preventing excessive weight gain is uncertain. Therefore, future research could expand the study population to women with BMIs in the normal to overweight range (18.5 to 29.9 kg/m2) with modification of weight-gain goals to be concordant with recent IOM recommendations [1]. Although women who chose to participate in any research trial are likely to be highly motivated, our intervention, though intensive, is not unlike common popular weight loss strategies that involve dietary modification, or weight loss programs that involve weekly group meetings, food records, and weigh-ins.-Therefore, we believe there is a great capacity for this type of intervention to be delivered broadly at a population level.
Our study has numerous strengths. We are unaware of any randomized trials of interventions to limit maternal gestational weight gain that have published information regarding neonatal body composition or growth in the first year of life, or that have provided physical or dietary assessments at one-year of life. Our study may be one of the first trials of its kind to provide estimates of neonatal and infant body composition and one-year follow up information for both the mother and her child. In addition, a substantial advantage of our particular group-based study design is that it informs the potential use of well-established community group-based weight intervention models during pregnancy. Finally, our study will help contribute to the body of research needed to establish appropriate gestational weight-gain recommendations for obese women to maximize both their health and the health of their offspring.
5.0 Conclusion
Healthy Moms is an NICHD funded single-center randomized trial of a group-based, diet and lifestyle weight-management intervention compared to a single individual dietary counseling session. This trial aims to improve dietary quality and limit gestational weight gain among obese women during pregnancy. This article reviews several design considerations for the trial that may prove beneficial to future randomized trials. We selected the strategies employed in Healthy Moms for their proven efficacy for weight management among nonpregnant adults and for their ease of dissemination should they prove efficacious in maintaining weight loss. Healthy Moms will generate important information about the effects of behavioral strategies relevant to both public health and clinical approaches on pregnancy outcomes.
Acknowledgments
Funding agency
This work was supported by a grant from the National Institute of Child Health and Human Development R01 HD0586-01 (PI, Victor J. Stevens).
We’d like to thank the Healthy Moms Study staff for their work on this trial. Clinic staff: Linda Kimmel, Judy Sanseri, Michelle Baker, Anzonia Haney; Intervention staff: Nina Scott and Chris Catlin; Study dieticians: Lonnie Issacson and Carol Young; System Developer: Donna Eubanks; Research Analyst: Padma Dandamudi; Recruitment: Barbara Strong, Donna White, and Cheryl Johnson; Data Coordination: Jane Wallace, Donna Gleason, and Sandra Larson; Administrative support: JaNice Brewster. Thanks also to Kevin Lutz for assistance with manuscript editing.
Abbreviations
- GWG
gestational weight gain
- BMI
body mass index
- CHR
Center for Health Research
- FFQ
food frequency questionnaire
- DASH
Dietary Approaches to Stop Hypertension
- IOM
Institute of Medicine
- KPNW
Kaiser Permanente Northwest
- EMR
electronic medical record. WLM, Weight Loss Maintenance trial
- ACOG
American College of Obstetricians and Gynecologists
- IPAQ
International Physical Activity Questionnaire
- EDS
Edinburgh Depression Survey
- SF-12
Short Form 12
Footnotes
Conflicts of interest
None of the authors reported any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Institute of Medicine and National Research Council. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity. 2007;15:986–93. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 4.Morin KH. Perinatal outcomes of obese women: a review of the literature. J Obstet Gynecol Neonatal Nurs. 1998;27:431–40. doi: 10.1111/j.1552-6909.1998.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 5.Sarwer DB, Allison KC, Gibbons LM, Markowitz JT, Nelson DB. Pregnancy and obesity: a review and agenda for future research. J Womens Health (Larchmt) 2006;15:720–33. doi: 10.1089/jwh.2006.15.720. [DOI] [PubMed] [Google Scholar]
