Abstract
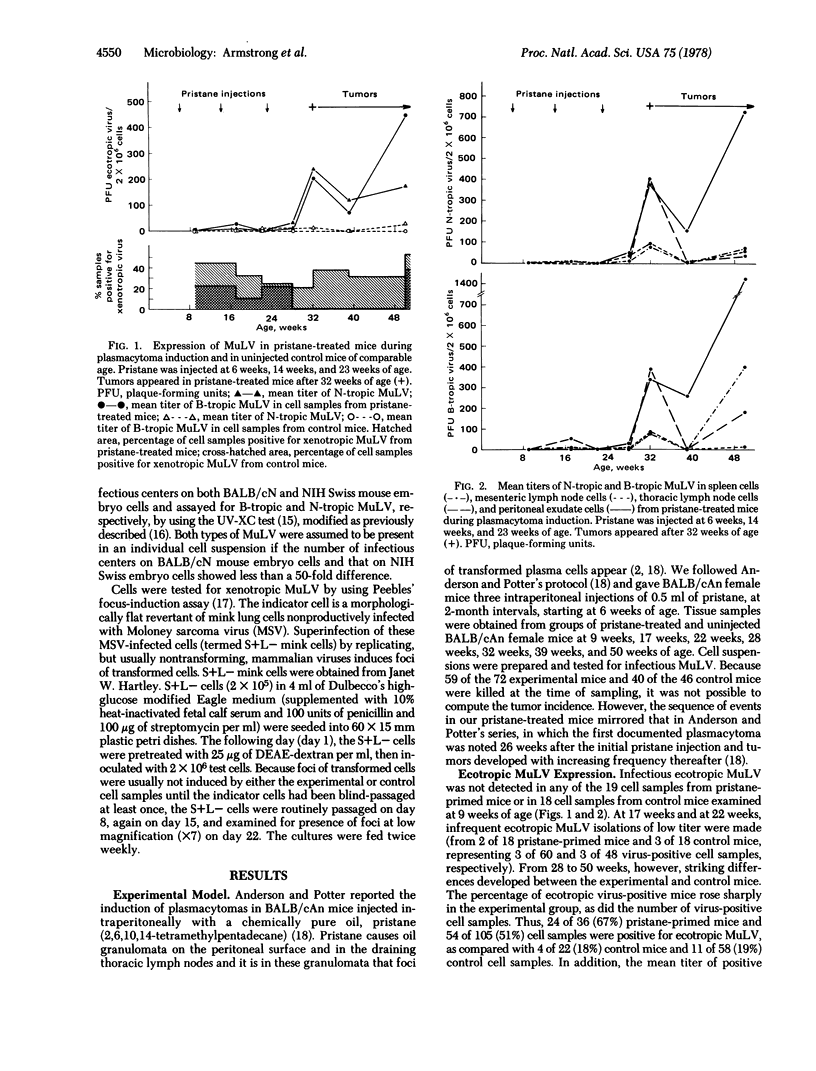
Plasmacytomas are induced in BALB/c mice by the intraperitoneal injection of pristane (2,6,10,14-tetra-methylpentadecane) after a latent period of six months and more [Anderson, P. N. & Potter, M. (1969) Nature 222, 994-995]. Spleen cells mesenteric lymph node cells, thoracic lymph node cells, and peritoneal exudate cells were prepared from pristane-treated and control uninjected BALB/c mice during the course of a 10-month period, and these cell suspensions were tested for the release of infectious murine leukemia viruses. Endogenous ecotropic and xenotropic murine leukemia viruses were expressed in pristane-treated mice during the latter part of the tumor induction period, in those cell populations in which transformed plasma cells appear, namely, peritoneal exudate cells and thoracic lymph node cells. The significance of preferential expression of both ecotropic and xenotropic murine leukemia virus in target cell populations following the administration of a carcinogen is discussed in terms of the possible formation of an oncogenic variant virus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Rabstein L. S. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970 Aug;30(8):2213–2222. [PubMed] [Google Scholar]
- Anderson P. N., Potter M. Induction of plasma cell tumours in BALB-c mice with 2,6,10,14-tetramethylpentadecane (pristane). Nature. 1969 Jun 7;222(5197):994–995. doi: 10.1038/222994a0. [DOI] [PubMed] [Google Scholar]
- Aoki T., Herberman R. B., Hartley J. W., Liu M., Walling M. J., Nunn M. Surface antigens on transplantable tumor cell lines producing mouse type C viruses. J Natl Cancer Inst. 1977 Apr;58(4):1069–1078. doi: 10.1093/jnci/58.4.1069. [DOI] [PubMed] [Google Scholar]
- Aoki T., Potter M., Sturm M. M. Analysis by immunoelectron microscopy of type-C viruses associated with primary and short-term transplanted mouse plasma cell tumors. J Natl Cancer Inst. 1973 Nov;51(5):1609–1617. doi: 10.1093/jnci/51.5.1609. [DOI] [PubMed] [Google Scholar]
- Aoki T., Todaro G. J. Antigenic properties of endogenous type-C viruses from spontaneously transformed clones of BALB-3T3. Proc Natl Acad Sci U S A. 1973 May;70(5):1598–1602. doi: 10.1073/pnas.70.5.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong M. Y., Gleichmann E., Gleichmann H., Beldotti L., Andre-Schwartz J., Schwartz R. S. Chronic allogeneic disease. II. Development of lymphomas. J Exp Med. 1970 Sep 1;132(3):417–439. doi: 10.1084/jem.132.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong M. Y., Ruddle N. H., Lipman M. B., Pierce S. K., Richards F. F. Role of endogenous murine leukemia virus in immunologically triggered lymphoreticular tumors. I. Development and use of oncogenic cellfree preparations serially passaged in vivo. J Natl Cancer Inst. 1977 Jan;58(1):67–72. doi: 10.1093/jnci/58.1.67. [DOI] [PubMed] [Google Scholar]
- Armstrong M. Y., Ruddle N. H., Lipman M. B., Richards F. F. Tumor induction by immunologically activated murine leukemia virus. J Exp Med. 1973 May 1;137(5):1163–1179. doi: 10.1084/jem.137.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong M. Y., Ruddle N. H., Richards F. F. Expression of endogenous murine leukemia viruses during the course of a protracted immunological disorder. J Exp Med. 1977 Apr 1;145(4):1060–1065. doi: 10.1084/jem.145.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer P., Baltimore D. Mechanism of restriction of ecotropic and xenotropic murine leukemia viruses and formation of pseudotypes between the two viruses. J Virol. 1977 Mar;21(3):965–973. doi: 10.1128/jvi.21.3.965-973.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R. Strategy for detection of cancer hazards to man. Nature. 1977 Feb 17;265(5595):589–596. doi: 10.1038/265589a0. [DOI] [PubMed] [Google Scholar]
