Abstract
Purpose
Despite the widespread use of antiemetics, nausea continues to be reported by over 70% of patients receiving chemotherapy.
Methods
In this double blind, multicenter trial, we randomly assigned 744 cancer patients to four arms: 1) placebo, 2) 0.5g ginger, 3) 1.0g ginger, or 4) 1.5g ginger. Nausea occurrence and severity were assessed at a baseline cycle and the two following cycles during which patients were taking their assigned study medication. All patients received a 5-HT3 receptor antagonist antiemetic on Day 1 of all cycles. Patients took three capsules of ginger (250mg) or placebo twice daily for six days starting three days before the first day of chemotherapy. Patients reported the severity of nausea on a 7-point rating scale (“1” = “Not at all Nauseated” and “7” = “Extremely Nauseated”) for Days 1-4 of each cycle. The primary outcomes were to determine the dose and efficacy of ginger at reducing the severity of chemotherapy-induced nausea on Day 1 of chemotherapy.
Results
A total of 576 patients were included in final analysis (91% female, mean age = 53). Mixed model analyses demonstrated that all doses of ginger significantly reduced acute nausea severity compared to placebo on Day 1 of chemotherapy (p=0.003). The largest reduction in nausea intensity occurred with 0.5g and 1.0g of ginger (p=0.017 and p=0.036, respectively). Anticipatory nausea was a key factor in acute chemotherapy-induced nausea (p<0.0001).
Conclusions
Ginger supplementation at daily dose of 0.5g-1.0g significantly aids in reduction of the severity of acute chemotherapy-induced nausea in adult cancer patients.
Keywords: chemotherapy, cancer, nausea, ginger
Introduction
Despite the widespread use of the 5-HT3 receptor antagonist antiemetics, ondansetron (Zofran®,) granistron (Kytril®), and dolasetron mesylate (Anzemet®), post-chemotherapy nausea and vomiting continue to be reported by over 70% of patients [12, 15]. Research also suggests that the 5-HT3 receptor antagonists are clinically more effective against emesis than they are against nausea [1, 11, 13, 14]. In general, there is still room for improvement in the control of nausea associated with chemotherapy for cancer.
Chemotherapy-induced nausea (CIN) can be categorized into three different types of nausea. Anticipatory nausea occurs before the start of chemotherapy in anticipation of the treatment and develops in 8-20% of patients [8, 22]. Acute nausea occurs within 24 hours post-chemotherapy, whereas delayed nausea occurs after 24 hours and up to five days post-chemotherapy. The majority of patients report the most severe nausea on Day 1 of chemotherapy and are less likely to have severe nausea on subsequent days if they do not experience it on Day 1 [21]. Recently, Schwartzberg et al demonstrated that patients who experience nausea and vomiting from their initial chemotherapy cycle of low emetogenic chemotherapy were 3.1 times more likely to experience nausea and vomiting at subsequent chemotherapy cycles compared to patient who did not experience nausea and vomiting with their initial chemotherapy cycle [30]. This rate is comparable to the rates of 3.8 and 3.7 for patients receiving moderate or highly emetogenic chemotherapy [30]. Therefore, it can be concluded that a patient who experiences nausea and vomiting at a chemotherapy cycle will be more likely to experience nausea and vomiting at subsequent chemotherapy cycle regardless of the type of chemotherapy (i.e., low, moderate or highly emetogenic).
Ginger, an ancient spice, is most notably known for its role as a flavoring agent for food in Asian and Indian recipes [31]. For over 2,500 years, the dried aromatic rhizome (underground stem) of ginger (Zingiber Officinale, Roscoe), has been used to treat gastrointestinal upsets, as well as joint and muscle pain. Ginger is listed as a food on the FDA's “generally regarded as safe” list. Research studies have demonstrated ginger's effectiveness against nausea associated with motion sickness, pregnancy, and surgery [2, 3, 10, 23, 34]. Previous clinical trials suggest that ginger may be effective against CIN, but design inadequacies, small numbers, and lack of dose-finding studies, limit their power and generalizability [17, 19, 24, 32, 35]. We conducted a randomized, double-blind, placebo-controlled, dose-finding clinical trial to determine if ginger was more effective than placebo in controlling acute CIN in cancer patients receiving 5-HT3 receptor antagonist antiemetics.
Methods
Patients and Study Design
Eligible patients were ≥ 18 years of age, able to understand English, had a diagnosis of cancer, may have received ≥ 1 chemotherapy cycle, and were scheduled for at least three additional chemotherapy cycles. Chemotherapy must have been given without concurrent radiation therapy or interferon and without planned interruption by radiation therapy or surgery. All patients must have experienced nausea of any severity in any chemotherapy cycle before study enrollment, as well as scheduled to receive a 5-HT3 receptor antagonist (e.g., Zofran®, Kytril®, Navoban®, or Anzemet®) plus dexamathasone at all chemotherapy cycles. Patients were not on coumadin or heparin for therapeutic anticoagulation, did not have a bleeding disorder, and had platelet count > 100,000/μl before the baseline cycle.
This study was a phase II/III, randomized, double-blind, placebo-controlled clinical trial conducted in 23 private practice oncology groups funded by the National Cancer Institute's Community Clinical Oncology Program (CCOP) and affiliated with the University of Rochester Cancer Center CCOP Research Base. The primary objective of the study was to determine if ginger supplementation reduced acute CIN (i.e., nausea on Day 1) in patients receiving standard 5-HT3 receptor antagonist antiemetics plus dexamethasone. The institutional review board of the University of Rochester and each participating site approved the protocol. Written informed consent was obtained from each patient. This study is registered on ClinicalTrials.gov, #NCT00040742.
