Abstract
Interleukin-7 is a critical cytokine for lymphoid development and a direct inhibitor of in vitro osteoclastogenesis in murine bone marrow cultures. To explore the role of IL-7 in bone, we generated transgenic mouse lines bearing the 2.3 Kb rat collagen 1A1 promoter driving the expression of human IL-7 specifically in osteoblasts. In addition we crossed these mice with IL-7 deficient mice to determine if the alterations in lymphopoiesis, bone mass and osteoclast formation observed in the IL-7 KO mice could be rescued by osteoblast-specific overexpression of IL-7. Here we show that mice overexpressing human IL-7 in the osteoblast lineage demonstrated increased trabecular bone volume in vivo by µCT and decreased osteoclast formation in vitro. Furthermore, targeted overexpression of IL-7 in osteoblasts rescued the osteopenic bone phenotype and B cell development of IL-7 KO mice but did not have an effect on T lymphopoiesis, which occurs in the periphery. The bone phenotypes in IL-7 KO mice and targeted IL-7 overexpressing mouse models were observed only in females. These results likely reflect both a direct inhibitory effects of IL-7 on osteoclastogenesis in vivo and gender specific differences in responses to IL-7.
Keywords: Bone, osteoclasts, lymphocytes, interleukin-7, mouse model
INTRODUCTION
Interleukin-7 (IL-7) is a critical cytokine for the development of B and T lymphocytes (1). It was initially identified as a pre-B cell growth factor, also known as lymphopoietin-1 (LP-1). It is produced by bone marrow stromal cells (2, 3), thymic epithelial cells and intestinal epithelial cells (4–7). Recombinant IL-7 supports the survival and growth of precursor B cells (2, 8–12) as well as T cells (13–15). The effects of IL-7 are mediated via the IL-7 receptor (IL-7R), which is composed of an exclusive IL-7Rα chain and a common γ chain shared by receptors for IL-2, IL-4, IL-9, IL-15, and IL-21. IL-7Rα is expressed on pre-B cells, but is not detected on mature B cell lines or primary mature B cells (16). It is also expressed on murine thymocytes, some mature T lineage lymphocytes and on bone marrowderived macrophages (16).
B cells are generated from hematopoietic stem cells (HSC), which reside in the liver during fetal life and in the bone marrow in the adult. IL-7 is known to facilitate the development to less mature (B220+IgM−) pre-B cells (3) and very early multipotential B220−IgM− cells, which proliferate in response to IL-7 in the bone marrow. However, IL-7 is ineffective as a regulator of the differentiation of B-linage cells beyond pre-B cell development since more mature B lineage cells lack IL-7R (12). In the bone marrow, the majority of B lineage cells (B220+) are IL-7 responsive. T lymphopoiesis is initiated in the thymus and IL-7 is essential for the survival and proliferation of early progenitors (CD4 and CD8 double negative cells) in this organ. IL-7 also participates in thymocyte differentiation by promoting rearrangement of T cell receptor γ and β chains (17, 18). Double negative T cells differentiate into CD4 and CD8 double positive cells and then into mature single CD4 or CD8 positive T cells by positive and negative selection. IL-7R expression is extinguished at the double positive state but reappears in single positive subsets in the periphery where it plays a fundamental role in the maintenance of homeostatic levels of naïve and memory T cells (19).
Osteoclastogenesis depends on interactions of hematopoietic and stromal cells. The latter provide the microenvironment that is essential for this process (20, 21). In addition, IL-7 production in osteoblasts is required for normal development of B lymphocytes in the bone marrow (22). B lymphoid lineage cells at early developmental stages express RANKL (23, 24) in both IL-7-dependent and independent pre-B cell populations (23). These results indicate that there is an interrelationship between RANKL and IL-7. In vivo and in vitro neutralization studies with an anti-IL-7 monoclonal antibody also indicated that in vivo neutralization of IL-7 inhibited ovariectomy-induced bone loss (25–27). In contrast, we have found that IL-7 knockout (IL-7 KO) mice lose trabecular but not cortical bone mass at a similar rate to wild type mice after ovariectomy (28). Administration of IL-7 in vivo to normal mice resulted in marked bone loss (29). However, it was shown that systemic administration of IL-7 up-regulated osteoclast formation in human peripheral blood cells by increasing osteoclastogenic cytokine production in T cells (30). It was also found that IL-7 did not induce bone resorption and bone loss in T cell-deficient nude mice (31). Treatment of mice with a neutralizing anti-IL-7 antibody prevented ovariectomy-induced proliferation of early T cell precursors in the thymus. These findings imply that ovariectomy up-regulates T cell development through IL-7, which may be a mechanism by which IL-7 regulates ovariectomy-induced bone loss (32). To determine the effects of IL-7 in vivo, several attempts have been made to over-express or block its activity. These include administration of IL-7, administration of antibodies against IL-7 or IL-7 receptor (33–35) or reconstitution of lethally irradiated mice with bone marrow cells that were retrovirally transduced to overexpress IL-7 (36). In these studies, administration of IL-7 induced an increase in B lymphopoiesis (31) and treatment with antibodies to IL-7 decreased the number of T cells (26, 34). In mice that were treated with anti-IL-7Rα, both T- and B-lymphopoiesis were perturbed; whereas, the numbers of mature B and T cells in the periphery remained unchanged (33).
In mice that overexpress IL-7 globally under the control of the major histocompatibility complex (MHC) class II Eα promoter, B cell development was dramatically affected. In these mice, the number of pro-, pre- and immature B cells in bone marrow was greatly increased, myeloid cells were virtually absent and T cell development was unaffected (37). In addition, at age 4–6 weeks the diameter of the femur was increased, the marrow cavity was enlarged and there was evidence of focal osteolysis (38, 39). In adult IL-7 overexpressing mice bone remodeling was extensive, which resulted in marrow cells infiltrating femoral muscles through holes in the cortical bone (40). Furthermore, the most recent report using IL-7 overexpressing mice indicated that osteopenia was observed at the age of 12 months and this was associated with an increased number of active osteoclasts on the surface of trabecular bone. These mice also exhibited increased osteoclastogenesis without an alteration in osteoblastic potential of bone marrow cultures (41).
In animals in which the IL-7 gene was inactivated (42), the size of the thymus and spleen was significantly reduced. In both IL-7 (IL-7 KO) and IL-7 receptor (IL-7R KO) deficient mice, which were generated by global gene inactivation, the number of B and T lymphocytes was markedly reduced. These animals also showed markedly decreased B-lymphopoiesis in their bone marrow, and an arrest in B cell development at the pro-B cell stage. However, some cells did undergo maturation in the absence of a functional IL-7 receptor or IL-7 (43–46). In our previous report micro-computed tomography and histomorphometric analysis demonstrated that IL-7 KO female mice had significantly decreased trabecular bone volume, trabecular numbers and increased trabecular space at the age of 3 months (28). Similarly, in vitro treatment of bone marrow cultures from WT mice with IL-7 decreased osteoclast (OCL) formation. Conversely, bone marrow cells from IL-7 KO mice showed a significant increase in OCL formation when cells were treated with M-CSF and RANKL (47).
