Abstract
Pharmacotherapy trials for treating tobacco dependence would benefit from behavioral interventions providing treatment consistent with clinical practice guidelines but not directing participants to treatments not evaluated in the trial. The Smoke Free and Living It© behavioral intervention manual includes participant and interventionist guides and is designed to provide both practical counseling and intra-treatment support. We utilized this intervention manual in a multicenter, randomized clinical trial of smokers with attention deficit hyperactivity disorder. In this study, we evaluated how the interventional manual performed in a “train-the-trainer” model requiring uniform counseling across 6 sites and 15 interventionists. We analyzed the skill-adherence of the interventionists and the intervention-adherence of the participants. The 255 randomized participants completed 9.3 ± 2.8 sessions (mean ± SD), with 157 participants (61.6%) completing all 11 of the sessions and 221 (86.7%) completing at least 6 of the 11 sessions. Of the 163 sessions for which the study interventionists were evaluated, 156 (95.7%) were rated as adherent to protocol and “meeting expectations” on at least 6 of 7 established criteria, illustrating that fidelity can be maintained with minimal supervision. The self-help and interventionists guides of the Smoke Free and Living It manual can thus be used to provide behavioral intervention with a high rate of adherence by both the interventionists and the participants. This manual meets the requirements of the United States Public Health Service Clinical Practice Guideline, can be adapted to specific research protocols, and provides a useful option for behavioral intervention during clinical trials for smoking cessation.
Keywords: behavioral intervention, USPHS Practice Guideline, treating tobacco dependence, clinical trials
1. Introduction
The World Health Organization [1] and the U.S. Public Health Service (USPHS) Clinical Practice Guideline [2] recommend that interventions for smokers include both behavioral and pharmacologic therapy because the combination results in higher tobacco abstinence rates [2, 3]. Behavioral interventions include motivational support, advice and guidance, and counseling. They range in intensity from self-help materials to individual cognitive-behavior therapy offered through specialized clinics [3], such as intensive residential programs [4, 5]. Behavioral interventions instruct individuals how to recognize high-risk smoking situations, develop alternative coping strategies, manage stress, improve problem-solving skills, and increase social support. Ideally, the intervention is tailored to a person’s situation because this increases the chances for success [6].
Numerous randomized, placebo-controlled clinical trials testing pharmacotherapies for smokers have used self-help pamphlets for the behavioral intervention [7]. One of the most widely used pamphlets is Clearing the Air [8], a comprehensive self-help pamphlet designed by the National Cancer Institute. This pamphlet provide smokers with information about available intervention options, including information on all available medications and national clinical programs (e.g., tobacco quit lines and web sites), as well as counseling topics which are evidence based recommendations. Although initially developed for use in a clinical setting, this pamphlet has been used to provide brief counseling intervention in clinical trials. However, in a randomized clinical trial, the participants’ use of medications or programs outside of the study protocol could introduce an uncontrolled bias and confound the results. Additional bias could be introduced in multisite trials if the intensity of the behavioral intervention differed between sites, potentially influencing both smoking abstinence and retention rates.
To address this, the Mayo Clinic Nicotine Research Program (NRP) developed the Smoke Free and Living It© manual [9], to be used in clinical trials to provide the brief counseling intervention. This has been used extensively in clinical trials [10-20]. The manual does not address the use of medications nor does it give information concerning treatment programs. It is left to the discretion of the site investigators whether to offer these other options to study participants at their last study visit. To help multiple sites deliver a consistent message, Smoke Free and Living It contains a guide for the study interventionists as well as a participant self-help guide. The participant guide is given to participants after they are randomized to treatment conditions. At each counseling visit, interventionists use key points from the interventionist’s guide as the basis for a 10-minute discussion of a single topic. The guide, along with uniform training and monitoring of the behavioral intervention delivery, can help reduce any bias introduced by differences in behavioral intervention, while meeting the USPHS Clinical Practice Guideline.
In order to determine whether the Mayo Clinic Smoke Free and Living It manual can be used properly and consistently in a “train-the-trainer” model of intervention standardization, we analyzed data from a multicenter, placebo-controlled study of smokers with attention deficit hyperactivity disorder (ADHD) that evaluated osmotic-release methylphenidate (OROS-MPH) or placebo for the treatment of ADHD in conjunction with nicotine patches to help with stopping smoking. Site interventionists were trained on the use of the manual through a train-the-trainer approach, and their skill-adherence to the required procedure was monitored throughout the study. Participant adherence with the behavioral interventions was also assessed.
