Abstract
Objective
Post stroke depression (PSD) occurs in the context of abrupt, often catastrophic disability that finds the patient and his/her family unprepared. We developed Ecosystem Focused Therapy (EFT), a systematic intervention aimed to increase the PSD patient’s and his/her ecosystem’s ability to address the “psychosocial storm” of PSD and utilize available treatments effectively and efficiently. This is a preliminary study of its efficacy.
Design
A total of 24 PSD patients were randomly assigned to receive weekly sessions of EFT or a comparison condition consisting of systematic Education on Stroke and Depression and their treatment (ESD) for 12 weeks.
Results
EFT may be more efficacious than ESD in reducing depressive symptoms and signs, in leading to a higher remission rate, and in ameliorating disability in PSD. Reduction of disability in the early part of the trial mediated later improvement in depressive symptomatology. Similarly, reduction in depressive symptoms and signs early on mediated later improvement in disability.
Conclusion
These encouraging findings require replication. Beyond its potential direct benefits in PSD, EFT may provide an appropriate context for efficient and timely administration of pharmacotherapy and of physical, speech, and occupational therapy thus maximizing their efficacy.
Keywords: Post stroke depression, ecosystem focused therapy
INTRODUCTION
Prolonged life span has increased the number of persons living with disability. Many such patients suffer from depression, which worsens their outcomes, undermines treatment adherence, and compromises their care (Lebowitz, et al. 1997). Stroke exemplifies the problems of aging adults living with disability occurring after an acute medical event and can serve as a model for development of interventions addressing their need (Alexopoulos, et al. 2008).
Stroke afflicts 700,000 Americans each year (Thom, et al. 2006). Approximately, 22% of stroke patients treated in acute and rehabilitation hospitals have major depression and 17% have minor depression (Robinson, et al. 2008). Disability, personal and family history of a psychiatric disorder, and high overall medical burden are risk factors of post-stroke depression (PSD) (Leentjens, et al. 2006; Robinson and Spalletta 2010; Tenev, et al. 2009).
Depression can devastate stroke survivors. PSD afflicts patients with significant stroke induced disability and cognitive impairment (Hackett and Anderson 2005). PSD patients are more likely to have impairments in executive functions, problem solving, psychomotor speed, attention, and memory than non-depressed stroke patients (Kauhanen, et al. 1999). Depression is a predictor of cognitive impairment for several years after stroke, especially in those with left-sided lesions (Downhill and Robinson 1994).
The currently available treatments leave many PSD patients depressed, disabled, and suffering. Antidepressants were found effective in established PSD (Robinson and Spalletta 2010) and may even prevent development of PSD in stroke victims (Robinson et al. 2008). In addition to reducing depressive symptoms, antidepressants may reduce mortality (Jorge, et al. 2003), motor impairment(Chollet, et al. 2011) and disability (Robinson 2003). Despite these encouraging findings, a Cochrane meta-analysis concluded that antidepressants are only weakly efficacious in PSD (Hackett, et al. 2008) suggesting that, while some patients improve, many derive limited, if any, benefit. A recent analysis of a large cohort of patients with incident stroke showed that antidepressant use was associated with 48% greater risk for ischemic or hemorrhagic stroke (Wu, et al. 2011). This finding is consistent with the results of the Women’s Health Initiative (Smoller, et al. 2009) and other studies (Chen, et al. 2008; Chen, et al. 2009). Intolerance of antidepressants by some stroke victims, inadequate dosages, and poor treatment adherence may further reduce the impact of antidepressants in PSD care. Much less research has been done in psychosocial interventions. A recent meta-analysis concluded that there is no convincing evidence of psychotherapy efficacy in PSD (Hackett et al. 2008). The limited efficacy of psychosocial interventions may be due to the fact that few, if any, have targeted the constellation of major contributors to PSD systematically.
PSD is unique in that stroke and depression and the resultant disability occur abruptly, often have catastrophic consequences and find the patient and his/her family unprepared. The intervention of this study focuses on the “psychosocial storm”, originating both from the patient’s sudden disability and the resultant change in patient’s needs and family life.