- Dunn C. Y., Aaronson S. A., Stephenson J. R. Interactions of chemical inducers and steroid enhancers of endogenous mouse type-C RNA viruses. Virology. 1975 Aug;66(2):579–588. doi: 10.1016/0042-6822(75)90230-5. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima K., Ikeda H., Hartley J. W., Stockert E., Rowe W. P., Old L. J. Changes in expression of murine leukemia virus antigens and production of xenotropic virus in the late preleukemic period in AKR mice. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4680–4684. doi: 10.1073/pnas.73.12.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R. G. Host range studies of FLOPC-1 murine myeloma C particles. J Virol. 1975 Nov;16(5):1137–1145. doi: 10.1128/jvi.16.5.1137-1145.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R. G. Intracisternal A particles from FLOPC-1 BALB/c myeloma: presence of high-molecular-weight RNA and RNA-dependent DNA polymerase. J Virol. 1976 May;18(2):745–756. doi: 10.1128/jvi.18.2.745-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Autoimmunity and neoplasia. The possible role of C-type viruses. Am J Clin Pathol. 1974 Aug;62(2):258–280. doi: 10.1093/ajcp/62.2.258. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J. S-tropic murine type-C viruses: frequency of isolation from continuous cell lines, leukemia virus preparations and normal spleens. Int J Cancer. 1974 May 15;13(5):587–598. doi: 10.1002/ijc.2910130503. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Chattopadhyay S. K., Teich N. M., Rowe W. P., Levine A. S. AKR murine leukemia virus genome: frequency of sequences in DNA of high-, low-, and non-virus-yielding mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3555–3559. doi: 10.1073/pnas.71.9.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERWIN R. M., ALGIRE G. H. Induction of plasma-cell neoplasms and fibrosarcomas in BALB/c mice carrying diffusion chambers. Proc Soc Exp Biol Med. 1959 Jul;101(3):437–439. doi: 10.3181/00379727-101-24970. [DOI] [PubMed] [Google Scholar]
- POTTER M., MACCARDLE R. C. HISTOLOGY OF DEVELOPING PLASMA CELL NEOPLASIA INDUCED BY MINERAL OIL IN BALB/C MICE. J Natl Cancer Inst. 1964 Sep;33:497–515. [PubMed] [Google Scholar]
- Peebles P. T. An in vitro focus-induction assay for xenotropic murine leukemia virus, feline leukemia virus C, and the feline--primate viruses RD-114/CCC/M-7. Virology. 1975 Sep;67(1):288–291. doi: 10.1016/0042-6822(75)90427-4. [DOI] [PubMed] [Google Scholar]
- Peters R. L., Hartley J. W., Spahn G. J., Rabstein L. S., Whitmire C. E., Turner H. C., Huebner R. J. Prevalence of the group-specific (gs) antigen and infectious virus expressions of the murine C-type RNA viruses during the life span of BALB-cCr mice. Int J Cancer. 1972 Sep 15;10(2):283–289. doi: 10.1002/ijc.2910100208. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M. Immunoglobulin-producing tumors and myeloma proteins of mice. Physiol Rev. 1972 Jul;52(3):631–719. doi: 10.1152/physrev.1972.52.3.631. [DOI] [PubMed] [Google Scholar]
- Potter M., Sklar M. D., Rowe W. P. Rapid viral induction of plasmacytomas in pristane-primed BALB-c mice. Science. 1973 Nov 9;182(4112):592–594. doi: 10.1126/science.182.4112.592. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Yau P., Dobbertin D. C., Sweeney T. K., Thach S. S., Brendler T., Thach R. E. Relationships between intracisternal type A and extracellular oncornavirus-like particles produced in murine MOPC-460 myeloma cells. J Virol. 1976 Apr;18(1):344–355. doi: 10.1128/jvi.18.1.344-355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pincus T. Quantitative studies of naturally occurring murine leukemia virus infection of AKR mice. J Exp Med. 1972 Feb 1;135(2):429–436. doi: 10.1084/jem.135.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Stephension J. R., Reynolds R. K., Tronick S. R., Aaronson S. A. Distribution of three classes of endogenous type-C RNA viruses among inbred strains of mice. Virology. 1975 Oct;67(2):404–414. doi: 10.1016/0042-6822(75)90442-0. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Type-C RNA virus gene expression in human tissue. J Virol. 1974 Dec;14(6):1584–1596. doi: 10.1128/jvi.14.6.1584-1596.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