Randomization was stratified by CCOP site. Within each site, a computer-generated random numbers table with block size eight was used to randomly assign patients to one of four treatment arms (Placebo, 0.5g, 1.0g, and 1.5g Ginger). The randomization assigned patients to the four arms in the ratio 1:1:1:1.
Study Medication
The ginger and placebo capsules were manufactured by Aphios Corporation in Woburn, MA. Ginger capsules contained a purified liquid extract of ginger root (Zingiber officinale) with concentrated 8.5mg of combined gingerols, zingerone, and shogoal content, equivalent to 250mg of ginger root, in extra virgin olive oil containing other excipients to improve solubilization and increase bioavailability. The placebo capsules consisted of only the extra virgin olive oil containing a higher level of excipients in order to match the weight of the ginger capsules. Both the ginger and placebo products were double encapsulated in size “0”, white, opaque, hard gelatin capsules with a nitrogen cap. The double encapsulation and nitrogen cap masked any difference in smell or color between the two products. The capsules were packaged in blister packs containing six capsules (ginger or placebo) and were grouped into morning and evening doses (3 capsules each). The contents of the blister packs for each study arm were: arm 1 (placebo) = 3 placebo capsules twice daily; arm 2 (0.5g daily ginger dose) = 2 placebo capsules and 1 ginger capsule twice daily; arm 3 (1.0g daily ginger dose) = 1 placebo capsule and 2 ginger capsules twice daily; arm 4 (1.5g daily ginger dose) = 3 ginger capsules twice daily.
Procedures
The days surrounding each chemotherapy infusion were identified for study purposes as follows: Days -3 to -1 were before chemotherapy, Day 1 was the day of chemotherapy infusion, and Days 2-4 were post-chemotherapy. All patients received 5-HT3 antiemetics plus dexamathasone on Day 1 of all chemotherapy cycles. All patients took the study medication twice daily for six days, starting three days before the start chemotherapy for study cycles 2 and 3. Compliance was measured by pill counts at the end of each study cycle. Nausea and emesis were measured using a modified four-day patient report diary developed by Burish[5] and Carey[6] and used by us in previous large clinical trials[13, 14, 26, 28]. Patients reported the severity of nausea four times each day (morning, afternoon, evening, and night) over three four-day periods (Days 1 – 4) during each cycle (baseline, study cycle 2 and study cycle 3) using a 7-point semantic rating scale anchored by “1” = “Not at all Nauseated” and by “7” = “Extremely Nauseated.” Anti-nausea medication use and number of vomiting episodes were also recorded. A 13-item Symptom Inventory was used to assess potential side effects of ginger, as well as anticipatory nausea, on an 11-point scale anchored by 0 (“Not Present) and 10 (“As Bad As You Can Imagine”) [7, 29]. The Symptom Inventory was completed once during the baseline cycle (i.e., Day 4) and three times during study cycles 2 and 3 (i.e., before starting the study medication (Day -4), before Day 1 of chemotherapy (i.e., Day -1), and after completion of study medication (Day 4)). Anticipatory nausea was analyzed using the nausea item on the Symptom Inventory completed prior to chemotherapy (i.e., Day -1). Quality of life was assessed using the 27-item Functional Assessment of Chronic Illness Therapy-General (FACIT-G) at baseline and follow-up assessments (i.e., Day 4).
Statistical Analysis
All statistical tests were performed at the two-tailed 5% level of significance and data were analyzed on an “intent-to-treat” basis. Acute nausea severity was measured as the average and maximum of the evening and night Day 1 responses. In order to analyze acute nausea severity accurately, we had to ensure that patients received chemotherapy before rating their nausea severity on Day 1 in the four-day diary. We excluded the morning and afternoon nausea responses on Day 1 because it was unlikely that a patient would have received chemotherapy prior to either of these rating times. Delayed nausea severity was measured as the average and maximum of all the values for Days 2 and 3. The follow-up phase nausea severity was measured as the average and maximum of the responses for Day 4. Average nausea severity (NAv) is indicated as the primary outcome measure in the protocol, but the maximum nausea severity (NMx) (i.e. nausea at its worst) was considered to be more relevant from the patient's point of view. Mixed model analyses and Type 3 tests of fixed effects using the Kenward-Roger degrees of freedom procedure [16] were used to examine the change from baseline NAv and NMx on Day 1. The fixed effects in the model were Dose, Cycle and Dose*Cycle interaction and the random effects consisted of subject-subject and within-subject variation. Under the missing-at-random assumption, missing values should not cause substantial bias using this method of analysis; however we checked this with multiple imputation (multiple imputation by chained equations, MICE [33]) and the changes in the results were minor. Contrasts were used to estimate and test the effect size of placebo versus ginger at any dose. Mean differences between each ginger dose and placebo were also estimated and tested using the Tukey-Kramer procedure for multiple comparisons. Similar analyses were used to examine the secondary outcomes delayed nausea, quality of life, and vomiting. To examine the effects of anticipatory nausea, the pre-chemotherapy nausea symptom inventory item score was added as a covariate in the above mixed model. Computations were performed with SAS Version 9.2 (PROC MIXED) and R Version 2.9.1 (MICE package Version 1.21).
