Abstract
Mutations in NIPA1 (non-imprinted in Prader-Willi/Angelman syndrome) have been described as a cause of autosomal dominant hereditary spastic paraplegia (HSP) known as SPG6 (spastic paraplegia-6). We present the first neuropathological description of a patient with a NIPA1 mutation, and clinical phenotype of complicated HSP with motor neuron disease-like syndrome and cognitive decline. Postmortem examination revealed degeneration of lateral corticospinal tracts and dorsal columns with motor neuron loss. TDP-43 immunostaining showed widespread spinal cord and cerebral skein-like and round neuronal cytoplasmic inclusions. We ruled out NIPA1 mutations in 419 additional cases of motor neuron disease. These findings suggest that hereditary spastic paraplegia due to NIPA1 mutations could represent a TDP-43 proteinopathy.
INTRODUCTION
Hereditary spastic paraplegia (HSP) is a genetically heterogeneous group of disorders characterized by progressive spasticity and lower limb weakness. “Complicated” forms present additional features including ataxia, peripheral neuropathy, extrapyramidal signs, dementia, and epilepsy [13]. The underlying pathology shows axonal degeneration in the terminal portions of the longest spinal tracts [2], however, the mechanisms of neurodegeneration are not well understood. HSP can be autosomal dominant, autosomal recessive, X-linked, or present as apparently sporadic [10]. Mutations in NIPA1 (non-imprinted in Prader-Willi/Angelman syndrome) cause a form of autosomal dominant HSP known as SPG6 (spastic paraplegia-6) [28].
TDP-43 has been identified as a major component in pathological inclusions in amyotrophic lateral sclerosis (ALS) and frontotemporal degeneration with motor neuron disease (FTD-MND) [25], which, like HSP, show degeneration of the pyramidal motor system. Here we report a case of complicated HSP with a NIPA1 mutation and widespread TDP-43 pathology.
CLINICAL HISTORY
A 13-year-old female developed leg spasticity, bladder dysfunction and weakness, which slowly progressed for decades interfering with ambulation. At age 53 she developed upper extremity weakness, bilateral facial weakness and increasing bladder and bowel dysfunction. She reported hoarseness, oropharyngeal dysphagia, dyspnea and orthopnea. Progressive cognitive decline with impaired attention, poor memory and personality change ensued. Family history information was limited by small paternal family size and early ages of death, however, her father had progressive personality changes, aggressiveness, word-finding difficulties and poor sentence formation since age 61, along with a gait disturbance and mild tremor. He died at age 71, but there was no autopsy nor was genetic material available.
Neurological examination at age 54 identified mild executive dysfunction with decreased phonetic word output, abnormal digit-span and attentional impairment, consistent with frontal lobe involvement. Cranial nerves were notable for slow tongue rapid movements. Motor exam demonstrated bilateral hand intrinsic muscle atrophy and 4/5 weakness. Marked leg spasticity was noted with spontaneous and evocable triple flexion. Reflexes were increased throughout, with up-going toes. Sensory exam showed reduced vibration sensation in toes and ankles, with normal proprioception, pin and temperature sensation.
Laboratory evaluation, including a comprehensive metabolic panel, thyroid function, CBC with differential, autoantibodies and B12, was negative or within normal limits. Creatine kinase was mildly elevated (310 U/L). A brain MRI showed non-specific periventricular white matter lesions. Nerve conduction exams demonstrated absent peroneal motor response with reduced tibial motor amplitude (0.5 mV) and velocity (39 m/sec). Sural sensory response was absent. There was mild chronic denervation in proximal arm and forearm muscles, but marked chronic partial denervation in the intrinsic hand muscles. Tibialis anterior motor unit amplitudes were large, with reduced recruitment and activation, suggesting a superimposed upper motor neuron process.
Serial examinations over the next 2 years demonstrated progressive lower motor neuron weakness (arms, legs, axial and respiratory muscles), tongue fasciculations, and cognitive decline. The patient died from neuromuscular respiratory failure 3 years after presentation of upper extremity weakness.
RESULTS
DNA sequencing of the entire coding region of NIPA1 (SPG6) demonstrated a guanine to adenosine substitution at position 341 (c.316G>A) resulting in a glycine to arginine change (p.G106R). In addition, variants in SPG3A and SPG4 (Athena Diagnostics, Inc), TARDBP mutations and C9orf72 expansion were ruled out.
