Abstract
The kidney is essential for the maintenance of normal calcium and phosphorus homeostasis. Calcium and inorganic phosphorus are filtered at the glomerulus, and are reabsorbed from tubular segments by transporters and channels which are regulated by 1α,25-dihydroxyvitamin (1α,25(OH)2D) and parathyroid hormone (PTH). The kidney is the major site of the synthesis of 1α,25(OH)2D under physiologic conditions, and is one of the sites of 24,25-dihydroxyvitamin D (24,25(OH)2D) synthesis. The activity of the 25(OH)D-1α-hydroxylase, the mixed function oxidase responsible for the synthesis of 1α,25(OH)2D, is regulated by PTH, 1α,25(OH)2D, fibroblast growth factor 23 (FGF23), inorganic phosphorus and other growth factors. Additionally, the vitamin D receptor which binds to, and mediates the activity of 1α,25(OH)2D, is widely distributed in the kidney. Thus, the kidney by regulating multiple transport and synthetic processes is indispensible in the maintenance of mineral homeostasis in physiological states.
Keywords: 1α,25-Dihydroxyvitamin D; calcium; phosphorus; tubular reabsorption; epithelial calcium channel; plasma membrane calcium pump; calbindin; hydroxylase
1. INTRODUCTION
The kidney has a unique function in mineral homeostasis. Both calcium and phosphorus are filtered, reabsorbed and excreted in the urine to a varying degree, generally in amounts that reflect the endogenous requirements of the two substances. The vitamin D-endocrine system plays a key role in controlling the renal excretion of both calcium and phosphorus. The reabsorption of calcium in the kidney is controlled by several factors, including 1α,25-dihydroxyvitamin D (1α,25(OH)2D) [1–5]. The kidney is the major site of synthesis of 1α,25(OH)2D, the active, hormonal form of vitamin D [6–8]. The renal 25-hydroxyvitamin D3 1α–hydroxylase (1α–hydroxylase) and 25-hydroxyvitamin D-24-hydroxylase (24-hydroxylase), and other 1α,25-dihydroxyvitamin D3 and vitamin D analog metabolizing enzymes are expressed in kidney tissue [9–11]. The kidney expresses the vitamin D receptor (VDR) [11, 12]. The kidney expresses several 1α,25(OH)2D-dependent proteins that are important in calcium reabsorption e.g. the plasma membrane calcium pump (PMCa) [13–15], the epithelial calcium channel (ECaC) [16], the sodium calcium exchanger [17], and the calbindins [13–15]. Finally, the kidney has an important role in the control of plasma phosphate, which following filtration in the glomerulus is reabsorbed in nephron segments at a rate influenced by many of the same hormones and factors involved in calcium regulation [18–23].
To understand how vitamin D influences the efficiency of calcium and phosphorus reabsorption, a brief review of normal calcium and phosphorus handling by the kidney follows.
2. CALCIUM AND PHOSPHORUS FILTRATION AND REABSORPTION IN THE KIDNEY
1.1 Calcium handling by the kidney
On account of protein-binding, slightly more than half of total plasma calcium (plasma concentration 10 mg/dL or 2.5 mM) is filtered at the glomerulus [24]. The concentration of calcium in the glomerular filtrate is the same as that of plasma ultra-filterable calcium [25–27]. Assuming a glomerular filtration rate of 140 L per day and an ultrafiltrable calcium of 5.5 mg/dL, about 8000 mg of calcium are filtered by the kidney in 24 hours; 98% of the filtered load of calcium is reabsorbed, resulting in an excreted calcium of about 150–200 mg/24 h [1, 24]. Fifty to sixty percent of the filtered load of calcium is reabsorbed in the proximal tubule [2, 3, 28] by sodium-dependent, para-cellular mechanisms. Inhibition of sodium-potassium ATPase activity by ouabain reduces the amount of calcium reabsorbed in the proximal tubule, as does the substitution of sodium with lithium [29]. A reduction of tubular sodium reabsorption by volume expansion inhibits proximal tubule calcium reabsorption, while an increase in sodium reabsorption associated with volume contraction, enhances proximal tubule calcium reabsorption [2, 29]. Importantly, calcium reabsorption in the proximal tubule is not altered by thiazide diuretics, hormones such as PTH or 1α,25(OH)2D3, or by hydrogen ions [2, 3, 28, 29]. Vitamin D-dependent proteins that play a role in trans-cellular calcium transport, such as the calbindins, ECaCs, and the PMCa pump are either not expressed in the proximal tubule, or are expressed in low amounts when compared with the amounts expressed in the distal tubule. Minimal amounts of calcium are absorbed in the descending loop and the thin ascending limb of the loop of Henle. About 20% of the filtered load of calcium is reabsorbed in the thick ascending limb of the loop of Henle; another 10–15% of filtered calcium is reabsorbed in the distal tubule with the remaining 5% being reabsorbed in the collecting duct [2, 3, 28, 29]. The movement of calcium in the distal nephron is energy dependent and occurs against a concentration gradient; furthermore, the tubular lumen is electro-negative and becomes progressively more so towards the end of the distal tubule [30, 31]. In the distal nephron, calcium reabsorption can be dissociated from sodium reabsorption by thiazide diuretics which inhibit sodium reabsorption but enhance calcium reabsorption [30, 31]. In contrast to the proximal tubule, where hydrogen ion have no effect on calcium reabsorption, in the distal nephron hydrogen ions inhibit calcium reabsorption.
1.2 Phosphate handling by the kidney
Inorganic phosphate in the serum is freely filtered at the glomerulus [18–20]. About 80% of filtered phosphorus is reabsorbed in the proximal tubule [18]. The amount of phosphorus reabsorbed in the proximal tubule is greatest in the first half of the proximal tubule with some further phosphorus reabsorption occurring in the pars recta [18]. Little or no phosphorus reabsorption occurs in the loop of Henle or the distal tubule. The reabsorption of phosphate is sodium-dependent and is mediated by sodium-phosphate co-transporters (Na-Pi IIa,SLC34A1; Na-Pi IIc, SLC34A3, and Pit-2, SLC20A2) [32, 33]. Na-Pi IIa activity is increased by a low phosphate diet and decreased by PTH.[34–37] The recently described phosphatonins, fibroblast growth factor-23 (FGF-23), MEPE and secreted frizzled related protein-4 (sFRP-4), inhibit sodium dependent phosphate transport [23, 38]. In opossum kidney (OK) cells, NaPi II is internalized from the cell membrane in response to FGF-23 and sFRP-4, similar to the effects of PTH [39, 40]. Additional factors involved in phosphorus reabsorption are noted in Table 1.
Table 1.
Factors that alter renal phosphate excretion
| Increase | Decrease |
|---|---|
| 1. High phosphate diet | 1. Low-phosphate diet |
| 2. Parathyroid hormone | 2. Parathyroidectomy |
| 3. Calcitonin | 3. Thyroxine |
| 4. Chronic vitamin D | 4. Acute vitamin D |
| 5. Glucagon | 5. Insulin |
| 6. Glucocorticoids | 6. Growth hormone |
| 7. Volume expansion | 7. Volume contraction |
| 8. Increased pCO2 | 8. Decreased pCO2 |
| 9. Chronic Acidosis | |
| 10. Starvation | |
| 11. Diuretics | |
| 12. “Phosphatonin” | |
| FGF-23 | |
| sFRP-4 |
3. ROLE OF THE KIDNEY IN THE METABOLISM OF 25OH D
3.1. The 25-hydroxyvitamin D3-1α-hydroxylase and the synthesis of 1α,25(OH)2D3
25-hydroxyvitamin D3-1α-hydroxylase is a multi-component, cytochrome P-450 containing enzyme present in mitochondria of renal proximal tubular cells which transfers electrons from NADPH to the cytochrome P450, Cyp27B1 [7, 41–56]. The latter, using molecular oxygen, converts 25-hydroxyvitamin D3 (25(OH)D3) to 1α,25(OH)2D3 and water. Nephrectomy greatly decreases circulating 1α,25(OH)2D3 concentration in vivo except during pregnancy, granuloma-forming diseases, and lymphomas associated with the ectopic production of 1α,25(OH)2D3 [57–60]. While the kidney is the major site of 1α,25(OH)2D3 production, 25(OH)D3-1α-hydroxylase activity has been found in vitro in several other cell types [49, 61–72]. In vitro, chick renal epithelial cells in culture, mammalian nephron segments and homogenates derived from avian and mammalian (mostly rodent) renal cells metabolize 25(OH)D3 to 1α,25(OH)2D3 [73–77]. Proximal and distal tubular segments synthesize 1α,25(OH)2D3 [78, 79]. Zehnder et al have demonstrated the presence of 1α-hydroxylase mRNA and protein in the distal convoluted tubule, cortical collecting duct, thick ascending limb of the loop of Henle, and Bowman’s capsule [79]. Recent experiments in which the 25(OH)D3-1α-hydroxylase cytochrome P450 gene (Cyp27B1) was deleted in mice, point to the central role of this enzyme in vitamin D metabolism [80]. Table 2 summarizes some of the key factors known to regulate the activity of this enzyme in vivo and in vitro. The major regulators appear to be PTH, inorganic phosphorus and 1α,25(OH)2D3 itself. Fibroblast growth factor 23 (FGF 23), and its binding protein Klotho that is produced mainly in the distal tubule, and which is essential in mediating FGF 23 activity, inhibit 25(OH)D3-1α-hydroxylase activity and regulate the production of 1α,25(OH)2D3 [81–93]. Indeed, FGF 23 may mediate the effects of changing dietary phosphorus on 1α,25(OH)2D3 production. Regulators such as 1α,25(OH)2D3 which alter the expression of the Cyp27B1 gene have reciprocal effects on the expression of the Cyp24A1 gene [94] that are mediated via vitamin D-response elements in the promoters of the respective genes [95, 96]. In cell culture, the extra-renal 25(OH)D3-1α-hydroxylase is regulated by nitric oxide and by activation of toll-like receptors [66–72].
TABLE 2.
Effect of increased level or activity of various factors on 1α,25(OH)2D3 concentration or 1α-hydroxylase activity
| Factor | Animals | Humans | Ref. |
|---|---|---|---|
| Parathyroid hormone | ↑ | ↑ | [27, 75, 291–299] |
| Serum inorganic phosphorus | ↓ | ↓ | [127, 300–302] |
| 1α,25(OH)2D3 | ↓ | ↓ | [291, 303] |
| Calcium (direct) | ? | ↓ | [304, 305] |
| Calcitonin | ↑,↓,0 | ↑ | [24, 75, 291, 292, 306, 307] |
| Hydrogen ion | ↓ | 0 | [293, 308, 309] |
| Sex steroids | ↑ | ↑ | [126, 310] |
| Prolactin | ↑ | 0 | [311–313] |
| Growth hormone and insulin-like growth factor-1 | ↑ | ↑,↓,0 | [225, 305, 314–319] |
| Glucocorticoids | ↓,0 | ↑,↓,0 | [183, 320–323] |
| Thyroid hormone | ? | ↓* | [324–326] |
| Fibroblast growth factor 23/Klotho axis | ↓ | ? | [81–93] |
| Frizzled related protein 4 | ↓ | ? | [23] |
| Pregnancy | ↑ | ↑* | [327, 328] |
↑ Stimulation or increase; ↓, suppression or decrease; 0, no effect; ?, effect not known.
Effects may be secondary to changes in calcium, phosphorus or parathyroid hormone. (With permission, modified from Kumar R[94])
3.2 Modeling Cyp27B1 and Computational Docking Studies
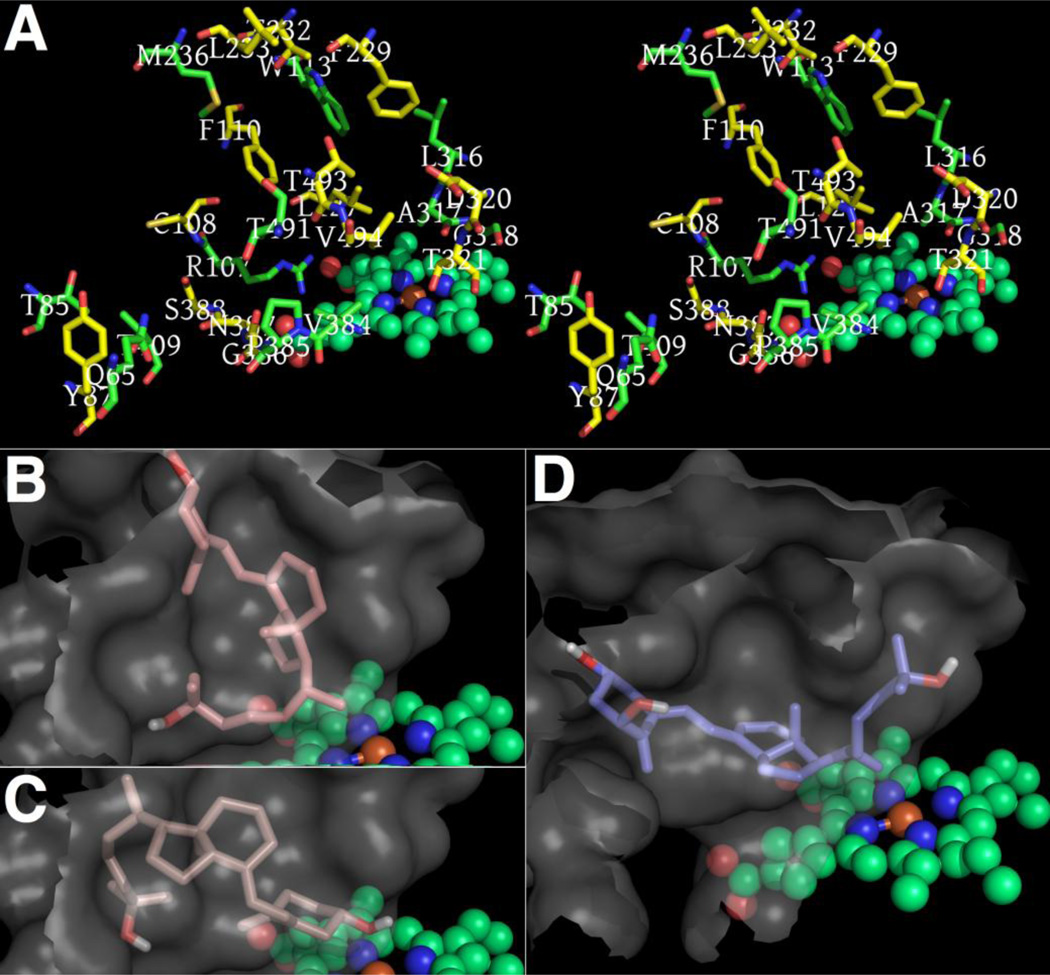
Given its critical role in the synthesis of 1α,25(OH)2D3, it is important to determine how the Cyp27B1 cytochrome P450 binds 25(OH)D3. Two energy-minimized homology models for Cyp27B1 were created by manual rebuilding from Cyp24A1 (open-cavity form, PDB ID 3K9V) [97] and Cyp2R1 (closed, PDB 3C6G [98] crystal structures, guided by I-TASSER and PHYRE2 modeling [99–101]. Figure 1A shows putative Cyp27B1 residues of the substrate binding cavity. The “yellow-carbon” Cyp27B1 residues differ from Cyp24A1’s homologous amino acids.