Results
Patient Characteristics
From June 2002 to December 2008, a total of 744 cancer patients were enrolled and randomized into one of the four treatment arms (Figure 1). Of these 744 patients, 662 completed the baseline measures prior to the study medication being administered. Of the 662 patients who completed the baseline cycle, 562 (85%) completed Study Cycle 2 (i.e., first cycle of study medication) and 471 (71%) completed Study Cycle 3 (i.e., second cycle of study medication). The analyses reported herein were conducted on 576 (87%) patients who provided evaluable data at either Study Cycle 2 or 3. The baseline characteristics were equivalent across treatment groups (Table 1). The majority of patients were white (91%), female (93%) with a mean age of 53 years and the most common types of cancer were breast (74%), gastrointestinal (8%), and lung (6%) cancers. Overall reasons for non-completion of the study included: changed mind about participation (n=81), incomplete study forms (n=36), gastrointestinal symptoms (n=32), other medical reasons (n=31), off chemotherapy (n=22), and chemotoxicity (n=19). The two most prominent reasons for dropout at baseline were changed mind about participation (n=36; 43%) and incomplete study forms (n=11, 13%), which were also the major causes for dropout for Study Cycles 2 and 3 (n=45; 23% and n=25; 13%, respectively). There were no meaningful or statistically significant mean differences between the four treatment arms at baseline for NAv (mean range = 2.2 to 2.6), NMx (mean range = 2.5 to 2.9), or quality of life (mean range = 71 to 72). Compliance to study medication did not significantly differ between treatment arms or study cycle (93% compliance = placebo; 83% compliance = 0.5g ginger; 90% compliance = 1.0g ginger; and 84% compliance = 1.5g ginger). In the four-day diary, patients were asked to record the number of times they used antiemetic medication after their chemotherapy treatment. The antiemetic medications were categorized into four types of medication: Type 1 (granisetron, odansetron, dolasetron mesylate, tropisetron), Type 2 (prochlorperazine), Type 3 (dexamethasone), and Type 4 (metochlopramide). There were no significant differences between treatment arms in regards to the use of antiemetics. Overall, the majority of patients did report taking antiemetic medications and the placebo arm tended to report more use of antiemetic medications compared to the ginger arms.
Figure 1. Patient Flow Diagram.
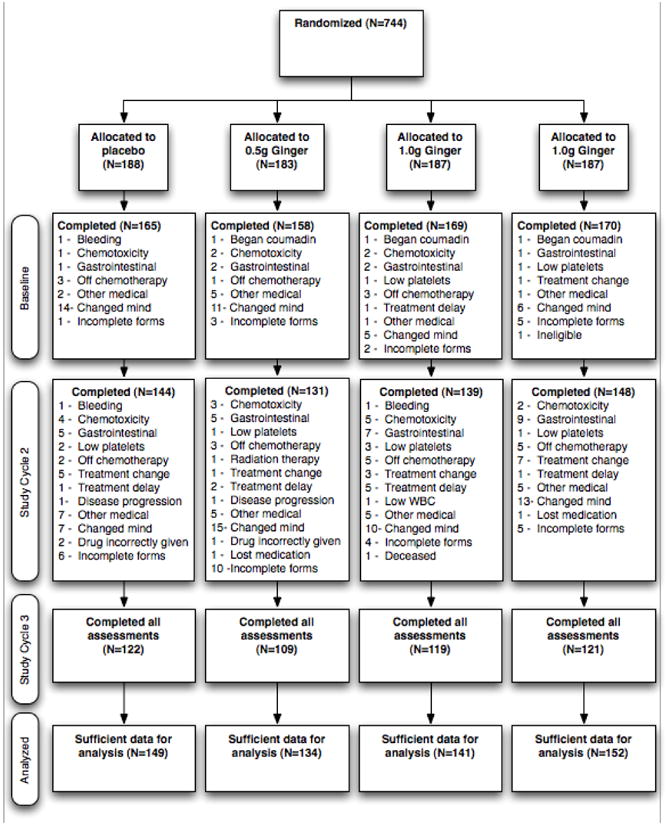
A total of 744 patients were consented and randomized into one of four treatment arms (placebo, 0.5g Ginger, 1.0g Ginger, or 1.5g Ginger). There was no significant difference in the dropout rate between treatment arms or study cycle. Only 469 patients fully completed the study, although data was evaluable for 576 patients and included in the analyses.
Table 1. Baseline Characteristics of Patients (N = 576).