Postmortem examination revealed diffusely atrophic spinal cord and a grossly unremarkable 1217 gram brain. Microscopic exam of the spinal cord showed extensive pial fibrosis and adhesions throughout, with focal leptomeningeal calcifications. Anterior roots appeared atrophic with focal adhesions; however the number of myelinated and unmyelinated fibers appeared within normal limits. Peripheral nerves and dorsal root ganglia were not available for examination. There was widespread motor neuron loss with moderate to severe gliosis in the anterior horns, most prominent in the thoracic, cervical and sacral cord with relative sparing of the lumbar segments. Bunina bodies were present in residual motor neurons of the spinal cord. Kluver-Barrera stains highlighted degeneration of lateral corticospinal tracts, anterior corticospinal tracts and dorsal columns throughout the spinal cord. Axonal loss was concomitant with myelin loss, as demonstrated by immunohistochemistry (IHC) for heaviest neurofilament protein and myelin basic protein (antibodies developed at CNDR). Moderate neuronal loss was noted in the substantia nigra, with occasional extracellular pigment. The motor cortex and other neocortical areas such as the middle frontal gyrus also showed moderate neuronal loss and gliosis. Scattered areas of white matter pallor with reactive astrocytes were noted in the subcortical white matter of the frontal, temporal and parietal lobes, without any evidence of frank infarction. These areas of rarefaction were associated with small and medium-sized vessels with rigid, thickened walls, and occasional perivascular hemosiderin–laden macrophages; changes consistent with hyaline arteriolosclerosis. Vessels with medial calcific degeneration were identified in the putamen and thalamic sections.
TDP-43 IHC (Proteintech Group Inc, Chicago, IL, USA; 1:4,500 dilution) revealed scattered skein-like and round cytoplasmic inclusions in residual anterior horn motor neurons (Figure 1). Immunoreactive oligodendroglial cytoplasmic inclusions were also present in the spinal gray and white matter. Additional motor neurons without definitive inclusions showed cleared-out nuclei with cytoplasmic diffusely granular TDP-43 immunoreactivity. A moderate density of cytoplasmic TDP-43 positive neuronal and glial inclusions as well as TDP-43 positive neuritic threads was identified in the substantia nigra, limbic system (amygdala, entorhinal cortex, CA1/subiculum and cingulate gyrus), basal ganglia, and neocortex (including angular gyrus, superior and middle temporal gyri, middle frontal gyrus and motor cortex). In these areas, many glial cells and neurons showed cleared nuclei with diffuse granular TDP-43 staining. The TDP-43 immunoreactive inclusions (as well as the diffuse cytoplasmic staining) were also identified with antibodies against phosphorylated TDP-43 (409–410, developed at CNDR). The morphology of the TDP-43 pathology in the cortex, consisting of moderate numbers of neuronal cytoplasmic inclusions and short dystrophic neurites involving all cortical layers, would correspond with a type B classification of FTLD-TDP [20] but the presence of abundant cells with diffuse cytoplasmic TDP-43 staining and nuclear clearing is an additional salient feature in this case. Otherwise, there was a low density of tau pathology in limbic regions and no evidence of senile plaques, Lewy bodies or other tau pathology.
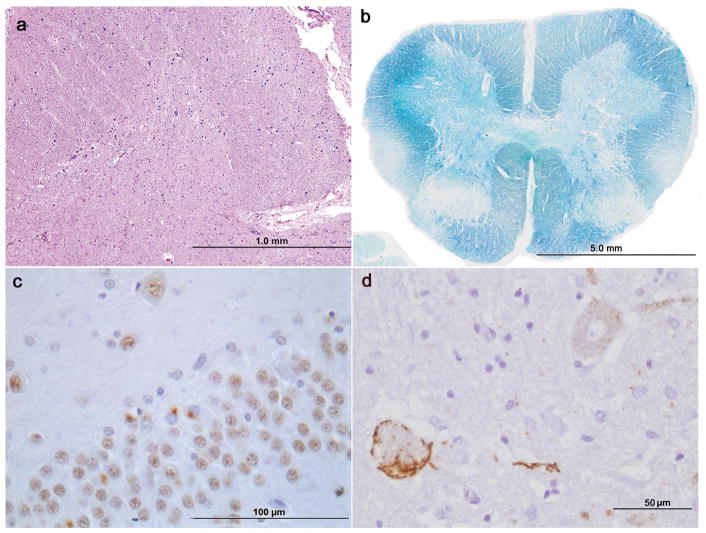
Figure 1. Neuropathological findings.