The impact of the “psychosocial storm” depends on the interaction between the severity of the patient’s clinical state and the strengths of his/her “ecosystem”. The patient becomes deskilled by the abrupt loss of strength, coordination, language, executive functions, behavioral disorganization, lack of motivation, fear for their life, and hopelessness caused by stroke and PSD, but also by the tasks of a demanding rehabilitation. These factors combined with lack of readiness often lead to a feeling of incompetence and helplessness (self-efficacy) fueling the experience of stress and promoting depression (Areán, et al. 2008; Ashby, et al. 1999; Kraaij, et al. 2002; Moos, et al. 2006).
The family of the PSD patient experiences a similar upheaval. Most family goals and tasks suddenly become anachronistic. The family needs to reengineer itself and learn how to meet the new demands of the disabled stroke victim. The patient may need assistance both with daily routine and with facilitating (e.g. driving, waiting) physical, occupational, and speech therapies. Family members play a role even when they do not live with the PSD patient (coordination of caregivers, finances, relocation). The pessimism and resignation of the PSD patient often becomes contagious and immobilizes those expected to help. Without guidance, even committed family members may be unable to help effectively and become a hindrance to the patients’ recovery. Finally, physical, occupational, and speech therapists need to understand the psychosocial context of the PSD patient and his/her “ecosystem” and coordinate their interventions (dose, timing, barriers) so that they are not excessively demanding and disorienting to patients and families.
We developed Ecosystem Focused Therapy (EFT), an intervention that targets the “psychosocial storm” experienced by the PSD patient and his/her family through five integrated components: 1) It offers to patients and families an action-oriented, “new perspective” about recovery and the new physical state; 2) it helps patients to form a treatment “adherence enhancement structure”; 3) it provides a “problem solving structure” to the patient focusing on solvable problems, valued by the patient, and pertinent to daily function; 4) it helps the family “reengineer its goals, involvement, and plans” to accommodate the patient’s disability and its impact on the family (e.g. finances, time commitment); and 5) it “coordinates care with specialized therapists” to arrive at a synergistic approach increasing patient participation in treatment and rehabilitation and utilization of community resources.
This paper is a preliminary report on the efficacy of EFT in reducing depression and disability in PSD patients. The comparison condition was a manualized intervention consisting of Education on Stroke and Depression (ESD). We examined whether EFT is more efficacious than ESD in reducing depressive symptoms and signs as well as disability. We explored whether improvement of disability mediates the differential efficacy of EFT and ESD in depression. We also explored whether improvement in depressive symptoms and signs mediates improvement in disability.
METHODS
Patients consecutively admitted to a rehabilitation hospital for treatment after stoke were screened for depression using the Patient Health Questionnaire (PHQ-9) (Kroenke, et al. 2001). Those with a PHQ-9 score of 10 of greater were invited to sign informed consent approved by the Institutional Review Board of Weill-Cornell Medical College and Burke Rehabilitation Center. They were include in this study if they were aged 60 years or older and had an ischemic, embolic or hemorrhagic stroke, and a diagnosis of unipolar major depression by DSM-IV. Exclusion criteria were moderately severe dementia (MiniMental Examination (MMSE) score < 20), greater than moderate aphasia (NIH Stroke Scale: Best Language > 1), expectation to be discharged to a nursing home, psychotic depression (by DSM-IV), suicidal intent or plan, and inability to speak English. Use of antidepressants or rehabilitation treatment for stroke related disability were not exclusion criteria.
Diagnostic evaluation was conducted with the SCID-R (First 1995) and an in person evaluation by clinician investigators. Severity of depression was quantified with the Hamilton Depression Rating Scale (HAM-D)(Hamilton 1960). Disability was rated with the World Health Organization Disability Assessment Schedule II (WHODAS-II)(Epping-Jordan and Ustun 2000). This instrument assesses six domains of function: 1) Understanding and communicating; 2)getting around; 3) self-care; 4) getting along with others; 5) household and work activities; and 6) participation in society. Impact on quality of life related to stroke was evaluated with the Stroke Impact Scale (Duncan, et al. 1999). Overall cognitive impairment was assessed with the Dementia Rating Scale (Mattis 1989). Memory was evaluated with the Hopkins Verbal Learning Test (Brandt 1991). Response inhibition was tested with the Stroop Color Word Test(Golden 1978) and semantic fluency with the Animal Naming Test (Goodglass and Kaplan 1983). The HAM-D and WHODAS were administered weekly after the initial evaluation during the 12-week study.
The subjects were randomly assigned to EFT or ESD using random numbers. Twelve weekly EFT or ESD sessions of approximately 45 minute duration were offered. The first session was held at the Rehabilitation Hospital; inpatients had their first session one or two days prior to discharge. All subsequent sessions were conducted at the subjects’ homes.