| 0 Grams Ginger N=149 | 0.5 Grams Ginger N=134 | 1 Gram Ginger N=141 | 1.5 Grams Ginger N=152 | ||
|---|---|---|---|---|---|
| Age | |||||
| Mean (Std. Error) | 53 (0.87) | 54 (1.0) | 52 (0.9) | 52 (0.8) | |
| Gender | |||||
| Women | 135 (91%) | 122 (91%) | 122 (87%) | 142 (93%) | |
| Men | 14 (9%) | 12 (9%) | 19 (13%) | 10 (7%) | |
| Race | |||||
| White | 137 (93%) | 126 (94%) | 133 (95%) | 142 (93%) | |
| Non-White | 11 (7%) | 8 (6%) | 7 (5%) | 10 (7%) | |
| Education | |||||
| College Grad | 60 (41%) | 47 (35%) | 55 (39%) | 54 (36%) | |
| H.S. Grad | 82 (55%) | 78 (58%) | 81 (57%) | 95 (62%) | |
| Some / No HS | 6 (4%) | 9 (7%) | 5 (4%) | 3 (2%) | |
| Previous surgery | |||||
| Yes | 131 (88%) | 115 (86%) | 126 (89%) | 129 (85%) | |
| No | 18 (12%) | 19 (14%) | 15 (11%) | 23 (15%) | |
| Previous chemotherapy | |||||
| Yes | 81 (54%) | 75 (57%) | 85 (60%) | 82 (54%) | |
| No | 68 (46%) | 57 (43%) | 56 (40%) | 70 (46%) | |
| Previous radiation therapy | |||||
| Yes | 13 (9%) | 7 (5%) | 13 (9%) | 8 (5%) | |
| No | 136 (91%) | 125 (95%) | 128 (91%) | 144 (95%) | |
| Tumor site | |||||
| Alimentary | 14 (10%) | 11 (8%) | 10 (7%) | 8 (5%) | |
| Breast | 107 (72%) | 100 (75%) | 103 (73%) | 117 (77%) | |
| Genitourinary | 2 (1%) | 3 (2%) | 3 (2%) | 2 (2%) | |
| Gynecologic | 11 (7%) | 6 (5%) | 9 (7%) | 5 (3%) | |
| Hematologic | 3 (2%) | 4 (3%) | 9 (6%) | 8 (5%) | |
| Lung | 10 (7%) | 7 (5%) | 6 (4%) | 10 (7%) | |
| Other | 2 (1%) | 3 (2%) | 1 (1%) | 2 (1%) | |
| Marital status | |||||
| Married | 107 (72%) | 96 (72%) | 103 (73%) | 114 (75%) | |
| Not Married | 42 (28%) | 38 (28%) | 38 (27%) | 38 (25%) | |
| Baseline average nausea, Day1 | |||||
| Mean (Std. Error) | 2.2 (0.13) | 2.6 (0.16) | 2.5 (0.15) | 2.4 (0.14) | |
| Baseline nausea at its worst | |||||
| Mean (Std. Error) | 2.5 (0.14) | 2.9 (0.18) | 2.8 (0.17) | 2.7 (0.16) | |
| Quality of life(FACT-G) | |||||
| Mean (Std. Error) | 72 (1.3) | 72 (1.3) | 71 (1.3) | 72 (1.3) | |
Ginger effects on chemotherapy-related nausea
The primary objective of this clinical trial was to determine if ginger was more effective than placebo at reducing nausea severity on Day 1 of chemotherapy (i.e., acute nausea). Initially, the protocol called for ANCOVA on NAv on Day 1 ratings from the daily diary, with baseline as the covariate. However, the distribution of the baseline and post-treatment outcome were positively skewed and using baseline as covariate in ANCOVA would give high-baseline subjects disproportionate influence. Change scores showed no skewness.
Consequently, mixed model analyses on change scores were performed to combine the information in both study cycles, obtain more power, and evaluate any differences in the two cycles. The mixed model analyses across both study cycles 2 and 3, using NAv and NMx, revealed that all doses of ginger significantly reduced acute CIN in both study cycles compared to placebo (p = 0.013 and 0.003, respectively; Figure 2). Differences in the least-squares means showed that 0.5g and 1.0g daily ginger were the most effective at reducing acute CIN (Table 2).
Figure 2. Ginger reduces severity of acute chemotherapy-induced nausea.
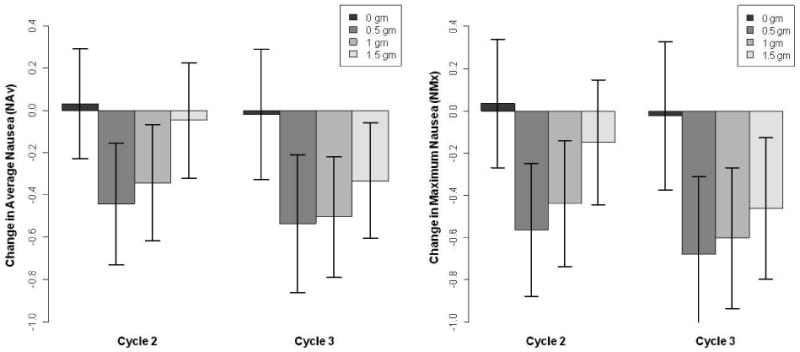
The boxplots represent the mean change in average nausea severity (NAv in left panel) and maximum nausea severity (NMx in right panel) for each treatment arm (i.e., ginger dose) on Day 1 of chemotherapy (i.e., acute CIN). Each shaded bar is a different treatment arm. All doses of ginger significantly reduced nausea severity on Day 1 of chemotherapy compared to placebo. The largest reduction in acute nausea occurred with 0.5g and 1.0g of ginger daily. Although Study Cycle 3 appears to show a greater reduction in nausea, there was no significant difference in mean nausea change between the two study cycles.
Table 2. Mixed Model Analyses for Nausea on Day 1 of Chemotherapy.
| Average Nausea (NAv) | Nausea at its Worst (NMx) | |||||
|---|---|---|---|---|---|---|
| P (overall)a | 0.028 | 0.012 | ||||
| Change | SEb | P-value | Change | SE | P-value | |
| Placebo vs. Any Ginger | -0.350 | -0.140 | 0.013 | -0.470 | 0.160 | 0.003 |
| Ginger Dose | LSMEANc | SE | P-valued | LSMEAN | SE | P-value |
| 0.0 gram | 0.015 | 0.121 | 0.024 | 0.137 | ||
| 0.5 gram | -0.441 | 0.127 | 0.046 | -0.566 | 0.145 | 0.017 |
| 1.0 gram | -0.402 | 0.124 | 0.076 | -0.506 | 0.141 | 0.036 |
| 1.5 grams | -0.158 | 0.120 | 0.738 | -0.269 | 0.137 | 0.431 |
Global test for the differences in group means.