Hematoxylin and eosin staining reveals extensive motor neuron loss and gliosis in anterior horn of the cervical spinal cord (a). Kluver-Barrera stain for myelin reveals mild myelin pallor of the lateral corticospinal tract and dorsal column in the lumbar spinal cord (b). TDP-43 IHC shows typical cytoplasmic inclusions in the dentate gyrus of the hippocampus (c). Phosphorylated TDP-43 IHC highlights skein-like cytoplasmic inclusions in residual motor neurons of the cervical spinal cord as well as diffuse granular cytoplasmic immunoreactivity in other motor neurons (d).
IHC for NIPA1 (A-12, Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA; 1:500 dilution) did not show pathologic cytoplasmic inclusions. However when compared with control tissue, there was an apparent change in frontal cortex and spinal cord in that the normal controls showed granular polarized cytoplasmic labeling in neurons which contrasted with the decreased, diffuse non-granular cytoplasmic staining in the test case, suggesting a potential subcellular mislocalization of NIPA1. In addition, NIPA1 expression appeared decreased in the frontal cortex compared with the controls (Figure 2). These differences were not seen in the hippocampus. NIPA1 also showed strong granular staining of endothelial cells in all cases, suggesting a potential association of the protein with membrane-bound organelles such as endoplasmic reticulum and vesicular trafficking systems.
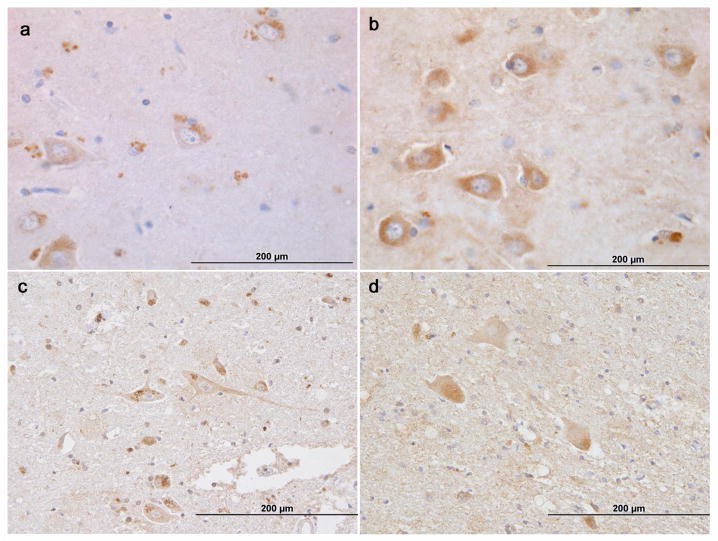
Figure 2. NIPA1 IHC.
Sections of frontal cortex (middle frontal gyrus; a, b) and cervical spinal cord (c, d) from a control brain (a, c) and the current case with NIPA1 mutation (b, d). Granular, polarized NIPA1 labeling in neurons, glial cells and endothelial cells is seen in the control, whereas decreased degree of staining with diffusion throughout the cytoplasm is seen in the mutated NIPA1 case.
To examine whether NIPA1 mutations could be an unrecognized cause of motor neuron disease (MND), 386 cases of ALS and 33 FTD-MND seen by neurologists in the University of Pennsylvania Health System were screened for mutations. This cohort included 103 autopsy-proven cases and 44 familial cases. All subjects signed informed consent and the study was conducted under Institutional Review Board approval. DNA was extracted from peripheral blood or brain tissue following the manufacturer’s protocols (Flexigene (Qiagen) or QuickGene DNA whole blood kit L (Autogen) for blood, and QIAsymphony DNA Mini Kit (Qiagen) for brain tissue. Genotyping was performed using real-time allelic discrimination with custom Applied Biosystem (ABI) TaqMan probes on the ABI 7500 fast real-time instrument using standard conditions. The following single nucleotide missense mutations were genotyped (custom assay ID is given for each): p.T45R;c.134C>G (Assay ID-AHI1OKK), p.G106R;c.316G>A (Assay ID-AHHSQEC) and p.G106R;c.316G>C (Assay ID-AHGJR74). Data were analyzed using ABI 7500 Software v2.0.1. No mutations were identified in these 419 cases.