Figure 1.
A. Stereo view of the Cyp27B1 model’s substrate cavity residues above the heme cofactor. B. Highest-scoring 25(OH)D3 dockings in the Cyp27B1 homology model did not seem plausible, though possible binding modes, see C, were among the top 10 results. D. In contrast, top 3 and many lower scoring dockings of 1α,25(OH)2D3 inside the Cyp24A1 crystal structure seemed credible.
Cyp27B1 models, the Cyp24A1 and Cyp2R1 structures and all ligand atom coordinates used were parameterized by Autodock Tools (http://autodock.scripps.edu/resources/adt), with heme Fe charge explored from +0.2 to 1.0, both with & without a partially-charged oxygen bound to the Fe atom. Control Autodock Vina computations [102], chosen for high binding mode accuracy, correctly oriented & positioned Chaps within 0.59 Å RMSD of that in the Cyp24A1 crystal (chain A) [97] and 25(OH)D3 within 0.99 Å RMSD of the 25(OH)D3 observed in the Cyp2R1 crystal [103]. The search box covered all molecular extents, with num_mode and exhaustiveness at 500. Predictions concerning the plausibility of the binding modes, and signal-noise level of calculations, were based on computed free energy of binding, clustering of binding modes, proximity to key residues identified in literature, and distances between the known hydroxylated carbon atoms and heme Fe.
Irrespective of whether the Cyp27B1 model was derived from Cyp24A1 (open cavity) or Cyp2R1 (closed) structures, the 25(OH)D3 docking modes deemed most plausible were not among those top ranked by Autodock Vina’s binding-energy scoring function. Compare the highest-rank 25(OH)D3 pose, −9.2 kcal/mol, in Figure 1B to the pose in Figure 1C (9th in rank) that we find somewhat promising at −8.1 kcal/mol. Allowing cavity residues of Cyp27B1 to be flexible during computations did not improve the likely validity of top-scored poses. Interestingly, we found top binding modes for the product of Cyp27B1 metabolism, 1α,25(OH)2D3, more believable as the molecules were oriented with A-rings closest to Cyp27B1’s heme Fe, though not in positions able to explain catalysis (best score −9.5 kcal/mol, not shown). Our top poses of 1α,25(OH)2D3 docked to the Cyp24A1 crystal structure, with best at −9.0 kcal/mol in Figure 1D, are acceptable predictions that appear nearly equivalent to a pose published by Rhieu et al. (Fig10 in [103]). Template-bias prevented our two homology models of Cyp27B1 from being useful prediction tools for understanding ligand-binding. Crystal structures of Cyp27B1 will be required to definitively establish its structure.
3.3 The 25(OH)D3-24-hydroxylase and the synthesis of 24,25-dihydroxyvitamin D3
The 25(OH)D3-24-hydroxylase enzyme is a multi-component cytochrome P450 enzyme expressed in the kidney as well as many other tissues.[104–125]. The renal 24-hydroxylase is regulated by calcium and phosphorus such that elevated or normal calcium levels induce the 24-hydroxylase activity, whereas, low calcium levels inhibit it [116–118, 126]. Similarly, elevated serum phosphorus concentrations increase 24-hydroxylase activity and low phosphorus diets decrease the enzyme activity [127]. 24-hydroxylase enzyme activity and mRNA expression in the kidney is up-regulated by 1α,25(OH)2D3 [128–134]. The effect of 1α,25(OH)2D3 is blunted in vitro and in vivo by parathyroid hormone [128–131]. We used antibodies against the 24-hydroxylase cytochrome P-450 to examine the distribution of the enzyme in the human kidney, and found high concentrations of the cytochrome P-450 in distal tubular cells and lower amounts in the proximal tubule [11]. 24-Hydroxylase activity, however, is expressed primarily in the proximal tubule but is also present in more distal segments [135, 136]. It is responsible for the conversion of 25(OH)D3 and 1α,25(OH)2D3 to 24,25-dihydroxyvitamin D3 (24,25(OH)2D3) and 1α,24,25-trihydroxyvitamin D3, respectively. There is conflicting evidence as to whether 25(OH)D3 or 1α,25(OH)2D3 is the preferred substrate for 24-hydroxylase [137]. Some have reported 1α,25(OH)2D3 is the preferred substrate with a Km approximately 10-fold lower than that for 25(OH)D3 [138, 139], while others have found Km values substantially lower for 25(OH)D3 [140]. It has been suggested that 24,25(OH)2D3 has unique properties and actions in cartilage and bone [124, 125, 141, 142], but others have not confirmed these observations [143–149].
3.4 The Structure of the 25(OH)D3-Cyp24A1
Recently, the structure of the Cyp24A1 in association with CHAPS was reported [97]. The crystal structure shows an open cleft leading to the active-site heme prosthetic group. The entrance to the cleft is flanked by conserved hydrophobic residues on helices A' and G'. The determinants of adrenodoxin recognition are conserved residues from helices K, K'', and L.
3.5 Other vitamin D metabolizing enzymes present in the kidney
The kidney is also capable of transforming 25(OH)D3 to several other compounds listed in Table 3 [150–170]. The specific physiological roles of these various metabolites are not known with certainty. Several polar metabolites of 1α,25(OH)2D3 are formed in the liver, including calcitroic acid and the glucuronide and sulfate conjugates of the hormone; these and small amounts of unchanged dihydroxylated and tri-hydroxylated metabolites of vitamin D are excreted in the urine [171–185]. Many of the transformations that occur with 25(OH)D3 also occur in the case of 1α,25(OH)2D3.
Table 3.
The metabolism of 25OHD3 by the kidney
| 25OHD3 |  |
24R, 25(OH)2D3 | 24-keto-25(OH)D3 | |
| 25S, 26(OH)2D3 | 25(OH)D3-lactone | |||
| 23S, 25(OH)2D3 | 23-keto-25(OH)D3 |
4. EFFECTS OF VITAMIN D, 25(OH)D3 AND 1α,25(OH)2D3 ON THE RENAL HANDLING OF CALCIUM AND PHOSPHORUS
In vitamin D deficiency urine calcium concentrations are low, whereas, with vitamin D excess or intoxication hypercalciuria is frequently present [1]. The excretion of calcium and phosphorus in the urine, however, also reflects the decreased or increased calcium absorption in the intestine, the presence of hypo- or hyper-calcemia, and the presence of diminished or elevated concentrations of circulating PTH. Short term infusions of vitamin D3, 25(OH)D3 and 1α,25(OH)2D3 decrease phosphate, calcium and sodium clearance relative to the clearance of inulin in parathyroidectomzed dogs [186–189]. We performed similar studies examining the effects of 25(OH)D3 on renal bicarbonate and phosphate reabsorption [190]. Unlike these earlier studies, we observed that phosphate and bicarbonate reabsorption increased only in intact animals but not in parathyroidectomized animals suggesting the need for PTH. Our observations are similar to those of others [191]. Yamamoto et al. examined the effects of 1α,25(OH)2D3 in vitamin D-deficient rats, vitamin D-deficient rats supplemented with dietary calcium to normalize plasma calcium and PTH levels, and vitamin D-replete rats following thyro-parathyroidectomy and the infusion of graded amounts of calcium [192]. Urinary calcium excretion was lower in vitamin D-replete rats than in vitamin D-deficient rats suggesting that vitamin D increased the efficiency of renal calcium reabsorption in the absence of PTH. In a second group of experiments, rats treated in the manner noted above, were TPTX and infused with PTH. The results of this experiment show that a lower dose of PTH is needed to exhibit a comparable effect on renal calcium reabsorption in vitamin D-replete rats when compared to vitamin D-deficient rats. This finding may be explained by in vitro studies of distal convoluted tubule cells in which 1α,25(OH)2D3 increased PTH/PTHrp receptor mRNA levels [193]. Micropuncture experiments have demonstrated that 25(OH)D3 exerts its anti-phosphaturic and hypocalciuric effects in the distal tubule [194, 195]. These effects occur shortly after the administration of 25(OH)D3 and are therefore independent of conversion of 25(OH)D3 to 1α,25(OH)2D3. 1α,25(OH)2D3 enhances the reabsorption of calcium in distal tubular segments of the nephron [196]. These studies are consistent with the localization of many vitamin D responsive proteins exclusively in the distal nephron. In vitro studies show that vitamin D deficiency is associated with decreased calcium uptake in luminal and basolateral membranes derived from distal nephron segments [197]. Cultured rabbit connecting tubule cells show an increase in the transport of calcium when treated with 1α,25(OH)2D3 [198]. ECaC1 (TRPV5, present in the apical membrane of the distal convoluted tubule) and ECaC2 (TRPV6, localized to the principal cells of the cortical and medullary collecting ducts) protein and mRNA are diminished in vitamin D deficient rats and increased by 1α,25(OH)2D3 [199–201]. 1α,25(OH)2D3 increases mRNA and protein expression of the PMCa pump which is involved with active transport of calcium through the basolateral membrane of distal tubule cells [202].
A synthesis of the experimental results suggest that 1α,25(OH)2D3 has effects on the distal tubular reabsorption of calcium through several mechanisms (Figure 2). Expression of proteins responsible for distal tubule calcium uptake, intracellular trafficking, and basolateral transport of calcium are responsive to 1α,25(OH)2D3.
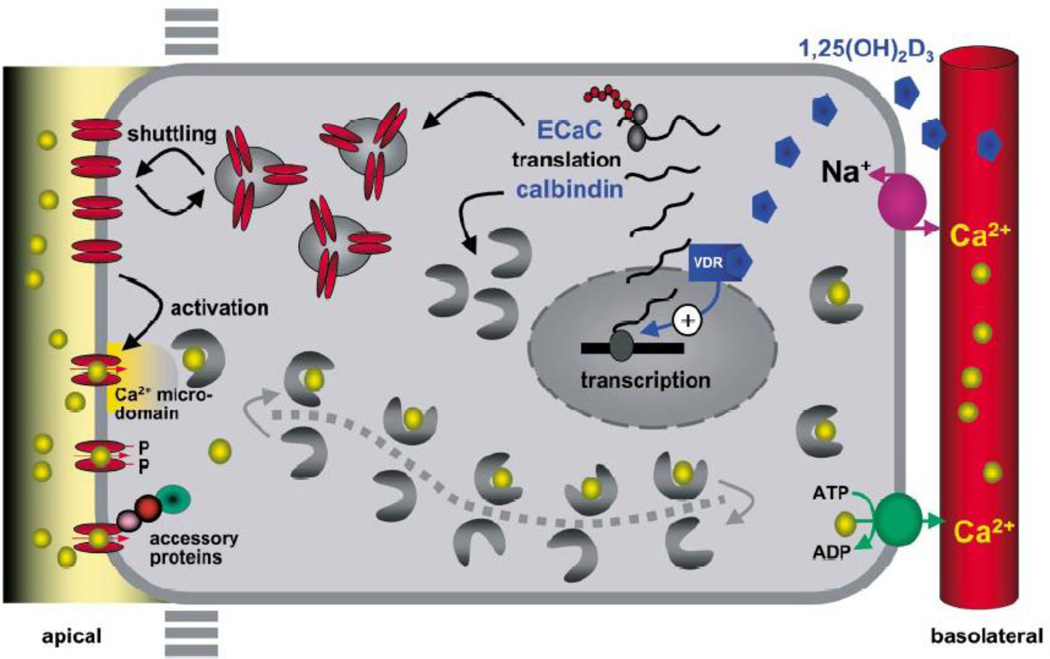
Figure 2.