Standard Error.
Means adjusted for Dose and Cycle.
Testing each dose versus reference (0 grams).
The secondary objectives of the study were to determine the effects of ginger on delayed nausea, anticipatory nausea, and quality of life in patients receiving chemotherapy. Mixed model analyses were used to examine delayed nausea for all time intervals on Day 2 and Day 3 and follow-up nausea on Day 4 when patients were no longer on study medication. Despite the significant reduction in acute nausea on Day 1 (i.e., acute) in all the ginger arms compared to placebo, the significance of ginger supplementation weakens for delayed (Days 2 and 3) and follow-up nausea (Day 4) (Figures 3 and 4). These data suggest that patients reported more severe delayed nausea compared to acute nausea. Overall, no significant differences were observed in vomiting or quality of life (FACIT-G), between the three ginger arms and placebo. The majority of patients did not report episodes of vomiting (mean incidence = 0.5). In contrast, type 3 tests of fixed effects revealed that anticipatory nausea (p < 0.0001) is also a factor in acute CIN.
Figure 3. Average nausea severity (NAv) over time for each study cycle.
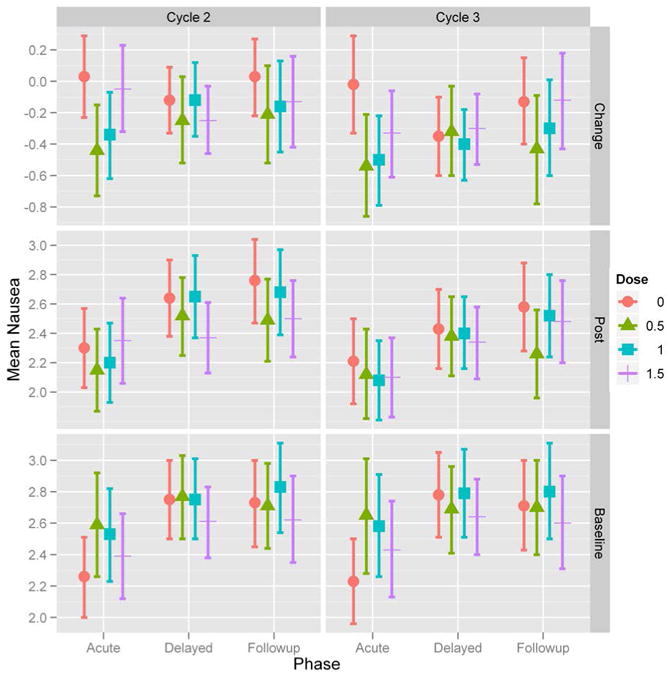
Treatment arms are: placebo (•); 0.5g ginger (▴); 1.0g ginger (▪); 1.5g ginger (┼). Acute phase represents evening and night of Day 1 diary nausea responses, delayed phase represents all diary nausea responses on Day 2 and Day 3, and follow-up phase represents all diary nausea responses on Day 4. The top two graphs show the mean change in NAv severity for acute, delayed, and follow-up phases during study cycle 2 (left) and study cycle 3 (right). The middle two graphs show the NAv severity for acute, delayed, and follow-up phases during study cycle 2 and 3. The bottom two graphs show the NAv severity for acute, delayed, and follow-up phases during the baseline cycle for patients that continued to study cycle 2 and study cycle 3.
Figure 4. Maximum nausea severity (NMX) over time for each study cycle.
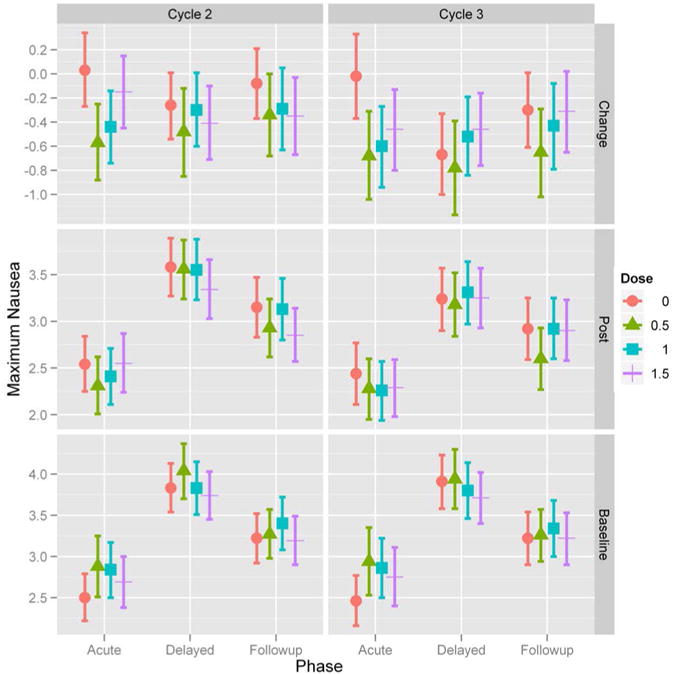
Treatment arms are: placebo (•); 0.5g ginger (▴); 1.0g ginger (▪); 1.5g ginger (┼). Acute phase represents evening and night of Day 1 diary nausea responses, delayed phase represents all diary nausea responses on Day 2 and Day 3, and follow-up phase represents all diary nausea responses on Day 4. The top two graphs show the mean change in NMx severity for acute, delayed, and follow-up phases during study cycle 2 (left) and study cycle 3 (right). The middle two graphs show the NMx severity for acute, delayed, and follow-up phases during study cycle 2 and 3. The bottom two graphs show the NMx severity for acute, delayed, and follow-up phases during the baseline cycle for patients that continued to study cycle 2 and study cycle 3.
