Abstract
Purpose
Aging is believed to affect epigenetic marking of brain DNA with 5-methylcytosine (5mC) and possibly via the 5mC to 5-hydroxymethylcytosine (5hmC) conversion by TET (ten-eleven translocation) enzymes. We investigated the impact of aging on hippocampal DNA 5-hydroxymethylation including in the sequence of aging-susceptible 5-lipoxygenase (5-LOX) gene.
Methods
Hippocampal samples were obtained from C57BL6 mice. Cellular 5hmC localization was determined by immunofluorescence. The global 5mC and 5hmC contents were measured with the corresponding ELISA. The 5-LOX 5hmC content was measured using a glucosyltransferase/enzymatic restriction digest assay. TET mRNA was measured using qRT-PCR.
Results
Global hippocampal 5hmC content increased during aging as did the 5hmC content in the 5-LOX gene. This occurred without alterations of TET1–3 mRNAs and without changes in the content of 8-hydroxy-2-deoxy-guanosine, a marker of non-enzymatic DNA oxidation.
Conclusions
The aging-associated increase of hippocampal 5hmC content (global and 5-LOX) appears to be unrelated to oxidative stress. It may be driven by an altered activity but not by the increased expression of the three TET enzymes. Global 5hmC content was increased during aging in the absence of 5mC decrease, suggesting that 5hmC could act as an epigenetic marker and not only as an intermediary in DNA demethylation. Further research is needed to elucidate the functional implications of the impact of aging on hippocampal cytosine hydroxymethylation.
Keywords: Epigenetic, 5-methylcytosine (5mC), 5-hydroxymethylcytosine (5hmC), 5-lipoxygenase (5-LOX), aging, hippocampus, ten-eleven translocation (TET)
1. Introduction
In addition to the dynamic and coordinated electrical activity of a multitude of cells, the dynamic and coordinated regulation of gene transcription is a hallmark of neuronal activity and brain functioning. Mechanisms of epigenetic marking of the genome, both nuclear (Riccio, 2010) and mitochondrial (Manev et al., in press), including neuronal activity-modified nuclear DNA methylation in the adult brain (Guo et al., 2011a), are emerging as key players in synaptic plasticity, learning and memory (Day and Sweatt, 2011; Miller and Sweatt 2007), and in the pathobiology of neurological and psychiatric disorders (Franklin and Mansuy, 2011; Guidotti and Grayson, 2011). Genomic DNA methylation that occurs at the 5’ carbon position of a base cytosine to form 5-methylcytosine (5mC) is catalyzed by DNA methyltransferases (DNMTs). This 5mC marking of DNA is a gene regulation mechanism (Bird, 2002), typically viewed as a pathway to gene silencing, although recent data suggest a more complex role for 5mC, which also includes stimulation of gene expression (Wu et al., 2010).
Both increases and decreases of DNA methylation have been observed during aging (Richardson, 2003). In the human brain, distinct DNA methylation changes have been shown to correlate with chronological age (Hernandez et al., 2011). In mice, changes in DNA methylation patterns have been documented in models of brain aging in vivo (Chouliaras et al., 2011; Dzitoyeva et al., 2009; Takasugi, 2011) and neuronal aging in vitro (Imbesi et al., 2009). Furthermore, pathological DNMT activity and aberrant 5mC formation have been linked to neurodegeneration and apoptotic neuronal death (Chestnut et al., 2011; Hernandez and Singleton, 2011). In these studies, neuroprotection was provided by DNMT inhibitors (Chestnut et al., 2011).
Another epigenetic mark, 5-hydroxymethylcytosine (5hmC), appears to be particularly susceptible to developmental and aging-associated modifications (for review, see Flax and Soloway, 2011). Although 5hmC can be produced by an action of free radicals on 5mC in artificial conditions (Castro et al., 1996), recently, a pathway was discovered (Tahiliani et al., 2009) that includes TET (ten-eleven translocation) enzymes (TET1, TET2, TE3) which catalyze the conversion of 5mC into 5hmC. Measurements of 8-hydroxy-2-deoxy-guanosine (8-OH-dG) are used as evidence of non-enzymatic DNA oxidation (Izzotti et al., 1999). In the brain, the formation of 5hmC has been demonstrated in the absence of 8-OH-dG alterations (Kriaucionis and Heintz, 2009). The exact nature of the physiological implications of 5mC to 5hmC conversion is currently being investigated. On one hand, 5hmC is viewed as an intermediary in DNA demethylation (Nabel and Kohli, 2011). On the other, it has been proposed that, similar to 5mC DNA marking, genomic DNA 5hmC marking may function as an epigenetic mechanism by itself (Branco et al., 2011). For example, 5hmC located in gene bodies was found to be associated with higher levels of gene transcription (Jin et al., 2011; Song et al., 2011). In the brain, 5hmC appears to be particularly abundant in the cerebellum (e.g., in Purkinje neurons) (Kriaucionis and Heintz, 2009) and the hippocampus (Münzel et al., 2010). The pattern of 5hmC localization in DNA extracted from human and mouse brain is highly susceptible to aging-associated modifications (Münzel et al., 2011; Song et al., 2011; Szulwach et al., 2011). It has been observed that brain 5hmC accumulates at discrete loci and that 5hmC interacts with DNA methyl-binding protein MeCP2, which is an important transcriptional regulator (Szulwach et al., 2011). Thus, aging-associated 5hmC alterations appear to be a likely participant in neuroplasticity of aging brain.
In this work, we used a mouse model of aging to investigate the impact of aging on hippocampal DNA 5-hydroxymethylation. In addition to measurements of global DNA modifications, we investigated DNA modifications in the mouse 5-lipoxygenase (5-LOX) gene. In mice, hippocampal 5-LOX expression increases during aging (Chinnici et al., 2007). Furthermore, in mouse models of aging-associated Alzheimer’s disease (AD) an overexpression of brain 5-LOX is associated with worsening of AD-like phenotypes (Chu et al., 2012) whereas 5-LOX knockdown reduces AD-like pathology (Firuzi et al., 2008).