2. Methods
2.1 Study design
The study was conducted by the National Institute on Drug Abuse Clinical Trials Network (CTN 0029) between December 2005 and January 2008. Participants were recruited at 6 study sites, located in Cambridge, Massachusetts; Columbus, Ohio; New York City, New York (2 sites); Portland, Oregon; and Rochester, Minnesota, and 255 current smokers with ADHD were enrolled. The 6 sites represented 3 areas of focus (2 were smoking research facilities; 2 were ADHD research facilities; and 2 were mental health research facilities)[21].
Once a participant was determined to be eligible for study participation, s/he was randomized to receive either OROS-MPH or matching placebo. After a 2-week ramp-up period, the participants continued to take the maximum dose of 72 mg/day OROS-MPH for the subsequent 9 weeks. The target smoking quit day was midway between week 4 and week 5 (Day 27). On that day, participants began using 21-mg nicotine patches. They continued using the patches and OROS-MPH at these doses until week 12. At week 12, the OROS-MPH was stopped and nicotine patch tapering began: 14-mg nicotine patches (one patch per day) were used for weeks 12 and 13, and 7-mg patches were used for the final week (week 14). The study design, methods, and results have previously been reported [18].
Study participation consisted of weekly clinic visits during the 11-week active treatment phase. During each of these 11 clinic visits, participants underwent a brief counseling session (5-10 minutes in length) in addition to other clinical trial procedures. Topics discussed during these counseling sessions consisted of a mix of problem-solving skills (e.g., “day before quit day/making not smoking easier,” “triggers/withdrawal,” “managing stress,” “weight and exercise,” “time management”) and support (e.g., “benefits of quitting smoking,” “rewarding yourself for not smoking,” “self image”) (see Table 1).
Table 1.
Timeline for administering Intervention modules
| Study week |
Intervention Discussion module |
Administered by |
|---|---|---|
| 1 | Nicotine Addiction & Congratulatory Message by Physician | Physician |
| 2 | Benefits of Quitting Smoking | Interventionist |
| 3 | Rewarding Yourself for not Smoking | Interventionist |
| 4 | Day Before Quit Day & Making not Smoking Easier | Interventionist |
| 5-9a | Triggers/Withdrawal | Interventionist |
| Managing Stress | Interventionist | |
| Weight/Exercise | Interventionist | |
| Self Image | Interventionist | |
| Time Management | Interventionist | |
| 10 | Maintenance | Interventionist |
| 11 | Stopping Study Medication & Focus on the Future | Interventionist |
These modules were completed in the order listed or rearranged to best meet the needs of a given participant.
2.2. Smoke Free and Living It
Since 1998, the Smoke Free and Living It intervention manual has been used in clinical trials involving over 7500 smokers. The manual serves both as a self-help guide and as the basis for brief counseling based on the USPHS Clinical Practice Guideline. The topics covered in the manual were developed from issues identified by past smokers who were trying to quit smoking through the Mayo Clinic Nicotine Dependence Center (Treatment and Research Programs). The topics included in this manual, which are designed as brief interventions (duration not to exceed 10 minutes), consist of both problem-solving skills and support topics. The manual is designed to be formatted for each individual study protocol; therefore, the number of chapters (i.e., topics) is equal to the number of visits that include a behavioral intervention. The topics are updated for each study to reflect the most current clinical updates and guidelines. An interventionist guide accompanies the patient manual to assist the interventionist in the delivery of each counseling topic. This assures that all intervention topics are covered in a consistent manner. Key points are listed for each topic, and discussion points are available as time allows. We chose to use this manual because of its past success [10-20] in helping smokers through their tobacco dependence treatment. This intervention indicates to the participant that the study staff supports their decision to stop smoking and establishes a relationship between the participant and study staff that can improve tobacco abstinence.