The principles and components of EFT were outlined in the Introduction. Briefly1, the EFT therapist describes the prognosis of depression, its interaction with disability, the role of rehabilitation, and valued and rewarding activities that are still possible, and corrects patient misconceptions. EFT uses education and direct suggestions for developing an adherence enhancement structure taking into consideration the patients’ cognitive and behavioral limitations. The family and/or professional caregivers participate based on need. The EFT therapist provides training in problem solving, helping patients select as an initial target a solvable problem that they value and is pertinent to his/her current daily functioning. EFT helps the family reengineer its goals and plans to accommodate the patient’s disability and its impact on the family. The EFT therapist works with specialized therapists to arrive to a synergistic approach to motivate the patient, and to help the patient and family develop a plan for participation in rehabilitation and make use of community resources, e.g. support groups, exercise programs and recreational services.
Not all PSD patients need all EFT components equally at all times. Each patient and ecosystem has different strengths and limitations at the outset. Moreover, the changing needs of the PSD patient and his/her “ecosystem” as the clinical state changes requires retargeting of the EFT components. For this reason, each EFT session begins with structured questions about persisting and/or emerging problems, thus guiding the therapist to utilize the most pertinent EFT components in each session.
ESD, the comparison condition to EFT, was designed as a treatment with “active therapeutic ingredients” and limited overlap with EFT. ESD is home-delivered and imparts education about depression, stroke, and the role of available treatments, thus, mimicking what a good clinician does in educating the PSD patient and family in a structured way. Comprehending illness-related information is a process contaminated by pessimism, denial, misconceptions, and stigma. For this reason, each ESD session begins by assessing “where the PSD patient and family” are and consists of a discussion and education material (printed or web-based) for which they have readiness to accept. ESD therapists do not engage in other interventions, e.g. linking mood changes to life events, weighing interpersonal options, role playing, focusing on dysfunctional thoughts, behavioral homework, or interpreting dreams or transference. ESD sessions are of similar duration to EFT sessions, thus controlling for therapist exposure.
Four therapists were trained and offered both EFT and ESD to the subjects of this study. Training consisted of reading and discussing the Manuals with the rest of the research team and supervised treatment of three practice cases of EFT and three cases of ESD. All EFT and ESD sessions were audiotaped, including those of practice cases and of the subjects of the treatment trial. Reviewers, who were not members of the research team, rated 20 EFT and 20 ESD sessions using the EFT and ESD Fidelity Scales (5 grades: 1=poor, 5=excellent). The mean rating of the EFT Rating Scale were: 4.4 for Perspective; 4.5 for Adherence Enhancement Structure; 4.4 for problem solving structure; 4.2 for Reengineering of Goals and Plans 4.2; 4.0 for Care Coordination; and 4.4 for the Global Rating. The mean ratings of the ESD Fidelity Scale were: 4.7 for Introducing ESD; 4.6 for Assessment of Educational Needs; 4.9 for Coverage of Stroke Topics; and 4.8 for the Global Rating.
Comparisons between the two treatment arms were based on intent to treat analysis. Baseline demographic and clinical characteristics of subjects of the EFT and ESD arms were compared using the Mann-Whitney test for continuous variables and chi square for dichotomous variables. The trajectories of depressive symptoms and signs (HAM-D) and of disability (WHODAS) during the 12 week trial were compared with mixed effects linear models. Odds Ratio was used to compare remission rates between treatment arms. Exploratory mediation analysis was conducted using a fixed effects regression model to assess the relationship between change in the mediator and subsequent change in outcome variables and compare this relationship between the two treatments.
RESULTS
A total of 174 stroke patients were screened and 54 had a PHQ-9 score equal to or greater than 10. Of the 54 subjects, 31 had major depression and 24 met study criteria and agreed to participate. They were aged 70.9 years (SD: 8.5) with had significant impairment in all Stroke Impact Domains and their WHODAS scores were consistent with moderately severe disability (Table 1). In 67% of participants, the index episode was their first episode and the severity of depression was within the mild to moderate range. There were no statistically significant differences between EFT and ESD treated subjects in demographic characteristics, age of depression onset, history of depressive severity of depression, quality of life (Stroke Impact Scale Domains), disability (WHODAS Domains), and cognitive test performance at baseline. An exception was the Stroop Color Word, a test of response inhibition, in which the EFT group performed worse than the ESD group (Table 1). Abnormal performance in the Stroop Color Word test has been associated with poor response of geriatric depression to citalopram(Alexopoulos, et al. 2005; Alexopoulos, et al. 2004) but not to problem solving therapy (Alexopoulos, et al. 2011; Arean, et al. 2010). Seven of the 12 subjects of the EFT arm and 5 of the 12 subjects of the ESD arm were treated with selective serotonin reuptake inhibitors (Fisher’s Exact Test p=0.46).