DISCUSSION
Families with HSP and NIPA1 mutations display widespread ages of onset and variable phenotypes, including clinical features similar to our patient such as peripheral neuropathy [8] and dysexecutive syndrome [16] (Table 1). Clinically, this patient exhibited complicated spastic paraplegia with progressive motor neuron-like syndrome which followed a course typical of that seen in MND/ALS and dysexecutive syndrome following a pattern consistent with frontotemporal degeneration. Her family history was significant for her father’s cognitive impairment suggestive of frontotemporal degeneration, suggesting that potentially he may have carried the same genetic mutation as the patient. We cannot entirely exclude that this patient with HSP developed superimposed sporadic ALS; however, HSP and ALS have overlapping clinical features that reflect dysfunction of the pyramidal system, suggesting a possible shared pathological basis for these two neurodegenerative disorders and thus supporting the hypothesis that all neurological deficits in this patient were caused by the underlying genetic mutation.
Table 1.
Clinical characteristics of all families reported with NIPA1 mutations.
| Mutation | Age of onset | Sensory symptoms | Bladder/bowel dysfunction | Cognitive impairment | Weakness/others | Family origin | Citation |
|---|---|---|---|---|---|---|---|
| c.316G>A p.G106R | 13 | Mild vibratory sensation impairment | Urinary urgency and incontinence, bowel incontinence | Mild dysexecutive syndrome, personality change | Proximal and distal limb weakness and atrophy, facial weakness | North American | Current study |
| 9–23 (mean 16.5) | No | Mild (4/14) | Mild (2/14) | Upper limb postural tremor, Epilepsy with GTCS* | British | Reed, 2005[29] | |
| 17–40 | No | No | No | No | Chinese | Chen, 2005[6] | |
| 20–27 (mean 23.75) | No | Urinary incontinence (3/4) | No | Weakness | Brazilian | Munhoz, 2006[22] | |
| 6 | Mild vibration deficits (3/4) | No | No | No | North American | Bien-Willner, 2006[3] | |
| 10 | Reduced vibration, temperature, pinprick and position sensation | Urinary urgency | No | Epilepsy with GTCS* Limb atrophy Facial dystonia |
Danish | Svenstrup, 2011[32] | |
| c.316G>C p.G106R | 13–35 | N/A | N/A | N/A | Weakness | Chinese | Chen, 2005[6] |
| 8–37 | Impaired vibration sense at the ankles 2/6 | Urinary retention and frequency (2/6) | Mild memory deficit (4/6), Dysexecutive syndrome (1/6) | Weakness (3/6), one described as severe | European (caucasian) | Klebe, 2007[16] | |
| 12–20 (mean 16) | No | Mild bladder disturbance (1/6) | No | Spinal cord atrophy on MRI | Chinese | Liu, 2008[18] | |
| 15–20 (mean 16.6) | Impaired vibration in lower limbs (3/7) | Urinary urgency | No | Lower limb wasting, Peripheral neuropathy | Chinese | Du, 2011[8] | |
| c.159C>G p.T45R | 12–35 (mean 22) | Mild vibratory sensation impairment, paresthesias | Urinary incontinence (3/31) | No | Proximal and distal lower extremity Dysmetria |
Irish | Fink, 1995[11] Rainier, 2003[28] |
| Late teens | Mild vibratory sensation impairment | Urinary urgency | No | Weakness | Iraqui | Rainier, 2003[28] | |
| c.298G>A p.A100T | Teens-49 | No | No | No | No | Japanese | Kaneko, 2006[15] |
Numbers in parenthesis refer to number of patients affected/number of patients described.
Generalized tonic-clonic seizures.
To our knowledge, this is the first neuropathological description of a patient with a NIPA1 mutation. Pathologically, HSP shows distal axonal degeneration of corticospinal tracts and dorsal columns [2], sometimes with motor neuron loss [26, 17]. This case demonstrates motor neuron and axonal loss throughout the spinal cord, including degeneration of dorsal columns and corticospinal tracts, consistent with HSP. In addition, widespread classic TDP-43 positive neuronal cytoplasmic inclusions were identified in lower motor neurons, substantia nigra, basal ganglia, limbic structures and neocortex (including motor cortex). Involvement of the frontal lobes and limbic system offers an etiological correlate for the cognitive impairment. Similar diffuse involvement of non-motor systems has been previously described in ALS, suggesting a continuum in TDP-43 proteinopathies [12]. These findings, albeit in a single case, suggest that TDP-43 may play a role in HSP with NIPA1 mutations.