Integrated model of active Ca2+ reabsorption in the distal part of the nephron. Apical entry of Ca2+ is facilitated by ECaC. Ca2+ then binds to calbindin-D28K, and this complex diffuses through the cytosol to the basolateral membrane, where Ca2+ is extruded by a Na+/Ca2+ exchanger and a plasma membrane Ca2+-ATPase. The individually controlled steps in the activation process of the rate limiting Ca2+ entry channel include 1α,25(OH)2D3-mediated transcriptional and translational activation, shuttling to the apical membrane, and subsequent activation of apically located channels by ambient Ca2+ concentration, direct phosphorylation and/or accessory proteins. (With permission from Hoenderop, et al [268])
5. DISTRIBUTION AND REGULATION OF VITAMIN D-DEPENDENT PROTEINS IN THE KIDNEY
Several vitamin D-dependent proteins are expressed in the kidney and many of these play a role in calcium and phosphate transport. The VDR, calbindins, PMCa pump, and the ECaCs (TRPV5 and TRPV6) are all found in renal tubule cells and act co-ordinately in the regulation of calcium transport in the nephron [5, 11, 14, 15, 28, 193, 199, 201, 203–206]. Additional vitamin D responsive proteins involved in renal calcium and phosphate transport are listed in Table 4.
Table 4.
Vitamin D responsive proteins in the kidney
| Vitamin D receptor |
| 24-hydroxylase |
| Plasma membrane calcium pump |
| Epithelial calcium channels, TRPV5 and TRPV6 |
| Calbindin D28K |
| Calbindin D9K |
| Calcium sensing receptor |
| PTH/PTHrp receptor |
| Sodium-phosphate co-transporter type 2 |
5.1 1α,25(OH)2D3 receptor (VDR) in the kidney
The VDR mediates many, if not all, of the effects of 1α,25(OH)2D3 in diverse organs [207–215]. The distribution of the VDR in the kidney has been assessed using a variety of techniques [135, 216–220]. A full discussion regarding its distribution is given in a separate contribution in this volume. Suffice it to say that the VDR is found in varying amounts in different species in proximal and distal tubules [11, 135, 217, 218, 221]. In the human kidney, we found that the VDR was present abundantly in the distal tubule and to a lesser extent in the proximal tubule. Cells expressing calbindin-D28K also express the calcium pump and the epithelial calcium channel [199, 222]. Interestingly, not all cells in the distal tubule expressed the receptor. Acid secreting cells do not express the VDR in significant amounts. Taken together, the results are consistent with the notion that the VDR is present in significant amounts in the distal tubule where it regulates the amount and the activity of several vitamin D-dependent proteins such as the plasma membrane calcium pump, epithelial calcium channel, and calbindin D28K. Although present in lesser amounts, the proximal tubule also expresses the VDR where it regulates the activity of 1α-hydroxylase and 24-hydroxylase. The VDR is detected in the developing rat kidney in vivo as early as day 15 post-coitum (p.c.) and in the cultured metanephros [12]. Significant amounts of the VDR are found in the metanephric mesenchyme and the ureteric bud. As the kidney matures, the VDR is observed in S-shaped and comma-shaped bodies and in the developing glomerulus, specifically in the parietal and visceral endothelial cells. VDR staining in the latter cells persists in the adult kidney as well. Similar patterns of VDR expression were found in the developing mouse kidney in vivo and in mouse metanephric cultures.
The VDR is regulated by several factors in diverse tissues [223]. Concentrations of the VDR in the kidney and parathyroid glands are mainly regulated by 1α,25(OH)2D3, PTH and dietary calcium. Exogenous administration of 1α,25(OH)2D3 in rats increases VDR levels in duodenal and renal tissues [224, 225]. When endogenous 1α,25(OH)2D3 concentrations are increased by adapting an animal to a low calcium diet (Table 5), VDR concentrations in the duodenum and kidney do not increase. The difference appears to be due to increases in the levels of PTH elicited by the low calcium diet and decreased PTH following 1α,25(OH)2D3 administration. Differences in Cyp24A1 expression (decreased in the presence of a low calcium diet, and increased following the administration of 1α,25(OH)2D3) might also contribute to this difference in VDR expression. PTH has been shown to down-regulate VDR in osteosarcoma cells as well as block up-regulation of VDR in rats infused with both PTH and 1α,25(OH)2D3 [226]. These results demonstrate the opposing effects of PTH and 1α,25(OH)2D3 on VDR expression in the kidney.
TABLE 5.
Effect of dietary calcium on unoccupied VDR content in rat duodenum and kidney
| Day | |||||
|---|---|---|---|---|---|
| Diet | 2 | 7 | 14 | 21 | |
| VDR | |||||
| Duodenum | 1% calcium | 341±26 | 197±17 | 202±17 | 259±26 |
| 0.02% calcium | 365±27 | 226±28 | 221±16 | 267±28 | |
| Kidney | 1% calcium | ND | 163±11 | 165±9 | 124±8 |
| 0.02% calcium | ND | 120±4* | 131±10* | 77±3* | |
| Calcium (mg/dL) | 1% calcium | 9.98±0.18 | 9.82±0.08 | 9.16±0.16 | 9.52±0.41 |
| 0.02% calcium | 9.45±0.12 | 9.15±0.17* | 9.09±0.14 | 8.70±0.29* | |
| Phosphorus (mg/dL) | 1% calcium | 8.40±0.17 | 8.88±0.22 | 8.73±0.17 | 7.92±0.29 |
| 0.02% calcium | 8.15±0.22 | 8.65±0.13 | 8.37±0.28 | 8.41±0.23 | |
| 1α, 25-(OH)2D3 (pg/mL) | 1% calcium | 153±11 | 113±13 | 139±9 | 160±32 |
| 0.02% calcium | 180±20 | 392±45** | 682±44** | 829±59** | |
Values are mean ± SEM.
Unoccupied vitamin D receptor content expressed as fmols [3H]1α,25(OH)2D3 bound per mg cytosol protein.
P<0.05;
P<0.001.
ND = not done.
Modified from Goff JP, et al[224]
In addition to the VDR, several vitamin D-dependent proteins are expressed in the kidney. The pattern of regulation of these proteins is of great interest, in as much as it casts light on the different mechanisms by which calcium is transported in the kidney. Those proteins involved in calcium transport include: calbindin D28K and calbindin D9K, the plasma membrane calcium pump, the epithelial calcium channel, and the calcium sensing receptor. Additionally, although not involved in the transport of calcium, the 24-hydroxylase and the sodium-phosphate type 2 co-transporter (NaPi 2) are also expressed in the kidney. All of these proteins appear to be regulated by 1α,25(OH)2D3.
5.2 Calbindins D and the kidney
The two forms of calbindin-D, namely, calbindin-D28K and calbindin-D9K are variably distributed in different tissues [227–244]. The proteins are classical E-F-hand proteins. Calbindin-D28K has 6-EF hands and the calbindin-D9K has 2 such motifs [245–264]. Calbindin D28K binds 4 moles of calcium per mole of protein and calbindin D9K two moles of calcium per mole of protein [227]. In the mouse kidney, both forms are present and are regulated by vitamin D. In other species, only calbindin-D28K is expressed in the kidney. Both proteins undergo conformational change upon binding to calcium [254, 256, 265]. We recently solved the structure of Ca2+-loaded calbindin-D28K using NMR spectroscopy [266]. The protein is comprised of a single, globular fold consisting of six distinct EF-hand subdomains, which coordinate Ca2+ in loops on EF1, EF3, EF4 and EF5. Ran-binding protein M, myo-inositol monophosphatase, and procaspase-3-derived peptides interact with the protein on a surface comprised of alpha5 (EF3), alpha8 (EF4) and the EF2–EF3 and EF4–EF5 loops. The Ca2+-dependent conformational change is probably what allows the protein to act as a modulator of effector proteins.
Calbindin D28K and calbindin-D9K are 1α,25(OH)2D3-regulated proteins [258–264, 267]. Their expression is reduced in the kidneys of 1α–hydroxylase knock-out mice [268]. Calbindin D28K and calbindin-D9K expression is normalized by treatment with 1α,25(OH)2D3, however, only calbindin D28K expression is increased when 1α–hydroxylase knock-out mice are fed a high calcium diet. In a mouse VDR-KO model, renal calbindin D9K expression is nearly abolished whereas calbindin D28K expression returns to control values as the animals age [269]. Calbindin-D9K induction by 1α,25(OH)2D3 in vitro is absent in VDR null cells [270]. In vitro, PTH has a synergistic effect with 1α,25(OH)2D3 on calbindin D9K expression [270]. In contrast, calbindin D28K expression is enhanced by PTH infusion without elevations in 1α,25(OH)2D3 concentrations [271]. Preincubation with calbindin D28K increases calcium uptake in luminal membranes while calbindin D9K preincubation increases calcium uptake in basolateral membranes [272, 273]. Calbindin D28K knock-out mice fed a high calcium (1%) diet have an elevated urinary calcium/creatinine ratio; curiously, no differences in serum calcium or PTH are noted compared to wild-type littermates [274, 275]. The elevated urinary calcium/creatinine ratio in calbindin D28K knock-out mice is not apparent after fasting [274]. This is consistent with the finding that fractional excretion of calcium is not altered in calbindin D28K knock-out mice fed a normal calcium diet [276]. The effects of calbindin D28K on renal calcium conservation in mice are modest, perhaps because of compensatory increases in calbindin D9K.
5.3. The plasma membrane calcium pump
In the adult human kidney, epitopes for the calcium-magnesium ATPase (calcium pump) are expressed in the basolateral membrane of distal tubular cells [14, 15, 28, 203, 204]. Similar patterns of expression were apparent in the rat [203] and rabbit kidney [277]. Not all cells of the distal tubule stained positively for the calcium pump; cells expressing acid secreting, carbonic anhydrase positive cells do not express the plasma membrane calcium pump, whereas the other cells of the distal tubule express the PMCa. Calbindin D28K is co-expressed with the PMCa pump. Our studies have been confirmed by others [277]. The PMCa pump is widely distributed in a large number of other calcium transporting tissues many of which display vitamin D-dependent calcium transport [13, 278–283].
The PMCa is regulated by 1α,25(OH)2D3. In MDBK (bovine distal tubule) cells, 1α,25(OH)2D3 treatment increases PMCa pump mRNA and protein [202]. Vitamin D deficiency decreases PMCa pump activity in the distal tubule of the kidney [272, 273]. We have shown that the PMCa pump mRNA and protein expression in the intestine increase after the administration of 1α,25(OH)2D3 [279, 284, 285]. The increase occurs within 3–6 hours and is dose-dependent. Dietary calcium and phosphorus depletion also increase the amount of PMCa pump expressed in the intestine. Thus, in the intestine and in intestinal cell basolateral membranes, 1α,25(OH)2D3 increases the synthesis of the PMCa pump. The mechanism by which up-regulation of PMCa pump activity occurs in the kidney and intestine is uncertain. 1α,25(OH)2D3-mediated increases in PMCa pump synthesis or stimulation by calbindin D9k or calbindin D28k are possibilities [272, 273]. There is also evidence that stimulation of the calcium sensing receptor (CaSR) decreases calcium absorption by inhibiting PMCa pump activity [286].
5.4. The epithelial calcium channel/vanilloid-receptor related transient receptor potential channels 5 and 6
Hoenderop et al described epithelial calcium channels (ECaCs) which are expressed in the apical membrane of the distal tubule and principal cells of the collecting duct and are distinct from previously described calcium channels [16, 200, 222, 287, 288]. There are at least two members in this family of calcium channels, ECaC-1/TRPV5 and ECaC-2/TRPV6 [288]. ECaC1/TRPV5 expression is limited to the kidney while ECaC2/TRPV6 is expressed in several other tissues [287–290]. The ECaCs/TRPV channels have six putative transmembrane spanning domains, including a pore-forming hydrophobic region between transmembrane domains 5 and 6 [16]. Several putative vitamin D response elements (VDRE) have been identified within the promoter region of the human TRPV5 channel. Hoenderop et al also demonstrated that ECaC1/TRPV5 mRNA and protein levels are increased to near control levels after vitamin D rescue in rats fed a vitamin D deficient diet [199]. In a study using 1α-hydroxylase knock-out mice, a greater than 50% reduction in ECaC-1/TRPV5 expression was found compared to control mice [268]. Additionally, renal ECaC-1/TRPV5 expression in 1α-hydroxylase KO mice is normalized after treatment with 1α,25(OH)2D3 [268]. Similar findings were seen when examining calbindin D28K expression which co-localizes to the same distal tubule cells as ECaC-1/TRPV5 [222, 268]. However, ECaC-1/TRPV5 is not regulated through vitamin D effects on calbindin D28K. Calbindin D28K KO mice and cyclosporine A induced down-regulation of calbindin D28K has no effect on ECaC-1/TRPV5 expression [274]. Others have suggested that calcium also regulates ECaC-1 expression. Quantitative PRC techniques showed reduced expression of ECaC-1 in VDR KO mice compared to control mice. When fed high calcium diets, VDR KO mice had normalization of ECaC-1 concentrations [290].
V. CONCLUSION
The kidney plays a vital role in the conservation of calcium and phosphorus. Besides being the site of synthesis of 1α,25(OH)2D3, the kidney responds to the hormone by increasing the efficiency of calcium and phosphorus reabsorption. Elements of the calcium transport systems including calbindin D28K, calbindin D9K, the epithelial calcium channel, and the plasma membrane calcium pump all localize to the distal portion of the nephron and are regulated directly or indirectly by 1α,25(OH)2D3.
HIGHLIGHTS.
The kidney is essential for the appropriate regulation of calcium and phosphorus homeostasis.