2.3 Behavioral intervention procedures
Behavioral intervention began on the day of randomization. The study physician covered the Nicotine Addiction module (Table 1), which reviews the powerful effect that nicotine, as delivered by cigarettes, has on the body. The study physician initialed the intervention and signed the Quit Date Contract, which the participant also signed. This was designed to indicate the physician’s support, and stressed the importance of the decision to stop smoking. Site interventionists started providing the behavioral intervention sessions at study week 2. The first 4 treatment modules were to be completed in the order that they appeared in the table of contents. These modules covered specific issues that many individuals report struggling with during the first weeks after stopping smoking. For study visits 5 through 9, the interventionists were allowed to tailor the sessions to meet the needs of each participant; the modules were covered in any order as concerns were identified by the interventionist. All topics were discussed by study completion. The final two sessions addressed Maintenance of Abstinence and Stopping Study Medication/Focus on the Future.
2.4 Training
This study used the “train-the-trainer” model [22], in which selected individuals attended an intensive training program and returned to train other interventionists at their home sites. Once certified, site trainers also had the primary responsibility for supervising the interventionists’ use of the Smoke Free and Living It manual [9] for counseling. All study interventionists were required to have a minimum of a bachelor’s degree, preferably in a behavioral science such as psychology or sociology, and were staff members of the clinical sites at which the study was conducted.
2.4.1. Train the trainer
Each site selected a lead interventionist to act as a site trainer, for a total of 6 site trainers. Each of the site trainers attended a centralized training program provided by staff from the Mayo Clinic NRP. This training consisted of 16 hours spread over 2 days and focused on learning about the version of the Smoke Free and Living It manual that was to be used in the study. Training materials included a participant counseling book, interventionist guide, DVD with samples of counseling sessions, and resource phone numbers for staff to use if they had questions or concerns. Training was completed in a uniform manner, which included viewing a video of counseling examples, observance of a “mock” counseling session, practice sessions, discussions, and final testing for certification.
2.4.2. Interventionist training
Once certified (see below), site trainers were able to provide training to additional study site interventionists. Onsite training was completed over a period of a week with at least 4 hours initially allotted for the trainer to work with the study staff. Time was allowed over the next few days for the staff to hold practice sessions with each other or the trainer. In the last step, the trainer observed the staff administering mock counseling sessions. After certification, the site trainer reviewed one videotape per interventionist on a monthly basis for quality assurance.
2.5 Certification
2.5.1. Site trainers
During training, each site trainer completed a mock counseling session (through the use of role-playing with each other or a Mayo Clinic Nicotine Dependence Center staff) rated by a Tobacco Treatment Specialist (TTS) [23] from the Mayo Clinic NRP for the purpose of certifying the candidate. Criteria used to assess the counseling skills included: (1) familiarity with intervention topics; (2) ability to effectively guide study participants through key points in 10 minutes; (3) ability to make and maintain eye contact; (4) ability to listen; (5) ability to identify individual needs and provide the appropriate intervention; (6) ability to remain nonjudgmental and encouraging; and (7) ability to recognize the opportunity for teaching versus the need to allow for more interaction and discussion within the remainder of the 10 minutes allowed. The trainees were evaluated on each of these criteria by using a 3-point scale: (1) meets expectations, (2) needs improvement, and (3) expectations not met and additional training is required. To be certified as an interventionist, the site trainer had to meet expectations on 6 of 7 of the established criteria. If this was not met, additional training was undertaken; certification was provided after the site trainer reviewed and rated additional intervention sessions, and the candidate met expectations on 6 of 7 criteria.
2.5.2. Interventionists
There were a total of 15 interventionists, ranging from 1 to 5 per site. Each interventionist completed a mock counseling session rated by the site trainer. A videotape of this session was reviewed by the site trainer or the TTS expert (where the site trainer had not achieved initial certification in time for study initiation). To be certified, the interventionist must have successfully met 6 of 7 of the counseling skills criteria in the judgment of the site trainer or TTS. If this goal was not achieved, more time was allowed for additional training. Of the 15 interventionists, only 1 required additional training. The additional training included specific feedback in the areas found to be deficient and review of the sample videos of study intervention sessions and the printed materials. After the additional training, another video was submitted for certification consideration.