2. Material and methods
2.1. Animals
Hippocampal samples were obtained from three cohorts of C57BL6 mice. In cohort A, mouse pups (2-day-old) and 2-week-old mice were obtained from Jackson Laboratories (Bar Harbor, ME). In cohort B, 2-month-old and 22-month-old male mice were obtained from the National Institute on Aging (Bethesda, MD). They were housed in groups of 4 to 6 in a temperature-controlled room and had free access to laboratory chow and water. Hippocampal samples were obtained following application of lethal anesthesia (160 mg/kg ketamine; Sigma, St. Louis, MO) and transcardial perfusion with 0.9% ice-cold saline to remove the circulating blood cells (i.e., until the outflow from the right atrium was clear). In cohort C, whole brains of 4-month-old and 24-month-old male mice were obtained from the Aged Rodent Tissue Bank (National Institute on Aging, Bethesda, MD). The main purpose of including this second cohort of young and old mice was to verify the reproducibility of observations made in cohort B. Animal procedures were conducted in accordance with the National Institutes on Health guidelines for the use of experimental animals and were approved by the University of Illinois Animal Care and Use Committee.
2.2. 5hmC immunofluorescence
The brains for immunofluorescence were obtained after a transcardial perfusion with 4% paraformaldehyde, postfixation overnight, and cryoprotection in 30% sucrose in a phosphate buffer saline at 4°C for a week (Chen et al., 2010). For visualizing the localization of 5hmC in the mouse hippocampus, 30 µm sagittal brain sections of 4-month old C57BL/6 mice were used. Sections were treated with 1N HCl for 15 min and incubated overnight at 4°C with primary antibodies [rabbit anti-5hmC (1:200, Active Motif)] in 10% donkey serum and 0.25% Triton X-100. For secondary detection, we used rhodamine-conjugated donkey anti-rabbit (1:200, Jackson Immunoresearch, West Grove, PA) antibodies. As a negative control, sections were processed in the absence of the primary antibody – no staining was observed (not shown). Images were captured using a fluorescence microscope (Carl Zeiss) equipped with a 5X objective lens.
2.3. Quantitative real time PCR (qRT-PCR) mRNA assay
Total RNA was extracted from brain samples with TRIzol Reagent (Invitrogen, Carlsbad, CA) and treated with DNase (Ambion Inc., Austin, TX). RNA was reverse transcribed with M-MLV Reverse Transcriptase (Invitrogen). The qRT-PCR was performed on a Stratagene Mx3005P QPCR System (Agilent Technologies, Santa Clara, CA) machine with a Maxima SYBR Green/ROX Master Mix (Fermentas Inc., Glen Burnie, MD). Data were normalized against a cyclophilin internal control and presented as a coefficient of variation, calculated with the formula 2−[ΔCt(target)− ΔC(input)] as previously described (Dzitoyeva et al., 2009). The list of primers used is reported in Table1.
Table 1.
The list of primers used for a qRT-PCR mRNA assay.
| Gene and PCR product size (nt) |
Primer sequence - forward | Primer sequence - reverse |
|---|---|---|
| Cyclophilin A; NM_008907 (162) | 5’-agcatacaggtcctggcatcttgt-3’ | 5’-aaacgctccatggcttccacaatg-3’ |
| TET1; NM_027384 (182) | 5’-acgctggaacaagtggtagccata-3’ | 5’-tgaacgtttgggtcttggaggtct-3’ |
| TET2; NM_001040400 (140) | 5’-gccctttgaatgaatccagcagca-3’ | 5’-tgccctcccaagactcttcatgtt-3’ |
| TET3; NM_183138 (179) | 5’-aaccagaacgccaaggtcagtagt-3’ | 5’-ttgatcttctctggcgtgctcagt-3’ |
2.4. Enzyme-linked immunosorbent assay (ELISA) of global DNA 5hmC and 5mC contents
The 5hmC content of extracted DNA was measured by a hydroxymethylated DNA quantification kit (Epigentek, Brooklyn, NY). Briefly, 100 ng DNA was bound to a 96-well plate. The hydroxymethylated fraction of DNA was detected using its respective capture and detection antibodies and quantified colorimetrically by reading the absorbance at 450 nm in a microplate spectrophotometer (Bio-Rad, Model 550, Hercules, CA). For measurement of 5mC contents, an alternative (methylated DNA quantification) kit from Epigentek was utilized. The results are expressed in units calculated according to the manufacturer’s manual.
2.5. ELISA assay of DNA 8-hydroxy-2-deoxy-guanosine (8-OH-dG) content
The DNA content of 8-OH-dG was measured with a kit (Cayman Chemical, Ann Arbor, MI) following the corresponding manual. Briefly, 600 ng DNA was digested by nuclease P1 for 30 min at 37°C and incubated with alkaline phosphatase for another 30 min. After boiling for 10 min, the DNA samples were loaded into a 96-well plate and incubated overnight at 4°C. The plate was developed for 2 h in the dark and read at 405 nm.