It is the site of synthesis of the active, hormonal form of vitamin D, 1α,25(OH)2D, as well as, the site of degradation of the hormone.
Many proteins involved in the transport of calcium are regulated by 1α,25(OH)2D.
Table 6.
Distribution of plasma membrane calcium pump in transporting epithelia as assessed by immunohistochemistry
| Tissue | Source | Cell Type | Location in Cell | Reference |
|---|---|---|---|---|
| Kidney | Rat, human | Distal convoluted tubule, principal cell | Basolateral | [14, 15, 28, 203, 204] |
| Intestine | Rat, chick | Absorptive cell | Basolateral | [278, 279] |
| Trophoblast | Rat, human | Syncytiotrophoblast | Basal | [280] |
| Choroid plexus | Cat Human | Choroid plexus Secretory cell | Apical | [281] |
| Shell gland | Chick | Principal cell | Apical | [282] |
| Bone | Human | Osteoblast | Not vectorially oriented | [13] |
| Bone | Chick | Osteoclast | Not vectorially oriented | [283] |
ACKNOWLEDGEMENTS
Supported by NIH grants AR058003 and AR060869 and a grant from the Ralph and Marion C. Falk Foundation (to RK).
ABBREVIATIONS
- 1α,25(OH)2D
1α,25-dihydroxyvitamin D
- ECaC
epithelial calcium channel
- PMCa
plasma membrane calcium pump
- VDR
vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Metabolic bone and stone disease. New York: Churchill Livingstone, Edinburgh; 1993. [Google Scholar]
- 2.Friedman PA, Gesek FA. Physiological Reviews. 1995;75:429–471. doi: 10.1152/physrev.1995.75.3.429. [DOI] [PubMed] [Google Scholar]
- 3.Borke JL, Penniston JT, Kumar R. Seminars in Nephrology. 1990;10:15–23. [PubMed] [Google Scholar]
- 4.Johnson JA, Kumar R. Current Opinion in Nephrology & Hypertension. 1994;3:424–429. doi: 10.1097/00041552-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R. Journal of Cellular Biochemistry. 1995;57:392–398. doi: 10.1002/jcb.240570304. [DOI] [PubMed] [Google Scholar]
- 6.Fraser DR, Kodicek E. Nature. 1970;228:764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- 7.Gray R, Boyle I, DeLuca HF. Science. 1971;172:1232–1234. doi: 10.1126/science.172.3989.1232. [DOI] [PubMed] [Google Scholar]
- 8.Shultz TD, Fox J, Heath H, 3rd, Kumar R. Proc Natl Acad Sci U S A. 1983;80:1746–1750. doi: 10.1073/pnas.80.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satomura K, Seino Y, Yamaoka K, Tanaka Y, Ishida M, Yabuuchi H, DeLuca HF. Kidney Int. 1988;34:712–716. doi: 10.1038/ki.1988.237. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, DeLuca HF. Am J Physiol. 1984;246:E168–E173. doi: 10.1152/ajpendo.1984.246.2.E168. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Schaefer J, Grande JP, Roche PC. American Journal of Physiology. 1994;266:F477–F485. doi: 10.1152/ajprenal.1994.266.3.F477. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JA, Grande JP, Roche PC, Sweeney WE, Jr, Avner ED, Kumar R. American Journal of Physiology. 1995;269:F419–F428. doi: 10.1152/ajprenal.1995.269.3.F419. [DOI] [PubMed] [Google Scholar]
- 13.Borke JL, Eriksen EF, Minami J, Keeting P, Mann KG, Penniston JT, Riggs BL, Kumar R. Journal of Clinical Endocrinology & Metabolism. 1988;67:1299–1304. doi: 10.1210/jcem-67-6-1299. [DOI] [PubMed] [Google Scholar]
- 14.Borke JL, Minami J, Verma A, Penniston JT, Kumar R. Journal of Clinical Investigation. 1987;80:1225–1231. doi: 10.1172/JCI113196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borke JL, Minami J, Verma AK, Penniston JT, Kumar R. Kidney International. 1988;34:262–267. doi: 10.1038/ki.1988.174. [DOI] [PubMed] [Google Scholar]
- 16.Muller D, Hoenderop JG, van Os CH, JMB R. Nephrology Dialysis Transplantation. 2001;16:1329–1335. doi: 10.1093/ndt/16.7.1329. [DOI] [PubMed] [Google Scholar]
- 17.Windhager EE, Frindt G, Milovanovic S. Ann N Y Acad Sci. 1991;639:577–591. doi: 10.1111/j.1749-6632.1991.tb17356.x. [DOI] [PubMed] [Google Scholar]
- 18.Berndt TJ, Knox FG. In: The Kidney : physiology and pathophysiology. Seldin DW, Giebisch GH, editors. New York: Raven Press; 1992. pp. 2511–2532. [Google Scholar]
- 19.Knox FG, Haramati A. In: The Kidney : physiology and pathophysiology. Seldin DW, Giebisch GH, editors. New York: Raven Press; 1985. pp. 1351–1396. [Google Scholar]
- 20.Knox FG, Haas JA, Berndt T, Marchand GR, Youngberg SP. American Journal of Physiology. 1977;233:F150–F153. doi: 10.1152/ajprenal.1977.233.2.F150. [DOI] [PubMed] [Google Scholar]
- 21.Schiavi SC, Moe OW. Current Opinion in Nephrology & Hypertension. 2002;11:423–430. doi: 10.1097/00041552-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Kumar R. Current Opinion in Nephrology & Hypertension. 2002;11:547–553. doi: 10.1097/00041552-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Berndt T, Craig TA, Bowe AE, Vassiliadis J, Reczek D, Finnegan R, Jan De Beur SM, Schiavi SC, Kumar R. Journal of Clinical Investigation. 2003 doi: 10.1172/JCI18563. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar R. In: Fluids and electrolytes. Kokko JP, Tannen RL, editors. Philadelphia: Saunders; 1996. pp. 391–419. [Google Scholar]
- 25.Neuman WF, Neuman MW. The chemical dynamics of bone mineral. Chicago: University of Chicago Press; 1958. [Google Scholar]
- 26.Moore EW. Journal of Clinical Investigation. 1970;49:318–334. doi: 10.1172/JCI106241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raman A. Clinical Biochemistry. 1971;4:141–146. doi: 10.1016/s0009-9120(71)91175-1. [DOI] [PubMed] [Google Scholar]
- 28.Kumar R, Penniston JT, Borke JL. New Physiol Sci. 1988;3:219–222. [Google Scholar]
- 29.Friedman PA. New Physiol Sci. 1988;3:17–21. [Google Scholar]
- 30.Costanzo LS, Windhager EE. Am J Physiol. 1978;235:F492–F506. doi: 10.1152/ajprenal.1978.235.5.F492. [DOI] [PubMed] [Google Scholar]
- 31.Costanzo LS, Windhager EE, Ellison DH. J Am Soc Nephrol. 2000;11:1562–1580. [PubMed] [Google Scholar]
- 32.Murer H, Hernando N, Forster I, Biber J. Curr Opin Nephrol Hypertens. 2001;10:555–561. doi: 10.1097/00041552-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Picard N, Capuano P, Stange G, Mihailova M, Kaissling B, Murer H, Biber J, Wagner CA. Pflugers Arch. doi: 10.1007/s00424-010-0841-1. [DOI] [PubMed] [Google Scholar]
- 34.Traebert M, Roth J, Biber J, Murer H, Kaissling B. American Journal of Physiology - Renal Fluid & Electrolyte Physiology. 2000;278:F148–F154. doi: 10.1152/ajprenal.2000.278.1.F148. [DOI] [PubMed] [Google Scholar]
- 35.Murer H, Lotscher M, Kaissling B, Levi M, Kempson SA, Biber J. Kidney Int. 1996;49:1769–1773. doi: 10.1038/ki.1996.264. [DOI] [PubMed] [Google Scholar]
- 36.Keusch I, Traebert M, Lotscher M, Kaissling B, Murer H, Biber J. Kidney International. 1998;54:1224–1232. doi: 10.1046/j.1523-1755.1998.00115.x. [DOI] [PubMed] [Google Scholar]
- 37.Lotscher M, Scarpetta Y, Levi M, Halaihel N, Wang H, Zajicek HK, Biber J, Murer H, Kaissling B. Journal of Clinical Investigation. 1999;104:483–494. doi: 10.1172/JCI3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamashita T, Konishi M, Miyake A, Inui K, Itoh N. Journal of Biological Chemistry. 2002;277:28265–28270. doi: 10.1074/jbc.M202527200. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien S, Bowe AE, Hernando N, Biber J, Murer H, Kumar R, Breusegem SY, Barry NP, Levi M, Pragnell M, Schiavi SC. Journal of the American Society of Nephrology. 2003 ASN abstract # 555303 (in press). [Google Scholar]
- 40.O'Brien S, Bowe AE, Pragnell M, Kumar R, Schiavi SC. Journal of Bone & Mineral Research. 2003 ASBMR Abstract. [Google Scholar]
- 41.Ghazarian JG, DeLuca HF. Archives of Biochemistry & Biophysics. 1974;160:63–72. doi: 10.1016/s0003-9861(74)80009-3. [DOI] [PubMed] [Google Scholar]
- 42.Ghazarian JG, Schnoes HK, DeLuca HF. Biochemistry. 1973;12:2555–2558. doi: 10.1021/bi00738a001. [DOI] [PubMed] [Google Scholar]
- 43.Gray RW, Omdahl JL, Ghazarian JG, DeLuca HF. The Journal of biological chemistry. 1972;247:7528–7532. [PubMed] [Google Scholar]
- 44.Pedersen JI, Ghazarian JG, Orme-Johnson NR, DeLuca HF. Journal of Biological Chemistry. 1976;251:3933–3941. [PubMed] [Google Scholar]
- 45.Yoon PS, DeLuca HF. Biochemistry. 1980;19:2165–2171. doi: 10.1021/bi00551a026. [DOI] [PubMed] [Google Scholar]
- 46.Yoon PS, Deluca HF. Arch Biochem Biophys. 1980;203:529–541. doi: 10.1016/0003-9861(80)90210-6. [DOI] [PubMed] [Google Scholar]
- 47.Yoon PS, DeLuca HF. Methods Enzymol. 1980;67:430–440. doi: 10.1016/s0076-6879(80)67052-9. [DOI] [PubMed] [Google Scholar]
- 48.Yoon PS, Rawlings J, Orme-Johnson WH, DeLuca HF. Biochemistry. 1980;19:2172–2176. doi: 10.1021/bi00551a027. [DOI] [PubMed] [Google Scholar]
- 49.Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, Portale AA. Molecular Endocrinology. 1997;11:1961–1970. doi: 10.1210/mend.11.13.0035. [DOI] [PubMed] [Google Scholar]
- 50.Fu GK, Portale AA, Miller WL. DNA Cell Biol. 1997;16:1499–1507. doi: 10.1089/dna.1997.16.1499. [DOI] [PubMed] [Google Scholar]
- 51.Monkawa T, Yoshida T, Wakino S, Shinki T, Anazawa H, Deluca HF, Suda T, Hayashi M, Saruta T. Biochem Biophys Res Commun. 1997;239:527–533. doi: 10.1006/bbrc.1997.7508. [DOI] [PubMed] [Google Scholar]
- 52.St-Arnaud R, Messerlian S, Moir JM, Omdahl JL, Glorieux FH. J Bone Miner Res. 1997;12:1552–1559. doi: 10.1359/jbmr.1997.12.10.1552. [DOI] [PubMed] [Google Scholar]
- 53.Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. Science. 1997;277:1827–1830. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- 54.Fraser DR, Kodicek E. Nature. 1970;228:764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- 55.Lawson DE, Fraser DR, Kodicek E, Morris HR, Williams DH. Nature. 1971;230:228–230. doi: 10.1038/230228a0. [DOI] [PubMed] [Google Scholar]
- 56.Shultz TD, Fox J, Heath H, 3rd, Kumar R. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:1746–1750. doi: 10.1073/pnas.80.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weisman Y, Vargas A, Duckett G, Reiter E, Root AW. Endocrinology. 1978;103:1992–1996. doi: 10.1210/endo-103-6-1992. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka Y, Halloran B, Schnoes HK, DeLuca HF. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:5033–5035. doi: 10.1073/pnas.76.10.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bell NH. In: Vitamin D : basic and clinical aspects. Kumar R, editor. Boston Hingham, MA: Nijhoff ; Distributors for North America Kluwer Boston; 1984. pp. 581–589. [Google Scholar]
- 60.Golconda MS, Larson TS, Kolb LG, Kumar R. Mayo Clinic Proceedings. 1996;71:32–36. doi: 10.4065/71.1.32. [DOI] [PubMed] [Google Scholar]