Table 1.
Demographic and Clinical Characteristics of Patients with Post Stroke Depression Treated with Ecosystem Focused Therapy (EFT) or Education on Stroke and Depression (ESD)
| ESD (N=12) | EFT (N=12) | Mann Whitney U | ||
|---|---|---|---|---|
|
|
||||
| DEMOGRAPHICS | Mean (SD) | Mean (SD) | z | p |
|
| ||||
| Gender | 58.3% Male | 50% Male | χ2=0.17, df=1, p=0.68 | |
|
|
||||
| Age | 69.4(9.58) | 72.3(7.44) | −0.87 | 0.39 |
|
|
||||
| Education | 15.4(2.84) | 14.3(3.25) | −0.74 | 0.46 |
|
|
||||
| DEPRESSION | ||||
|
| ||||
| Hamilton Depression Rating Scale1 | 22.3(6.89) | 20.4(9.19) | −1.11 | 0.27 |
|
|
||||
| Age of Onset | 57.8(16) | 63.6(14.9) | −1.14 | 0.26 |
|
|
||||
| Number of Episodes | 1.3(0.65) | 1.45(0.68) | −0.53 | 0.59 |
|
|
||||
| DISABILITY | ||||
|
| ||||
| WHODAS-II2 | ||||
|
|
||||
| Total | 32.7 (7.6) | 35.2(8.9) | −0.69 | 0.49 |
|
|
||||
| Life Activities | 3.1 (1.3) | 3.0(1.3) | −0.36 | 0.72 |
|
|
||||
| Getting Around | 6.8 (2.3) | 7.9 (2.9) | 1.3 | 0.19 |
|
|
||||
| Self-Care | 5.7 (2.6) | 6.2 (2.9) | 0.41 | 0.68 |
|
|
||||
| Getting Along with Others | 3.8 (1.5) | 3.8 (2.3) | −0.24 | 0.81 |
|
|
||||
| Understanding/Communicating | 4.4 (1.4) | 5.2(2.1) | 0.65 | 0.52 |
|
|
||||
| Work Activities | 2.2 (1.0) | 2.5 (1.4) | 0.54 | 0.59 |
|
|
||||
| Participation in Society | 6.8 (2.0) | 6.7(1.8) | −0.35 | 0.73 |
|
|
||||
| COGNITIVE FUNCTION | ||||
|
| ||||
| Mattis Dementia Rating Scale | ||||
|
|
||||
| Total Score | 129(3.7) | 118(19.0) | −0.71 | 0.48 |
|
|
||||
| Initiation/Perseveration | 29.5(3.8) | 29.5(7.2) | −0.15 | 0.89 |
|
|
||||
| Hopkins Verbal Learning Test – Revised | ||||
|
|
||||
| Immediate Recall | 17.3(4.36) | 16.3(5.76) | −0.15 | 0.88 |
|
|
||||
| Delayed Recall | 4.27(2.83) | 3.66(2.44) | −0.47 | 0.64 |
|
|
||||
| Animal Naming Test | 15(4.35) | 10.5(4.89) | −1.83 | 0.07 |
|
|
||||
| Stroop Color Word | 31.3 (8.2) | 14.6 (10.8) | −2.1 | 0.04 |
|
|
||||
| QUALITY OF LIFE | ||||
|
| ||||
| Stroke Impact Scale | ||||
|
|
||||
| Physical Problems | 50.6(18.7) | 50.9(18.5) | −0.36 | 0.72 |
|
|
||||
| Memory & Thinking | 75.5(15.1) | 81.5(15.8) | −0.87 | 0.38 |
|
|
||||
| Mood & Emotional Control | 56.0(18.6) | 63.1(24.9) | −1.19 | 0.27 |
|
|
||||
| Communication | 85.1(14.5) | 83.7(15.0) | −0.13 | 0.90 |
|
|
||||
| Typical Day Activities | 44.7(26.9) | 47.4(17.7) | −0.40 | 0.69 |
|
|
||||
| Mobility | 65.7(24.4) | 58.3(23.3) | −0.74 | 0.46 |
|
|
||||
| Hand Function | 30.4(39.5) | 35.9(34.9) | −0.41 | 0.68 |
|
|
||||
| ADLS/IADL | 59.7(19.2) | 60(20.0) | −0.15 | 0.88 |
|
|
||||
| Strength | 50.5(17.9) | 49.4(25.0) | −0.65 | 0.52 |
|
|
||||
| Overall Recovery | 48.7(26.5) | 54.0(19.2) | −0.47 | 0.64 |
24-item Total Score
World Health Organization Disability Assessment Schedule- II
The treatment and assessment procedures were well accepted by the subjects. In the EFT arm, two EFT subjects died (during the 7th and the 9th week of treatment respectively) and one subject was lost to follow-up after the 10th week of treatment. In the ESD arm, one subject decided to discontinue therapy after the 3rd week of treatment.