2.6. Sequence-specific DNA 5hmC assay
The 5hmC modifications of 5-LOX DNA sequences were quantified using an assay that involves a 5hmC-sensitive enzymatic restriction digest combined with glucosylation and qRT-PCR. The principle of this method has been recently described by Davis and Vaisvila (2011). We have modified this assay by selecting a different set of restriction enzymes. Hence, we identified multiple recognition sites for a number of 5hmC-sensitive restriction enzymes in the 5’UTR/promoter and first exon/intron regions of the mouse 5-LOX gene. The following enzymes were selected based on their published characteristics (REBASE Methylation Sensitivity: http://rebase.neb.com/rebase/rebms.html) and their recognition sequences are indicated in parentheses: TseI [GCWGC], NmeAIII [GCCGAG (21/19)], EciI [GGCGGA (11/9)], SfaNI [GCATC (5/9)], and EcoP15I [CAGCAG (25/27)] (New England Biolabs; Ipswich, MA). The principle of the method has been described by Davis and Vaisvila (2011). Prior to restriction digestion, DNA was glucosylated with T4 glucosyl transferase (New England Biolabs) and aliquots were digested with the indicated enzymes in separate reactions. Reaction conditions were selected according to the manufacturer’s instructions. Upon completion, the reaction mixes were diluted with water and an aliquot, approximately 100 ng of digested DNA, was used in qRT-PCR as described above. Undigested DNA was used as an input control. The following are the primers used in this assay. 5’UTR/promoter: forward 5’-agaaggagagaaggatgcgt-3’, reverse 5’-catgactccgggcaagtgagtgct-3’; first exon/intron: forward 5’-agcactcacttgcccggagtcatg-3’, reverse 5’-agtcatcaggaagtctagggtgcct-3’; these primers amplify a 230 nt and a 378 nt fragment, respectively. Data are presented as units of coefficient of variation (units) (Dzitoyeva et al., 2009).
2.7. Statistical analysis
For statistical analysis, we used SPSS software (version 18.0). Data were analyzed by ANOVA followed by an independent sample t-test. Results are expressed as the mean ± standard error mean (SEM). P<0.05 values were accepted as statistically significant.
3. Results
3.1. Hippocampal global 5hmC DNA content increases during development and aging
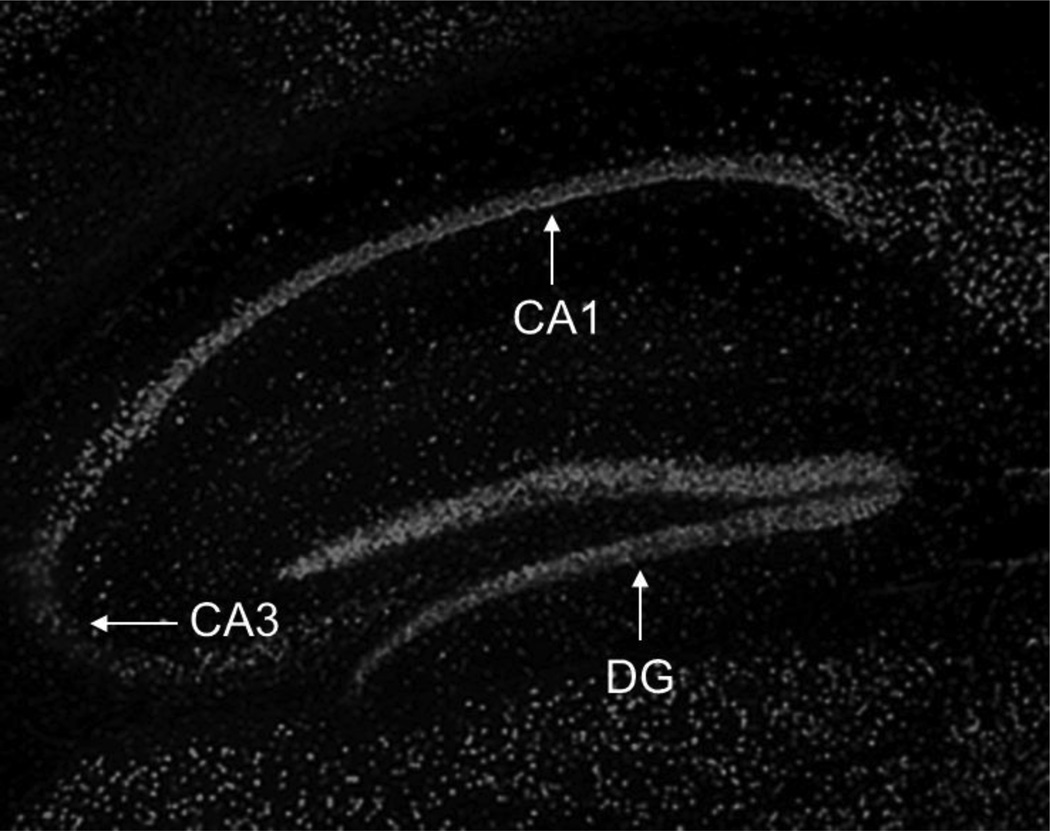
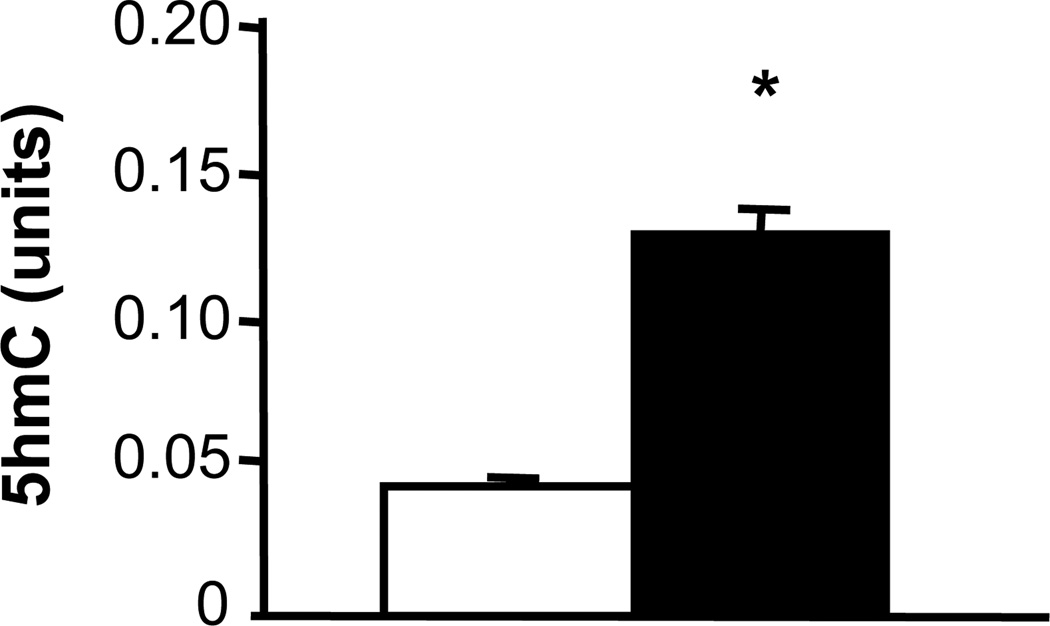