Adverse Events
A total of 24 adverse events were reported during the course of the study. Only nine of the reported adverse advents were considered to be related to study drug (i.e., ginger) and these patients withdrew from the study. These adverse reactions included gastrointestinal symptoms, such as Grade 2 heartburn, bruising/flushing, and rash.
Discussion
Nausea is a complicated symptom to research and effectively treat because it is a subjective and unobservable phenomenon [4]. Previous research has shown that perceived susceptibility and expectancy, as well as age, are important risk factors for nausea severity [27]. In this large, multisite clinical trial, ginger reduced the severity of acute nausea in cancer patients receiving chemotherapy. One key feature of this study was the implementation of the six-day course of ginger three days before the start of chemotherapy, an approach similar to the administration of ginger in prevention of motion sickness [10, 23]. Although our primary objective was to reduce acute CIN, the six-day course of ginger or placebo allowed us to evaluate anticipatory and delayed CIN. Our data suggests that anticipatory nausea contributes to the report of acute nausea. This phenomenon is by Roscoe et al [27]. The authors reported that the odds of cancer patients reporting severe nausea from chemotherapy was 2.85 times greater in patients who said they were susceptible to nausea compared to others who stated they were not susceptible [27].
We can only speculate that the mechanism by which ginger alleviates nausea is through a combination of anti-inflammatory and anti-spasmodic activities. Current antiemetic medications, such as 5-HT3, are receptor antagonists for specific neurotransmitters in the gastrointestinal tract [12]. Likewise, ginger can bind 5-HT3 receptors to enhance antiemetic effects and can increase detoxification enzymes to counteract oxidative damage to tissues [9]. We speculate that starting ginger three days before the start of chemotherapy may have primed the gut for an anti-nausea response through 5-HT3 receptor binding and induction of detoxification enzymes. Furthermore, a receptor-based mechanism of action could explain why the lower ginger doses were more effective than the highest dose. Hypothetically, a certain dose ginger (i.e., 1.0g) may saturate the receptors rendering higher doses ineffective. Similar to our findings, Lien et al published that 1.0g dose of ginger was more effective against motion sickness than 2.0g dose [18].
The main strengths of this study include the large sample size, double-blinded treatment, and evaluation of three types of CIN. Two main weaknesses of the study, which should be controlled for in future studies, were not controlling for chemotherapy regimens (i.e., high versus low emetogenic regimens) or the severity level of nausea before enrollment. Recent research demonstrated that patients are more likely to experience CINV from subsequent chemotherapy cycles if CINV is experienced during the initial or earlier cycle, regardless of chemotherapy regimen (i.e., low, moderate or highly emetogenic) [30]. Our study did not collect information on the type of chemotherapy regimen because we only enrolled patients that reported nausea from previous chemotherapy cycle. This study design enabled the investigation of presence of nausea without vomiting. The low incidence of emesis was expected since 5-HT3 receptor antagonist antiemetics [20] were used. However, we did not expect the NAv and NMx severity at baseline to be low considering patients reported nausea during a previous chemotherapy cycle. Limitations of the study results include the small effect size for nausea severity and the lack of effect on delayed nausea and quality of life. The effect size for nausea severity may have been larger if the study only enrolled patients with moderate to severe nausea with chemotherapy prior to enrollment. Furthermore, our data showed that patients reported more severe delayed nausea (Day 2 and Day 3) compared to acute nausea (Day 1) suggesting that delayed nausea is a more debilitating problem for patients than acute nausea. The inability to detect a significant difference on quality of life could be due to the inability to control delayed nausea. We speculate that a reduction in delayed nausea would significantly improve patient quality of life during chemotherapy.
Although some cancer patients may use ginger for prevention or treatment of CIN, little published work has addressed the efficacy of ginger for CIN. Since 1986, only five articles published the use of ginger for CINV in cancer patients receiving chemotherapy. In 1986, Pace et al studied 20 patients being treated for leukemia with cytosine arabinoside (ARA-C) and showed that patients who received ginger had significantly less severe nausea on the day of chemotherapy and on the following day than those taking the placebo capsules [24]. Similarly, Sontakke et al showed that 1g of ginger before and after chemotherapy was as good as metoclopramide at complete control of nausea [32]. In contrast, Zick et al showed no improvement in acute or delayed CIN with ginger (1.0g or 2.0g) in a randomized, double-blind, placebo-controlled study in 162 cancer patients [35]. Levine et al showed that a high protein diet with 1.0g of ginger reduced severity of delayed nausea and use of antiemetic medications [17]. However, the study contributed the reduction to the high protein diet and not necessarily to ginger supplementation. Most recently, Pillai et al demonstrated the ginger powder (1-2g daily) reduced the severity of acute and delayed CINV in children and young adults receiving highly emetogenic chemotherapy for sarcoma [25]. We conclude that ginger (Zingiber officinale), at dose of 0.5g to 1.0 g daily, significantly aids in the reduction of acute CIN in patients receiving standard antiemetics. Thus far, ginger has demonstrated a beneficial effect on acute nausea from chemotherapy, however the effectiveness of ginger for nausea associated with other medical conditions awaits further controlled trials.