In the present study we generated transgenic mice in a C57BL/6 background (Tg) that used the 2.3 Kb rat collagen 1α1 promoter to selectively express human IL-7 in osteoblast-lineage cells in order to explore the role that IL-7 expression in the local bone environment had on bone function. Furthermore, to determine if the alteration in bone mass, lymphopoiesis and osteoclast formation in IL-7 KO mice can be rescued by local expression of IL-7 in bone, we crossed IL-7 KO mice with mice that had targeted IL-7 production in osteoblast lineage cells.
MATERIALS AND METHODS
1. Generation of pOBCOL2.3-hIL-7 transgenic mice and breeding with IL-7 KO mice
Three founder lines were generated using the pOBCol 2.3-hIL-7 construct. The human IL-7 cDNA sequence in this construct was cloned by RT-PCR from a human bone marrow cDNA library using gene specific primers (forward: 5’-TTG CGG TCA TCA TGA CTA C-3’; reverse: 5’-TTC TAG GAA GCA TTC CAC TC-3’) (48). We generated two sets of specific primers to PCR genotype the transgene. Tg IL-7 mice (Line C, high Tg) were bred to existing IL-7 KO mice. Homozygosity was confirmed by backcrossing with WT or IL-7 KO mice. Specific PCR primers were generated based on the original literature (42). All animal procedures were conducted according to protocols approved by the University of Connecticut Health Center Animal Care Committee. Mice were housed in Thoren isolator cages at the institutional Center for Laboratory Animal Care, an AALAC accredited facility.
2. RNA extraction and RT-PCR
Total RNA was extracted from various tissues from WT and Tg IL-7 mice with TRI-reagent following the company recommended protocol (Molecular Research Center, Cincinnati, OH) (49). Total RNA was converted to cDNA by reverse transcriptase (Superscript II, GIBCO/BRL) and random hexamer and aliquots of RT mixture was used for PCR. PCR amplification was done using gene-specific PCR primers and Taq polymerase (Amplitaq, Applied Biosystems, Carlsbad, CA). Specific amplimer sets were designed from published mRNA sequences: human IL-7 (48) spanning exons II to VI (forward: 5’-CTG TTG CCA GTA GCA TCA T-3’; reverse: 5’-CTT TAG GAA ACA CAA GTC ATT C-3’), murine β-actin (50) (forward: 5’-GTG GGC CGC TCT AGG CAC CAA-3’; reverse: 5’-CTC TTT GAT GTC ACG CAC GAT TTC-3’). The amplified products were run in a 1.5 % agarose gel, stained with ethidium bromide and photographed under UV illumination.
3. Static and Dynamic Histomorphometry and µCT analysis
Bone histomorphometry was performed on mouse long bones (femur). Histomorphometric analysis was performed in the Center for Bone Histomorphometry at the University of Connecticut Health Center. Histomorphometric analysis was performed in a blinded, nonbiased manner using a computerized semiautomated Osteomeasure system with fluorescent and light microscopy. The bones from at least 6–14 mice per group were examined. Animals received intraperitoneal injections of 10 mg/kg of calcein 9 days and 2 days before sacrifice. For static histomorphometry, the femur from each mouse was removed and fixed in 4% paraformaldehyde in PBS prior to decalcification. Quantitation of osteoclasts was performed in paraffin embedded tissues by staining for TRAP (+) osteoclasts adjacent to bone. For dynamic histomorphometry, the femur from each mouse was removed and fixed in 70% ethanol at 4°C, dehydrated in progressive concentrations of ethanol, cleared in xylene and embedded in methylmethacrylate. Histomorphometric analyses of one section from each bone were performed. Statistical significance of the histomorphometric measurements was determined by one-way analysis of variance (ANOVA) and the Bonferroni post hoc test when the ANOVA demonstrates significant differences. The measurements, terminology and units, that were used for histomorphometric analysis, were those recommended by the Nomenclature Committee of the American Society of Bone and Mineral Research (51).
Trabecular morphometry within the metaphyseal region of distal femurs, and cortical morphometry of femora at mid-diaphysis, was quantified using conebeam micro-focus X-ray computed tomography (μCT40, Scanco Medical AG, Bassersdorf, Switzerland). Serial tomographic images were acquired at 55 kV and 145 μA, collecting 1000 projections per rotation at 300 msec integration time. Three-dimensional 16-bit grayscale images were reconstructed using standard convolution back-projection algorithms with Shepp and Logan filtering, and rendered within a 12.3 mm field of view at a discrete density of 578,704 voxels/mm3 (isometric 12-μm voxels). Segmentation of bone from marrow and soft tissue was performed in conjunction with a constrained Gaussian filter to reduce noise, applying hydroxyapatite-equivalent density thresholds of 710 mg/cm3 and 470 mg/cm3 for the cortical and trabecular compartments, respectively. Volumetric regions for trabecular analysis were selected within the endosteal borders to include the secondary spongiosa of femoral metaphyses located 960 μm (~6% of length) from the growth plate and extending 1 mm proximally. Trabecular morphometry was characterized by measuring the bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular spacing (Tb.Sp), and bone surface area (BS). Cortical morphometry in the femur was quantified within a 600 μm long segment (50 serial sections) extending distally from the diaphyseal mid-point between proximal and distal growth plates. Cross-sectional measurements included average cortical bone area and cortical thickness.
4. Bone Marrow Cell Culture
Bone marrow cells from WT, Tg IL-7, IL-7 KO and rescued IL-7 KO, all in a C57BL/6 background were used throughout the experiments. Mouse bone marrow cells from femur, tibia and humerus were isolated by a modification of previously published methods (21, 52–56). Marrow cells were collected into tubes, washed twice with α-MEM and cultured (1×106 cells/cm2) in α-MEM containing 10% heat inactivated fetal bovine serum (HIFBS). Cultures were fed every three days with fresh medium. M-CSF and RANKL (both at 30 ng/ml) or 1,25-dihydroxyvitamin D3 (10−8 M) were added to cultures as indicated in each experiment. Cells were fixed on day 5 of culture or at times indicated in each experiment with 2.5% glutaraldehyde in PBS for 30 min at room temperature before being stained for TRAP. Enzyme histochemistry for TRAP was performed with a commercial kit (SIGMA, St. Louis, MO). Osteoclast formation assays were performed in sextuplet cultures per treatment group.
5. Colony-forming unit granulocyte-macrophage (CFU-GM) assay
Bone marrow cells were prepared as described previously (57). Briefly, cells were suspended in αMEM with 10% HIFBS (Hyclone Laboratories, Logan, UT). Cells were then plated on 35mm tissue culture dish in 1 ml 1.5% methylcellulose supplemented with 20% HIFBS, 2% BSA (SIGMA, St. Louis, MO) and 1.0 ng/ml recombinant murine GM-CSF (R & D Systems, Minneapolis, MN) as the source of colony-stimulating activity. Assays were performed in sextuplets and cultures were maintained at 37°C for 6 days. The numbers of colonies (>40 cells) were counted at the end of incubation.