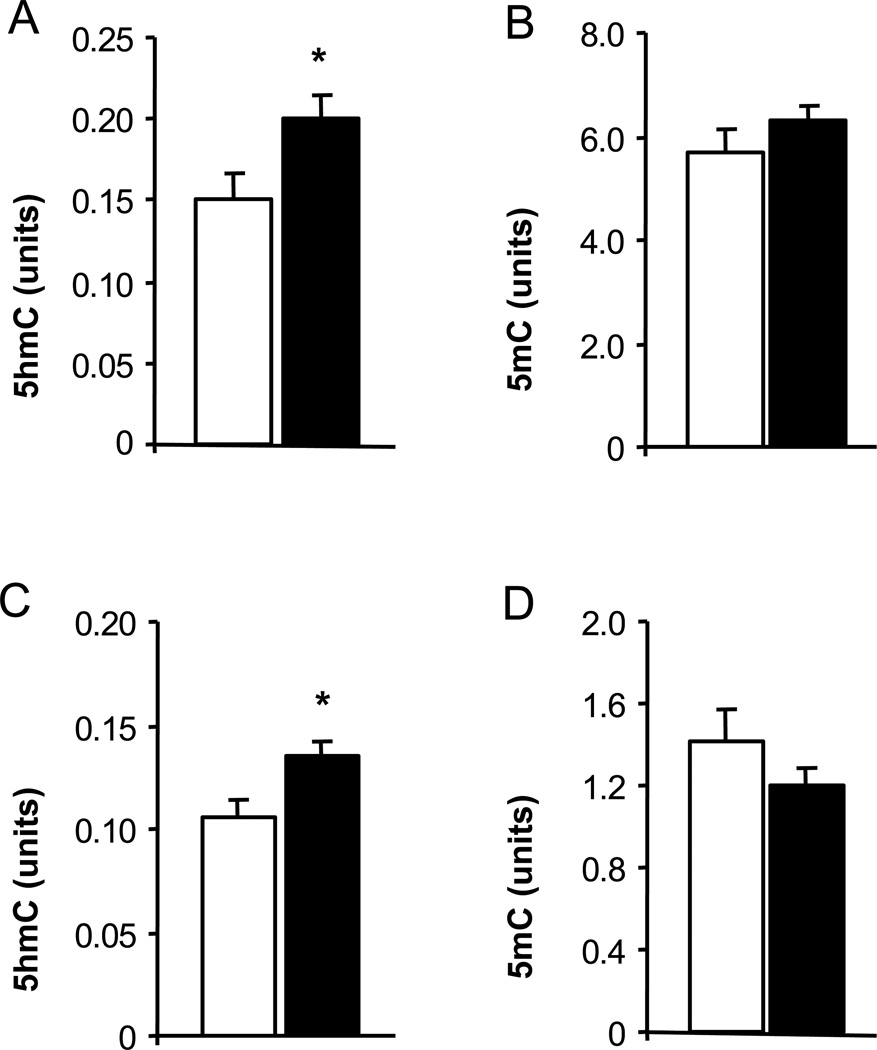
Using 5hmC immunostaining, we evaluated the distribution and localization of 5hmC immunoreactivity in the mouse hippocampus. We observed a heterogeneous 5hmC distribution with a strong 5hmC immunofluorescence in the dentate gyrus and in the CA1 region (Fig. 1). Previous work using a quantitative liquid chromatography-mass spectrometry method for 5hmC measurement found that hippocampal 5hmC content increases during mouse development; e.g. from post-natal day 1 to 90 days of age (Münzel et al., 2010). Using the ELISA method for global 5hmC quantification, we found significantly higher content of hippocampal 5hmC in samples from 2-week-old mice compared to samples from 2-day-old pups (Fig. 2). The effect of aging on hippocampal global 5hmC content was measured in two cohorts of young and old mice (Fig. 3). In both cohorts, 5hmC content was greater in the hippocampi of old vs. corresponding young mice, whereas the content of global 5mC did not differ between the age groups. Furthermore, the hippocampal content of global 8-OH-dG, a marker of non-enzymatic DNA oxidation (Nicolle et al., 2001), did not differ between the age groups (4-month-old: 14.4 ± 1,4; 24-month-old: 13.2 ± 1.0; pg/µg DNA; n = 5).
Fig. 1.
5hmC immunofluorescence in the hippocampus of adult mice. The fluorescence of the rhodamine-conjugated secondary antibody was captured at a 5X objective lens. No signal was obtained in the absence of the primary 5hmC antibody (not shown). Strong 5hmC immunolabelling was found in the dentate gyrus (DG) and in the CA1 region.
Fig. 2.
Global 5hmC content in the hippocampus increases during mouse development. DNA was extracted from hippocampal samples of 2-day-old mouse pups (open bar) and 2-week-old mice (filled bar). DNA was analyzed with a 5hmC ELISA assay. Results are expressed in units (mean ± SEM; n = 6; *P<0.05 compared to the corresponding 2-day-old pups).
Fig. 3.
Global 5hmC and 5mC content in the hippocampus of young and old mice. DNA was extracted from hippocampal samples of 2- and 22-month-old mice (A, B), and 4- and 24-month-old mice (C, D). DNA was analyzed with 5hmC and 5mC ELISA assays. Results (open bars, young mice; filled bars, old mice) are expressed in units (mean ± SEM; n = 5–6; *P<0.05 compared to the corresponding young group).