In addition to family history suggesting a potential autosomal dominant inheritance, the finding of a well-known HSP-causing mutation in NIPA1 supports this as the underlying cause of our patient’s neurodegenerative disorder. Further evidence suggests that variants in NIPA1 and other HSP-associated genes may be implicated in ALS. A genome-wide copy number variation analysis demonstrated an association of 15q microdeletions (including the NIPA1 locus) with ALS [4]. Furthermore, other HSP-associated genes cause MND phenotypes. Spatacsin (SPG11) mutations were found in 10 of 25 unrelated families with autosomal recessive ALS, including a case with classical ALS pathology (TDP-43 IHC not performed) [27]. A patient with juvenile ALS with a prolonged course carried a mutation in exon 1 of spastin (SPAST) [21]. All these findings expand the concept that mutations in some HSP-associated genes may also cause ALS.
It is well established that TDP-43 inclusions are found in patients with mutations in genes such as progranulin (GRN) [5,19], valosin-containing protein (VCP) [24], dynactin (DCTN1) [9], optineurin (OPTN) [14], angiogenin (ANG) [31] and chromosome 9 open reading frame 72 (C9orf72) [1, 7, 23, 30] In these patients, as in our case, there is no pathologic accumulation of the mutated protein. Thus, NIPA1 mutations may cause a MND phenotype with TDP-43 pathology. Nevertheless, screening of 419 patients with ALS or FTD-MND did not reveal any of the three previously identified NIPA1 mutations. This may reflect a limited statistical power in our study. However, it is also possible that while a point mutation in NIPA1 may be sufficient to cause HSP and MND with TDP-43 pathology, other molecular alterations may confer a susceptibility risk factor increasing neuronal vulnerability to additional insults. In fact, an ALS study identified deletions of NIPA1 as a risk factor [4]. NIPA1 encodes a transmembrane Mg2+ transporter protein, which may interfere with TDP-43 through perturbations in Mg2+ concentrations at the subcellular level. A gain-of-function dominant negative effect has been proposed as a mechanism for NIPA1 mutations in HSP [28]. In our case, IHC evidence of possible decreased expression and mislocalization suggests a potential loss of function, although further testing is needed.
In summary, we present the first neuropathological description of an HSP patient with a NIPA1 mutation, showing axonal degeneration of corticospinal tracts and dorsal columns of the spinal cord, lower motor neuron loss and TDP-43 pathology, suggesting a possible common pathway for motor neuron degeneration in HSP with NIPA1 mutations and ALS. In addition, we have not identified NIPA1 point mutations in 419 ALS and FTD-MND cases indicating that these are not an unrecognized common cause of ALS. Further studies on the role of TDP-43 in HSP with NIPA1 and other genetic causes are needed to illustrate the interaction of these two devastating disorders and potentially develop therapeutic targets.
Acknowledgments
The authors would like to acknowledge Robert Greene and Amanda Piarulli for their technical support in the genotyping process and Subhojit Roy and Mark Forman for the initial neuropathological evaluation of this case. The authors are grateful to the patient and her family for their generosity.
References
- 1.Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobagyi T, Shaw CE. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122(6):691–702. doi: 10.1007/s00401-011-0911-2. [DOI] [PubMed] [Google Scholar]
- 2.Behan WM, Maia M. Strumpell’s familial spastic paraplegia: genetics and neuropathology. J Neurol Neurosurg Psychiatry. 1974;37 (1):8–20. doi: 10.1136/jnnp.37.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bien-Willner R, Sambuughin N, Holley H, Bodensteiner J, Sivakumar K. Childhood-onset spastic paraplegia with NIPAL gene mutation. J Child Neurol. 2006;21 (11):974–977. doi: 10.1177/08830738060210111501. [DOI] [PubMed] [Google Scholar]
