Abstract
In 1954, Penfield and Jasper briefly described that percepts of unpleasant odor were elicited by intraoperative electrical stimulation of the olfactory bulb in patients with epilepsy. Since then, few peer-reviewed studies have reported such phenomena elicited by stimulation mapping via subdural electrodes implanted on the ventral surface of frontal lobe. Here, we determined what types of olfactory hallucinations could be reproduced by such stimulation in children with focal epilepsy. This study included 16 children (age range: 5 to 17 years), who underwent implantation of subdural electrodes to localize the presumed epileptogenic zone and eloquent areas. Pairs of electrodes were electrically stimulated and clinical responses were observed. In case a patient reported a perception, she/he was asked to describe its nature. We also described the stimulus parameters to elicit a given symptom. Eleven patients reported a perception of smell in response to electrical stimulation while the remaining five did not. Nine patients perceived an unpleasant smell (like bitterness, smoke, or garbage), while two perceived a pleasant smell (like strawberry or good food). Such olfactory hallucinations were induced by stimulation proximal to the olfactory bulb or tract on either hemisphere but not by that of orbitofrontal gyri lateral to the medial orbital sulci. The range of stimulus parameters employed to elicit olfactory hallucinations was comparable to those for other sensorimotor symptoms. Our systematic study of epileptic children replicated stimulation-induced olfactory hallucinations. We failed to provide evidence that a positive olfactory perception could be elicited by conventional stimulation of secondary olfactory cortex alone.
Keywords: Pediatric epilepsy surgery, Intracranial electrodes, Electrical stimulation, Functional cortical mapping, Odor, Smell, Orbitofrontal gyrus, Perception, Stimulus parameters, Presurgical evaluation
INTRODUCTION
The ability to perceive odors is important for our life. Smell of smoke, for example, helps us in detecting potential dangers in the environment. Studies of human anatomy and the effect of lesioning have suggested that the primary olfactory system includes the olfactory bulb and tract located on the ventral surface of frontal lobe, as well as the piriform cortex, amygdala and uncus in the anterior-medial temporal lobe [1,2]. Damage involving the primary olfactory system can result in complete or partial loss of odor perception (i.e.: anosmia or hyposmia) [2–4]. The orbitofrontal cortex is considered to be a part of the secondary olfactory cortex [5,6]; damage involving the orbitofrontal cortex can result in deficits in discriminating odor quality or impaired odor memory [7,8].
In 1954, Penfield and Jasper briefly described that intraoperative electrical stimulation of the olfactory bulb elicited transient perception of unpleasant smell (e.g.: burning rubber, stench or manure) in patients with epilepsy [9]; however, patient profile, sample size or stimulus parameters were not reported in detail. Since then, few peer-reviewed studies have reported such phenomena elicited by stimulation mapping via subdural electrodes implanted on the ventral surface of frontal lobe. Conversely, several studies using intracranial depth electrodes reported rare observations of olfactory hallucinations (e.g.: smells like burned wood or something to do with animals) elicited by stimulation of the anterior-medial temporal lobe [10–12]. In the present study, we determined whether and what types of olfactory hallucinations could be reproduced by electrical stimulation via subdural electrodes implanted on the ventral surface of frontal lobes. We also described the stimulus parameters and sites to elicit a given symptom and compared the stimulus intensity eliciting olfactory hallucinations to those eliciting other sensorimotor symptoms.
METHODS
Patients
This study has been approved by the Institutional Review Board at Wayne State University. Written informed consent was obtained by the guardian of each patient. The inclusion criteria consisted of: (i) patients with a history of focal epilepsy who underwent extraoperative subdural electrocorticography (ECoG) recording as a part of presurgical evaluation in Children’s Hospital of Michigan in Detroit between 2007 and 2011; (ii) functional cortical mapping via direct cortical stimulation via subdural electrodes implanted on the ventral surface of frontal lobe; (iii) capability to verbally report percepts elicited by electrical stimulation. The exclusion criteria consisted of a documented history of anosmia or hyposmia. This case-series study included a consecutive series of 16 children who satisfied the aforementioned criteria (12 males; age range: 5–17 years; median age: 13 years) (Table 1).
Table 1.