- 6.Hillier TA, Pedula KL, Vesco KK, Schmidt MM, Mullen JA, LeBlanc ES, et al. Excess gestational weight gain: Modifying fetal macrosomia risk associated with maternal glucose. Obstet Gynecol. 2008;112:1007–14. doi: 10.1097/AOG.0b013e31818a9779. [DOI] [PubMed] [Google Scholar]
- 7.Vesco KK, Sharma AJ, Dietz PM, Rizzo J, Callaghan WM, England L, et al. Newborn size among obese women with weight gain outside the 2009 institute of medicine recommendation. Obstet Gynecol. 2011 doi: 10.1097/AOG.0b013e3182113ae4. In press. [DOI] [PubMed] [Google Scholar]
- 8.Hedderson M, Weiss N, Sacks D, Pettitt D, Selby J, Quesenberry C, et al. Pregnancy Weight Gain and Risk of Neonatal Complications. Macrosomia, Hypoglycemia, and Hyperbilirubinemia. Obstet Gynecol. 2006;108:1153–61. doi: 10.1097/01.AOG.0000242568.75785.68. [DOI] [PubMed] [Google Scholar]
- 9.Vesco KK, Dietz PM, Rizzo J, Stevens VJ, Perrin NA, Bachman DJ, et al. Excessive gestational weight gain and postpartum weight retention among obese women. Obstet Gynecol. 2009;114:1069–75. doi: 10.1097/AOG.0b013e3181baeacf. [DOI] [PubMed] [Google Scholar]
- 10.Beyerlein A, Schiessl B, Lack N, von Kries R. Optimal gestational weight gain ranges for the avoidance of adverse birth weight outcomes: a novel approach. American Journal of Clinical Nutrition. 2009;90:1552–8. doi: 10.3945/ajcn.2009.28026. [DOI] [PubMed] [Google Scholar]
- 11.Cedergren MI. Optimal gestational weight gain for body mass index categories. Obstet Gynecol. 2007;110:759–64. doi: 10.1097/01.AOG.0000279450.85198.b2. [DOI] [PubMed] [Google Scholar]
- 12.Oken E, Kleinman KP, Belfort MB, Hammitt JK, Gillman MW. Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am J Epidemiol. 2009;170:173–80. doi: 10.1093/aje/kwp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margerison Zilko CE, Rehkopf D, Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. American Journal of Obstetrics & Gynecology. 2010;202:574–8. doi: 10.1016/j.ajog.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. International Journal of Obesity. 2008;32:495–501. doi: 10.1038/sj.ijo.0803710. [DOI] [PubMed] [Google Scholar]
- 15.Thornton YS, Smarkola C, Kopacz SM, Ishoof SB. Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. J Natl Med Assoc. 2009;101:569–77. doi: 10.1016/s0027-9684(15)30942-1. [DOI] [PubMed] [Google Scholar]
- 16.Jeffries K, Shub A, Walker SP, Hiscock R, Permezel M. Reducing excessive weight gain in pregnancy: a randomised controlled trial. Med J Aust. 2009;191:429–33. doi: 10.5694/j.1326-5377.2009.tb02877.x. [DOI] [PubMed] [Google Scholar]
- 17.Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr. 2010;91:373–80. doi: 10.3945/ajcn.2009.28166. [DOI] [PubMed] [Google Scholar]
- 18.Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstet Gynecol. 2009;113:305–12. doi: 10.1097/AOG.0b013e318195baef. [DOI] [PubMed] [Google Scholar]
- 19.Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. Am J Clin Nutr. 2011 doi: 10.3945/ajcn.110.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinter CA, Beck-Nielsen H, Jensen DM, Jorgensen JS, Ovesen P. The LIP (Lifestyle in Pregnancy) study. Diabetes Care. 2011;34:2502–7. doi: 10.2337/dc11-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–38. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 22.WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. 2011 http://www.wmanet/en/30publications/10policies/b3/ cited 2011 Aug. 25. [PubMed]
- 23.Institute of Medicine (U.S.) Subcommittee on Nutritional Status and Weight Gain During Pregnancy. Nutrition in Pregnancy. National Academy of Sciences; 1990. [Google Scholar]
- 24.Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes. 2006;30:391–9. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 25.ACOG Committee Opinion. Exercise during pregnancy and the postpartum period. 267. 2002. [Google Scholar]
- 26.ACOG Technical Bulletin. Nutrition during pregnancy. Int J Gynecol Obstet. 2007;179:67–74. [PubMed] [Google Scholar]
- 27.United States Department of Agriculture. My pyramid for moms. 2007 http://www.mypyramidgov/mypyramidmoms/index.html. cited 2008 Feb. 28.