6. Flow Cytometry
Long bones, spleen and thymus were harvested and single cell suspensions were prepared. For bone marrow, femur and tibia were removed of attached muscle and flushed with 10 ml of staining media containing 1X HBSS (GIBCO, Invitrogen Corp., Carlsbad, CA), 0.01 M HEPES (pH 7.4) and supplemented with 2% fetal bovine serum (FBS). Spleens and thymus were smashed gently between frosted glass slides in staining media. Red blood cells were subsequently lysed by hypotonic shock and the cell preparation filtered through a nylon mesh and counted prior to staining. The antibodies used for flow cytometric analysis were the following: anti-mouse B220/CD45R, anti-mouse IgM, anti-mouse AA4.1/CD93, anti-mouse CD23, anti-mouse CD3, anti-mouse TCRαβ and anti-mouse TCRγδ. All antibodies were used coupled to non-overlapping exclusive fluorochromes and were purchased from commercial sources (Pharmingen and eBioscience). Labeling cells for flow cytometry was performed by standard staining procedures in staining media. The cells were kept on ice at all times and dead cells were excluded by their ability to incorporate propidium iodide. Flow cytometric analysis was done on a FACSCalibur flow cytometry analyzer (BD Biosciences, San Jose, CA) and data analysis was performed using CellQuest software (BD Biosciences).
7. CTX, Osteocalcin and human IL-7 ELISA
Serum from WT, Tg IL-7, IL-7 KO and rescued IL-7 KO female mice that were fasted overnight, was obtained for c-terminal telopeptides (CTX) and osteocalcin measurement. Serum CTX and osteocalcin were measured by ELISA (RatLaps, Nordic Bioscience Diagnostics, Herlev, Denmark and mouse osteocalcin kit, Biomedical Technologies Inc., Stoughton, MA) according to manufacturer’s recommendations. Human IL-7 ELISA was performed using serum or conditioned medium from calvarial organ cultures of WT, Tg IL-7, IL-7 KO and rescued IL-7 KO mice by ELISA according to manufacturer’s recommendation (R and D Systems, Minneapolis, MN).
8. Statistical Analysis
Statistical analysis was performed by one way analysis of variance (ANOVA) and the Bonferroni post hoc test when ANOVA demonstrated significant differences. All experiments were repeated at least twice.
RESULTS
Overexpression of IL-7 in the osteoblast lineage alters bone marrow hematopoiesis and bone homeostasis
To explore the role of IL-7 in bone in vivo, we generated 3 transgenic mouse lines (A, B and C) in a C57BL/6 background (Tg IL-7). The construct that was used contained a 2.3 kb fragment of the rat collagen 1α1-5’ regulatory region together with 1.6 kb of the first intron, which selectively expressed human IL-7 in osteoblasts (Figure 1A, (58). Human IL-7 levels were detected by ELISA using a commercial kit in the medium of neonatal calvarial organ cultures from all three lines (Table 1). Line C (high Tg) produced the highest level of human IL-7 while line B (low Tg) had levels that were 470 fold lower. Line A was intermediate. Consistent with the known effect of IL-7 on B cell lymphopoiesis, there was an increase in the percentage of early B cells (B220+IgM−) in the bone marrow of line C (high Tg) mice compared to WT mice (41.7% vs. 27.4%, respectively) (Table 2). In contrast, line B (low Tg) mice had normal bone marrow B-lymphopoiesis (data not shown). Human IL-7 was minimally detectable in the serum of all Tg lines at the age of 7.5 weeks and was not detected in the serum of control (WT) mice (Table 1). However, human IL-7 level in the serum was greater at 3 weeks of age compared to 7.5 weeks of age for lines A (intermediate Tg) and C (high Tg) suggesting that the activity of the collagen promoter decreased as the mice aged.
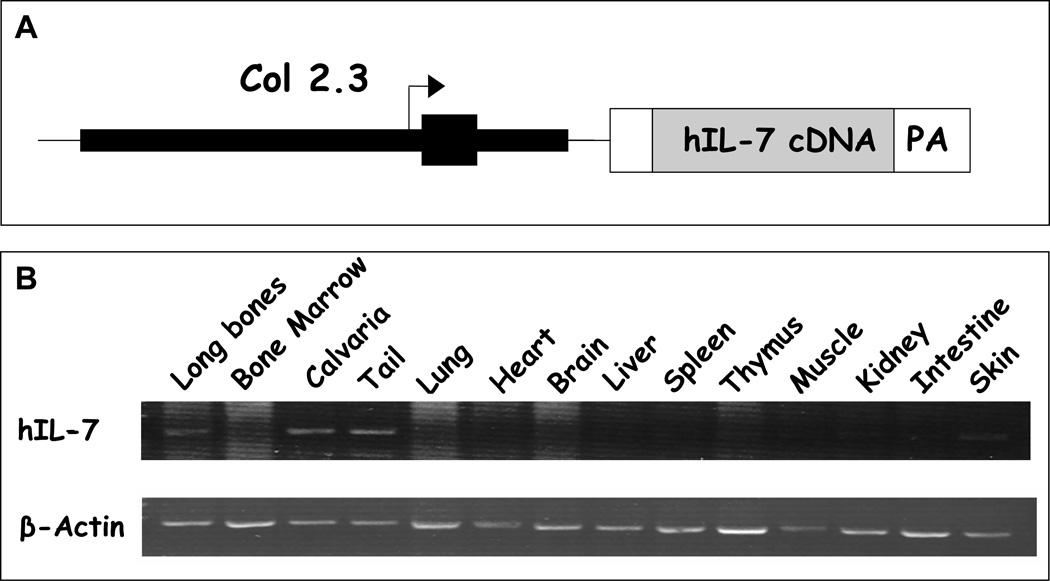
Figure 1.
A. pOBCOL2.3-hIL-7 construct. pOBCOL2.3-hIL-7 contains 2300bp of 5’flanking sequence of the rat col1α1 promoter, first exon and first intron driving human IL-7 cDNA. B. RT-PCR analysis of transgene expression. Selected tissues from pOBCOL2.3-hIL-7 transgenic mouse (high Tg Line C). Total RNA was extracted from 6 week old heterozygous female mouse. Ten micrograms of total RNA was reverse transcribed and 1/50 of cDNA mixture was used for PCR reaction for human IL-7 (hIL-7) and mouse actin.
Table 1.
Human IL-7 concentration (pg/ml) in conditioned medium from neonatal calvarial organ cultures and from serum of IL-7 over-expressing mice (at 3 weeks and 7.5 weeks old).
| Line A | Line B | Line C | |
|---|---|---|---|
| CM | 44.34±4.042 (4) | 0.30±0.027 (5) | 62.95±0.575 (3) |
| Serum at 3 week | 1.21±0.100 (8) | 0.06±0.040 (4) | 3.58±0.351 (9) |
| Serum at 7.5 week | 1.04±0.190 (8) | 0.09±0.062 (14) | 1.18±0.350 (8) |
Table 2.
Effect of human IL-7 overexpression in B lymphopoiesis in the bone marrow from WT and Tg IL-7 mice at 8 weeks of age.
| B220+ IgM− (%) | B220+ IgM+ (%) | |
|---|---|---|
| WT (4) | 27.4±2.30 | 12.9±1.10 |
| Tg IL-7 (4) | 41.7±3.68* | 15.2±1.17 |
Values represent mean±SEM.
Significant effect of Tg IL-7 bone marrow, p≤0.01.