Summary
| Patient number | Age at surgery (years) | Gender | Medications at the time of surgery | Placement of subdural electrodes | Surgical resection | Pathology | Olfactory symptom elicited by stimulation of the ventral surface of frontal lobe (stimulus intensity) | Motor symptom elicited by stimulation of the Rolandic area (stimulus intensity) | Somatosensory symptom elicited by stimulation of the Rolandic area (stimulus intensity) | Visual symptom elicited by stimulation of the occipital lobe (stimulus intensity) | Auditory or language symptom elicited by stimulation of the temporal and frontal lobes (stimulus intensity) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 5 | Male | OXC; VPA; LEV | Lt FTPO | Lt F | Cortical dysplasia | Bad smell (6 mA) | Hand twitching (6 mA) | Mouth tingling (6 mA) | Eye deviation without reporting a visual perception (6 mA) | Receptive aphasia (6 mA) |
| #2 | 8 | Male | OXC | Lt FTPO | Lt T | Tumor | Garbage like bad smell (4 mA) | Mouth twitching (3 mA); Hand twitching (3 mA); Leg twitching (3 mA) | Mouth tingling (3 mA); Hand tingling (3 mA) | Phosphene (3 mA) | Receptive aphasia (4 mA); Expressive aphasia (4 mA) |
| #3 | 8 | Male | CBZ; LCM | Rt FTPO | Rt FT | Cortical dysplasia | Not reported (6 mA) | Mouth twitching (6 mA); Hand twitching (3 or 6 mA); Leg twitching (3 mA) | Not reported (6 mA) | Eye deviation without reporting a visual perception (6 mA) | Not reported (6 mA) |
| #4 | 9 | Female | VPA; VGB | Lt FTPO | Lt F | Cortical tubers | Not reported (9 mA) | Mouth twitching (9 mA); Hand twitching (9 mA); Leg twitching (9 mA) | Mouth tingling (6 or 9 mA) | Phosphene (9 mA) | Expressive aphasia (9 mA) |
| #5 | 10 | Male | VPA; LCM | Rt FTPO | Rt PT | Cortical dysplasia | Bad smell (3 mA) | Hand twitching (3 or 6 mA); Leg twitching (3 mA) | Hand tingling (3 mA); Leg tingling (3 mA) | Phosphene (6 mA) | Not reported (6 mA) |
| #6 | 10 | Male | OXC | Rt FTPO | MSTs on Rt FP | Arachnoid cyst | Smells like smoke (3 mA) | Mouth twitching (6 mA); Hand twitching (3 or 6 mA) | Mouth tingling (3 or 6 mA); Hand tingling (6 mA) | Phosphene (3 mA) | Not reported (6 mA) |
| #7 | 11 | Female | OXC; LEV | Rt FTPO | Rt FT | Cortical dysplasia | Smells like sweet strawberries (6 mA) | Hand twitching (3 mA); Leg twitching (6 mA) | Mouth tingling (6 mA); Hand tingling (3 mA) | Phosphene (6 mA) | Hearing sounds (6 mA) |
| #8 | 12 | Male | VPA; LEV | Lt FTPO | Lt T | Gliosis | Smelling of smoke (3 mA) | Hand twitching (6 mA); Eye deviation (6 mA) | Mouth tingling (6 mA); Hand tingling (3 or 6 mA) | Phosphene (3 mA) | Receptive aphasia (6 mA) |
| #9 | 14 | Male | OXC; PHT | Lt FTP; Rt FTPO | Rt T | Gliosis | Bad smell (3 mA) | Mouth twitching (6 mA); Hand twitching (3 or 6 mA); Eye deviation (6 mA) | Mouth tingling (6 or 9 mA) | Phosphene (6 mA) | Expressive aphasia (6 mA) |