2.6. Ongoing supervision, training, and quality control
Interventionists were videotaped during each session. Study participants were informed that videotaping of the interventionist would occur during the trial for quality checks. A Microsoft Excel document was used to track completed intervention sessions and the random number generator function was used to select intervention sessions for site trainers to review in order to monitor and rate each interventionist on a monthly basis, contingent on the interventionist having active cases. This allowed trainers to ensure that all counseling was being conducted in accordance with the study protocol.
If no videotaped sessions with a participant were available for the interventionist in a given month, a mock counseling session was used for review instead. The same 3-point scale described above was used to ensure that study interventionists were consistent in administering the intervention and met 6 of 7 of the counseling skills criteria. If an interventionist failed to meet expectations in 3 consecutive sessions, the interventionist was required to attend additional training and repeat the certification process prior to being assigned any additional study participants.
Every 6 months, the same protocol-specific program was used to select sessions that had been rated by the site trainer and sent to a certified TTS for review as part of the quality assurance. Sites provided the Mayo Clinic NRP with a videotape of each interventionist administering counseling. This was either a live video session (with the camera focused on the interventionist) or a mock video session when no participant was available for intervention (some sites had delayed start-up times and had not recruited by the time of the videotape deadline). Mayo TTS staff reviewed the videotaped sessions using the same set of criteria that the site trainers used. Feedback was sent to each site trainer and interventionist after the tapes had been viewed. Concerns were addressed as appropriate. One concern was that the sessions were too long, and advice was given on how to reduce the duration of the session by focusing on key elements of the counseling. Another concern was a lack of confidence with the topics being discussed, which made the sessions too mechanical. The trainer was asked to aid the interventionist with the specific topics in order to increase confidence. After the concerns were addressed, a second tape was provided to the TTS staff for review.
2.7. Participant adherence to the intervention
Participants were also monitored for adherence with the behavioral program, with ratings on: (1) participant’s attendance; (2) Smoke Free and Living It homework completion (e.g., listing triggers, ideas of how to change their environment to support smoke-free living, information about their support system); and (3) participant’s participation in the topic being discussed. Adherence was rated individually and per counseling session as “not at all,” “slightly,” “moderately,” “considerably,” or “extremely” adherent.
2.8 Statistical analysis
In order to assess the generalizability of the intervention across different types of patient samples we used an approach similar to that used for assessing site-type differences in abstinence outcomes and improvement of ADHD symptoms[21]. Research sites were categorized as ADHD clinic, tobacco dependence clinic or mental health clinic, with 2 research sites included in each of these 3 site-types. In order to assess whether interventionist counseling skills differed across site-types an analysis was performed using data collected from the videotapes reviewed by the certified TTS as part of the quality assurance process. Using these data, a binary outcome was created to indicate whether the counselor met expectations for all 7 counseling skills. To accommodate repeated measures, this binary outcome was compared across site-types using General Estimating Equations (GEE). To assess whether participant adherence differed across site-types the subject participation ratings were used with a binary endpoint created to indicate “considerably” or “extremely” adherent. A GEE analysis that included only data from attended sessions was used to compare this binary endpoint across site-types. All analyses were performed using SAS 9.2[24].
3. Results
3.1 Interventionists adherence to skill training
Fifteen interventionists were trained and a total of 163 intervention sessions were reviewed by the certified TTS. In all but one instance, the certified TTS agreed with the supervisors’ assessment of the interventionists’ competency. Of the 163 sessions reviewed, 156 (95.7%) sessions were classified as adherent, indicating that the counselor was rated as “meets expectations” on at least 6 of the 7 evaluation criteria (Table 2). In addition, for 122 (74.8%) of the reviewed sessions the counselor was indicated to have met all 7 of the evaluation criteria. The percentage of sessions in which the counselor was rated as meeting all 7 evaluation criteria did not differ significantly across site-types (73.8%, 72.9% and 77.8% for mental health, ADHD, and tobacco dependence clinics respectively; GEE: df=2, chi-square=0.08, p=0.96).
Table 2.