Efficacy of EFT vs. ESD in Reducing Depressive Symptoms and Signs
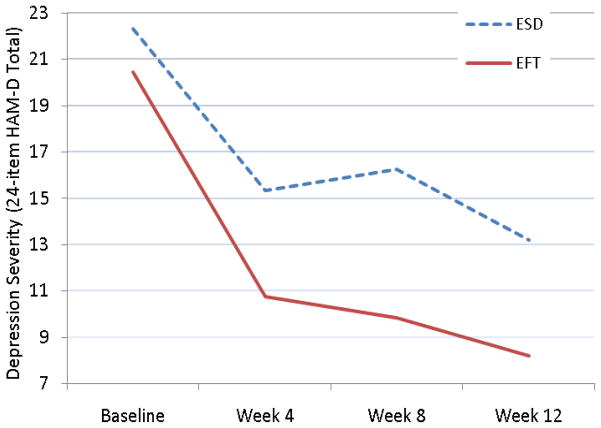
A mixed effects linear model showed an incremental contribution of the treatment by time interaction to greater decline in depressive symptoms and signs over time for EFT over ESD (LR χ2=3.7, df=1, p=.054) (Figure 1). At week 12, the observed mean Ham-D of EFT-treated participants was 8.2 (SD: 6.63) and of ESD participants 13.2 (SD: 5.37). The standardized between group effect size at week 12 was 0.83 (95% CI: −0.07–1.72).
Figure 1.
Trajectory of Depressive Symptoms in Subjects with Post-Stroke Major Depression Receiving Ecosystem Focused Therapy (EFT; N=12) or Education on Stroke and Depression (ESD; N=12)
HAM-D: Hamilton Depression Rating Scale
In the EFT group, 8 of the 12 (66.7%) subjects achieved remission of depression (HAM-D<10), while 2 of the 12 subjects (16.7%) remitted in the ESD group (Odds Ratio = 10; 95% CI = 1.44–69.26). The number needed to treat (NNT) was 2, i.e. 1 more remission occurred for every 2 patients receiving EFT rather than ESD.
To explore whether change in disability mediates improvement in depressive symptoms, we examined whether change in WHODAS scores between baseline and week 8 (mediator) was associated with improvement of HAM-D scores in subsequent weeks (from week 8 to 12). A fixed effects linear regression model showed that the association between WHODAS change and subsequent HAM-D change varied across intervention groups (R2=0.283) suggesting that greater WHODAS improvement (week 0 to 8) is associated with greater HAM-D improvement (week 8 to 12) in EFT but not ESD treated patients.
Efficacy of EFT vs. ESD in Reducing Disability
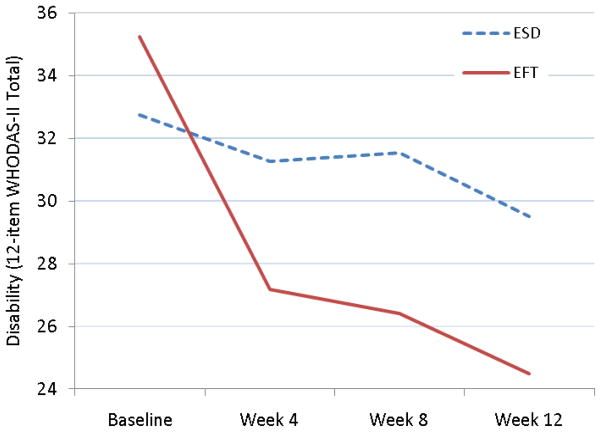
A mixed-effects linear model showed that the treatment by time interaction was associated with faster improvement in disability in EFT subjects (LR χ2=5.9, df=1, p=.015) (Figure 2). At week 12, the observed WHODAS mean of EFT-treated participants was 24.5 (SD: 8.54) and of ESD participants 29.5 (SD: 10.16). The standardized between group effect size at week 12 was 0.53 (95% CI: −0.36 to 1.43).