Acknowledgments
This research was supported by the National Cancer Institute of the National Institutes of Health PHS grants 1R25CA10618 (Cancer Control Research) and U10CA37420 (Community Clinical Oncology Program).
Footnotes
None of the authors have conflicts of interest to disclose.
References
- 1.Aapro M. 5-HT(3)-receptor antagonists in the management of nausea and vomiting in cancer and cancer treatment. Oncology. 2005;69:97–109. doi: 10.1159/000087979. [DOI] [PubMed] [Google Scholar]
- 2.Arfeen Z, Owen H, Plummer JL, Ilsley AH, Sorby-Adams RA, Doecke CJ. A double-blind randomized controlled trial of ginger for the prevention of postoperative nausea and vomiting. Anaesth Intensive Care. 1995;23:449–452. doi: 10.1177/0310057X9502300406. [DOI] [PubMed] [Google Scholar]
- 3.Bone ME, Wilkinson DJ, Young JR, McNeil J, Charlton S. Ginger root--a new antiemetic. The effect of ginger root on postoperative nausea and vomiting after major gynaecological surgery Anaesthesia. 1990;45:669–671. doi: 10.1111/j.1365-2044.1990.tb14395.x. [DOI] [PubMed] [Google Scholar]
- 4.Brearley SG, Clements CV, Molassiotis A. A review of patient self-report tools for chemotherapy-induced nausea and vomiting. Support Care Cancer. 2008;16:1213–1229. doi: 10.1007/s00520-008-0428-y. [DOI] [PubMed] [Google Scholar]
- 5.Burish TG, Carey MP, Krozely MG, Greco FA. Conditioned side effects induced by cancer chemotherapy: prevention through behavioral treatment. J Consult Clin Psychol. 1987;55:42–48. doi: 10.1037//0022-006x.55.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Carey MP, Burish TG. Etiology and treatment of the psychological side effects associated with cancer chemotherapy: a critical review and discussion. Psychol Bull. 1988;104:307–325. doi: 10.1037/0033-2909.104.3.307. [DOI] [PubMed] [Google Scholar]
- 7.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Figueroa-Moseley C, Jean-Pierre P, Roscoe JA, Ryan JL, Kohli S, Palesh OG, Ryan EP, Carroll J, Morrow GR. Behavioral interventions in treating anticipatory nausea and vomiting. J Natl Compr Canc Netw. 2007;5:44–50. doi: 10.6004/jnccn.2007.0006. [DOI] [PubMed] [Google Scholar]
- 9.Geiger JL. The essential oil of ginger, Zingiber officinale, and anaesthesia. The International Journal of Aromatherapy. 2005;15:7–14. [Google Scholar]
- 10.Grontved A, Brask T, Kambskard J, Hentzer E. Ginger root against seasickness. A controlled trial on the open sea. Acta Otolaryngol. 1988;105:45–49. doi: 10.3109/00016488809119444. [DOI] [PubMed] [Google Scholar]
- 11.Grunberg SM, Osoba D, Hesketh PJ, Gralla RJ, Borjeson S, Rapoport BL, du Bois A, Tonato M. Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity--an update. Support Care Cancer. 2005;13:80–84. doi: 10.1007/s00520-004-0718-y. [DOI] [PubMed] [Google Scholar]
- 12.Herrstedt J, Dombernowsky P. Anti-emetic therapy in cancer chemotherapy: current status. Basic Clin Pharmacol Toxicol. 2007;101:143–150. doi: 10.1111/j.1742-7843.2007.00122.x. [DOI] [PubMed] [Google Scholar]
- 13.Hickok JT, Roscoe JA, Morrow GR, Bole CW, Zhao H, Hoelzer KL, Dakhil SR, Moore T, Fitch TR. 5-Hydroxytryptamine-receptor antagonists versus prochlorperazine for control of delayed nausea caused by doxorubicin: a URCC CCOP randomised controlled trial. Lancet Oncol. 2005;6:765–772. doi: 10.1016/S1470-2045(05)70325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickok JT, Roscoe JA, Morrow GR, King DK, Atkins JN, Fitch TR. Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics: a University of Rochester James P. Wilmot Cancer Center Community Clinical Oncology Program Study of 360 cancer patients treated in the community. Cancer. 2003;97:2880–2886. doi: 10.1002/cncr.11408. [DOI] [PubMed] [Google Scholar]
- 15.Hickok JT, Roscoe JA, Morrow GR, Ryan JL. A Phase II/III Randomized, Placebo-Controlled, Double-Blind Clinical Trial of Ginger (Zingiber officinale) for Nausea Caused by Chemotherapy for Cancer: A Currently Accruing URCC CCOP Cancer Control Study. Support Cancer Ther. 2007;4:247–250. doi: 10.3816/SCT.2007.n.022. [DOI] [PubMed] [Google Scholar]
- 16.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 17.Levine ME, Gillis MG, Koch SY, Voss AC, Stern RM, Koch KL. Protein and ginger for the treatment of chemotherapy-induced delayed nausea. J Altern Complement Med. 2008;14:545–551. doi: 10.1089/acm.2007.0817. [DOI] [PubMed] [Google Scholar]