Counselor adherence checklist results (N = 163)a
| Meets expectations |
||
|---|---|---|
| Evaluation criteria | No. | (%) |
| 1. Familiarity with each of the intervention topics. | 160 | (98) |
| 2. Ability to effectively guide study participant through key points in the 10-minute time allowed. |
145 | (89) |
| 3. Ability to make and maintain eye contact. | 150 | (92) |
| 4. Ability to listen. | 163 | (100) |
| 5. Ability to identify individual needs and provide the appropriate intervention. | 155 | (95) |
| 6. Ability to remain non-judgmental and encouraging. | 163 | (100) |
| 7. Ability to recognize the opportunity for teaching vs. the need to allow for more interaction and discussion remaining within the 10-minute time allowed. |
152 | (93) |
Of the 163 sessions reviewed, 156 (95.7%) were classified as adherent, indicating that the counselor was rated as “Meets Expectations” on at least 6 of the 7 evaluation criteria.
3.2 Participant adherence with session participation and homework
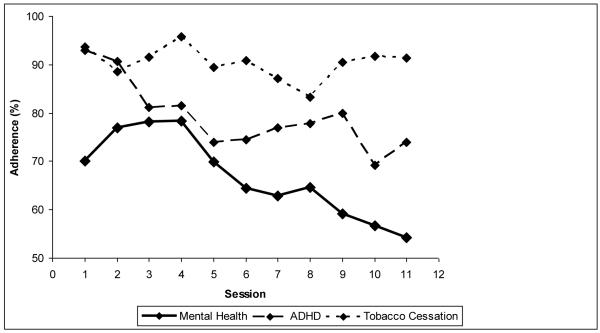
Of the 255 randomized participants, the mean ± SD number of sessions completed was 9.3 ± 2.8, with 157 (61.6%) completing all 11 of the sessions and 221 (86.7%) completing at least 6 of the 11 sessions. Among participants completing study visits with counseling sessions, the percentage rated “considerably” or “extremely” adherent declined significantly over time (df=1 chi-square=10.0, p=0.001) and differed significantly between site types (df=2, chi-square=14.2, p<0.001) with no evidence of a time by site-type interaction effect (df=2, chi-square=2.16, p=0.34); whereby the tobacco dependence sites had the best participant adherence and the mental health clinic sites had the lowest participant adherence (Figure 1).
Figure 1.
Participant Adherence by site specialty
The percentage of participants who were “considerably” or “extremely” adherent with behavioral session participation and homework is presented according to site-type.
4. Discussion
In the current study, we have shown that Mayo Clinic’s Smoke Free and Living It manual can be effectively implemented through a train-the-trainer approach. Fifteen interventionists across 6 geographically diverse study sites delivered counseling sessions to 255 participants. Approximately 95.7% of the counseling sessions reviewed adhered to the protocol, and participant adherence with the behavioral intervention was high, with 61.6% attending all 11 sessions. Our session attendance rate was similar to that reported in a previous study of young adult smokers in which 59% of participants attended 8 of 8 treatment sessions[25]. Our findings support the premise that training and ongoing quality control can maintain the skill level throughout the duration of a clinical trial [26]. Although some participants may have missed a study visit, and therefore a behavioral intervention session, the provision of the self-help guide provided all participants the opportunity to be exposed to each of the intervention topics. The use of the Smoke Free and Living It manual allowed us to provide study participants with problem-solving skills, coping skills, and social support, while keeping the main focus of the study on the pharmacological intervention being studied.
A recent literature review by Walters et al. [27] of 17 training programs for substance abuse treatment found that training of study staff improves the staff’s attitude, skill level, and knowledge, which in turn helps individual staff members to improve their proficiency in the delivery of the behavioral intervention. Though the improvements were in evidence immediately after the training, it was found that skills, attitude, knowledge, and proficiency declined unless additional contact was made after the training [27]. These results confirmed earlier findings from a study of motivational interviewing by Miller et al. [28], in which 104 licensed substance abuse professionals were randomized to one of five conditions (clinical workshop, workshop with practice feedback, workshop with individual coaching sessions, workshops with feedback and coaching, and waitlist group for self-guided training). A comparison of clinical proficiency immediately after training and after long-term follow up demonstrated that those who received the training had high levels of proficiency the day after the training, but without further training the proficiency fell below clinical trial criteria; in contrast, those who received feedback and/or coaching fully retained their proficiency [28]. These findings show that face-to-face training, ongoing supervision, and quality control of these skills are very much needed in clinical trials utilizing behavioral interventions [29-34], although not adequately reported in the current literature[35]. In this report we present the implementation of a train-the-trainer approach that incorporated initial interpersonal training and a periodic review of skill level.