- 28.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):S61–78. S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 29.Karanja N, Stevens VJ, Hollis JF, Kumanyika SK. Steps to soulful living (steps): a weight loss program for African-American women. Ethn Dis. 2002;12:363–71. [PubMed] [Google Scholar]
- 30.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 31.U.S.Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans. 6. Washington DC: U.S. Government Printing Office; 2005. [Google Scholar]
- 32.Karanja NM, Obarzanek E, Lin PH, McCullough ML, Phillips KM, Swain JF, et al. Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension Trial. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99:S19–S27. doi: 10.1016/s0002-8223(99)00412-5. [DOI] [PubMed] [Google Scholar]
- 33.Hollis JF, Gullion CM, Stevens VJ, Brantley PJ, Appel LJ, Ard JD, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. American Journal of Preventive Medicine. 2008;35:118–26. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ACOG Committee on Practice Bulletins. Chronic hypertension in pregnancy. Obstetrics & Gynecology. 2001;98(suppl-85) doi: 10.1016/s0029-7844(01)01471-5. [DOI] [PubMed] [Google Scholar]
- 35.Stevens VJ, Rossner J, Greenlick M, Stevens N, Frankel HM, Craddick S. Freedom from fat: a contemporary multi-component weight loss program for the general population of obese adults. J Am Diet Assoc. 1989;89:1254–8. [PubMed] [Google Scholar]
- 36.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–93. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 37.Obarzanek E, Vollmer WM, Lin PH, Cooper LS, Young DR, Ard J, et al. Effects of individual components of multiple behavior changes: The PREMIER trial. American Journal of Health Promotion. 2007;31:545–60. doi: 10.5555/ajhb.2007.31.5.545. [DOI] [PubMed] [Google Scholar]
- 38.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: The weight Loss Maintenance Randomized Controlled Trial. JAMA. 2008;299:1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 39.Stevens VJ, Corrigan SA, Obarzanek E, Bernauer E, Cook NR, Hebert P, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med. 1993;153:849–58. [PubMed] [Google Scholar]
- 40.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith WD, et al. Long-term weight loss and changes in blood pressure: Results of the Trials of Hypertension Prevention, Phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 41.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12 (Suppl):151S–62S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 42.PREMIER Intervention. 2007 http://www.kpchrorg/public/premier/intervention/Intervention.asp.
- 43.Martin GL, Pear J. Behavior modification: What is it and how to do it. 7. New York: Prentice Hall; 2003. [Google Scholar]
- 44.Institute of Medicine. Nutrition During Pregnancy. Washington D.C: National Academy Press; 1990. [Google Scholar]
- 45.Block G. Health habits and history questionnaire-diet history and other risk factors, personal computer system packet. Bethesda, MD: National Cancer Institute; 1989. [Google Scholar]
- 46.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America's Table Study. American Journal of Epidemiology. 2001;154:1089–99. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 47.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 48.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale (EPDS) British Journal of Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 49.Cox JL, Chapman G, Murray D, Jones P. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. Journal of Affective Disorders. 1996;39:185–9. doi: 10.1016/0165-0327(96)00008-0. [DOI] [PubMed] [Google Scholar]
- 50.Ware JE. SF-36 Health survey: Manual and interpretation guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 51.Flynn HA, Blow FC, Marcus SM. Rates and predictors of depression treatment among pregnant women in hospital-affiliated obstetrics practices. General Hospital Psychiatry. 2006;28:289–95. doi: 10.1016/j.genhosppsych.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Mckee MD, Cunningham M, Jankowski KR, Zayas L. Health-related functional status in pregnancy: relationship to depression and social support in a multi-ethnic population. Obstetrics & Gynecology. 2001;97:988–93. doi: 10.1016/s0029-7844(01)01377-1. [DOI] [PubMed] [Google Scholar]
- 53.Hueston WJ, Kasik-Miller S. Changes in functional health status during normal pregnancy. Journal of Family Practice. 1998;47:209–12. [PubMed] [Google Scholar]
- 54.Wells KB, Stewart A, Hays RD, Burnam MA, Rogers W, Daniels M, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. JAMA. 1989;262:914–9. [PubMed] [Google Scholar]
- 55.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;325:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 56.WHO growth reference values for birth onward. 2007 www.whoint/entity/childgrowth/standards/Chap_7pdf.
- 57.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322–8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2009;58:453–9. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]