3.2. Aging increases 5hmC content in hippocampal 5-LOX DNA sequences
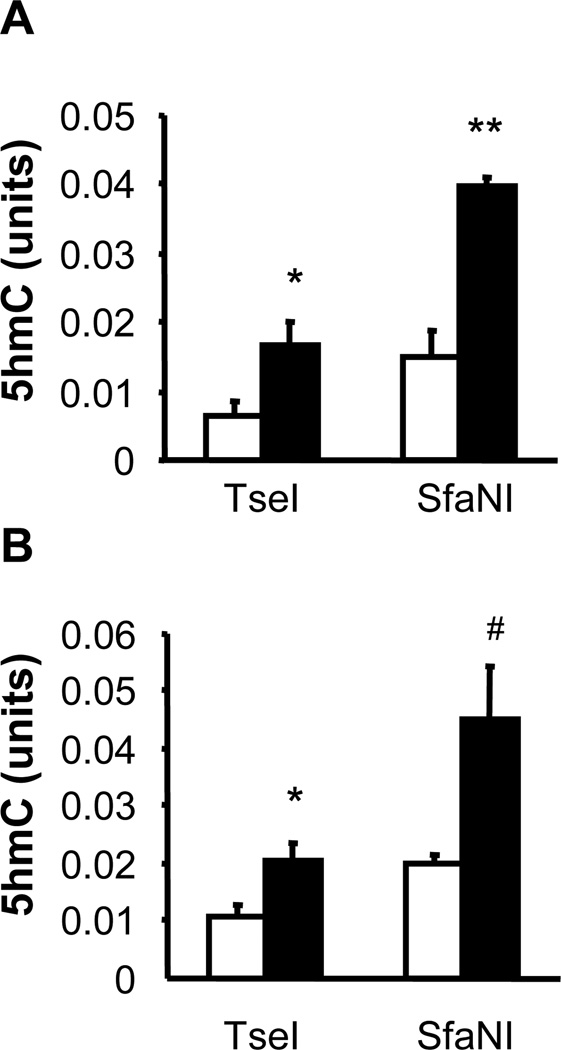
Previous reports found that 5-LOX expression in the brain increases during aging (Chinnici et al., 2007; Uz et al., 1998) and in Alzheimer’s disease (Firuzi et al., 2008; Ikonomovic et al., 2008; Wang et al., 2011), but the aging-affected methylation status of the mouse 5-LOX gene has not been consistently related with the rate of 5-LOX transcription (Dzitoyeva et al., 2009). It has been noticed that global 5hmC changes do not necessary reflect the same type of 5hmC changes in individual gene sequences. To test whether aging affects gene-specific 5hmC content, we analyzed two areas of the mouse 5-LOX gene, a 5’UTR/promoter region and a first exon/intron region. The selected 5-LOX 5’UTR/promoter region contains restriction sites for all five enzymes used in our assay of sequence-specific 5hmC content. Aging increased 5hmC content in the 5-LOX promoter in both cohorts of aging mice (Fig. 4). The increase was more prominent in 24-month-old vs. 4-month-old mice (in 4 out of 5 restriction sites; Fig. 4B) than in 22-month-old vs. 2-month-old mice (in 3 out of 5 restriction sites; Fig. 4A). The selected 5-LOX exon/intron region contains restriction sites for only two of the enzymes used in our assay. Also, in this region the 5hmC content increased during aging (Fig. 5).
Fig. 4.
Effect of aging on hippocampal 5hmC content in the 5’UTR/promoter sequence of the mouse 5-LOX gene. DNA was extracted from hippocampal samples of 2- and 22-month-old mice (A), and 4- and 24-month-old mice (B). The sequence-specific 5hmC content was assayed with the five indicated enzymes (TseI, SfaNI, EcoP15I, EciI, and NmeAIII) as described in the text. Results (open bars, young mice; filled bars, old mice) are expressed in units (mean ± SEM; n = 5–6; *P<0.05, **P<0.01 compared to the corresponding young group).
Fig. 5.
Effect of aging on hippocampal 5hmC content in the first exon/intron sequence of the mouse 5-LOX gene. DNA was extracted from hippocampal samples of 2- and 22-month-old mice (A), and 4- and 24-month-old mice (B). Sequence-specific 5hmC content was assayed with the two indicated enzymes (TseI and SfaNI) as described in the text. Results (open bars, young mice; filled bars, old mice) are expressed in units (mean ± SEM; n = 4–5; *P<0.05, **P<0.01 compared to the corresponding young group; #not significant; in 2 out of 5 samples we did not detect 5hmC with this assay).
3.3. Aging does not affect hippocampal mRNA content of TET enzymes
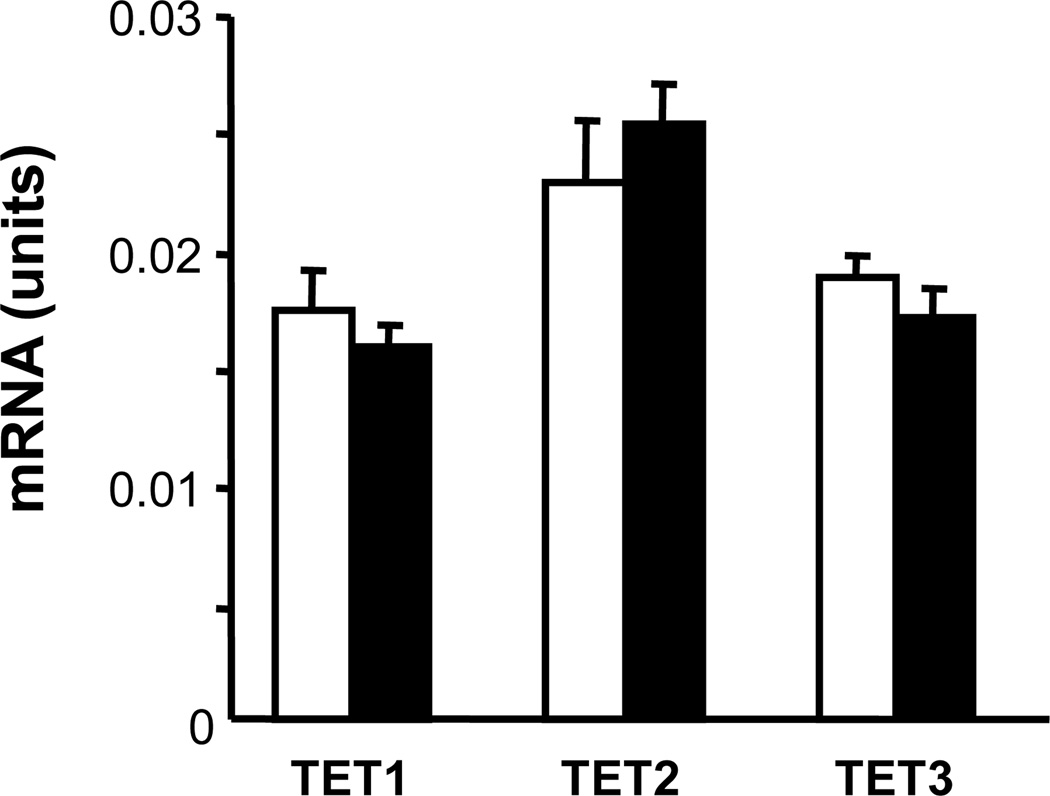
Since TET enzymes play a crucial role in the conversion of 5mC to 5hmC, we measured the effect of aging on TET1, TET2, and TET3 mRNAs in the hippocampus of young and old mice. The expression of none of the three TET mRNAs was significantly altered during aging (Fig. 6).