| #10 | 14 | Female | OXC | Lt FTPO | Lt T | Gliosis | Bad smell (6 mA) | Mouth twitching (6 mA); Hand twitching (3 or 6 mA); Eye deviation (6 mA) | Mouth tingling (6 mA) | Phosphene (3 mA) | Hearing sounds (6 mA); Receptive aphasia (6 mA); Expressive aphasia (6 mA) |
| #11 | 14 | Female | OXC; TPM | Lt FTPO | Lt T | Tumor | Smells like good food (6 mA) | Mouth twitching (3 mA); Hand twitching (3 mA) | Mouth tingling (3 mA); Hand tingling (3 or 6 mA) | Phosphene (6 mA) | Hearing sounds (6 mA); Receptive aphasia (6 mA); Expressive aphasia (3 or 6 mA) |
| #12 | 14 | Male | OXC; LEV | Lt FTP | Lt F | Gliosis | Bad smell (6 mA) | Mouth twitching (6 mA); Hand twitching (3 mA) | Mouth tingling (6 mA); Hand tingling (3 mA) | Not stimulated | Receptive aphasia (6 mA); Expressive aphasia (6 mA) |
| #13 | 15 | Male | LTG; LEV | Lt FTP | Lt T | Tumor | Not reported (6 mA) | Mouth twitching (3 mA) | Not reported (6 mA) | Not stimulated | Hearing sounds (3 mA); Receptive aphasia (3 mA); Expressive aphasia (3 mA) |
| #14 | 15 | Male | VPA | Lt FTPO | Lt T | Gliosis, Hippocampal sclerosis | Not reported (6 mA) | Mouth twitch (3 mA); Eye deviation (6 mA) | Mouth tingling (3 mA) | Phosphene (3 mA) | Receptive aphasia (3mA); Expressive aphasia (3mA) |
| #15 | 17 | Male | OXC; LEV | Lt FTPO | Lt T | Cortical dysplasia | Unpleasant, bitter smelling (3 mA) | Mouth twitching (3 mA); Hand twitching (3 mA); Eye deviation (6 mA) | Not reported (6 mA) | Phosphene (3 mA) | Hearing sounds (6 mA); Receptive aphasia (6 mA); Expressive aphasia (6 mA) |
| #16 | 17 | Male | OXC; LTG | Lt FTPO | Lt F | Gliosis | Not reported (6 mA) | Hand twitching (3 mA); Leg twitching (3 mA) | Mouth tingling (3 mA); Leg tingling (3 mA) | Not reported (6 mA) | Expressive aphasia (3 mA) |
LEV: Levetiracetam. LTG: Lamotrigine. LCM: Lacosamide. CBZ: Carbamazepine. OXC: Oxcarbazepine. TPM: Topiramate. VPA: Valproate. VGB: Vigabatrin. Lt: Left. Rt: Right. F: Frontal. T: Temporal. O: Occipital. P: Parietal. MSTs: Multiple subpial transactions.
Subdural electrode placement and video-ECoG recording
For extraoperative ECoG recording, platinum grid electrodes (10 mm intercontact distance, 4 mm diameter) were surgically implanted (Figure 1) [13]. All electrode plates were stitched to adjacent plates and/or the edge of dura mater, to avoid movement of subdural electrodes after placement. In addition, intraoperative pictures were taken with a digital camera before dural closure, to confirm the spatial accuracy of electrode display on the three-dimensional brain surface reconstructed from MRI [14,15]. Chronic subdural ECoG recordings were performed for 2–5 days, and anti-epileptic medications were discontinued or reduced until a sufficient number of habitual seizures were captured. Seizure-onset zones were visually determined (Table 1) [13].
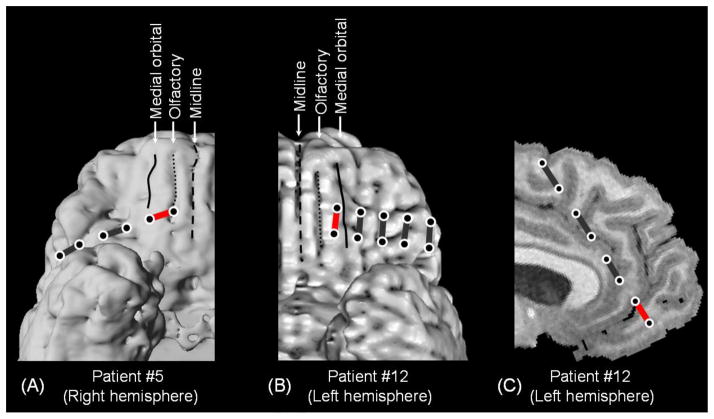
Figure 1. Olfactory hallucinations elicited by electrical stimulation in two patients.