- 61.Schuster I, Egger H, Bikle D, Herzig G, Reddy GS, Stuetz A, Stuetz P, Vorisek G. Steroids. 2001;66:409–422. doi: 10.1016/s0039-128x(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 62.Diaz L, Arranz C, Avila E, Halhali A, Vilchis F, Larrea F. Journal of Clinical Endocrinology & Metabolism. 2002;87:3876–3882. doi: 10.1210/jcem.87.8.8730. [DOI] [PubMed] [Google Scholar]
- 63.Zehnder D, Evans KN, Kilby MD, Bulmer JN, Innes BA, Stewart PM, Hewison M. American Journal of Pathology. 2002;161:105–114. doi: 10.1016/s0002-9440(10)64162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howard GA, Turner RT, Sherrard DJ, Baylink DJ. Journal of Biological Chemistry. 1981;256:7738–7740. [PubMed] [Google Scholar]
- 65.Dusso AS, Finch J, Brown A, Ritter C, Delmez J, Schreiner G, Slatopolsky E. Journal of Clinical Endocrinology & Metabolism. 1991;72:157–164. doi: 10.1210/jcem-72-1-157. [DOI] [PubMed] [Google Scholar]
- 66.Adams JS, Beeker TG, Hongo T, Clemens TL. J Bone Miner Res. 1990;5:1265–1269. doi: 10.1002/jbmr.5650051212. [DOI] [PubMed] [Google Scholar]
- 67.Adams JS, Gacad MA, Diz MM, Nadler JL. J Clin Endocrinol Metab. 1990;70:595–600. doi: 10.1210/jcem-70-3-595. [DOI] [PubMed] [Google Scholar]
- 68.Adams JS, Ren SY. Endocrinology. 1996;137:4514–4517. doi: 10.1210/endo.137.10.8828516. [DOI] [PubMed] [Google Scholar]
- 69.Adams JS, Ren SY, Arbelle JE, Horiuchi N, Gray RW, Clemens TL, Shany S. Endocrinology. 1994;134:2567–2573. doi: 10.1210/endo.134.6.8194484. [DOI] [PubMed] [Google Scholar]
- 70.Adams JS, Ren SY, Arbelle JE, Shany S, Gacad MA. Endocrinology. 1995;136:2262–2269. doi: 10.1210/endo.136.5.7536666. [DOI] [PubMed] [Google Scholar]
- 71.Adams JS, Sharma OP, Gacad MA, Singer FR. J Clin Invest. 1983;72:1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu S, Chun R, Gacad MA, Ren S, Chen H, Adams JS. Endocrinology. 2002;143:4135. doi: 10.1210/en.2002-220568. [DOI] [PubMed] [Google Scholar]
- 73.Henry HL. In: Vitamin D: Basic and clinical aspects. Kumar R, editor. Boston: Nijhoff ; Distributors for North America Kluwer Boston; 1984. pp. 152–174. [Google Scholar]
- 74.Turner RL. In: Vitamin D: Basic and clinical aspects. Kumar R, editor. Boston: Nijhoff ; Distributors for North America Kluwer Boston; 1984. pp. 175–196. [Google Scholar]
- 75.Rasmussen H, Wong M, Bikle D, Goodman DB. Journal of Clinical Investigation. 1972;51:2502–2504. doi: 10.1172/JCI107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trechsel U, Bonjour JP, Fleisch H. Journal of Clinical Investigation. 1979;64:206–217. doi: 10.1172/JCI109441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henry HL. Journal of Biological Chemistry. 1979;254:2722–2729. [PubMed] [Google Scholar]
- 78.Bland R, Zehnder D, Hughes SV, Ronco PM, Stewart PM, Hewison M. Kidney International. 2001;60:1277–1286. doi: 10.1046/j.1523-1755.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- 79.Zehnder D, Bland R, Walker EA, Bradwell AR, Howie AJ, Hewison M, Stewart PM. Journal of the American Society of Nephrology. 1999;10:2465–2473. doi: 10.1681/ASN.V10122465. [DOI] [PubMed] [Google Scholar]
- 80.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N. Journal of Biological Chemistry. 2003;278:2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 82.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 83.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Biochem Biophys Res Commun. 2004;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 86.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Nature. 2006;444:770–774. doi: 10.1681/01.asn.0000926868.48235.3d. [DOI] [PubMed] [Google Scholar]
- 87.Kuro-o M. Pflugers Arch. 2010;459:333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 88.Kuro-o M. Pediatr Nephrol. 2010;25:583–590. doi: 10.1007/s00467-009-1260-4. [DOI] [PubMed] [Google Scholar]
- 89.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Journal of Biological Chemistry. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Medici D, Razzaque MS, Deluca S, Rector TL, Hou B, Kang K, Goetz R, Mohammadi M, Kuro OM, Olsen BR, Lanske B. J Cell Biol. 2008;182:459–465. doi: 10.1083/jcb.200803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Razzaque MS, Lanske B. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 93.Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumar R. Physiological Reviews. 1984;64:478–504. doi: 10.1152/physrev.1984.64.2.478. [DOI] [PubMed] [Google Scholar]
- 95.Turunen MM, Dunlop TW, Carlberg C, Vaisanen S. Nucleic Acids Res. 2007;35:2734–2747. doi: 10.1093/nar/gkm179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumar R, Iachini DN, Neilsen PM, Kaplan J, Michalakas J, Anderson PH, May BK, Morris HA, Callen DF. Molecular and cellular endocrinology. 325:46–53. doi: 10.1016/j.mce.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 97.Annalora AJ, Goodin DB, Hong WX, Zhang Q, Johnson EF, Stout CD. J Mol Biol. 2010;396:441–451. doi: 10.1016/j.jmb.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strushkevich N, Usanov SA, Plotnikov AN, Jones G, Park HW. J Mol Biol. 2008;380:95–106. doi: 10.1016/j.jmb.2008.03.065. [DOI] [PubMed] [Google Scholar]
- 99.Kelley LA, Sternberg MJ. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 100.Roy A, Kucukural A, Zhang Y. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y. Proteins. 2007;69(Suppl 8):108–117. doi: 10.1002/prot.21702. [DOI] [PubMed] [Google Scholar]
- 102.Trott O, Olson AJ. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rhieu SY, Annalora AJ, Gathungu RM, Vouros P, Uskokovic MR, Schuster I, Palmore GT, Reddy GS. Arch Biochem Biophys. 2011;509:33–43. doi: 10.1016/j.abb.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rubin LP, Yeung B, Vouros P, Vilner LM, Reddy GS. Pediatric Research. 1993;34:98–104. doi: 10.1203/00006450-199307000-00023. [DOI] [PubMed] [Google Scholar]
- 105.Staal A, van den Bemd GJ, Birkenhager JC, Pols HA, van Leeuwen JP. Bone. 1997;20:237–243. doi: 10.1016/s8756-3282(96)00371-7. [DOI] [PubMed] [Google Scholar]
- 106.Armbrecht HJ, Hodam TL, Boltz MA, Partridge NC, Brown AJ, Kumar VB. Endocrinology. 1998;139:3375–3381. doi: 10.1210/endo.139.8.6134. [DOI] [PubMed] [Google Scholar]
- 107.Kurahashi I, Matsunuma A, Kawane T, Abe M, Horiuchi N. Endocrine Journal. 2002;17:109–118. doi: 10.1385/ENDO:17:2:109. [DOI] [PubMed] [Google Scholar]
- 108.Nishimura A, Shinki T, Jin CH, Ohyama Y, Noshiro M, Okuda K, Suda T. Endocrinology. 1994;134:1794–1799. doi: 10.1210/endo.134.4.8137744. [DOI] [PubMed] [Google Scholar]
- 109.Kreutz M, Andreesen R, Krause SW, Szabo A, Ritz E, Reichel H. Blood. 1993;82:1300–1307. [PubMed] [Google Scholar]
- 110.Demers C, Lemay J, Hendy GN, Gascon-Barre M. Journal of Molecular Endocrinology. 1997;18:37–48. doi: 10.1677/jme.0.0180037. [DOI] [PubMed] [Google Scholar]
- 111.Theodoropoulos C, Demers C, Delvin E, Menard D, Gascon-Barre M. Clinical Endocrinology. 2003;58:489–499. doi: 10.1046/j.1365-2265.2003.01743.x. [DOI] [PubMed] [Google Scholar]
- 112.Furuichi T, Kawata S, Asoh Y, Kumaki K, Ohyama Y. Life Sciences. 1998;62:453–459. doi: 10.1016/s0024-3205(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 113.Akeno N, Saikatsu S, Kawane T, Horiuchi N. Endocrinology. 1997;138:2233–2240. doi: 10.1210/endo.138.6.5170. [DOI] [PubMed] [Google Scholar]
- 114.Tanaka Y, Lorenc RS, DeLuca HF. Archives of Biochemistry & Biophysics. 1975;171:521–526. doi: 10.1016/0003-9861(75)90061-2. [DOI] [PubMed] [Google Scholar]
- 115.Tanaka Y, DeLuca HF, Ikekawa N, Morisaki M, Koizumi N. Archives of Biochemistry & Biophysics. 1975;170:620–626. doi: 10.1016/0003-9861(75)90157-5. [DOI] [PubMed] [Google Scholar]
- 116.Holick MF, Baxter LA, Schraufrogel PK, Tavela TE, DeLuca HF. Journal of Biological Chemistry. 1976;251:397–402. [PubMed] [Google Scholar]
- 117.Holick MF, Schnoes HK, DeLuca HF, Gray RW, Boyle IT, Suda T. Biochemistry. 1972;11:4251–4255. doi: 10.1021/bi00773a009. [DOI] [PubMed] [Google Scholar]
- 118.Knutson JC, DeLuca HF. Biochemistry. 1974;13:1543–1548. doi: 10.1021/bi00704a034. [DOI] [PubMed] [Google Scholar]
- 119.Kumar R, Schnoes HK, DeLuca HF. Journal of Biological Chemistry. 1978;253:3804–3809. [PubMed] [Google Scholar]
- 120.Garabedian M, Du Bois MB, Corvol MT, Pezant E, Balsan S. Endocrinology. 1978;102:1262–1268. doi: 10.1210/endo-102-4-1262. [DOI] [PubMed] [Google Scholar]
- 121.Kulkowski JA, Chan T, Martinez J, Ghazarian JG. Biochemical & Biophysical Research Communications. 1979;90:50–57. doi: 10.1016/0006-291x(79)91588-2. [DOI] [PubMed] [Google Scholar]
- 122.DeLuca H. Nutrition Reviews. 1979;37:161–193. doi: 10.1111/j.1753-4887.1979.tb06660.x. [DOI] [PubMed] [Google Scholar]
- 123.Boyle IT, Gray RW, DeLuca HF. Proceedings of the National Academy of Sciences of the United States of America. 1971;68:2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ornoy A, Goodwin D, Noff D, Edelstein S. Nature. 1978;276:517–519. doi: 10.1038/276517a0. [DOI] [PubMed] [Google Scholar]
- 125.Henry HL, Taylor AN, Norman AW. Journal of Nutrition. 1977;107:1918–1926. doi: 10.1093/jn/107.10.1918. [DOI] [PubMed] [Google Scholar]
- 126.Tanaka Y, Castillo L, DeLuca HF. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:2701–2705. doi: 10.1073/pnas.73.8.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tanaka Y, Deluca HF. Archives of Biochemistry & Biophysics. 1973;154:566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- 128.Shinki T, Jin CH, Nishimura A, Nagai Y, Ohyama Y, Noshiro M, Okuda K, Suda T. Journal of Biological Chemistry. 1992;267:13757–13762. [PubMed] [Google Scholar]
- 129.Zierold C, Mings JA, DeLuca HF. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13572–13576. doi: 10.1073/pnas.241516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zierold C, Reinholz GG, Mings JA, Prahl JM, DeLuca HF. Archives of Biochemistry & Biophysics. 2000;381:323–327. doi: 10.1006/abbi.2000.1964. [DOI] [PubMed] [Google Scholar]
- 131.Matsumoto T, Kawanobe Y, Ogata E. Biochimica et Biophysica Acta. 1985;845:358–365. doi: 10.1016/0167-4889(85)90199-5. [DOI] [PubMed] [Google Scholar]
- 132.Armbrecht HJ, Boltz MA. FEBS Letters. 1991;292:17–20. doi: 10.1016/0014-5793(91)80823-l. [DOI] [PubMed] [Google Scholar]
- 133.Gray RW, Omdahl JL, Ghazarian JG, Horst RL. Steroids. 1990;55:395–398. doi: 10.1016/0039-128x(90)90097-u. [DOI] [PubMed] [Google Scholar]
- 134.Chen ML, Boltz MA, Armbrecht HJ. Endocrinology. 1993;132:1782–1788. doi: 10.1210/endo.132.4.7681765. [DOI] [PubMed] [Google Scholar]
- 135.Kawashima H, Torikai S, Kurokawa K. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:1199–1203. doi: 10.1073/pnas.78.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang W, Friedman PA, Kumar R, Omdahl JL, May BK, Siu-Caldera ML, Reddy GS, Christakos S. American Journal of Physiology. 1999;276:E793–E805. doi: 10.1152/ajpendo.1999.276.4.E793. [DOI] [PubMed] [Google Scholar]