Figure 2.
Trajectory of Disability in Subjects with Post-Stroke Major Depression Receiving Ecosystem Focused Therapy (EFT; N=12) or Education on Stroke and Depression (ESD; N=12)
WHODAS-II: World Health Organization Disability Assessment Schedule-II
To examine whether change in depressive symptoms and signs mediates improvement in disability, we examined whether change in HAM-D scores from baseline to week 8 (mediator) was associated with reduction in WHODAS scores in subsequent weeks (week 8 to 12). A linear regression model, showed that the association between HAM-D change and subsequent WHODAS change varied across intervention groups (R2=0.279) suggesting that greater HAM-D decline is followed by greater WHODAS reduction in EFT but not ESD treated patients.
DISCUSSION
This preliminary study suggests that EFT is more efficacious than ESD in reducing depressive symptoms and signs, in leading to a higher remission rate, and in ameliorating disability in PSD. The benefits of EFT were above and beyond those of community-based clinical care since patients of both arms received unrestricted controlled care by their physicians and the comparison condition consisted of information on depression, stroke and their treatment. Reduction of disability in the early part of the trial mediated later improvement in depressive symptomatology. Similarly, reduction in depressive symptoms and signs early on mediated later improvement in disability. Thus, changes in the course of depression and of disability of PSD patients appear to be intertwined.
Disability is the single strongest correlate of PSD (Robinson and Spalletta 2010). Depression occurring soon after stroke is a predictor of greater functional impairment during follow-up, ranging from 6 weeks to 2 years in 83% of studies (Parikh, et al. 1990; Robinson and Spalletta 2010). Disability in PSD patients is associated with poor quality of life, social support, physical functioning, self-esteem, perceived control and pessimism in stroke patients (Raju, et al. 2010; Teoh, et al. 2009). However, remission of PSD over the first few months after stroke results in greater improvement in activities of daily living (Bilge, et al. 2008; Chemerinski, et al. 2001).
The beneficial effect of EFT on depression and disability is consistent with its intent. EFT is designed to help PSD patients develop a new perspective and adaptation skills and change their “ecosystem” (family, specialized therapists) so as to accommodate the patients’ new state. It is based on the “model of adaptive functioning”, in which adaptive behavior is a function of the person’s competence as well as the demands of the environment (Lawton 1982). Accordingly, the EFT therapist works with the patient and the “ecosystem” to set goals above the current level of performance but within reach and continuously “calibrate the environment” to the PSD patient’s competence level. Thus, EFT can benefit the PSD patient directly by increasing “behavioral activation” (engagement in valued and rewarding activities) and “self-efficacy” (sense of competence and empowerment). In addition, EFT may have indirect benefits by enhancing adherence to rehabilitation and other treatments and thus reducing both depression and disability. The putative mechanisms of rehabilitation therapies after stroke include increase of brain plasticity resulting in a new functional architecture (Ward 2005a). Thus, behavioral enrichment may help recovery of function directly and by enhancing the effects of specific types of rehabilitative therapies (Ward 2005b).
The principal limitation of this study is its small number of subjects, permitting only tentative conclusions. Another limitation is that the raters could not be blinded to the treatment condition although they were unaware of the study hypotheses. Finally, the study used the same therapists to deliver EFT and ESD. They received similar training in each treatment, were certified based on high standards, and their sessions were audiotaped and rated for adherence to the manuals of EFT and ESD. Despite these precautions, greater therapist allegiance to one of the two treatments may have influenced their performance. For these reasons, replication is necessary.
In summary, this study provides preliminary evidence that EFT reduces both depressive symptoms and signs and disability in PSD. Beyond its direct benefits, providing an ecosystem-based context for a timely administration of pharmacotherapy and of physical, speech, and occupational therapy can maximize their efficacy.