(A) In patient #5, a 1×6 strip electrode was implanted on the ventral surface of the right frontal lobe. Stimulation of a pair denoted by a red bar (but not pairs denoted by black bars) elicited an olfactory hallucination. (B and C) In patient #12, a 2×5 strip electrode was implanted on the ventral surface of the left frontal lobe. A 1×8 strip electrode was implanted on the medial surface of the left frontal lobe; the anterior end of this electrode was located on the gyrus rectus on the left side. Stimulation of pairs denoted by red bars (but not pairs denoted by black bars) elicited an olfactory hallucination. Circles: electrode sites. Line: medial orbital sulcus. Dotted line: olfactory sulcus where the olfactory tract is located. Broken line: midline.
Coregistration of subdural electrodes to the individual three-dimensional MRI
MRI including a T1-weighted spoiled gradient echo image as well as fluid-attenuated inversion recovery image was preoperatively obtained [16]. Planar x-ray images (lateral and anteroposterior) were acquired with the subdural electrodes in place for electrode localization on the brain surface [14, 17–19]; three metallic fiducial markers were placed at anatomically well-defined locations on the patient’s head for co-registration of the x-ray image with the MRI. A three-dimensional surface image was created with the location of electrodes directly defined on the brain surface [16,17,19]. The accuracy of this procedure was reported previously as 1.2 ± 0.7 mm with a maximal misregistration of 2.7 mm [17], and was confirmed by intraoperative digital photographs of lateral surface showing in situ locations of the subdural electrodes [14,20,21].
Electrical stimulation protocol
As a component of the clinical management of patients with epilepsy, functional cortical mapping with electrical stimulation was performed using a method similar to those described previously [22–26]. To minimize the risk of stimulation-induced seizures, a loading dose of phenytoin (20 mg/kg) was intravenously administered prior to the mapping session. To minimize the risk of feeling scared or overwhelmed, each patient was informed prior to the stimulation study that she/he might have a transient sensorimotor, auditory, visual, olfactory, gustatory, or language symptom. Each patient was aware of the timing of each stimulation trial but unaware of the location of stimulated sites. A train of repetitive electrical stimuli was delivered to a pair of subdural electrodes using the Grass stimulator (Astro-Med, Inc, West Warwick, RI), and clinical symptoms elicited by stimulation were observed by at least two investigators.
The stimulus frequency was 50 Hz, the pulse duration was 300 μsec, and the train duration ranged up to 5 sec. To minimize the risk of charge accumulation in the patient’s body, biphasic stimulus pulses were used. To determine the presence of after-discharges, video-ECoG was recorded continuously during the entire mapping session. When a clinical symptom was elicited, the train of stimuli was immediately terminated. Stimulus intensity was initially set to 3 mA and was increased to 6 and 9 mA in a stepwise manner until a clinical symptom or after-discharge was observed. Once the after-discharge threshold was determined, stimulus intensity above that threshold was no longer utilized. Sites at which stimulation consistently (at least twice) elicited a clinical symptom were classified as eloquent areas specific to a given symptom. Sites were declared ‘not proven to be eloquent’ if after-discharges were elicited without a symptom, or a symptom failed to be elicited by maximal stimuli. When both clinical symptom and after-discharges were noted, another train of stimuli with the same or 1 mA smaller intensity was given until either clinical symptom or after-discharges subsided.
RESULTS
The results are summarized in Table 1, Figures 1 and 2. Eleven patients reported a perception of smell in response to electrical stimulation. Nine of the eleven patients perceived a bad or unpleasant smell (like bitterness, smoke, or garbage). The remaining two patients perceived a pleasant smell (like strawberry or good food). A total of 18 out of 22 trials (82%) elicited an olfactory hallucination, when stimulation involved areas medial to the medial orbital sulci (Figure 1). As presented in Table 1, the stimulus intensities employed to elicit olfactory hallucinations ranged 3 to 6 mA. This range was comparable to those for other sensorimotor symptoms in the majority of study patients.