- 137.Omdahl JL, Morris HA, May BK. Annual Review of Nutrition. 2002;22:139–166. doi: 10.1146/annurev.nutr.22.120501.150216. [DOI] [PubMed] [Google Scholar]
- 138.Akiyoshi-Shibata M, Sakaki T, Ohyama Y, Noshiro M, Okuda K, Yabusaki Y. European Journal of Biochemistry. 1994;224:335–343. doi: 10.1111/j.1432-1033.1994.00335.x. [DOI] [PubMed] [Google Scholar]
- 139.Burgos-Trinidad M, DeLuca HF. Biochimica et Biophysica Acta. 1991;1078:226–230. doi: 10.1016/0167-4838(91)90562-e. [DOI] [PubMed] [Google Scholar]
- 140.Taniguchi T, Eto TA, Shiotsuki H, Sueta H, Higashi S, Iwamura T, Okuda KI, Setoguchi T. Journal of Bone & Mineral Research. 2001;16:57–62. doi: 10.1359/jbmr.2001.16.1.57. [DOI] [PubMed] [Google Scholar]
- 141.Seo EG, Norman AW. Journal of Bone & Mineral Research. 1997;12:598–606. doi: 10.1359/jbmr.1997.12.4.598. [DOI] [PubMed] [Google Scholar]
- 142.Henry HL, Norman AW. Science. 1978;201:835–837. doi: 10.1126/science.684411. [DOI] [PubMed] [Google Scholar]
- 143.St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay MB, Glorieux FH. Endocrinology. 2000;141:2658–2666. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- 144.Kobayashi Y, Taguchi T, Terada T, Oshida J, Morisaki M, Ikekawa N. Tetrahedron. 1979;22:2023–2026. [Google Scholar]
- 145.Yamada S, Ohmori M, Takayama H. Tetrahedron. 1979;21:1859–1862. [Google Scholar]
- 146.Halloran BP, DeLuca HF, Yamada S, Takayama H, Jee WS. Calcified Tissue International. 1981;33:489–497. doi: 10.1007/BF02409479. [DOI] [PubMed] [Google Scholar]
- 147.Tanaka Y, DeLuca HF, Kobayashi Y, Taguchi T, Ikekawa N, Morisaki M. Journal of Biological Chemistry. 1979;254:7163–7167. [PubMed] [Google Scholar]
- 148.Mathews CHE, Parfitt AM, Brommage R, De Luca HF. American Society for Bone and Mineral Research (abstract) 1983:S66. [Google Scholar]
- 149.Tanaka Y, Pahuja DN, Wichmann JK, De Luca HF, Kobayashi Y, Taguchi T, Ikekawa N. Archives of Biochemistry & Biophysics. 1982;218:134–141. doi: 10.1016/0003-9861(82)90328-9. [DOI] [PubMed] [Google Scholar]
- 150.Takasaki Y, Horiuchi N, Takahashi N, Abe E, Shinki T, Suda T, Yamada S, Takayama H, Horikawa H, Masumura T, Sugahara M. Biochemical & Biophysical Research Communications. 1980;95:177–181. doi: 10.1016/0006-291x(80)90720-2. [DOI] [PubMed] [Google Scholar]
- 151.Takasaki Y, Suda T, Yamada S, Takayama H, Nishii Y. Biochemistry. 1981;20:1681–1686. doi: 10.1021/bi00509a042. [DOI] [PubMed] [Google Scholar]
- 152.Wichmann JK, Schnoes HK, DeLuca HF. Biochemistry. 1981;20:7385–7391. doi: 10.1021/bi00529a010. [DOI] [PubMed] [Google Scholar]
- 153.Redel J, Bazely N, Tanaka Y, DeLuca HF. FEBS Letters. 1978;94:228–230. doi: 10.1016/0014-5793(78)80943-0. [DOI] [PubMed] [Google Scholar]
- 154.Partridge JJ, Shiuey SJ, Chadha NK, Baggiolini EG, Blount JF. Journal Am Chem Society. 1981;103:1253–1255. [Google Scholar]
- 155.Cesario M, Guilhem J, Pascard C, Redel J. Tetrahedron. 1978;12:1097–1098. [Google Scholar]
- 156.Cesario M, Guilhem J, Pascard C, Redel J. Tetrahedron. 1980;21:1588. [Google Scholar]
- 157.Horst RL, Littledike ET. Biochemical & Biophysical Research Communications. 1980;93:149–154. doi: 10.1016/s0006-291x(80)80258-0. [DOI] [PubMed] [Google Scholar]
- 158.Gray RW, Caldas AE, Weber JL, Ghazarian JG. Biochemical & Biophysical Research Communications. 1978;82:212–218. doi: 10.1016/0006-291x(78)90585-5. [DOI] [PubMed] [Google Scholar]
- 159.Tanaka Y, Shepard RA, DeLuca HF, Schnoes HK. Biochemical & Biophysical Research Communications. 1978;83:7–13. doi: 10.1016/0006-291x(78)90390-x. [DOI] [PubMed] [Google Scholar]
- 160.Horst RL. Biochemical & Biophysical Research Communications. 1979;89:286–293. doi: 10.1016/0006-291x(79)90976-8. [DOI] [PubMed] [Google Scholar]
- 161.Wichmann JK, DeLuca HF, Schnoes HK, Horst RL, Shepard RM, Jorgensen NA. Biochemistry. 1979;18:4775–4780. doi: 10.1021/bi00589a002. [DOI] [PubMed] [Google Scholar]
- 162.Ishizuka S, Ishimoto S, Norman AW. FEBS Letters. 1982;138:83–87. doi: 10.1016/0014-5793(82)80400-6. [DOI] [PubMed] [Google Scholar]
- 163.Tanaka Y, Wichmann JK, Schnoes HK, DeLuca HF. Biochemistry. 1981;20:3875–3879. doi: 10.1021/bi00516a032. [DOI] [PubMed] [Google Scholar]
- 164.Horst RL, Pramanik BC, Reinhardt TA, Shiuey SJ, Partridge JJ, Uskokovic MR, Napoli JL. Biochemical & Biophysical Research Communications. 1982;106:1006–1011. doi: 10.1016/0006-291x(82)91811-3. [DOI] [PubMed] [Google Scholar]
- 165.Partridge JJ, Chadha NK, Shiuey SJ, Wovkulich PM, Uskokovic MR, Napoli JL, Horst RL. In: Vitamin D: Endocrinological aspects and their clinical applications. Norman AW, editor. New York: Walter de Gruyter; 1982. pp. 1073–1078. [Google Scholar]
- 166.Morris DS, Williams DH, Norris AF. Journal of Organic Chemistry. 1981;46:3422–3428. [Google Scholar]
- 167.Morris DS, Williams DH, Norris AF. Journal Chem Soc Chem Comm. 1981;9:424–425. [Google Scholar]
- 168.Tanaka Y, Wichmann JK, Paaren HE, Schnoes HK, DeLuca HF. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:6411–6414. doi: 10.1073/pnas.77.11.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Horst RL, Reinhardt TA, Napoli JL. Biochemical & Biophysical Research Communications. 1982;107:1319–1325. doi: 10.1016/s0006-291x(82)80142-3. [DOI] [PubMed] [Google Scholar]
- 170.Horst RL, Reinhardt TA, Pramanik BC, Napoli JL. Biochemistry. 1983;22:245–250. doi: 10.1021/bi00271a002. [DOI] [PubMed] [Google Scholar]
- 171.Kumar R. In: Vitamin D: Basic and clinical aspects. Kumar R, editor. Boston: Martinus Nirjhoff; 1984. pp. 69–90. [Google Scholar]
- 172.Frolik CA, DeLuca HF. Journal of Clinical Investigation. 1972;51:2900–2906. doi: 10.1172/JCI107114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Frolik CA, DeLuca HF. Journal of Clinical Investigation. 1973;52:543–548. doi: 10.1172/JCI107214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kumar R, Harnden D, DeLuca HF. Biochemistry. 1976;15:2420–2423. doi: 10.1021/bi00656a027. [DOI] [PubMed] [Google Scholar]
- 175.Esvelt RP, Schnoes HK, DeLuca HF. Biochemistry. 1979;18:3977–3983. doi: 10.1021/bi00585a021. [DOI] [PubMed] [Google Scholar]
- 176.Ohnuma N, Kruse JR, Popjak G, Norman AW. Journal of Biological Chemistry. 1982;257:5097–5102. [PubMed] [Google Scholar]
- 177.Ohnuma N, Norman AW. Journal of Biological Chemistry. 1982;257:8261–8271. [PubMed] [Google Scholar]
- 178.Reinhardt TA, Napoli JL, Beitz DC, Littledike ET, Horst RL. Biochemical & Biophysical Research Communications. 1981;99:302–307. doi: 10.1016/0006-291x(81)91745-9. [DOI] [PubMed] [Google Scholar]
- 179.Kumar R, Nagubandi S, Mattox VR, Londowski JM. Journal of Clinical Investigation. 1980;65:277–284. doi: 10.1172/JCI109669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kumar R, Nagubandi S, Londowski JM. Digestive Diseases & Sciences. 1981;26:242–246. doi: 10.1007/BF01391637. [DOI] [PubMed] [Google Scholar]
- 181.Litwiller RD, Mattox VR, Jardine I, Kumar R. Journal of Biological Chemistry. 1982;257:7491–7494. [PubMed] [Google Scholar]
- 182.Gray RW, Caldas AE, Wilz DR, Lemann J, Jr, Smith GA, DeLuca HF. Journal of Clinical Endocrinology & Metabolism. 1978;46:756–765. doi: 10.1210/jcem-46-5-756. [DOI] [PubMed] [Google Scholar]
- 183.Seeman E, Kumar R, Hunder GG, Scott M, Heath H, 3rd, Riggs BL. Journal of Clinical Investigation. 1980;66:664–669. doi: 10.1172/JCI109902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wiesner RH, Kumar R, Seeman E, Go VL. Journal of Laboratory & Clinical Medicine. 1980;96:1094–1100. [PubMed] [Google Scholar]
- 185.Harnden D, Kumar R, Holick MF, Deluca HF. Science. 1976;193:493–494. doi: 10.1126/science.941020. [DOI] [PubMed] [Google Scholar]
- 186.Puschett JB, Beck WS., Jr Science. 1975;190:473–475. doi: 10.1126/science.1166316. [DOI] [PubMed] [Google Scholar]
- 187.Puschett JB, Beck WS, Jr, Jelonek A, Fernandez PC. Journal of Clinical Investigation. 1974;53:756–767. doi: 10.1172/JCI107614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Puschett JB, Moranz J, Kurnick WS. Journal of Clinical Investigation. 1972;51:373–385. doi: 10.1172/JCI106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Puschett JB, Fernandez PC, Boyle IT, Gray RW, Omdahl JL, DeLuca HF. Proceedings of the Society for Experimental Biology & Medicine. 1972;141:379–384. doi: 10.3181/00379727-141-36781. [DOI] [PubMed] [Google Scholar]
- 190.Siegfried D, Kumar R, Arruda, Kurtzman N. Influence of vitamin D on bicarbonate reabsorption. In: Massry SG, editor. Proceedings of the 2nd International Congress on Phosphate. Heidelberg: Plenum press; 1976. [Google Scholar]
- 191.Popovtzer MM, Robinette JB, DeLuca HF, Holick MF. Journal of Clinical Investigation. 1974;53:913–921. doi: 10.1172/JCI107632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yamamoto M, Kawanobe Y, Takahashi H, Shimazawa E, Kimura S, Ogata E. Journal of Clinical Investigation. 1984;74:507–513. doi: 10.1172/JCI111448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sneddon WB, Barry EL, Coutermarsh BA, Gesek FA, Liu F, Friedman PA. Cellular Physiology & Biochemistry. 1998;8:261–277. doi: 10.1159/000016288. [DOI] [PubMed] [Google Scholar]
- 194.Winaver J, Sylk DB, Robertson JS, Chen TC, Puschett JB. Mineral Electrolyte Metab. 1980;4:178–188. [PubMed] [Google Scholar]
- 195.Sutton RAL, NLM W, Dirks JS. Dirks, 25-hydroxyvitamin D3 (25(OH)D3): Enhancement of distal tubular calcium reabsorption in the dog, 8th annual meeting of the american society of nephrology; 1975. p. 8. [Google Scholar]
- 196.Harris CA, Seely JF. Clinical Res. 1979;27:417A. [Google Scholar]
- 197.Bouhtiauy I, Lajeunesse D, Brunette MG. Endocrinology. 1993;132:115–120. doi: 10.1210/endo.132.1.8419116. [DOI] [PubMed] [Google Scholar]
- 198.Bindels RJ, Hartog A, Timmermans J, Van Os CH. American Journal of Physiology. 1991;261:F799–F807. doi: 10.1152/ajprenal.1991.261.5.F799. [DOI] [PubMed] [Google Scholar]
- 199.Hoenderop JG, Muller D, Van Der Kemp AW, Hartog A, Suzuki M, Ishibashi K, Imai M, Sweep F, Willems PH, Van Os CH, Bindels RJ. Journal of the American Society of Nephrology. 2001;12:1342–1349. doi: 10.1681/ASN.V1271342. [DOI] [PubMed] [Google Scholar]
- 200.Nijenhuis T, Hoenderop JG, van der Kemp AW, Bindels RJ. J Am Soc Nephrol. 2003;14:2731–2740. doi: 10.1097/01.asn.0000094081.78893.e8. [DOI] [PubMed] [Google Scholar]
- 201.Woudenberg-Vrenken TE, Bindels RJ, Hoenderop JG. Nature reviews. 2009;5:441–449. doi: 10.1038/nrneph.2009.100. [DOI] [PubMed] [Google Scholar]