The minimum standard of care for smoking cessation clinical trials includes a brief behavioral intervention [2]. The aim of the behavioral intervention is to provide smokers who are trying to stop smoking with needed problem-solving skills, training, and social support. Individual, group, and telephone counseling are effective, and their effectiveness increases with treatment intensity. Practical counseling (behavioral intervention) (problem solving/skills training) and social support delivered as part of treatment are especially effective, and clinicians should use these when counseling patients making a quit attempt [2]. In our analysis we found that participant adherence to the intervention was rated higher in sites with tobacco dependence research expertise. In an earlier study, it was found that these tobacco dependence clinics also had the highest tobacco abstinence [21]. Whether these differences were due to interventionist skill or to differences in the patient populations at the various types of sites (i.e., smoking, mental health, ADHD) is unclear. However, these findings suggest that participant adherence may play a role in the relative effectiveness of Smoke Free and Living It and, thus, future research should report on participant adherence in trials using Smoke Free and Living It.
This study has several limitations inherent in the initial study design. One limitation to this study report is the lack of detailed assessment of the counseling skill criteria and the participant adherence criteria. The skills assessment tool that we utilized did not allow for an analysis of the quality of the delivered intervention beyond what was demonstrated as an acquired minimal skill set. The assessment scheme did allow for standardization of the intervention but only insofar as ensuring that counselors not meeting minimum criteria received additional feedback and instruction. Although a more intensive assessment would have provided more information for assessing differences in the quality of the intervention delivered between counselors, the utility of this additional information needs to be weighed against the increased the amount of staff and participant burden required to collect it.
This study included sites which were experienced in recruiting a variety of different patient populations (i.e. participants who smoked or had ADHD or other addictions). Site differences observed in participant adherence rates could be partially related to site staff experience with different patient populations. Future studies which include brief behavioral interventions should consider that although the behavioral intervention may be minimal, it could potentially produce bias in multisite studies. We believe that train-the-trainer is a reasonable approach to training study staff located at different sites while acknowledging that intervention effectiveness could vary depending on the level of experience of site personnel with different patient populations. All sites selected for this clinical trial were experienced in research conduct, however, the sites without experience with tobacco dependence might have benefited from additional education in tobacco dependence. A needs-based approach which considers site experience and expertise may enhance the train-the-trainer methodology. Future quality assessments in multisite studies should emphasize the quality of intervention beyond a minimal therapist skills set.
A challenge in clinical trials for smoking cessation is to include basic brief counseling while focusing the study on the pharmacologic intervention. The present results suggest that interventionists in a multi-site clinical trial were successfully trained to meet a minimum quality threshold in delivering the Smoke Free and Living It intervention manual which provides a minimal behavioral intervention for trials of pharmacotherapy for tobacco dependence. This manual can be used as a self-help guide, an interventionist guide, or both, and can be tailored to a specific research protocol, regardless of the size of the study or number of study sites. This manual meets the standard of care set by the USPHS Clinical Practice Guideline and therefore could be part of the basic tool kit for clinical trials for smoking cessation.
Acknowledgments
A special thanks to the exceptional research staff at each of the study sites, the coordinating staff at the University of Cincinnati and Richard Morris from the Mayo Clinic Nicotine Research Program, for their patience and persistence in helping to collect, compile, and organize these data. Finally, the authors wish to thank the study participants who participated in this clinical trial.
The clinical trial in which we evaluated the implementation of Smoke Free and Living It was supported by the following grants from the National Institute on Drug Abuse: U10-DA015831 and K24 DA022288 to Harvard University (Dr Weiss); U10-DA013035 to New York State Psychiatric Institute (Dr. Nunes); U10-DA013046 to New York University (Dr. Rotrosen); U10-DA013036 to Oregon Health and Science University (Dr. McCarty); and U10-DA013732 to the University of Cincinnati (Dr. Somoza). The study medication and matching placebo were provided by McNeil Consumer & Specialty Pharmaceuticals at no cost.
Abbreviations
- ADHD
Attention Deficit Hyperactivity Disorder
- NRP
Nicotine Research Program
- OROS-MPH
osmotic-release oral system methylphenidate
- SD
standard deviation
- TTS
Tobacco Treatment Specialist
- USPHS
United States Public Health Service
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].World Health Organization Guidelines for Controlling and Monitoring the Tobacco Epidemic. 1988.