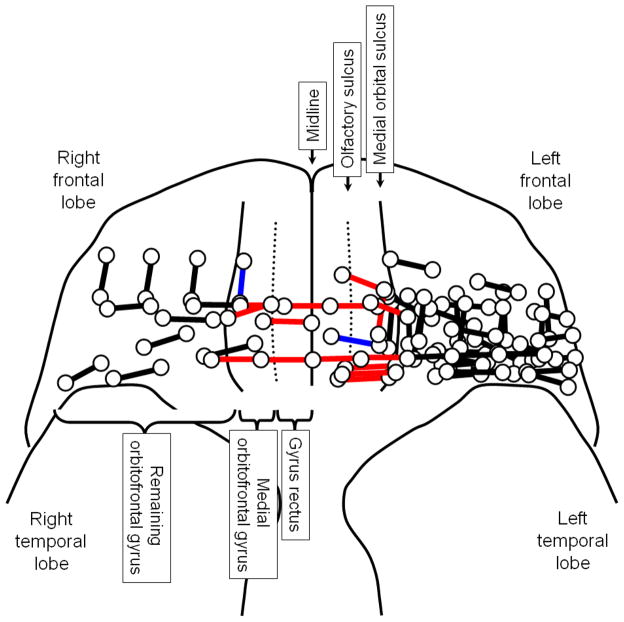
Figure 2. Summary of electrical stimulation mapping.
Circles: electrode sites. Red bars: sites of which stimulation elicited an unpleasant smell. Blue bars: sites of which stimulation elicited a pleasant smell. Black bars: sites of which stimulation failed to elicit an olfactory hallucination. Dotted lines: olfactory sulci where the olfactory tracts lie in. The medial surface of gyrus rectus is not shown. Patient #12 had a single electrode located on the medial surface of gyrus rectus, as presented in Figure 1.
Conversely, none of 39 trials elicited an olfactory hallucination, when stimulation involved areas lateral to the medial orbital sulci alone. The Fisher exact probability test revealed that the probability of a trial eliciting an electrically-induced olfactory hallucination was greater when stimulation involved areas medial to the medial orbital sulci (p<0.0001). In other words, olfactory hallucinations were induced by stimulation proximal to the olfactory bulbs or tracts on either hemisphere but not by that of orbitofrontal gyri lateral to the medial orbital sulci, the structures considered to be a part of the secondary olfactory cortex. Two patients (patients #4 and #9) had seizure onset zones involving the orbitofrontal cortex; neither patient had olfactory aura preceding complex partial seizures.
A total of five patients failed to report any olfactory hallucinations elicited by stimulation (Table 1). In two patients (patients #4 and #16), stimulation did not involve areas medial to the medial orbital sulci. In the remaining three patients (patients #3, #13 and #14), stimulation involved areas immediately medial to the medial orbital sulcus and lateral to the olfactory sulcus.
DISCUSSION
We were able to reproduce olfactory hallucinations elicited by electrical stimulation proximal to the olfactory bulb or tract on either hemisphere (Figure 2). Taking into account the size of subdural electrodes as well as the inherent spread of the stimulus current [27], the positive perception of odors can be explained by the effects of direct stimulation of olfactory bulb or tract. Unlike the previous study of intraoperative stimulation [9], we were able to study children as young as 5 years old and provided the exact stimulus parameters. We found that the range of stimulus parameters employed to elicit olfactory hallucinations was within those for other sensorimotor symptoms. We don’t know why the perceived smells in the majority of patients were of unpleasant nature. This could be attributed to the notion that mammalian olfaction is so highly conserved that a predominantly aversive response is built in as a protective reflex to improve the survival [28]. The stimulus parameters could be associated with the unpleasant nature of olfactory hallucinations. In the present study, we used macro-electrodes, which are designed to stimulate a large number of neural cells and fibers. Stimulation using micro-electrodes with very low stimulus intensity might be able to stimulate odor-specific cells or fibers.
When stimulation involved sites lateral to the medial orbital sulci alone, none of 39 stimulation trials elicited an olfactory hallucination. In other words, we failed to elicit positive perception of odors by stimulation of the secondary olfactory cortex alone. This finding does not indicate the lack of olfactory functions in that structure, partly because patients were not assigned an olfactory task during stimulation trials. The present study is not designed to localize the sites of which stimulation causes failure to conduct a given task. A previous study of monkeys using single-neuron recording from the orbitofrontal cortex reported that a small subset of neurons responded to odors, with some differentially responding to different odors [29]. A study of healthy adults using positron emission tomography reported that the orbitofrontal cortices were activated by an odor memory recognition task [30]. Further studies using olfactory tasks involving discrimination or memory of distinctive odors during stimulation trials are warranted to determine the functions of the orbitofrontal cortex. A number of recent studies have indicated that humans have an extraordinary sense of smell [31]. Behavioral studies, for example, reported that healthy adults detected the scent of fear in human sweat more accurately than chance [32,33].