- 202.Glendenning P, Ratajczak T, Dick IM, Prince RL. Archives of Biochemistry & Biophysics. 2000;380:126–132. doi: 10.1006/abbi.2000.1908. [DOI] [PubMed] [Google Scholar]
- 203.Borke JL, Caride A, Verma AK, Penniston JT, Kumar R. American Journal of Physiology. 1989;257:F842–F849. doi: 10.1152/ajprenal.1989.257.5.F842. [DOI] [PubMed] [Google Scholar]
- 204.Johnson JA, Kumar R. Seminars in Nephrology. 1994;14:119–128. [PubMed] [Google Scholar]
- 205.Canaff L, Hendy GN. Journal of Biological Chemistry. 2002;277:30337–30350. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- 206.Wald H, Dranitzki-Elhalel M, Backenroth R, Popovtzer MM. Pflugers Archiv - European Journal of Physiology. 1998;436:289–294. doi: 10.1007/s004240050634. [DOI] [PubMed] [Google Scholar]
- 207.Ozono K, Sone T, Pike JW. Journal of Bone & Mineral Research. 1991;6:1021–1027. doi: 10.1002/jbmr.5650061002. [DOI] [PubMed] [Google Scholar]
- 208.Pike JW. Annual Review of Nutrition. 1991;11:189–216. doi: 10.1146/annurev.nu.11.070191.001201. [DOI] [PubMed] [Google Scholar]
- 209.Hughes MR, Malloy PJ, O'Malley BW, Pike JW, Feldman D. Journal of Receptor Research. 1991;11:699–716. doi: 10.3109/10799899109066437. [DOI] [PubMed] [Google Scholar]
- 210.Feldman D, Malloy PJ. Molecular & Cellular Endocrinology. 1990;72:C57–C62. doi: 10.1016/0303-7207(90)90137-w. [DOI] [PubMed] [Google Scholar]
- 211.Malloy PJ, Hochberg Z, Tiosano D, Pike JW, Hughes MR, Feldman D. Journal of Clinical Investigation. 1990;86:2071–2079. doi: 10.1172/JCI114944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hughes M, Malloy P, Kieback D, McDonnell D, Feldman D, Pike JW, O'Malley B. Advances in Experimental Medicine & Biology. 1989;255:491–503. doi: 10.1007/978-1-4684-5679-0_52. [DOI] [PubMed] [Google Scholar]
- 213.Ritchie HH, Hughes MR, Thompson ET, Malloy PJ, Hochberg Z, Feldman D, Pike JW, O'Malley BW. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:9783–9787. doi: 10.1073/pnas.86.24.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sone T, Scott RA, Hughes MR, Malloy PJ, Feldman D, O'Malley BW, Pike JW. Journal of Biological Chemistry. 1989;264:20230–20234. [PubMed] [Google Scholar]
- 215.Hughes MR, Malloy PJ, Kieback DG, Kesterson RA, Pike JW, Feldman D, O'Malley BW. Science. 1988;242:1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- 216.Stumpf WE, Sar M, Narbaitz R, Reid FA, DeLuca HF, Tanaka Y. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:1149–1153. doi: 10.1073/pnas.77.2.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF. Science. 1979;206:1188–1190. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- 218.Kawashima H, Kurokawa K. Journal of Biological Chemistry. 1982;257:13428–13432. [PubMed] [Google Scholar]
- 219.Liu L, Ng M, Iacopino AM, Dunn ST, Hughes MR, Bourdeau JE. Journal of the American Society of Nephrology. 1994;5:1251–1258. doi: 10.1681/ASN.V551251. [DOI] [PubMed] [Google Scholar]
- 220.Liu L, Khastgir A, McCauley JM, Dunn ST, Morrissey JH, Christakos S, Hughes MR, Bourdeau JE. American Journal of Physiology. 1996;270:F677–F681. doi: 10.1152/ajprenal.1996.270.4.F677. [DOI] [PubMed] [Google Scholar]
- 221.Solvsten H, Fogh K, Svendsen M, Kristensen P, Astrom A, Kumar R, Kragballe K. Journal of Investigative Dermatology. Symposium Proceedings. 1996;1:28–32. [PubMed] [Google Scholar]
- 222.Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ. Journal of Biological Chemistry. 1999;274:8375–8378. doi: 10.1074/jbc.274.13.8375. [DOI] [PubMed] [Google Scholar]
- 223.Kumar R. Nephrology Dialysis Transplantation. 1996;11(Suppl 3):6–10. doi: 10.1093/ndt/11.supp3.6. [DOI] [PubMed] [Google Scholar]
- 224.Goff JP, Reinhardt TA, Beckman MJ, Horst RL. Endocrinology. 1990;126:1031–1035. doi: 10.1210/endo-126-2-1031. [DOI] [PubMed] [Google Scholar]
- 225.Uhland-Smith A, DeLuca HF. Biochimica et Biophysica Acta. 1993;1176:321–326. doi: 10.1016/0167-4889(93)90061-s. [DOI] [PubMed] [Google Scholar]
- 226.Reinhardt TA, Horst RL. Endocrinology. 1990;127:942–948. doi: 10.1210/endo-127-2-942. [DOI] [PubMed] [Google Scholar]
- 227.Gross M, Kumar R. American Journal of Physiology. 1990;259:F195–F209. doi: 10.1152/ajprenal.1990.259.2.F195. [DOI] [PubMed] [Google Scholar]
- 228.Desplan C, Heidmann O, Lillie JW, Auffray C, Thomasset M. Journal of Biological Chemistry. 1983;258:13502–13505. [PubMed] [Google Scholar]
- 229.Desplan C, Thomasset M, Moukhtar M. Journal of Biological Chemistry. 1983;258:2762–2765. [PubMed] [Google Scholar]
- 230.Fullmer CS, Wasserman RH. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:4772–4776. doi: 10.1073/pnas.84.14.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hofmann T, Kawakami M, Hitchman AJ, Harrison JE, Dorrington KJ. Canadian Journal of Biochemistry. 1979;57:737–748. doi: 10.1139/o79-092. [DOI] [PubMed] [Google Scholar]
- 232.Horrocks WD. In: Advances in Inorganic Biochemistry. Eichorn GL, Marzitti LG, editors. New York: Elsevier; 1982. pp. 201–261. [Google Scholar]
- 233.Hunziker W. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:7578–7582. doi: 10.1073/pnas.83.20.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hunziker W, Schrickel S. Molecular Endocrinology. 1988;2:465–473. doi: 10.1210/mend-2-5-465. [DOI] [PubMed] [Google Scholar]
- 235.Kumar R, Wieben E, Beecher SJ. Molecular Endocrinology. 1989;3:427–432. doi: 10.1210/mend-3-2-427. [DOI] [PubMed] [Google Scholar]
- 236.Lomri N, Perret C, Gouhier N, Thomasset M. Gene. 1989;80:87–98. doi: 10.1016/0378-1119(89)90253-9. [DOI] [PubMed] [Google Scholar]
- 237.Parmentier M, Lawson DE, Vassart G. European Journal of Biochemistry. 1987;170:207–215. doi: 10.1111/j.1432-1033.1987.tb13688.x. [DOI] [PubMed] [Google Scholar]
- 238.Takagi T, Nojiri M, Konishi K, Maruyama K, Nonomura Y. FEBS Letters. 1986;201:41–45. doi: 10.1016/0014-5793(86)80567-1. [DOI] [PubMed] [Google Scholar]
- 239.Tsarbopoulos A, Gross M, Kumar R, Jardine I. Biomedical & Environmental Mass Spectrometry. 1989;18:387–393. doi: 10.1002/bms.1200180605. [DOI] [PubMed] [Google Scholar]
- 240.Wilson PW, Harding M, Lawson DE. Nucleic Acids Research. 1985;13:8867–8881. doi: 10.1093/nar/13.24.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yamakuni T, Kuwano R, Odani S, Miki N, Yamaguchi Y, Takahashi Y. Nucleic Acids Research. 1986;14:6768. doi: 10.1093/nar/14.16.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Darwish HM, Krisinger J, Strom M, DeLuca HF. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:6108–6111. doi: 10.1073/pnas.84.17.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fullmer CS, Wasserman RH. Journal of Biological Chemistry. 1981;256:5669–5674. [PubMed] [Google Scholar]
- 244.MacManus JP, Watson DC, Yaguchi M. Biochemical Journal. 1986;235:585–595. doi: 10.1042/bj2350585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Szebenyi DM, Obendorf SK, Moffat K. Nature. 1981;294:327–332. doi: 10.1038/294327a0. [DOI] [PubMed] [Google Scholar]
- 246.Elms TN, Taylor AN. Journal of Dental Research. 1987;66:1431–1434. doi: 10.1177/00220345870660090401. [DOI] [PubMed] [Google Scholar]
- 247.Dorrington KJ, Kells DI, Hitchman AJ, Hartison JE, Hofmann T. Canadian Journal of Biochemistry. 1978;56:492–499. doi: 10.1139/o78-076. [DOI] [PubMed] [Google Scholar]
- 248.O'Neil JD, Dorrington KJ, Hofmann T. Canadian Journal of Biochemistry & Cell Biology. 1984;62:434–442. doi: 10.1139/o84-059. [DOI] [PubMed] [Google Scholar]
- 249.Chiba K, Ohyashiki T, Mohri T. Journal of Biochemistry. 1983;93:487–493. doi: 10.1093/oxfordjournals.jbchem.a134203. [DOI] [PubMed] [Google Scholar]
- 250.Vogel HJ, Drakenberg T, Forsen S, O'Neil JD, Hofmann T. Biochemistry. 1985;24:3870–3876. doi: 10.1021/bi00336a009. [DOI] [PubMed] [Google Scholar]
- 251.Shelling JG, Sykes BD. Journal of Biological Chemistry. 1985;260:8342–8347. [PubMed] [Google Scholar]
- 252.Bredderman PJ, Wasserman RH. Biochemistry. 1974;13:1687–1694. doi: 10.1021/bi00705a021. [DOI] [PubMed] [Google Scholar]
- 253.Fullmer CS, Edelstein S, Wasserman RH. Journal of Biological Chemistry. 1985;260:6816–6819. [PubMed] [Google Scholar]
- 254.Gross MD, Nelsestuen GL, Kumar R. Journal of Biological Chemistry. 1987;262:6539–6545. [PubMed] [Google Scholar]
- 255.Gross MD, Kumar R, Hunziker W. Journal of Biological Chemistry. 1988;263:14426–14432. [PubMed] [Google Scholar]
- 256.Veenstra TD, Gross MD, Hunziker W, Kumar R. Journal of Biological Chemistry. 1995;270:30353–30358. doi: 10.1074/jbc.270.51.30353. [DOI] [PubMed] [Google Scholar]
- 257.Kumar R, Hunziker W, Gross M, Naylor S, Londowski JM, Schaefer J. Archives of Biochemistry & Biophysics. 1994;308:311–317. doi: 10.1006/abbi.1994.1044. [DOI] [PubMed] [Google Scholar]
- 258.Wasserman RH, Corradino RA, Fullmer CS, Taylor AN. Vitamins & Hormones. 1974;32:299–324. doi: 10.1016/s0083-6729(08)60017-5. [DOI] [PubMed] [Google Scholar]
- 259.Christakos S, Brunette MG, Norman AW. Endocrinology. 1981;109:322–324. doi: 10.1210/endo-109-1-322. [DOI] [PubMed] [Google Scholar]
- 260.Taylor AN, Wasserman RH. Archives of Biochemistry & Biophysics. 1967;119:536–540. doi: 10.1016/0003-9861(67)90488-2. [DOI] [PubMed] [Google Scholar]
- 261.Taylor AN, McIntosh JE, Bourdeau JE. Kidney International. 1982;21:765–773. doi: 10.1038/ki.1982.95. [DOI] [PubMed] [Google Scholar]
- 262.Roth J, Thorens B, Hunziker W, Norman AW, Orci L. Science. 1981;214:197–200. doi: 10.1126/science.7025212. [DOI] [PubMed] [Google Scholar]
- 263.Roth J, Brown D, Norman AW, Orci L. American Journal of Physiology. 1982;243:F243–F252. doi: 10.1152/ajprenal.1982.243.3.F243. [DOI] [PubMed] [Google Scholar]
- 264.Thomasset M, Parkes CO, Cuisinier-Gleizes P. American Journal of Physiology. 1982;243:E483–E488. doi: 10.1152/ajpendo.1982.243.6.E483. [DOI] [PubMed] [Google Scholar]
- 265.Venters RA, Benson LM, Craig TA, Bagu J, Paul KH, Kordys DR, Thompson R, Naylor S, Kumar R, Cavanagh J. Analytical biochemistry. 2003;317:59–66. doi: 10.1016/s0003-2697(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 266.Kojetin DJ, Venters RA, Kordys DR, Thompson RJ, Kumar R, Cavanagh J. Nature structural & molecular biology. 2006;13:641–647. doi: 10.1038/nsmb1112. [DOI] [PubMed] [Google Scholar]
- 267.Hemmingsen C, Staun M, Nielsen PK, Olgaard K. Pharmacology & Toxicology. 2002;91:111–115. doi: 10.1034/j.1600-0773.2002.910304.x. [DOI] [PubMed] [Google Scholar]
- 268.Hoenderop JG, Dardenne O, Van Abel M, Van Der Kemp AW, Van Os CH, St - Arnaud R, Bindels RJ. FASEB Journal. 2002;16:1398–1406. doi: 10.1096/fj.02-0225com. [DOI] [PubMed] [Google Scholar]
- 269.Li YC, Bolt MJ, Cao LP, Sitrin MD. American Journal of Physiology - Endocrinology & Metabolism. 2001;281:E558–E564. doi: 10.1152/ajpendo.2001.281.3.E558. [DOI] [PubMed] [Google Scholar]