- 4.Blauw HM, Al-Chalabi A, Andersen PM, van Vught PW, Diekstra FP, van Es MA, Saris CG, Groen EJ, van Rheenen W, Koppers M, Van’t Slot R, Strengman E, Estrada K, Rivadeneira F, Hofman A, Uitterlinden AG, Kiemeney LA, Vermeulen SH, Birve A, Waibel S, Meyer T, Cronin S, McLaughlin RL, Hardiman O, Sapp PC, Tobin MD, Wain LV, Tomik B, Slowik A, Lemmens R, Rujescu D, Schulte C, Gasser T, Brown RH, Jr, Landers JE, Robberecht W, Ludolph AC, Ophoff RA, Veldink JH, van den Berg LH. A large genome scan for rare CNVs in amyotrophic lateral sclerosis. Hum Mol Genet. 2010;19 (20):4091–4099. doi: 10.1093/hmg/ddq323. [DOI] [PubMed] [Google Scholar]
- 5.Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, 3rd, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM, Mackenzie IR. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171(1):227–240. doi: 10.2353/ajpath.2007.070182. S0002-9440(10)61957-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S, Song C, Guo H, Xu P, Huang W, Zhou Y, Sun J, Li CX, Du Y, Li X, Liu Z, Geng D, Maxwell PH, Zhang C, Wang Y. Distinct novel mutations affecting the same base in the NIPA1 gene cause autosomal dominant hereditary spastic paraplegia in two Chinese families. Hum Mutat. 2005;25(2):135–141. doi: 10.1002/humu.20126. [DOI] [PubMed] [Google Scholar]
- 7.Dejesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du J, Hu YC, Tang BS, Chen C, Luo YY, Zhan ZX, Zhao GH, Jiang H, Xia K, Shen L. Expansion of the phenotypic spectrum of SPG6 caused by mutation in NIPA1. Clin Neurol Neurosurg. 2011;113 (6):480–482. doi: 10.1016/j.clineuro.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Farrer MJ, Hulihan MM, Kachergus JM, Dachsel JC, Stoessl AJ, Grantier LL, Calne S, Calne DB, Lechevalier B, Chapon F, Tsuboi Y, Yamada T, Gutmann L, Elibol B, Bhatia KP, Wider C, Vilarino-Guell C, Ross OA, Brown LA, Castanedes-Casey M, Dickson DW, Wszolek ZK. DCTN1 mutations in Perry syndrome. Nat Genet. 2009;41(2):163–165. doi: 10.1038/ng.293. ng.293 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fink JK. Hereditary spastic paraplegia. Curr Neurol Neurosci Rep. 2006;6 (1):65–76. doi: 10.1007/s11910-996-0011-1. [DOI] [PubMed] [Google Scholar]
- 11.Fink JK, Wu CT, Jones SM, Sharp GB, Lange BM, Lesicki A, Reinglass T, Varvil T, Otterud B, Leppert M. Autosomal dominant familial spastic paraplegia: tight linkage to chromosome 15q. Am J Hum Genet. 1995;56 (1):188–192. [PMC free article] [PubMed] [Google Scholar]
- 12.Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, Xie SX, Kwong LK, Elman L, McCluskey L, Clark CM, Malunda J, Miller BL, Zimmerman EA, Qian J, Van Deerlin V, Grossman M, Lee VM, Trojanowski JQ. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009;66(2):180–189. doi: 10.1001/archneurol.2008.558. 66/2/180 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding AE. Classification of the hereditary ataxias and paraplegias. Lancet. 1983;1(8334):1151–1155. doi: 10.1016/s0140-6736(83)92879-9. S0140-6736(83)92879-9 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Ito H, Nakamura M, Komure O, Ayaki T, Wate R, Maruyama H, Nakamura Y, Fujita K, Kaneko S, Okamoto Y, Ihara M, Konishi T, Ogasawara K, Hirano A, Kusaka H, Kaji R, Takahashi R, Kawakami H. Clinicopathologic study on an ALS family with a heterozygous E478G optineurin mutation. Acta Neuropathol. 2011;122(2):223–229. doi: 10.1007/s00401-011-0842-y. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko S, Kawarai T, Yip E, Salehi-Rad S, Sato C, Orlacchio A, Bernardi G, Liang Y, Hasegawa H, Rogaeva E, St George-Hyslop P. Novel SPG6 mutation p.A100T in a Japanese family with autosomal dominant form of hereditary spastic paraplegia. Mov Disord. 2006;21 (9):1531–1533. doi: 10.1002/mds.21005. [DOI] [PubMed] [Google Scholar]
- 16.Klebe S, Lacour A, Durr A, Stojkovic T, Depienne C, Forlani S, Poea-Guyon S, Vuillaume I, Sablonniere B, Vermersch P, Brice A, Stevanin G. NIPA1 (SPG6) mutations are a rare cause of autosomal dominant spastic paraplegia in Europe. Neurogenetics. 2007;8 (2):155–157. doi: 10.1007/s10048-006-0074-9. [DOI] [PubMed] [Google Scholar]