Acknowledgments
This work was supported by NIH grants NS47550 and NS64033 (to E. Asano). We are grateful to Harry T. Chugani, MD, Sarah Minarik, RN, BSN, and Carol Pawlak, REEG/EPT at Children’s Hospital of Michigan, Wayne State University for the collaboration and assistance in performing the studies described above.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Price JL. Olfactory system. In: Paxinos G, editor. The Human Nervous System. Academic Press; San Diego, CA: 1990. pp. 979–998. [Google Scholar]
- 2.West SE, Doty RL. Influence of epilepsy and temporal lobe resection on olfactory function. Epilepsia. 1995;36:531–542. doi: 10.1111/j.1528-1157.1995.tb02565.x. [DOI] [PubMed] [Google Scholar]
- 3.Zusho H. Posttraumatic anosmia. Arch Otolaryngol. 1982;108:90–92. doi: 10.1001/archotol.1982.00790500026006. [DOI] [PubMed] [Google Scholar]
- 4.Duvall AJ, 3rd, Porto DP, Lyons D, Boies LR., Jr Frontal sinus fractures. Analysis of treatment results. Arch Otolaryngol Head Neck Surg. 1987;113:933–935. doi: 10.1001/archotol.1987.01860090031014. [DOI] [PubMed] [Google Scholar]
- 5.Dade LA, Zatorre RJ, Jones-Gotman M. Olfactory learning: convergent findings from lesion and brain imaging studies in humans. Brain. 2002;125:86–101. doi: 10.1093/brain/awf003. [DOI] [PubMed] [Google Scholar]
- 6.Gottfried JA, Zald DH. On the scent of human olfactory orbitofrontal cortex: meta-analysis and comparison to non-human primates. Brain Res Brain Res Rev. 2005;50:287–304. doi: 10.1016/j.brainresrev.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Zatorre RJ, Jones-Gotman M. Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain. 1991;114:71–84. [PubMed] [Google Scholar]
- 8.Jones-Gotman M, Zatorre RJ. Odor recognition memory in humans: role of right temporal and orbitofrontal regions. Brain Cogn. 1993;22:182–198. doi: 10.1006/brcg.1993.1033. [DOI] [PubMed] [Google Scholar]
- 9.Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. Little, Brown & Co; Oxford, England: 1954. [Google Scholar]
- 10.Gloor P, Olivier A, Quesney LF, Andermann F, Horowitz S. The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Ann Neurol. 1982;12:129–144. doi: 10.1002/ana.410120203. [DOI] [PubMed] [Google Scholar]
- 11.Fish DR, Gloor P, Quesney FL, Olivier A. Clinical responses to electrical brain stimulation of the temporal and frontal lobes in patients with epilepsy. Pathophysiological implications. Brain. 1993;116:397–414. doi: 10.1093/brain/116.2.397. [DOI] [PubMed] [Google Scholar]
- 12.Bartolomei F, Barbeau E, Gavaret M, Guye M, McGonigal A, Régis J, Chauvel P. Cortical stimulation study of the role of rhinal cortex in déjà vu and reminiscence of memories. Neurology. 2004;63:858–864. doi: 10.1212/01.wnl.0000137037.56916.3f. [DOI] [PubMed] [Google Scholar]
- 13.Asano E, Juhász C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009;132:1038–1047. doi: 10.1093/brain/awp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalal SS, Edwards E, Kirsch HE, Barbaro NM, Knight RT, Nagarajan SS. Localization of neurosurgically implanted electrodes via photograph-MRI-radiograph coregistration. J Neurosci Methods. 2008;174:106–115. doi: 10.1016/j.jneumeth.2008.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu HC, Nagasawa T, Brown EC, Juhasz C, Rothermel R, Hoechstetter K, Shah A, Mittal S, Fuerst D, Sood S, Asano E. Gamma-oscillations modulated by picture naming and word reading: intracranial recording in epileptic patients. Clin Neurophysiol. 2011;122:1929–1942. doi: 10.1016/j.clinph.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alkonyi B, Juhász C, Muzik O, Asano E, Saporta A, Shah A, Chugani HT. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87:77–87. doi: 10.1016/j.eplepsyres.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Stockhausen HM, Thiel A, Herholz K, Pietrzyk U. A convenient method for topographical localization of intracranial electrodes with MRI and a conventional radiograph. Neuroimage. 1997;5:S514. [Google Scholar]