- 270.Cao LP, Bolt MJ, Wei M, Sitrin MD, Chun Li Y. Archives of Biochemistry & Biophysics. 2002;400:118–124. doi: 10.1006/abbi.2002.2775. [DOI] [PubMed] [Google Scholar]
- 271.Hemmingsen C, Staun M, Lewin E, Nielsen PK, Olgaard K. Journal of Bone & Mineral Research. 1996;11:1086–1093. doi: 10.1002/jbmr.5650110807. [DOI] [PubMed] [Google Scholar]
- 272.Bouhtiauy I, Lajeunesse D, Christakos S, Brunette MG. Kidney International. 1994;45:461–468. doi: 10.1038/ki.1994.60. [DOI] [PubMed] [Google Scholar]
- 273.Bouhtiauy I, Lajeunesse D, Christakos S, Brunette MG. Kidney International. 1994;45:469–474. doi: 10.1038/ki.1994.61. [DOI] [PubMed] [Google Scholar]
- 274.Lee CT, Huynh VM, Lai LW, Lien YH. Kidney International. 2002;62:2055–2061. doi: 10.1046/j.1523-1755.2002.00670.x. [DOI] [PubMed] [Google Scholar]
- 275.Sooy K, Kohut J, Meyer M, Yeh C, Hou T, Christakos S. J Bone Min Res. 1999;14(Suppl 1):S211. [Google Scholar]
- 276.Lutz W, Berndt T, Knox FG, Kumar R. FASEB Journal. 2000;14:A343. [Google Scholar]
- 277.Doucet A, Katz AI. American Journal of Physiology. 1982;242:F346–F352. doi: 10.1152/ajprenal.1982.242.4.F346. [DOI] [PubMed] [Google Scholar]
- 278.Borke JL, Caride A, Verma AK, Penniston JT, Kumar R. Pflugers Archiv - European Journal of Physiology. 1990;417:120–122. doi: 10.1007/BF00370781. [DOI] [PubMed] [Google Scholar]
- 279.Wasserman RH, Smith CA, Brindak ME, De Talamoni N, Fullmer CS, Penniston JT, Kumar R. Gastroenterology. 1992;102:886–894. doi: 10.1016/0016-5085(92)90174-w. [DOI] [PubMed] [Google Scholar]
- 280.Borke JL, Caride A, Verma AK, Kelley LK, Smith CH, Penniston JT, Kumar R. American Journal of Physiology. 1989;257:c341–c346. doi: 10.1152/ajpcell.1989.257.2.C341. [DOI] [PubMed] [Google Scholar]
- 281.Borke JL, Caride AJ, Yaksh TL, Penniston JT, Kumar R. Brain Research. 1989;489:355–360. doi: 10.1016/0006-8993(89)90870-6. [DOI] [PubMed] [Google Scholar]
- 282.Wasserman RH, Smith CA, Smith CM, Brindak ME, Fullmer CS, Krook L, Penniston JT, Kumar R. Histochemistry. 1991;96:413–418. doi: 10.1007/BF00315999. [DOI] [PubMed] [Google Scholar]
- 283.Kumar R, Haugen JD, Medora M, Yamakawa K, Hruska KA. Journal of Bone & Mineral Research. 1993;8:S386. doi: 10.1002/jbmr.5650080415. [DOI] [PubMed] [Google Scholar]
- 284.Cai Q, Chandler JS, Wasserman RH, Kumar R, Penniston JT. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1345–1349. doi: 10.1073/pnas.90.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wasserman RH, Chandler JS, Meyer SA, Smith CA, Brindak ME, Fullmer CS, Penniston JT, Kumar R. Journal of Nutrition. 1992;122:662–671. doi: 10.1093/jn/122.suppl_3.662. [DOI] [PubMed] [Google Scholar]
- 286.Blankenship KA, Williams JJ, Lawrence MS, McLeish KR, Dean WL, Arthur JM. American Journal of Physiology - Renal Fluid & Electrolyte Physiology. 2001;280:F815–F822. doi: 10.1152/ajprenal.2001.280.5.F815. [DOI] [PubMed] [Google Scholar]
- 287.Muller D, Hoenderop JG, Meij IC, van den Heuvel LP, Knoers NV, den Hollander AI, Eggert P, Garcia-Nieto V, Claverie-Martin F, Bindels RJ. Genomics. 2000;67:48–53. doi: 10.1006/geno.2000.6203. [DOI] [PubMed] [Google Scholar]
- 288.Hoenderop JG, Nilius B, Bindels RJ. Annual Review of Physiology. 2002;64:529–549. doi: 10.1146/annurev.physiol.64.081501.155921. [DOI] [PubMed] [Google Scholar]
- 289.Peng JB, Hediger MA. Current Opinion in Nephrology & Hypertension. 2002;11:555–561. doi: 10.1097/00041552-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 290.Weber K, Erben RG, Rump A, Adamski J. Biochemical & Biophysical Research Communications. 2001;289:1287–1294. doi: 10.1006/bbrc.2001.6121. [DOI] [PubMed] [Google Scholar]
- 291.Murayama A, Takeyama K, Kitanaka S, Kodera Y, Kawaguchi Y, Hosoya T, Kato S. Endocrinology. 1999;140:2224–2231. doi: 10.1210/endo.140.5.6691. [DOI] [PubMed] [Google Scholar]
- 292.Shinki T, Ueno Y, DeLuca HF, Suda T. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8253–8258. doi: 10.1073/pnas.96.14.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Adams ND, Gray RW, Lemann J., Jr Calcified Tissue International. 1979;28:233–238. doi: 10.1007/BF02441241. [DOI] [PubMed] [Google Scholar]
- 294.Bilezikian JP, Canfield RE, Jacobs TP, Polay JS, D'Adamo AP, Eisman JA, DeLuca HF. New England Journal of Medicine. 1978;299:437–441. doi: 10.1056/NEJM197808312990902. [DOI] [PubMed] [Google Scholar]
- 295.Boyle IT, Gray RW, Omdahl JL, DeLuca HF. Calcium control of the in vivo biosynthesis of 1,25-dihydroxyvitamin D3: Nicolaysen's endogenous factor. In: S T, editor. Proceeding of the International Symposium on Endocrinology. London: Heinemann; 1972. pp. 468–476. [Google Scholar]
- 296.Drezner MK, Neelon FA, Haussler M, McPherson HT, Lebovitz HE. Journal of Clinical Endocrinology & Metabolism. 1976;42:621–628. doi: 10.1210/jcem-42-4-621. [DOI] [PubMed] [Google Scholar]
- 297.Fraser DR, Kodicek E. Nature - New Biology. 1973;241:163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- 298.Garabedian M, Holick MF, Deluca HF, Boyle IT. Proceedings of the National Academy of Sciences of the United States of America. 1972;69:1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Omdahl JL, Gray RW, Boyle IT, Knutson J, DeLuca HF. Nature - New Biology. 1972;237:63–64. doi: 10.1038/newbio237063a0. [DOI] [PubMed] [Google Scholar]
- 300.Gray RW, Wilz DR, Caldas AE, Lemann J., Jr Journal of Clinical Endocrinology & Metabolism. 1977;45:299–306. doi: 10.1210/jcem-45-2-299. [DOI] [PubMed] [Google Scholar]
- 301.Yoshida T, Yoshida N, Monkawa T, Hayashi M, Saruta T. Endocrinology. 2001;142:1720–1726. doi: 10.1210/endo.142.5.8119. [DOI] [PubMed] [Google Scholar]
- 302.Zhang MY, Wang X, Wang JT, Compagnone NA, Mellon SH, Olson JL, Tenenhouse HS, Miller WL, Portale AA. Endocrinology. 2002;143:587–595. doi: 10.1210/endo.143.2.8627. [DOI] [PubMed] [Google Scholar]
- 303.Kawashima H, Torikai S, Kurokawa K. Nature. 1981;291:327–329. doi: 10.1038/291327a0. [DOI] [PubMed] [Google Scholar]
- 304.Bland R, Walker EA, Hughes SV, Stewart PM, Hewison M. Endocrinology. 1999;140:2027–2034. doi: 10.1210/endo.140.5.6683. [DOI] [PubMed] [Google Scholar]
- 305.Lund B, Sorensen OH, Bishop JE, Norman AW. Journal of Clinical Endocrinology & Metabolism. 1980;50:480–484. doi: 10.1210/jcem-50-3-480. [DOI] [PubMed] [Google Scholar]
- 306.Yoshida N, Yoshida T, Nakamura A, Monkawa T, Hayashi M, Saruta T. Journal of the American Society of Nephrology. 1999;10:2474–2479. doi: 10.1681/ASN.V10122474. [DOI] [PubMed] [Google Scholar]
- 307.Adams ND, Gray RW, Lemann J., Jr Journal of Clinical Endocrinology & Metabolism. 1979;48:1008–1016. doi: 10.1210/jcem-48-6-1008. [DOI] [PubMed] [Google Scholar]
- 308.Sauveur B, Garabedian M, Fellot C, Mongin P, Balsan S. Calcified Tissue Research. 1977;23:121–124. doi: 10.1007/BF02012776. [DOI] [PubMed] [Google Scholar]
- 309.Weber HP, Gray RW, Dominguez JH, Lemann J., Jr Journal of Clinical Endocrinology & Metabolism. 1976;43:1047–1055. doi: 10.1210/jcem-43-5-1047. [DOI] [PubMed] [Google Scholar]
- 310.Castillo L, Tanaka Y, DeLuca HF, Sunde ML. Archives of Biochemistry & Biophysics. 1977;179:211–217. doi: 10.1016/0003-9861(77)90105-9. [DOI] [PubMed] [Google Scholar]
- 311.Adams ND, Garthwaite TL, Gray RW, Hagen TC, Lemann J., Jr Journal of Clinical Endocrinology & Metabolism. 1979;49:628–630. doi: 10.1210/jcem-49-4-628. [DOI] [PubMed] [Google Scholar]
- 312.Kumar R, Cohen WR, Epstein FH. New England Journal of Medicine. 1980;302:1143–1145. doi: 10.1056/NEJM198005153022010. [DOI] [PubMed] [Google Scholar]
- 313.Spanos E, Colston KW, Evans IM, Galante LS, Macauley SJ, Macintyre I. Molecular & Cellular Endocrinology. 1976;5:163–167. doi: 10.1016/0303-7207(76)90080-0. [DOI] [PubMed] [Google Scholar]
- 314.Eskildsen PC, Lund B, Sorensen OH, Bishop JE, Norman AW. Journal of Clinical Endocrinology & Metabolism. 1979;49:484–486. doi: 10.1210/jcem-49-3-484. [DOI] [PubMed] [Google Scholar]
- 315.Gertner JM, Horst RL, Broadus AE, Rasmussen H, Genel M. Journal of Clinical Endocrinology & Metabolism. 1979;49:185–188. doi: 10.1210/jcem-49-2-185. [DOI] [PubMed] [Google Scholar]
- 316.Menaa C, Vrtovsnik F, Friedlander G, Corvol M, Garabedian M. Journal of Biological Chemistry. 1995;270:25461–25467. doi: 10.1074/jbc.270.43.25461. [DOI] [PubMed] [Google Scholar]
- 317.Condamine L, Menaa C, Vrtovsnik F, Vztovsnik F, Friedlander G, Garabedian M. Journal of Clinical Investigation. 1994;94:1673–1679. doi: 10.1172/JCI117512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kumar R, Merimee TJ, Silva P, Epstein FH. The effect of chronic growth hormone excess or deiciency on plasma 1,25-dihydroxyvitamin D levels in man. In: Norman AW, Schaefer K, von Herrath D, Grigoleit HG, Coburn JW, De Luca HF, Mawer EB, Suda T, editors. Proceedings of the fourth workshop on Vitamin D. New York: de Gruyter, Elmsford; 1979. pp. 1005–1009. [Google Scholar]
- 319.Bianda T, Glatz Y, Bouillon R, Froesch ER, Schmid C. Journal of Clinical Endocrinology & Metabolism. 1998;83:81–87. doi: 10.1210/jcem.83.1.4484. [DOI] [PubMed] [Google Scholar]
- 320.Carre M, Ayigbede O, Miravet L, Rasmussen H. Proceedings of the National Academy of Sciences of the United States of America. 1974;71:2996–3000. doi: 10.1073/pnas.71.8.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Favus MJ, Walling MW, Kimberg DV. Journal of Clinical Investigation. 1973;52:1680–1685. doi: 10.1172/JCI107349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Feher JJ, Wasserman RH. Endocrinology. 1979;104:547–551. doi: 10.1210/endo-104-2-547. [DOI] [PubMed] [Google Scholar]
- 323.Akeno N, Matsunuma A, Maeda T, Kawane T, Horiuchi N. Journal of Endocrinology. 2000;164:339–348. doi: 10.1677/joe.0.1640339. [DOI] [PubMed] [Google Scholar]
- 324.Bouillon R, Muls E, De Moor P. Journal of Clinical Endocrinology & Metabolism. 1980;51:793–797. doi: 10.1210/jcem-51-4-793. [DOI] [PubMed] [Google Scholar]
- 325.Pahuja DN, De Luca HF. Archives of Biochemistry & Biophysics. 1982;213:293–298. doi: 10.1016/0003-9861(82)90465-9. [DOI] [PubMed] [Google Scholar]
- 326.Jastrup B, Mosekilde L, Melsen F, Lund B, Sorensen OH. Metabolism: Clinical & Experimental. 1982;31:126–132. doi: 10.1016/0026-0495(82)90123-8. [DOI] [PubMed] [Google Scholar]
- 327.Kumar R, Cohen WR, Silva P, Epstein FH. Journal of Clinical Investigation. 1979;63:342–344. doi: 10.1172/JCI109308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Pike JW, Parker JB, Haussler MR, Boass A, Toverud SV. Science. 1979;204:1427–1429. doi: 10.1126/science.451573. [DOI] [PubMed] [Google Scholar]