Acknowledgments
Grant support: P30 MH085943 and by the Sanchez Foundation
The authors thank Tim Clark, MA, Dorothy Tagarelli, LMSW, and Sophie Rutimann, MSed, for their help in the conduct of this study.
Footnotes
The Ecosystem Focused Treatment (EFT) Manual is available upon request gsalexop@med.cornell.edu
ClinicalTrials.gov Identifier: NCT00944762
Disclosures: Dr. Alexopoulos received grant support from Forest Pharmaceuticals and has been a member of speakers’ bureaus of Forest, Lilly, Bristol Meyers Squibb, Merck, Astra Zeneca, Avanir, and Novartis. He holds equity of Johnson and Johnson. No other authors report conflicts of interests.
References
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Murphy C, Heo M. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29:2278–2284. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Raue PJ, Kiosses DN, Mackin RS, Kanellopoulos D, McCulloch C, Arean PA. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Arch Gen Psychiatry. 2011;68:33–41. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Raue PJ, Sirey JA, Arean PA. Developing an intervention for depressed, chronically medically ill elders: a model from COPD. Int J Geriatr Psychiatry. 2008;23:447–453. doi: 10.1002/gps.1925. [DOI] [PubMed] [Google Scholar]
- Areán P, Alexopoulos G, Chu J. Cognitive Behavioral Case Management for Depressed Low-income Older Adults. In: Gallagher-Thompson D, Steffen A, Thompson L, editors. Handbook of Behavioral and Cognitive Therapies with Older Adults. New York: Springer; 2008. pp. 219–232. [Google Scholar]
- Arean PA, Raue P, Mackin RS, Kanellopoulos D, McCulloch C, Alexopoulos GS. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. Am J Psychiatry. 2010;167:1391–1398. doi: 10.1176/appi.ajp.2010.09091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Bilge C, Kocer E, Kocer A, Turk Boru U. Depression and functional outcome after stroke: the effect of antidepressant therapy on functional recovery. Eur J Phys Rehabil Med. 2008;44:13–18. [PubMed] [Google Scholar]
- Brandt J. Clinical Neuropsychologist. Swets & Zeitlinger; 1991. The Hopkins verbal learning test: development of a new memory test with six equivalent forms; pp. 125–142. [Google Scholar]
- Chemerinski E, Robinson RG, Kosier JT. Improved recovery in activities of daily living associated with remission of poststroke depression. Stroke. 2001;32:113–117. doi: 10.1161/01.str.32.1.113. [DOI] [PubMed] [Google Scholar]
- Chen Y, Guo JJ, Li H, Wulsin L, Patel NC. Risk of cerebrovascular events associated with antidepressant use in patients with depression: a population-based, nested case-control study. Ann Pharmacother. 2008;42:177–184. doi: 10.1345/aph.1K369. [DOI] [PubMed] [Google Scholar]
- Chen Y, Guo JJ, Patel NC. Hemorrhagic stroke associated with antidepressant use in patients with depression: does degree of serotonin reuptake inhibition matter? Pharmacoepidemiol Drug Saf. 2009;18:196–202. doi: 10.1002/pds.1699. [DOI] [PubMed] [Google Scholar]
- Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, Bejot Y, Deltour S, Jaillard A, Niclot P, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- Downhill JE, Jr, Robinson RG. Longitudinal assessment of depression and cognitive impairment following stroke. J Nerv Ment Dis. 1994;182:425–431. doi: 10.1097/00005053-199408000-00001. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan JA, Ustun TB. The WHODAS-II: leveling the playing field for all disorders. WHO Mental Health Bulletin. 2000;6:5–6. [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured clinical interview for DSM-IV - patient version (SCID-P) Washington: American Psychiatric Press; 1995. [Google Scholar]
- Golden CJ. The Stroop Color and Word Test (Manual) Chicago: Stoetling; 1978. [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Hackett ML, Anderson CS. Predictors of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:2296–2301. doi: 10.1161/01.STR.0000183622.75135.a4. [DOI] [PubMed] [Google Scholar]
- Hackett ML, Anderson CS, House A, Xia J. Interventions for treating depression after stroke. Cochrane Database Syst Rev. 2008:1–89. doi: 10.1002/14651858.CD003437.pub3. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge RE, Robinson RG, Arndt S, Starkstein S. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160:1823–1829. doi: 10.1176/appi.ajp.160.10.1823. [DOI] [PubMed] [Google Scholar]