- 18.Miller KJ, Makeig S, Hebb AO, Rao RP, denNijs M, Ojemann JG. Cortical electrode localization from X-rays and simple mapping for electrocorticographic research: The “Location on Cortex” (LOC) package for MATLAB. J Neurosci Methods. 2007;162:303–308. doi: 10.1016/j.jneumeth.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Muzik O, Chugani DC, Zou G, Hua J, Lu Y, Lu S, Asano E, Chugani HT. Multimodality Data Integration in Epilepsy. Int J Biomed Imaging. 2007 doi: 10.1155/2007/13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutka JT, Otsubo H, Kitano S, Sakamoto H, Shirasawa A, Ochi A, Snead OC., 3rd Utility of digital camera-derived intraoperative images in the planning of epilepsy surgery for children. Neurosurgery. 1999;45:1186–1191. doi: 10.1097/00006123-199911000-00033. [DOI] [PubMed] [Google Scholar]
- 21.Wellmer J, von Oertzen J, Schaller C, Urbach H, König R, Widman G, Van Roost D, Elger CE. Digital photography and 3D MRI-based multimodal imaging for individualized planning of resective neocortical epilepsy surgery. Epilepsia. 2002;43:1543–1550. doi: 10.1046/j.1528-1157.2002.30002.x. [DOI] [PubMed] [Google Scholar]
- 22.Jayakar P, Lesser RP. Extraoperative Methods. In: Engel J Jr, Pedley TA, editors. Epilepsy: a comprehensive textbook. Lippincott-Raven; Philadelphia: 1997. pp. 1785–1793. [Google Scholar]
- 23.Haseeb A, Asano E, Juhász C, Shah A, Sood S, Chugani HT. Young patients with focal seizures may have the primary motor area for the hand in the postcentral gyrus. Epilepsy Res. 2007;76:131–139. doi: 10.1016/j.eplepsyres.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zijlmans M, van Eijsden P, Ferrier CH, Kho KH, van Rijen PC, Leijten FS. Illusory shadow person causing paradoxical gaze deviations during temporal lobe seizures. J Neurol Neurosurg Psychiatry. 2009;80:686–688. doi: 10.1136/jnnp.2008.154310. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs J, Zijlmans M, Zelmann R, Olivier A, Hall J, Gotman J, Dubeau F. Value of electrical stimulation and high frequency oscillations (80–500 Hz) in identifying epileptogenic areas during intracranial EEG recordings. Epilepsia. 2010;51:573–582. doi: 10.1111/j.1528-1167.2009.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima K, Brown EC, Rothermel R, Carlson A, Matsuzaki N, Shah A, Atkinson M, Mittal S, Fuerst D, Sood S, Asano E. Multimodality language mapping in patients with left-hemispheric language dominance on Wada test. Clin Neurophysiol. doi: 10.1016/j.clinph.2012.01.027. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yousif N, Liu X. Modeling the current distribution across the depth electrode-brain interface in deep brain stimulation. Expert Rev Med Devices. 2007;4:623–631. doi: 10.1586/17434440.4.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ache BW, Young JM. Olfaction: diverse species, conserved principles. Neuron. 2005;48:417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Rolls ET, Baylis LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J Neurosci. 1994;14:5437–5452. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–745. doi: 10.1016/s0896-6273(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 31.Zelano C, Sobel N. Humans as an animal model for systems-level organization of olfaction. Neuron. 2005;48:431–454. doi: 10.1016/j.neuron.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Chen D, Haviland-Jones J. Human olfactory communication of emotion. Percept Mot Skills. 2000;91:771–781. doi: 10.2466/pms.2000.91.3.771. [DOI] [PubMed] [Google Scholar]
- 33.Ackerl K, Atzmueller M, Grammer K. The scent of fear. Neuro Endocrinol Lett. 2002;23:79–84. [PubMed] [Google Scholar]