Fig. 6.
TET mRNA content in the hippocampus of young and old mice. A qRT-PCR assay was used with hippocampal samples of 4-month-old (open bars) and 24-month-old (filled bars) mice. Results are expressed in units (mean ± SEM; no significant differences were observed).
4. Discussion
In this work, we demonstrated that aging increases not only the global 5hmC content in hippocampal DNA but also 5hmC content in selected DNA sequences of the mouse 5-LOX gene. Generally, increased nucleic acid oxidation has been documented in aging-associated neurological disorders (Moreira et al., 2008). Although 5hmC can be produced by an action of free radicals on 5mC in artificial conditions (Castro et al., 1996), no evidence has been provided to support a direct physiological role for oxidative stress in the formation of 5hmC in the DNA of living systems. Using 8-OH-dG as a marker of oxidative DNA damage it was found that 8-OH-dG levels are increased in DNA samples extracted from total brain homogenates of old vs. young mice (Izzotti et al., 1999). Subsequent studies have shown that aging affects brain 8-OH-dG content in a region- and cell type-specific manner; in the hippocampus 8-OH-dG changes are most prominent in the dentate gyrus and in area CA1 (Nicolle et al., 2001). Furthermore, during normal hippocampal aging, increased DNA oxidation was evidenced by elevated levels of 8-OH-dG only in the hippocampi of a subset of old subjects; i.e., only in subjects with learning impairment whereas the hippocampal 8-OH-dG content did not differ between young and behaviorally unimpaired old subjects (Nicolle et al., 2001). The latter finding is similar to our observation of unaltered hippocampal 8-OH-dG content in old mice. Our findings that hippocampal 5hmC content increased without concomitant alterations of hippocampal 8-OH-dG content suggest that aging-associated 5hmC elevations can occur in the absence of non-specific oxidative DNA modifications.
Recent research has attributed the origin of 5hmC in genomic DNA to TET enzymatic activity (Branco et al., 2011; Ito et al., 2010, 2011; Tahiliani et al., 2009). TET proteins (TET1, TET2, TET3) are Fe(II)2-oxoglutarate-dependent dioxygenases that oxidize 5mC. A TET1-mediated hydroxylation of 5mC has recently been shown to be capable of promoting active DNA demethylation in the adult brain (Guo et al., 2011b). Hence, TET-produced 5hmC may serve as an intermediary for the removal of methylated cytosines (Bhutani et al., 2011; Nabel and Kohli, 2011). In our experimental conditions, an increase in 5hmC was not accompanied by a 5mC decrease. This may have occurred because no subsequent demethylation had taken place in these hippocampal samples during aging. Alternatively, as revealed by our immunolocalization of hippocampal 5hmC, this DNA marker appears not to be uniformly distributed in the hippocampus. Instead, 5hmC immunolabelling is most prominently present in the dentate gyrus and CA1 cells; similar localization of hippocampal 5hmC has been observed by others (Münzel et al., 2011). Thus, if the aging-induced 5hmC conversion was accompanied by a simultaneous 5mC reduction in a subset of 5hmC-positive cells, this 5mC reduction easily could have been missed by measurements of global 5mC content in DNA samples extracted from the entire hippocampus.
Our assay of global 5hmC measurements confirmed the previous results obtained with a different quantification technique (Münzel et al., 2010), which revealed significantly higher 5hmC levels in the hippocampus of 90-day-old mice compared to newborn mice. Similar to our results from our aging model, which revealed 5hmC elevations without concomitant changes (e.g., reduction) of 5mC levels, these authors noted that also during development, hippocampal 5hmC increases in the absence of 5mC changes (Münzel et al., 2010).
The expression rates of various TET enzymes are influenced by developmental stages. TET1 appears to be the primary TET enzyme in embryonic stem cells and TET3 is the most abundant TET enzyme in oocytes and zygotes (Branco et al., 2011). We found transcripts of all three TET enzymes in mouse hippocampus. However, the mRNA levels of these enzymes were not affected by aging. Thus, if TET enzymes are involved in the observed aging-increased 5hmC levels, their involvement would be through altered enzyme activity rather than through increased TET expression. Further research is needed to explore this possibility.