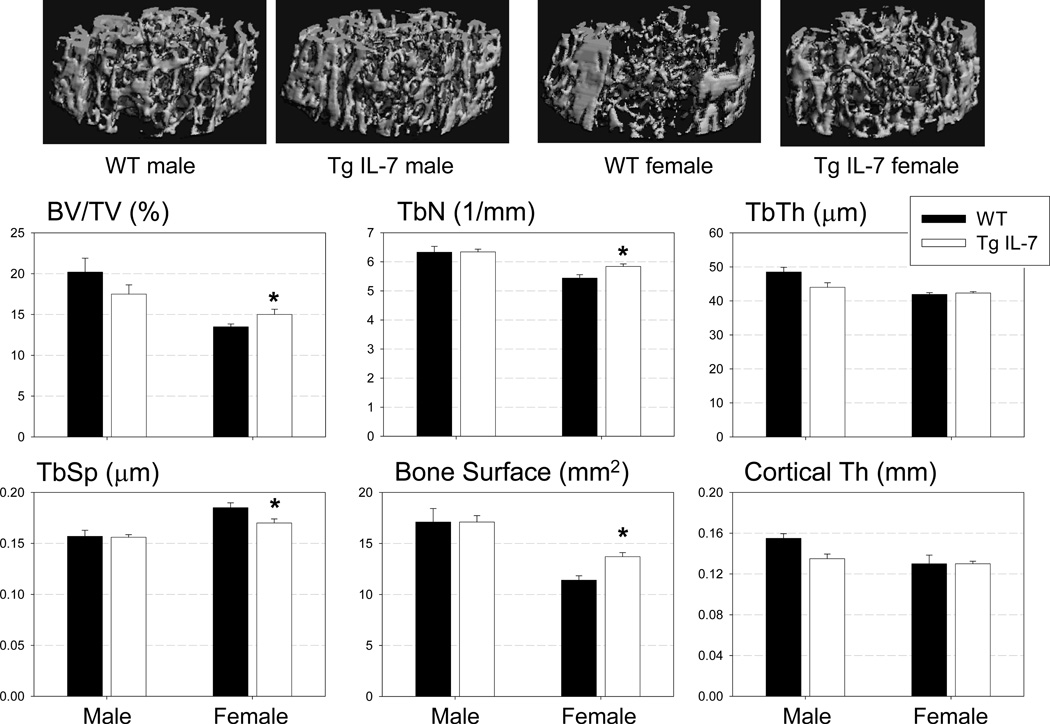
To evaluate the tissue specific expression of the transgene, we performed RT-PCR for the human IL-7 gene in multiple tissues that were isolated from line C (high Tg) female mice. As shown in figure 1B there was strong expression of human IL-7 in the long bones, calvaria and tail. Transgene expression was not detected in the bone marrow, lung, heart, brain, liver, spleen, thymus, muscle, kidney and intestine. WT littermate showed no human IL-7 expression in any tissue type examined (data not shown). As shown in figure 2, microCT image analysis of line C (high Tg) mice demonstrated increased trabecular bone mass (BV/TV, 15%) and trabecular numbers (Tb N, 7%) with decreased trabecular spacing (Tb SP, 10%). These changes were observed in female, but not in male mice at 7.5 weeks of age. Similarly, the trabecular bone mass of line A (intermediate Tg) female mice was increased at 7.5 weeks relative to control WT mice. There was also a significant decrease in cortical thickness in male line A (intermediate Tg) mice (Supplemental Figure 1). Line B (low Tg) mice had no statistical changes in trabecular bone mass, trabecular number or trabecular spacing in either males or females (Supplemental Figure 2).
Figure 2. Increased trabecular bone mass in high Tg (Line C) mice.
Upper panel: Representative images of microCT scan of WT and Tg IL-7 femurs. Increased trabecular bone mass (BV/TV), trabecular number (Tb.N) and bone surface and decreased trabecular space (Tb. Sp) in females but not in males at 7.5 weeks. There was no significant difference in trabecular thickness (TbTh) and cortical thickness between two groups. Values represent mean±SEM. N=4–7/group. *, Significant effect of Tg IL-7 mice, p≤0.05.
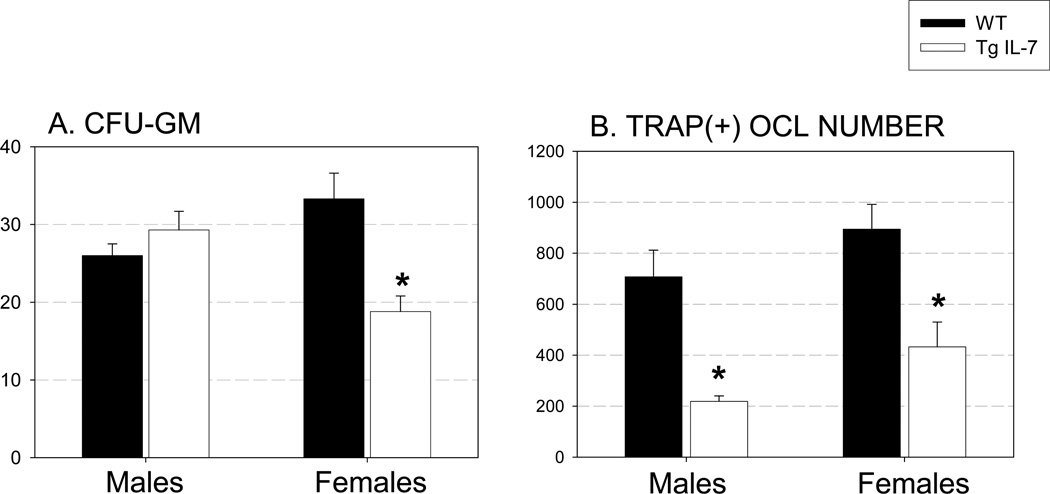
Next we examined the formation of CFU-GM in bone marrow cells, isolated from WT and Tg IL-7 mice. This assay is indicative of myeloid precursors with osteoclastic developmental potential (57). We found a 44% decrease in colony formation in line C (high Tg) females compared with wild type controls (Figure 3A). To investigate if locally produced IL-7 had effects on OCL formation in vitro, we cultured bone marrow from line C (high Tg) and WT littermates with M-CSF and RANKL (both at 30 ng/ml) for 5 days (Figure 3B). The number of OCL formed in cultured female bone marrow cells was significantly greater (by 26%) than in male bone marrow cells. Bone marrow cultures from line C (high Tg) mice showed decreased TRAP (+) OCL number from both males and females (30% to 50% inhibition) compared to cells from WT littermates. 1,25 (OH)2 vitamin D3 (10−8 M) treatment of bone marrow cells from line C (high Tg) and WT littermates demonstrated a significant inhibition of OCL formation at day 6 in both male and female line C (high Tg) cells (36% and 23%, respectively) compared to WT (Supplemental figure 3).
Figure 3. Osteoblast specific IL-7 overexpression decreased osteoclast precursor population and OCL formation.
A. CFU-GM. Bone marrow cells from Tg IL-7 female mice demonstrated a significant decrease in CFU-GM. Bone marrow cells were cultured in semisolid methylcellulose to examine the number of osteoclast precursor cells. B. TRAP(+) OCL number. Bone marrow cells from Tg IL-7 mice demonstrated a significant decrease in TRAP(+) OCL formation when stimulated with M-CSF and RANKL (both at 30 ng/ml). Bone marrow cells were cultured for 5 days. Values represent mean±SEM. Experiments were repeated at least twice and representative experiment is shown. *, Significant effect of Tg IL-7 bone marrow, p≤0.01.