- [2].Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update, Clinical Practice Guideline. Executive Summary. U.S. Department of Health and Human Services. Public Health Service; Rockville, MD: 2008. [Google Scholar]
- [3].World Health Organization Tobacco Free Initiative (TFI): Smoking Cessation. 2010.
- [4].Hays JT, Croghan IT, Schroeder DR, Burke MV, Ebbert JO, McFadden DD, et al. Residential treatment compared with outpatient treatment for tobacco use and dependence. Mayo Clin Proc. 2011;86:203–9. doi: 10.4065/mcp.2010.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hays JT, Wolter TD, Eberman KM, Croghan IT, Offord KP, Hurt RD. Residential (inpatient) treatment compared with outpatient treatment for nicotine dependence. Mayo Clin Proc. 2001;76:124–33. doi: 10.1016/S0025-6196(11)63117-0. [DOI] [PubMed] [Google Scholar]
- [6].Volkow N. Research Report Series. Tobacco Addiction. National Institute of Health, National Institute on Drug Abuse; 2009. [Google Scholar]
- [7].Lancaster T, Stead L, Silagy C, Sowden A. Effectiveness of interventions to help people stop smoking: findings from the Cochrane Library. BMJ. 2000;321:355–8. doi: 10.1136/bmj.321.7257.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].National Cancer Institute . Clearing the Air: Quit Smoking Today. National Institutes of Health, National Cancer Institute; Bethesda, MD: 2010. [Google Scholar]
- [9].Mayo Clinic Nicotine Research Program . Smoke Free and Living It. Mayo Foundation for Medical Education and Research - Nicotine Dependence Center - Research Program; 2000. [Google Scholar]
- [10].Hurt RD, Dale LC, Fredrickson PA, Caldwell CC, Lee GA, Offord KP, et al. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up. One-year outcome and percentage of nicotine replacement. JAMA. 1994;271:595–600. [PubMed] [Google Scholar]
- [11].Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, Schroeder DR. High-dose nicotine patch therapy. Percentage of replacement and smoking cessation. JAMA. 1995;274:1353–8. [PubMed] [Google Scholar]
- [12].Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- [13].Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. a randomized, controlled trial. Ann Intern Med. 2001;135:423–33. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- [14].Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. J Clin Oncol. 2003;21:914–20. doi: 10.1200/JCO.2003.08.160. [DOI] [PubMed] [Google Scholar]
- [15].Croghan GA, Sloan JA, Croghan IT, Novotny P, Hurt RD, DeKrey WL, et al. Comparison of nicotine patch alone versus nicotine nasal spray alone versus a combination for treating smokers: a minimal intervention, randomized multicenter trial in a nonspecialized setting. Nicotine Tob Res. 2003;5:181–7. doi: 10.1080/1462220031000073252. [DOI] [PubMed] [Google Scholar]
- [16].Croghan IT, Hurt RD, Dakhil SR, Croghan GA, Sloan JA, Novotny P, et al. Randomized comparison of a nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clin Proc. 2007;82:186–95. doi: 10.4065/82.2.186. [DOI] [PubMed] [Google Scholar]
- [17].Rigotti NA, Gonzales D, Dale LC, Lawrence D, Chang Y. A randomized controlled trial of adding the nicotine patch to rimonabant for smoking cessation: efficacy, safety and weight gain. Addiction. 2009;104:266–76. doi: 10.1111/j.1360-0443.2008.02454.x. [DOI] [PubMed] [Google Scholar]
- [18].Winhusen TM, Somoza EC, Brigham GS, Liu DS, Green CA, Covey LS, et al. Impact of attention-deficit/hyperactivity disorder (ADHD) treatment on smoking cessation intervention in ADHD smokers: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:1680–8. doi: 10.4088/JCP.09m05089gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wilcox CS, Oskooilar N, Erickson JS, Billes SK, Katz BB, Tollefson G, et al. An open-label study of naltrexone and bupropion combination therapy for smoking cessation in overweight and obese subjects. Addict Behav. 2010;35:229–34. doi: 10.1016/j.addbeh.2009.10.017. [DOI] [PubMed] [Google Scholar]