The aging-associated increase of global 5hmC content observed in our study is the reflection of the net-effect of aging on 5hmC content in the entire DNA. This net effect is likely a result of multiple and possibly even bidirectional changes in 5hmC content within individual genes and various other DNA sequences. In brain DNA, a number of 5hmC loci have been identified both in gene promoters and in gene bodies (Kinney et al., 2011; Jin et al., 2011; Song et al., 2011). As an example, we selected the 5-LOX gene, a known target of aging (Chinnici et al., 2007; Chu and Praticò, 2009; Uz et al., 1998), and found that both the promoter and the gene body contained higher levels of 5hmC in old vs. young hippocampal samples. The nature of the association between 5hmC levels and gene expression is currently unclear. It was suggested that 5hmC may have a limited impact on the transcription of its directly associated genes (Xu et al., 2011). On the other hand, it was noted that 5hmC levels inversely correlate with MeCP2 levels and it was suggested that an interaction of methyl-binding proteins with 5hmC differs from their interaction with 5mC (Szulwach et al., 2011) indicating a possible mechanism of 5hmC-modified gene expression. In contrast to the human 5-LOX gene, which is regulated by epigenetic DNA methylation mechanisms (Katryniok et al., 2010; Zhang et al., 2004), the role of DNA methylation in the regulation of the mouse 5-LOX gene, which contains only a few CpG dinucleotides in the promoter region, is less clear (Dzitoyeva et al., 2009). On the other hand, it is possible that DNA hydroxymethylation of the 5-LOX gene may be less species-specific than DNA methylation. Namely, Szulwach et al. (2011) compared the 5hmC content of mouse and human cerebellum and found strong conservation of 5hmC targeting. Further studies are needed to evaluate the putative functional implications of the mouse 5-LOX DNA hydroxymethylation found in our experiments and to verify whether similar mechanisms apply to human 5-LOX gene and whether DNA hydroxymethylation plays a role in the recently observed association between 5-LOX and the payhobiology of Alzheimer’s disease (Chu et al., 2012; Firuzi et al., 2008).
In conclusion, our results point to a stimulatory effect of aging on hippocampal global 5hmC content that appears to be unrelated to oxidative stress. This 5hmC increase occurred in the absence of 5mC changes, suggesting that 5hmC could act as an epigenetic marker and not only as an intermediate molecule in DNA demethylation. Further research is needed to elucidate the functional implications of the impact of aging on hippocampal cytosine hydroxymethylation, including in specific DNA sequences such as the 5-LOX gene.
Acknowledgements
This research was supported by the National Institutes of Health (NIH) grant R01AG015347 from the National Institute on Aging (NIA) to H.M. NIH and NIA had no role in the preparation, review, or approval of this manuscript.
Abbreviations
- AD
Alzheimer’s disease
- DG
dentate gyrus
- DNMTs
DNA methyltransferases
- 8-OH-dG
8-hydroxy-2-deoxy-guanosine
- 5hmC
5-hydroxymethylcytosine
- 5-LOX
5-lipoxygenase
- 5mC
5-methylcytosine
- qRT-PCR
quantitative real time PCR
- TET
ten-eleven translocation
Footnotes
The content is solely the responsibility of the authors and does not necessary represent the official views of the NIA.
References
- Bhutani N, Burns DM, Blau HM. DNA demethylation dynamics. Cell. 2011;146:866–872. doi: 10.1016/j.cell.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet. 2011 doi: 10.1038/nrg3080. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Castro GD, Díaz Gómez MI, Castro JA. 5-Methylcytosine attack by hydroxyl free radicals and during carbon tetrachloride promoted liver microsomal lipid peroxidation: structure of reaction products. Chem Biol Interact. 1996;99:289–299. doi: 10.1016/0009-2797(95)03680-6. [DOI] [PubMed] [Google Scholar]
- Chen H, Dzitoyeva S, Manev H. 5-Lipoxygenase in mouse cerebellar Purkinje cells. Neuroscience. 2010;171:383–389. doi: 10.1016/j.neuroscience.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ. Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci. 2011;31:16619–16636. doi: 10.1523/JNEUROSCI.1639-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnici CM, Yao Y, Praticò D. The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol Aging. 2007;28:1457–1462. doi: 10.1016/j.neurobiolaging.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Chouliaras L, van den Hove DL, Kenis G, Keitel S, Hof PR, van Os J, Steinbusch HW, Schmitz C, Rutten BP. Prevention of age-related changes in hippocampal levels of 5-methylcytidine by caloric restriction. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.06.003. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Giannopoulos PF, Ceballos-Diaz C, Golde TE, Praticò D. Adeno-associated virus-mediated brain delivery of 5-lipoxygenase modulates the AD-like phenotype of APP mice. Mol Neurodegener. 2012;7:1. doi: 10.1186/1750-1326-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Praticò D. The 5-lipoxygenase as a common pathway for pathological brain and vascular aging. Cardiovasc Psychiatry Neurol. 2009;2009:174657. doi: 10.1155/2009/174657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T, Vaisvila R. High sensitivity 5-hydroxymethylcytosine detection in Balb/C brain tissue. J Vis Exp. 2011;48:2661. doi: 10.3791/2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzitoyeva S, Imbesi M, Ng LW, Manev H. 5-Lipoxygenase DNA methylation and mRNA content in the brain and heart of young and old mice. Neural Plast. 2009;2009:209596. doi: 10.1155/2009/209596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Praticò D. 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer's disease. FASEB J. 2008;22:1169–1178. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flax JD, Soloway PD. Methylation on the mind. Nat Neurosci. 2011;14:1494–1496. doi: 10.1038/nn.2988. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Mansuy IM. The involvement of epigenetic defects in mental retardation. Neurobiol Learn Mem. 2011;96:61–67. doi: 10.1016/j.nlm.