- 18.Lien HC, Sun WM, Chen YH, Kim H, Hasler W, Owyang C. Effects of ginger on motion sickness and gastric slow-wave dysrhythmias induced by circular vection. Am J Physiol Gastrointest Liver Physiol. 2003;284:G481–489. doi: 10.1152/ajpgi.00164.2002. [DOI] [PubMed] [Google Scholar]
- 19.Meyer K, Schwartz J, Crater D, Keyes B. Zingiber officinale (ginger) used to prevent 8-Mop associated nausea. Dermatol Nurs. 1995;7:242–244. [PubMed] [Google Scholar]
- 20.Morrow GR, Hickok JT, Rosenthal SN. Progress in reducing nausea and emesis. Comparisons of ondansetron (Zofran), granisetron (Kytril), and tropisetron (Navoban) Cancer. 1995;76:343–357. doi: 10.1002/1097-0142(19950801)76:3<343::aid-cncr2820760302>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Morrow GR, Roscoe JA, Hickok JT, Banerjee TK, Issel B, Kirshner JJ. Time of first occurrence, severity, and persistence of nausea following initial chemotherapy in 322 patients: A URCC CCOP study. Supportive Care in Cancer. 2001;9:290. [Google Scholar]
- 22.Morrow GR, Roscoe JA, Kirshner JJ, Hynes HE, Rosenbluth RJ. Anticipatory nausea and vomiting in the era of 5-HT3 antiemetics. Support Care Cancer. 1998;6:244–247. doi: 10.1007/s005200050161. [DOI] [PubMed] [Google Scholar]
- 23.Mowrey DB, Clayson DE. Motion sickness, ginger, and psychophysics. Lancet. 1982;1:655–657. doi: 10.1016/s0140-6736(82)92205-x. [DOI] [PubMed] [Google Scholar]
- 24.Pace JC. Oral ingestion of encapsulated ginger and reported self-care actions for the relief of chemotherapy-associated nausea and vomiting. University of Alabama, City; 1986. Oral ingestion of encapsulated ginger and reported self-care actions for the relief of chemotherapy-associated nausea and vomiting. Editor (ed)ˆ(eds) Book. [Google Scholar]
- 25.Pillai AK, Sharma KK, Gupta YK, Bakhshi S. Anti-emetic effect of ginger powder versus placebo as an add-on therapy in children and young adults receiving high emetogenic chemotherapy. Pediatr Blood Cancer. 2011;56:234–238. doi: 10.1002/pbc.22778. [DOI] [PubMed] [Google Scholar]
- 26.Roscoe JA, Hickok JT, Morrow GR. Patient expectations as predictor of chemotherapy-induced nausea. Ann Behav Med. 2000;22:121–126. doi: 10.1007/BF02895775. [DOI] [PubMed] [Google Scholar]
- 27.Roscoe JA, Morrow GR, Colagiuri B, Heckler CE, Pudlo BD, Colman L, Hoelzer K, Jacobs A. Insight in the prediction of chemotherapy-induced nausea. Support Care Cancer. 2010;18:869–876. doi: 10.1007/s00520-009-0723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Pierce HI, Flynn PJ, Kirshner JJ, Moore DF, Atkins JN. The efficacy of acupressure and acustimulation wrist bands for the relief of chemotherapy-induced nausea and vomiting. A University of Rochester Cancer Center Community Clinical Oncology Program multicenter study. J Pain Symptom Manage. 2003;26:731–742. doi: 10.1016/s0885-3924(03)00254-9. [DOI] [PubMed] [Google Scholar]
- 29.Rosenthal DI, Mendoza TR, Chambers MS, Asper JA, Gning I, Kies MS, Weber RS, Lewin JS, Garden AS, Ang KK, X SW, Cleeland CS. Measuring head and neck cancer symptom burden: the development and validation of the M. D. Anderson symptom inventory, head and neck module. Head Neck. 2007;29:923–931. doi: 10.1002/hed.20602. [DOI] [PubMed] [Google Scholar]
- 30.Schwartzberg L, Szabo S, Gilmore J, Haislip S, Jackson J, Jain G, Balu S, Buchner D. Likelihood of a subsequent chemotherapy-induced nausea and vomiting (CINV) event in patients receiving low, moderately or highly emetogenic chemotherapy (LEC/MEC/HEC) Curr Med Res Opin. 2011;27:837–845. doi: 10.1185/03007995.2011.556603. [DOI] [PubMed] [Google Scholar]
- 31.Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Sontakke S, Thawani V, Naik MS. Ginger as an antiemetic in nausea and vomiting induced by chemotherapy: A randomized, cross-over, double-blind study. Indian Journal of Pharmacology. 2003;35:32–36. [Google Scholar]
- 33.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Statistical Methods in Medical Research. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 34.Visalyaputra S, Petchpaisit N, Somcharoen K, Choavaratana R. The efficacy of ginger root in the prevention of postoperative nausea and vomiting after outpatient gynaecological laparoscopy. Anaesthesia. 1998;53:506–510. doi: 10.1046/j.1365-2044.1998.00369.x. [DOI] [PubMed] [Google Scholar]
- 35.Zick SM, Ruffin MT, Lee J, Normolle DP, Siden R, Alrawi S, Brenner DE. Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Support Care Cancer. 2009;17:563–572. doi: 10.1007/s00520-008-0528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