- [20].Croghan I, Hurt R, Ebbert J, Croghan G, Polk O, Stella P, et al. Racial differences in smoking abstinence rates in a multicenter, randomized, open-label trial in the United States. J Public Health. 2010;18:59–68. doi: 10.1007/s10389-009-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Covey LS, Hu MC, Green CA, Brigham G, Hurt RD, Adler L, et al. An exploration of site effects in a multisite trial of OROS-methylphenidate for smokers with attention deficit/hyperactivity disorder. Am J Drug Alcohol Abuse. 2011;37:392–9. doi: 10.3109/00952990.2011.596979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Orfaly RA, Frances JC, Campbell P, Whittemore B, Joly B, Koh H. Train-the-trainer as an educational model in public health preparedness. J Public Health Manag Pract. 2005;(Suppl):S123–7. doi: 10.1097/00124784-200511001-00021. [DOI] [PubMed] [Google Scholar]
- [23].Stevens S, Burke M. Training specialists to treat tobacco use and dependence (“Formazione degli specialisti nel trattamento della dipendenza e del consumo di tabacco”) Tabaccologia. 2010;1:5–7. [Google Scholar]
- [24].SAS Institute Inc. SAS/STAT User’s Guide. Version 9.2 SAS Institute; Cary, NC: 2001. [Google Scholar]
- [25].Ames SC, Werch CE, Ames GE, Lange LJ, Schroeder DR, Hanson AC, et al. Integrated smoking cessation and binge drinking intervention for young adults: a pilot investigation. nn Behav Med. 2010;40:343–9. doi: 10.1007/s12160-010-9222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Martino S. Strategies for training counselors in evidence-based treatments. Addict Sci Clin Pract. 2010;5:30–9. [PMC free article] [PubMed] [Google Scholar]
- [27].Walters ST, Matson SA, Baer JS, Ziedonis DM. Effectiveness of workshop training for psychosocial addiction treatments: a systematic review. J Subst Abuse Treat. 2005;29:283–93. doi: 10.1016/j.jsat.2005.08.006. [DOI] [PubMed] [Google Scholar]
- [28].Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol. 2004;72:1050–62. doi: 10.1037/0022-006X.72.6.1050. [DOI] [PubMed] [Google Scholar]
- [29].Sholomskas DE, Syracuse-Siewert G, Rounsaville BJ, Ball SA, Nuro KF, Carroll KM. We don’t train in vain: a dissemination trial of three strategies of training clinicians in cognitive-behavioral therapy. J Consult Clin Psychol. 2005;73:106–15. doi: 10.1037/0022-006X.73.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Martino S, Ball SA, Nich C, Frankforter TL, Carroll KM. Community program therapist adherence and competence in motivational enhancement therapy. Drug Alcohol Depend. 2008;96:37–48. doi: 10.1016/j.drugalcdep.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rakovshik SG, McManus F. Establishing evidence-based training in cognitive behavioral therapy: A review of current empirical findings and theoretical guidance. Clin Psychol Rev. 2010;30:496–516. doi: 10.1016/j.cpr.2010.03.004. [DOI] [PubMed] [Google Scholar]
- [32].Madson MB, Campbell TC. Measures of fidelity in motivational enhancement: a systematic review. J Subst Abuse Treat. 2006;31:67–73. doi: 10.1016/j.jsat.2006.03.010. [DOI] [PubMed] [Google Scholar]
- [33].Beidas RS, Kendall PC. Training Therapists in Evidence-Based Practice: A Critical Review of Studies From a Systems-Contextual Perspective. Clin Psychol (New York) 2010;17:1–30. doi: 10.1111/j.1468-2850.2009.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Herschell AD, Kolko DJ, Baumann BL, Davis AC. The role of therapist training in the implementation of psychosocial treatments: a review and critique with recommendations. Clin Psychol Rev. 2010;30:448–66. doi: 10.1016/j.cpr.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Perepletchikova F, Treat TA, Kazdin AE. Treatment integrity in psychotherapy research: analysis of the studies and examination of the associated factors. J Consult Clin Psychol. 2007;75:829–41. doi: 10.1037/0022-006X.75.6.829. [DOI] [PubMed] [Google Scholar]