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Grayson DR. A neurochemical basis for an epigenetic vision of psychiatric disorders (1994–2009) Pharmacol Res. 2011;64:344–349. doi: 10.1016/j.phrs.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Ma DK, Mo H, Ball MP, Jang MH, Bonaguidi MA, Balazer JA, Eaves HL, Xie B, Ford E, Zhang K, Ming GL, Gao Y, Song H. Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat Neurosci. 2011a;14:1345–1351. doi: 10.1038/nn.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011b;145:423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez DG, Singleton AB. Using DNA methylation to understand biological consequences of genetic variability. Neurodegener Dis. 2011 doi: 10.1159/000333097. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez DG, Nalls MA, Gibbs JR, Arepalli S, van der Brug M, Chong S, Moore M, Longo DL, Cookson MR, Traynor BJ, Singleton AB. Distinct DNA methylation changes highly correlated with chronological age in the human brain. Hum Mol Genet. 20:1164–1172. doi: 10.1093/hmg/ddq561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Abrahamson EE, Uz T, Manev H, Dekosky ST. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer's disease. J Histochem Cytochem. 2008;56:1065–1073. doi: 10.1369/jhc.2008.951855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbesi M, Dzitoyeva S, Ng LW, Manev H. 5-Lipoxygenase and epigenetic DNA methylation in aging cultures of cerebellar granule cells. Neuroscience. 2009;164:1531–1537. doi: 10.1016/j.neuroscience.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Cartiglia C, Taningher M, De Flora S, Balansky R. Age-related increases of 8-hydroxy-2'-deoxyguanosine and DNA-protein crosslinks in mouse organs. Mutat Res. 1999;446:215–223. doi: 10.1016/s1383-5718(99)00189-8. [DOI] [PubMed] [Google Scholar]
- Jin SG, Wu X, Li AX, Pfeifer GP. Genomic mapping of 5-hydroxymethylcytosine in the human brain. Nucleic Acids Res. 2011;39:5015–5024. doi: 10.1093/nar/gkr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katryniok C, Schnur N, Gillis A, von Knethen A, Sorg BL, Looijenga L, Rådmark O, Steinhilber D. Role of DNA methylation and methyl-DNA binding proteins in the repression of 5-lipoxygenase promoter activity. Biochim Biophys Acta. 2010;1801:49–57. doi: 10.1016/j.bbalip.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kinney SM, Chin HG, Vaisvila R, Bitinaite J, Zheng Y, Estève PO, Feng S, Stroud H, Jacobsen SE, Pradhan S. Tissue-specific distribution and dynamic changes of 5-hydroxymethylcytosine in mammalian genomes. J Biol Chem. 2011;286:24685–24693. doi: 10.1074/jbc.M110.217083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Dzitoyeva S, Chen H. Mitochondrial DNA: A blind spot in neuroepigenetics. Biomol Concepts. doi: 10.1515/bmc-2011-0058. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G. Nucleic acid oxidation in Alzheimer disease. Free Radic Biol Med. 2008;44:1493–1505. doi: 10.1016/j.freeradbiomed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Münzel M, Globisch D, Brückl T, Wagner M, Welzmiller V, Michalakis S, Müller M, Biel M, Carell T. Quantification of the sixth DNA base hydroxymethylcytosine in the brain. Angew Chem Int Ed Engl. 2010;49:5375–5377. doi: 10.1002/anie.201002033. [DOI] [PubMed] [Google Scholar]
- Münzel M, Globisch D, Carell T. 5-Hydroxymethylcytosine, the sixth base of the genome. Angew Chem Int Ed Engl. 2011;50:6460–6468. doi: 10.1002/anie.201101547. [DOI] [PubMed] [Google Scholar]
- Nabel CS, Kohli RM. Molecular biology. Demystifying DNA demethylation. Science. 2011;333:1229–1230. doi: 10.1126/science.1211917. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience. 2001;107:415–431. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- Riccio A. Dynamic epigenetic regulation in neurons: enzymes, stimuli and signaling pathways. Nat Neurosci. 2010;13:1330–1337. doi: 10.1038/nn.2671. [DOI] [PubMed] [Google Scholar]
- Richardson B. Impact of aging on DNA methylation. Ageing Res Rev. 2003;2:245–261. doi: 10.1016/s1568-1637(03)00010-2. [DOI] [PubMed] [Google Scholar]
- Song CX, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, Li Y, Chen CH, Zhang W, Jian X, Wang J, Zhang L, Looney TJ, Zhang B, Godley LA, Hicks LM, Lahn BT, Jin P, He C. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol. 2011;29:68–72. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulwach KE, Li X, Li Y, Song CX, Wu H, Dai Q, Irier H, Upadhyay AK, Gearing M, Levey AI, Vasanthakumar A, Godley LA, Chang Q, Cheng X, He C, Jin P. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat Neurosci. 2011;14:1607–1616. doi: 10.1038/nn.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi M. Progressive age-dependent DNA methylation changes start before adulthood in mouse tissues. Mech Ageing Dev. 2011;132:65–71. doi: 10.1016/j.mad.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Uz T, Pesold C, Longone P, Manev H. Aging-associated up-regulation of neuronal 5-lipoxygenase expression: putative role in neuronal vulnerability. FASEB J. 1998;12:439–449. doi: 10.1096/fasebj.12.6.439. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Zhou B, Mao WW, Yin M. Overexpression of 5-lipoxygenase increases the neuronal vulnerability of PC12 cells to aβ(42) Yakugaku Zasshi. 2011;131:1843–1853. doi: 10.1248/yakushi.131.1843. [DOI] [PubMed] [Google Scholar]
- Wu H, Coskun V, Tao J, Xie W, Ge W, Yoshikawa K, Li E, Zhang Y, Sun YE. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science. 2010;329:444–448. doi: 10.1126/science.1190485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wu F, Tan L, Kong L, Xiong L, Deng J, Barbera AJ, Zheng L, Zhang H, Huang S, Min J, Nicholson T, Chen T, Xu G, Shi Y, Zhang K, Shi YG. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol Cell. 2011;42:451–464. doi: 10.1016/j.molcel.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chen CQ, Manev H. DNA methylation as an epigenetic regulator of neural 5-lipoxygenase expression: evidence in human NT2 and NT2-N cells. J Neurochem. 2004;88:1424–1430. doi: 10.1046/j.1471-4159.2003.02275.x. [DOI] [PubMed] [Google Scholar]