In summary, these results demonstrate that the transgene is active and, as expected, its expression modulates B-lymphocyte lineage development in vivo. Because of the similarity in the bone phenotype of lines A (intermediate Tg) and C (high Tg), these results also show that changes in their bone mass were the result of transgenic hIL-7 overexpression and not the site of transgene insertion into the genome.
Exclusive expression of IL-7 by osteoblastic cells in the IL-7 deficient background recovers cellularity in bone marrow but not fully in the periphery
Because transgenic mice overexpressing IL-7 in osteoblastic lineage cells also express endogenous murine IL-7 at multiple sites in mice, this model does not provide a clear answer regarding the role that IL-7 production in the bone microenvironment has on osteoclast development or activity. To explore this, we crossed the line C mice (high Tg IL-7) into an IL-7 KO background to generate IL-7 KO/Tg IL-7 and studied the extent to which this double mutant rescued the IL-7 deficient phenotype. Initial analyses of cellularity (Table 3) indicated that rescued IL-7 KO mice recovered their splenic cellularity but maintained low thymic cellularity. This is consistent with the recovery of B cell lymphopoiesis in the bone marrow, and an inability of the transgene to rescue T cell lymphopoiesis in the thymus. To confirm this we examined the B- and T-lymphocyte compartments in the mice by flow cytometric analysis.
Table 3.
Total cell number of bone marrow, spleen and thymus from WT, Tg IL-7, IL-7 KO, Rescued IL-7 KO mice.
| WT | Tg IL-7 | IL-7 KO | Rescued IL-7 KO |
|
|---|---|---|---|---|
| Bone Marrow | 2.4±1.44 | 2.4±0.65 | 1.8±0.23 | 1.8±0.33 |
| Spleen | 11.0±2.89 | 19.2±0.90* | 2.6±1.10+ | 11.8±2.28# |
| Thymus | 19.0±0.47 | 14.5±0.75 | 0.18±0.045+ | 0.17±0.080a |
Values represent mean±SEM ×107 cells, N=4/group.
Significant effect of Tg IL-7 mice, p≤0.05.
Significant effect of IL-7 KO mice, p≤0.01.
Significant effect of rescued IL- KO mice compared to IL-7 KO mice, p≤0.05.
Significant effect of rescued IL-KO mice compared to WT mice, p≤0.05.
Exclusive expression of IL-7 by osteoblastic cells in the IL-7 deficient background recovers B cell lymphopoiesis but does not rescue defects in T cell development
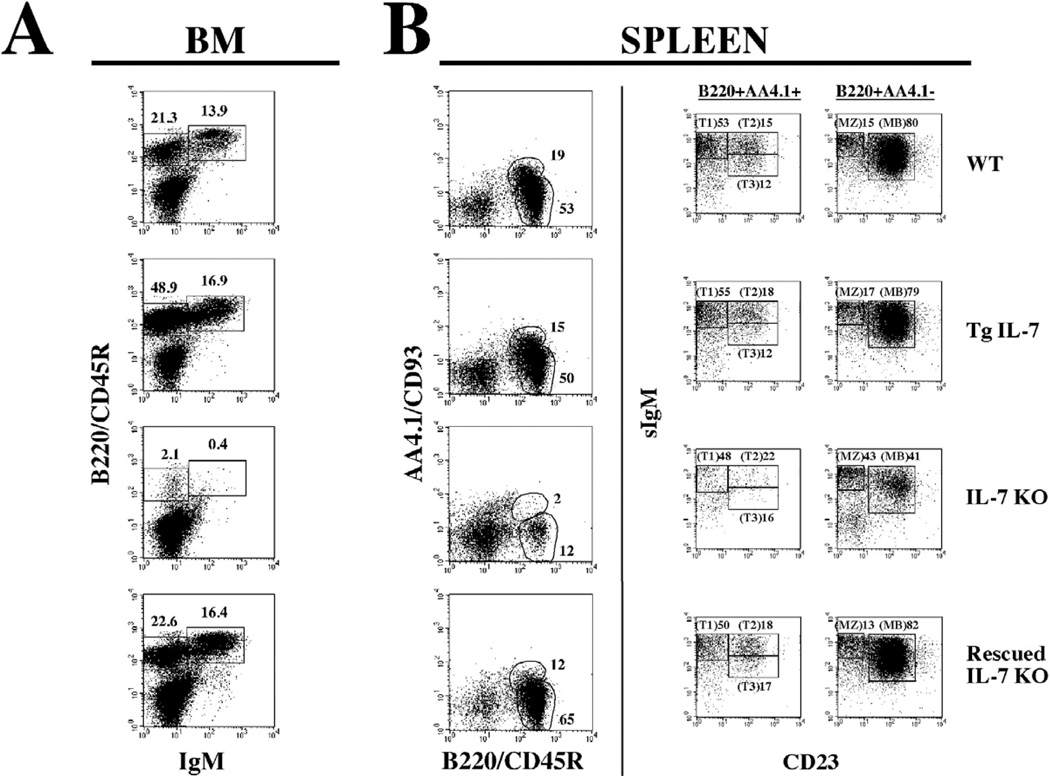
Analyses of B cell development in the bone marrow was done by evaluating the distribution of early B cells (B220+IgM−) vs. more mature B cells (B220+IgM+). As shown in figure 4, early B cells (B220+IgM−) were increased in the bone marrow of Tg IL-7 mice (WT = 21.3%, Tg IL-7 = 48.9%). In contrast, IL-7 KO mice had minimal bone marrow B-lymphopoiesis (IL-7 KO = 2.1%). However, rescued IL-7 KO mice (IL-7 KO/Tg IL-7) had levels of early B cells that were comparable to wild type controls (IL-7 KO/Tg IL-7 = 22.6%). A similar effect was observed in the distribution of more mature B cells. The migration of B cells from the bone marrow to the periphery was analyzed by scoring splenic populations for their expression of B220/CD45R vs. AA4.1/CD93. This combination of antibodies identifies transitional B cell intermediates as B220+AA4.1+ and marginal zone plus mature B lymphocytes as B220+AA4.1−. Transitional B cell intermediates are cells that recently emigrated to the spleen from the bone marrow. This population in extremely underrepresented in the IL-7 KO mice and, as seen in Figure 4, was at near normal levels in the rescued IL-7 KO double mutant. This population can be further subdivided in three sequential stages of early splenic B cell development when dissected in the context of IgM and CD23. These three populations (T1, T2, and T3) are present in the rescued IL-7 KO mice, indicating that migration of early B cells from the bone marrow is critical for their progression through a process that appears to depend on IL-7 production in the periphery. The analysis of more mature cells in the spleen (B220+AA4.1−) also showed a recovery in the rescued IL-7 KO mice, as expected given the rescue of splenic cellularity. This is evident in the CD23+IgM+ population that increased from 41% in the IL-7 KO mice to 82% in the rescued IL-7 KO mice. However, the population of marginal zone B cells (B220+AA4.1+CD23−IgM+) did not change in any of the models. We believe this is because these cells are generated during fetal life in an IL-7 independent fashion.
Figure 4. B cell lymphopoiesis is rescued in IL-7 KO mice after exclusive expression of IL-7 by osteoblasts.