- Kauhanen M, Korpelainen JT, Hiltunen P, Brusin E, Mononen H, Maatta R, Nieminen P, Sotaniemi KA, Myllyla VV. Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke. 1999;30:1875–1880. doi: 10.1161/01.str.30.9.1875. [DOI] [PubMed] [Google Scholar]
- Kraaij V, Arensman E, Spinhoven P. Negative life events and depression in elderly persons: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2002;57:P87–94. doi: 10.1093/geronb/57.1.p87. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP. Competence, environmental press, and the adaptation of older people. In: Lawton MP, Windley PG, Byerts TO, editors. Aging and the Environments. New York: Springer; 1982. [Google Scholar]
- Lebowitz BD, Pearson JL, Schneider LS, Reynolds CF, 3rd, Alexopoulos GS, Bruce ML, Conwell Y, Katz IR, Meyers BS, Morrison MF, et al. Diagnosis and treatment of depression in late life. Consensus statement update. JAMA. 1997;278:1186–1190. [PubMed] [Google Scholar]
- Leentjens AF, Aben I, Lodder J, Verhey FR. General and disease-specific risk factors for depression after ischemic stroke: a two-step Cox regression analysis. Int Psychogeriatr. 2006;18:739–748. doi: 10.1017/S1041610206003486. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale. Odessa: Psychological Assessment Resources; 1989. [Google Scholar]
- Moos RH, Brennan PL, Schutte KK, Moos BS. Older adults’ coping with negative life events: common processes of managing health, interpersonal, and financial/work stressors. Int J Aging Hum Dev. 2006;62:39–59. doi: 10.2190/ENLH-WAA2-AX8J-WRT1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh RM, Robinson RG, Lipsey JR, Starkstein SE, Fedoroff JP, Price TR. The impact of post-stroke deprssion on recovery in activities of daily living over two year follow-up. Arch Neurol. 1990;47:785–789. doi: 10.1001/archneur.1990.00530070083014. [DOI] [PubMed] [Google Scholar]
- Raju RS, Sarma PS, Pandian JD. Psychosocial problems, quality of life, and functional independence among Indian stroke survivors. Stroke. 2010;41:2932–2937. doi: 10.1161/STROKEAHA.110.596817. [DOI] [PubMed] [Google Scholar]
- Robinson RG. Poststroke depression: prevalence, diagnosis, treatment, and disease progression. Biol Psychiatry. 2003;54:376–387. doi: 10.1016/s0006-3223(03)00423-2. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Jorge RE, Moser DJ, Acion L, Solodkin A, Small SL, Fonzetti P, Hegel M, Arndt S. Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2008;299:2391–2400. doi: 10.1001/jama.299.20.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RG, Spalletta G. Poststroke depression: a review. Can J Psychiatry. 2010;55:341–349. doi: 10.1177/070674371005500602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Allison M, Cochrane BB, Curb JD, Perlis RH, Robinson JG, Rosal MC, Wenger NK, Wassertheil-Smoller S. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative study. Arch Intern Med. 2009;169:2128–2139. doi: 10.1001/archinternmed.2009.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenev VT, Robinson RG, Jorge RE. Is family history of depression a risk factor for poststroke depression? Meta-analysis. Am J Geriatr Psychiatry. 2009;17:276–280. doi: 10.1097/JGP.0b013e3181953b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh V, Sims J, Milgrom J. Psychosocial predictors of quality of life in a sample of community-dwelling stroke survivors: a longitudinal study. Topics in stroke rehabilitation. 2009;16:157–166. doi: 10.1310/tsr1602-157. [DOI] [PubMed] [Google Scholar]
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Ward NS. Neural plasticity and recovery of function. Prog Brain Res. 2005a;150:527–535. doi: 10.1016/S0079-6123(05)50036-0. [DOI] [PubMed] [Google Scholar]
- Ward NS. Plasticity and the functional reorganization of the human brain. Int J Psychophysiol. 2005b;58:158–161. doi: 10.1016/j.ijpsycho.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Wu CS, Wang SC, Cheng YC, Gau SS. Association of cerebrovascular events with antidepressant use: a case-crossover study. Am J Psychiatry. 2011;168:511–521. doi: 10.1176/appi.ajp.2010.10071064. [DOI] [PubMed] [Google Scholar]