- 17.Kuru S, Sakai M, Konagaya M, Yoshida M, Hashizume Y. Autopsy case of hereditary spastic paraplegia with thin corpus callosum showing severe gliosis in the cerebral white matter. Neuropathology. 2005;25 (4):346–352. doi: 10.1111/j.1440-1789.2005.00620.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu SG, Zhao JJ, Zhuang MY, Li FF, Zhang QJ, Huang SZ, Che FY, Lu de G, Liu SE, Teng JJ, Ma X. Clinical and genetic study of SPG6 mutation in a Chinese family with hereditary spastic paraplegia. J Neurol Sci. 2008;266 (1–2):109–114. doi: 10.1016/j.jns.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Mackenzie IR. The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol. 2007;114(1):49–54. doi: 10.1007/s00401-007-0223-8. [DOI] [PubMed] [Google Scholar]
- 20.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122(1):111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer T, Schwan A, Dullinger JS, Brocke J, Hoffmann KT, Nolte CH, Hopt A, Kopp U, Andersen P, Epplen JT, Linke P. Early-onset ALS with long-term survival associated with spastin gene mutation. Neurology. 2005;65(1):141–143. doi: 10.1212/01.wnl.0000167130.31618.0a. 65/1/141 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Munhoz RP, Kawarai T, Teive HA, Raskin S, Sato C, Liang Y, St George-Hyslop PH, Rogaeva E. Clinical and genetic study of a Brazilian family with spastic paraplegia (SPG6 locus) Mov Disord. 2006;21(2):279–281. doi: 10.1002/mds.20775. [DOI] [PubMed] [Google Scholar]
- 23.Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, Wszolek ZK, Ferman TJ, Josephs KA, Boylan KB, Rademakers R, Dickson DW. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122(6):673–690. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann M, Mackenzie IR, Cairns NJ, Boyer PJ, Markesbery WR, Smith CD, Taylor JP, Kretzschmar HA, Kimonis VE, Forman MS. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol. 2007;66(2):152–157. doi: 10.1097/nen.0b013e31803020b9. 00005072-200702000-00007 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. doi: 10.1126/science.1134108. 314/5796/130 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Nomura H, Koike F, Tsuruta Y, Iwaki A, Iwaki T. Autopsy case of autosomal recessive hereditary spastic paraplegia with reference to the muscular pathology. Neuropathology. 2001;21 (3):212–217. doi: 10.1046/j.1440-1789.2001.00388.x. [DOI] [PubMed] [Google Scholar]
- 27.Orlacchio A, Babalini C, Borreca A, Patrono C, Massa R, Basaran S, Munhoz RP, Rogaeva EA, St George-Hyslop PH, Bernardi G, Kawarai T. SPATACSIN mutations cause autosomal recessive juvenile amyotrophic lateral sclerosis. Brain. 2010;133(2):591–598. doi: 10.1093/brain/awp325. awp325 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rainier S, Chai JH, Tokarz D, Nicholls RD, Fink JK. NIPA1 gene mutations cause autosomal dominant hereditary spastic paraplegia (SPG6) Am J Hum Genet. 2003;73 (4):967–971. doi: 10.1086/378817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed JA, Wilkinson PA, Patel H, Simpson MA, Chatonnet A, Robay D, Patton MA, Crosby AH, Warner TT. A novel NIPA1 mutation associated with a pure form of autosomal dominant hereditary spastic paraplegia. Neurogenetics. 2005;6 (2):79–84. doi: 10.1007/s10048-004-0209-9. [DOI] [PubMed] [Google Scholar]
- 30.Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Holtta-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seilhean D, Cazeneuve C, Thuries V, Russaouen O, Millecamps S, Salachas F, Meininger V, Leguern E, Duyckaerts C. Accumulation of TDP-43 and alpha-actin in an amyotrophic lateral sclerosis patient with the K17I ANG mutation. Acta Neuropathol. 2009;118(4):561–573. doi: 10.1007/s00401-009-0545-9. [DOI] [PubMed] [Google Scholar]
- 32.Svenstrup K, Moller RS, Christensen J, Budtz-Jorgensen E, Gilling M, Nielsen JE. NIPA1 mutation in complex hereditary spastic paraplegia with epilepsy. Eur J Neurol. 2011 Sep;18(9):1197–9. doi: 10.1111/j.1468-1331.2011.03359.x. [DOI] [PubMed] [Google Scholar]