A. Bone marrow cell suspensions from WT, Tg IL-7, IL-7 KO and rescued IL-7 KO mice were stained with anti-mouse IgM and with anti-B220/CD45R antibodies and analyzed by flow cytometry as described. Gates were drawn to identify immature (B220/CD45R+IgM−) and mature (B220/CD45R+IgM+) B cells. The numbers on top correspond to the percent of gated cells in the total population. B. Splenocytes were dissected by their reactivities against anti-B220/CD45R and anti-AA4.1/CD93. Populations were gated into B220/CD45R+AA4.1/CD93+ (transitional B cells) and B220/CD45R+AA4.1/CD93− (mature B cells) populations (left panels). Gated populations were further dissected by their reactivities against anti-IgM and anti-CD23 (right panels). The numbers correspond to the percent of cells within the gate for total splenocytes (right panels) or for the correspondent gated populations (left panels).
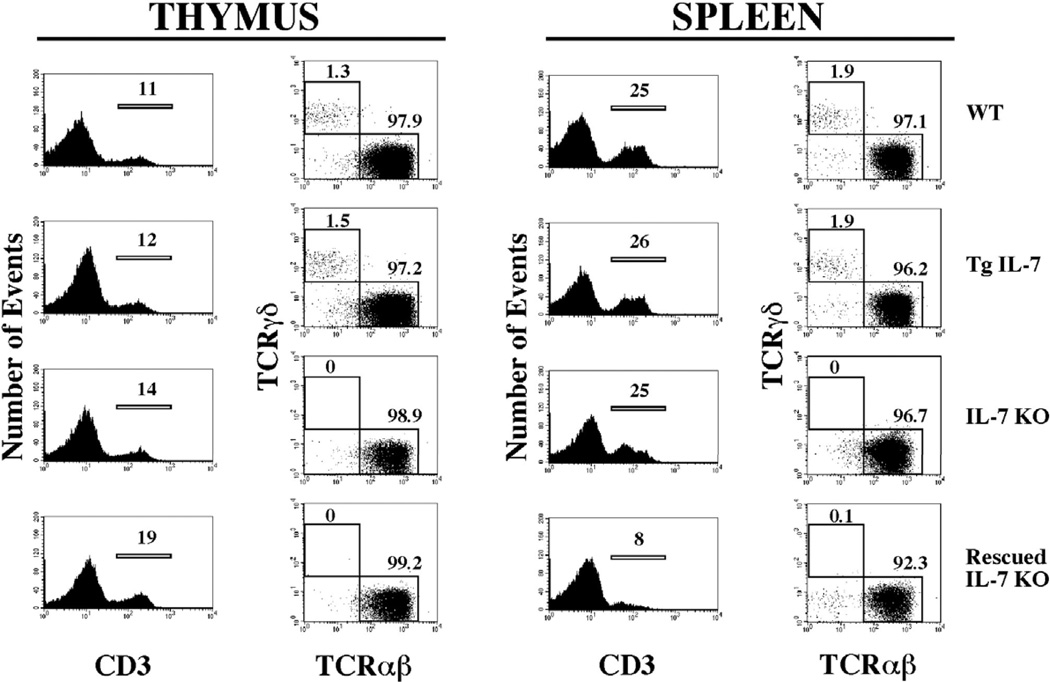
T cell development in the thymus and periphery is regulated by IL-7 at various levels including modulation of T cell receptor rearrangement, control of programmed cell death, expansion during thymopoiesis, and maintenance of homeostatic levels of T cells in periphery. All of these roles of IL-7 are reflected in the IL-7 KO mice by a profound decrease in T cell numbers both in the thymus and the periphery. This also explains the low cellularity observed in the thymus and partially in the spleen. To test if expression of IL-7 in the bone marrow space has an impact on T cell development in an IL-7 KO background, we analyzed the distribution of T cells in rescued IL-7 KO and WT mice. Figure 5 shows the results for thymus and spleen. The relative distribution of T cells in the thymus, evaluated by cells expressing CD3, was comparable between the four groups analyzed. However, this does not apply to absolute numbers, as IL-7 KO and the rescued IL-7 KO mice had markedly fewer cells, indicating that expansion of developing T cell is not rescued by the transgene (Table 3). In addition, the development of γδ T cells, an IL-7 dependent process in the thymus, was also not rescued (Figure 5). In spleen results were similar with the only difference being that the relative number of CD3 positive cells decreased in the rescued IL-7 KO mice (from ~25% to 8%) as a consequence of normalization of the B cell compartment. In summary, the analyses of lymphopoiesis argue strongly that the rescue effect of the IL-7 transgene was restricted to developmental processes occurring in the bone marrow microenvironment and the periphery.
Figure 5. T cell lymphopoiesis remains deficient in IL-7 KO mice after selective expression of IL-7 by osteoblasts.
Cell suspensions from thymus and spleen from WT, Tg IL-7, IL-7 KO and rescued IL-7 KO mice were stained with anti-CD3, anti-TCRαβ and anti-TCR-γδ antibodies coupled to distinct fluorochromes, and analyzed by flow cytometry as described. Histograms on the left showed the percentage of cells reactive to anti-CD3 and plots in the right show the distribution of TCRαβ and TCRγδ within CD3 gated populations.
Exclusive expression of IL-7 by osteoblastic cells rescues the majority of the bone parameters affected by global IL-7 deficiency
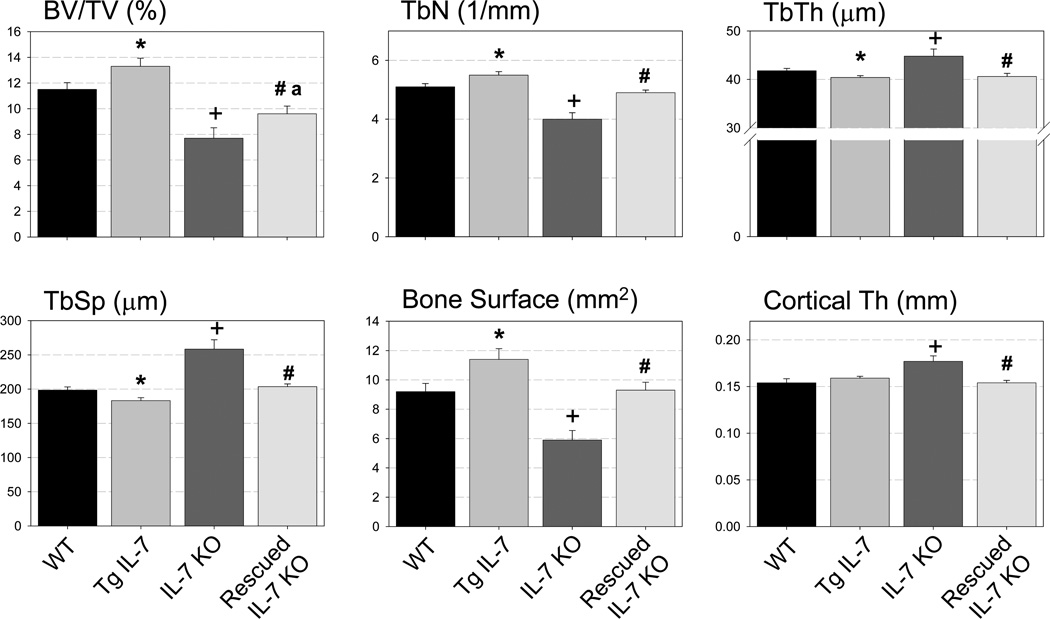
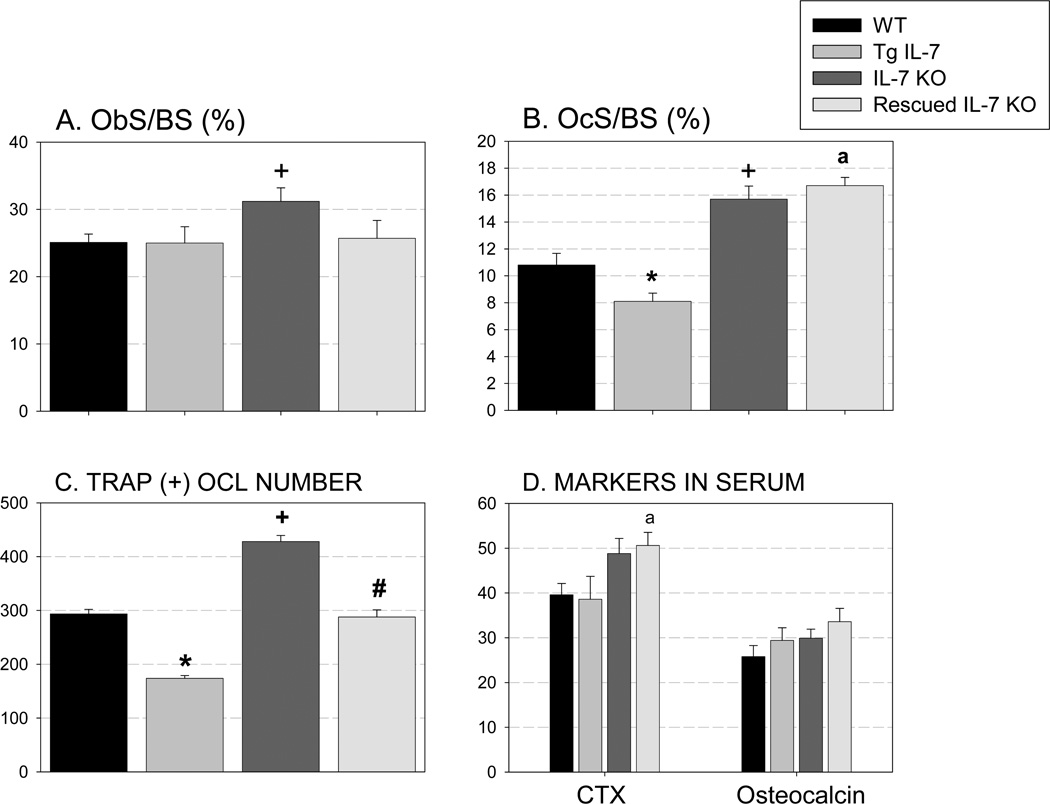
Our analysis of the Tg IL-7 mice indicated that overexpression of this cytokine in the bone marrow space affected bone parameters preferentially in female mice. Thus, we performed micro-CT and static and dynamic histomorphometric analyses of 8-week-old female mice comparing WT, Tg IL-7, IL-7 KO and rescued IL-7 KO mice. Compared to WT mice (Figure 6) there were significant differences in trabecular bone volume in Tg IL-7 mice (16% increase), in IL-7 KO mice (33% decrease) and in rescued IL-7 KO mice (17% decrease). Importantly, rescued IL-7 KO mice had significantly greater trabecular bone mass than did IL-7 KO mice, indicating a partial rescue. The parameters of trabecular number, thickness and bone surface showed a similar pattern to that of trabecular bone volume in the four groups. Additionally, we found a significant increase in cortical thickness in IL-7 KO mice. However, in rescued IL-7 KO mice this value was equivalent to WT mice. We also found a significant increase in osteoblast number (ObS/BS) and osteoclast number (OcS/BS) in IL-7 KO mice (24% and 45%, respectively) compared to WT (Figure 7A and B). In rescued IL-7 KO mice the osteoblast number was equivalent to that of WT. Unexpectedly and despite a decrease in osteoclast number in the Tg IL-7 mice, this parameter was elevated in IL-7 KO mice and remained elevated in rescued IL-7 KO mice. To investigate the function of osteoblast in these models, we performed dynamic histomorphometric analysis of female femurs at 8 weeks of age. As shown in supplemental figure 4, there was a trend for an increase in bone formation rate (BFR, p=0.07) and a significant increase in mineral apposition rate (MAR) in rescued IL-7 KO mice compared to WT or IL-7 KO mice. In Tg IL-7 mice, there was trend toward increase in MAR and BFR compared to WT. These results indicate that the expression of IL-7 in osteoblasts influences B-lymphopoiesis as reported by Wu et. al. (22). We speculated that the increased B lineage cells in the bone marrow may, in turn, influence osteoblasts and bone formation as well as there being direct effect of IL-7 on osteoblasts.
Figure 6. Exclusive expression of IL-7 by osteoblastic cells rescues the majority of the bone parameters affected by a global IL-7 deficiency.
Trabecular bone volume (BV/TV), trabecular number (TbN), trabecular thickness (TbTh), trabecular space (TbSp), bone surface and cortical thickness of femurs from WT, Tg IL-7, IL-7 KO and rescued IL-7 KO female mice at 8 weeks of age were measured by microCT analysis. Eight to 18 bones per group were evaluated. Data represent mean±SEM. *, Significant effect of Tg IL-7 mice, p≤0.05. +, Significant effect of IL-7 KO mice, p≤0.01. #, Significant effect of rescued IL-KO mice compared to IL-7 KO mice, p≤0.05. a, Significant effect of rescued IL-KO mice compared to WT mice, p≤0.05.
Figure 7.
Static histomorphometric analysis of femurs from 8 week of female mice shows regulation of osteoclast number in vivo in response to IL-7 overexpression (A and B). Osteoblast surface per bone surface and osteoclast surface per bone surface were evaluated after the sections were stained with TRAP. Nine to 12 bones per group were examined. C. TRAP(+) OCL number Bone marrow cells from WT, Tg IL-7, IL-7 KO and rescued mice were cultured with or without M-CSF and RANKL (both at 30 ng/ml) for 5 days in vitro and TRAP stained. Experiments were repeated at least twice and representative experiment is shown. D. Serum levels of CTX and osteocalcin (pg/ml) were measured by ELISA. N=4–13. Values represent mean±SEM. *, Significant effect of Tg IL-7 compared to WT, p≤0.05. +, significant effect of IL-7KO compared to WT mice, p≤0.01. #, Significant effect of rescued IL-7 KO mice compared to IL-7 KO mice, p≤0.05. a, Significant effect of rescued IL-7 KO mice compared to WT mice, p≤0.05.
To investigate if locally produced IL-7 had effects on osteoclastogenesis in vitro, we cultured bone marrow cells from WT, Tg IL-7, IL-7 KO and rescued IL-7 KO mice in media containing M-CSF and RANKL for 4 days (Figure 7C). Bone marrow cultures from Tg IL-7 mice showed decreased OCL formation while cells from IL-7 KO mice showed increased osteoclast formation compared to WT littermate cells. Bone marrow cells from rescued IL-7 KO mice showed OCL formation that was similar to that seen in WT cells. In addition to in vitro osteoclast formation, we measured serum markers for bone resorption (CTX) and formation (osteocalcin) in the mice (Figure 7D). There was no significant effect of Tg IL-7 mice on CTX levels in serum. However, we observed a trend toward an increase in CTX levels in the serum of IL-7 KO mice that remained elevated in rescued IL-7 KO mice. Serum osteocalcin levels in these mice groups were comparable.
These results indicate that transgenic expression of IL-7 in osteoblast-lineage cells in vivo can rescue the majority of the bone phenotype of IL-7 KO mice. However, the notable exception was the persistence of an increase in osteoclast number and bone resorption in vivo, indicating that IL-7 production in organs outside the bone marrow microenvironment, influences in vivo osteoclastogenesis through unknown mechanisms.
DISCUSSION
The role of IL-7 in lymphopoiesis is well established. However, its pleiotropic effects on non-lymphoid cells through indirect modulation of lymphocyte populations or direct effects on non-lymphoid target populations is less well understood. Our previously published reports demonstrated that IL-7 could act as a regulator of osteoclastogenesis. Initially we observed that IL-7 added to bone marrow cultures from WT animals directly inhibited the process (47) while cultures from animals deficient in IL-7 showed increased osteoclast formation when compared to similar cultures from WT mice (28, 47). Our current studies have been designed to provide answers to the role of IL-7 on osteoclastogenesis in vivo. The initial analysis of transgenic mice overexpressing IL-7 in a bone specific fashion showed an expected hematopoietic response, which was represented by expansion of the B cell compartment. In addition, these mice demonstrated high bone mass together with a poor osteoclastogenic activity in vivo. Interestingly, this bone phenotype was significant in females but not in males, suggesting that IL-7 has effects on non lymphoid compartments in a gender-dependent manner. These results are in contrast with reports evaluating the impact of systemic administration of IL-7 or global overexpression of the cytokine in transgenic systems. In these latter cases IL-7 stimulated osteoclastogenesis that in the long term translated into an osteopenic phenotype. Subsequent studies have proposed that this response was mediated by the induction of the osteoclastogenic cytokine RANKL by activated T cells and/or decreased levels of the RANK decoy receptor osteoprotegerin (OPG) (31).
However, these effects were not elicited in our localized IL-7 overexpressing mouse model. We found that the main hematopoietic phenotype in the Tg IL-7 (high IL-7 expressing line) was an exacerbated B cell lymphopoiesis in the bone marrow without a major impact on IL-7 responsive cells in the periphery. This also affected the relative proportion of CFU-GM colonies. From these results we hypothesize that overexpression of IL-7 in the bone marrow microenvironment changes the distribution or the differentiation of osteoclast progenitors by disturbing the balance of lineage development in situ. The availability of transgenic mice expressing the human cytokine at different levels also allowed us to conclude that there was a dose effect among the different lines, reaffirming the validity of our model. At 7.5 weeks old, female Tg IL-7 (both high and intermediate Tg Lines) showed increased trabecular bone mass and trabecular numbers by microCT, while the low Tg mice (Line B) had no changes in any parameter examined in either males or females (Supplemental figure 2). Importantly, all three lines express minimally detectable (0.09–1.18 pg/ml) levels of human IL-7 in serum but higher amounts in the conditioned medium from cultured calvaria (0.3–63 pg/ml). Levels of human IL-7 in the serum of transgenic lines A (intermediate Tg) and C (high Tg) were similar to murine IL-7 levels in the serum of WT mice, which we measured in the 6 to 60 pg/ml range. This is in marked contrast with the peripheral levels of IL-7 in mice overexpressing the cytokine gene under the control of the MHC II promoter where IL-7 levels were 10–30 times higher in the bone marrow, lymph nodes and spleen compared to WT mice (59).
As mentioned, our IL-7 overexpression model produced a profound effect on B cell development. This was more evident upon crossing these mice with IL-7 deficient mice to generate animals in which B cell development was rescued with high efficiency. In these animals the T cell deficiency, characteristic of IL-7 deficiency, was not rescued and animals still had compromised thymopoiesis with decreased generation of αβ and γδ T cells. This was not completely unexpected, as it is known that multiple cytokines, including IL-7, act in the localized thymic environment to regulate thymopoiesis through their association with extracellular matrix components, which modulate their concentration in a topologically restricted manner. In fact, none of the IL-7 transgenic mice had increased peripheral levels of IL-7. Osteoblasts are known participants in the generation of hematopoietic niches, supporting B cell lymphopoiesis (60, 61), and one mechanism through which they influence hematopoiesis is their ability to produce cytokines such as IL-7 (22). On the contrary, it has been reported that IL-7 decreased osteoblast function and facilitated the uncoupling of osteoblast and osteoclast (25). However, our study and a previously reported study (25) examined two completely different systems. Our study examined the local effect of IL-7 while Weitzmann et al. examined the global effect of IL-7 by administration of the cytokine intraperitoneally. Our goal in the current study was to examine the direct effect of IL-7 on bone.
Regarding the bone phenotype of these models, IL-7 deficient mice have decreased trabecular bone and a higher number of osteoclasts on their bone surfaces. Interestingly, trabecular bone parameters reverted to WT values when the cytokine was expressed exclusively in the bone marrow microenvironment. Moreover, local overexpression of IL-7 in rescued IL-7 KO mice also decreased the elevated osteoblast number observed in IL-7 KO mice. However, the elevated osteoclast phenotype and increased bone resorption of IL-7 KO mice persisted in rescued IL-7 KO mice. This result implies that additional effects of IL-7 at extramedullary sites, possibly in the thymus, influence this phenomenon. The lack of T cells in the IL-7 deficient and in the IL-7 rescued transgenic mice could account for some of the observed phenotype. In general, T cells have a protective role on bone turnover under physiological conditions. Activated CD8+ T cells are reported to inhibit osteoclastogenesis in vitro (62, 63) and our previous report demonstrated that in vivo depletion of CD4 and CD8 T cells in vivo generated an increased number of osteoclast in response to 1,25-dihydroxyvitamin D3 in vitro (64).
All these observations indicate a possible link between B cell development and bone homeostasis. This has been proposed by various investigators based on reports documenting age related bone loss, which was concurrent with a decline in B lymphocytes (65). It has also been shown that there is a decrease in the pre-B cell populations with age in the bone marrow (66) and subsequent work has revealed that this phenomenon was due to a decline in the responsiveness of pro-B cells to IL-7 (67).
One unexpected finding of our studies was the observation that only female IL-7 transgenic mice had increased trabecular bone volume in vivo and decreased CFU-GM compare to WT littermates. The reasons for these gender differences are not clear. They could be due to differential hormonal control of hematopoiesis since there are compelling data that estrogen regulates B cell lymphopoiesis in mice (68, 69). In contrast, there was a significant difference in osteoclast formation in vitro in both males and females in response to M-CSF and RANKL or vitamin D3 treatment. Possible explanation for this discrepancy is that CFU-GM may not precisely represent the entire osteoclast pool in the bone marrow. We reported that osteoclast progenitors from mouse bone marrow were unable to differentiate into osteoclasts when they were treated with GM-CSF in single cell cloning experiments (70). Rather, these osteoclast precursor cells, which were exposed to GM-CSF, developed dendritic cell-like characteristics in vitro.
We postulate that the phenotypes of IL-7 deficient and IL-7 rescued mice are product of the interactions between myeloid and B cells. These two lineages develop in the context of overlapping microenvironments and there is evidence of a possible regulation by B cells of osteoclast progenitor generation and/or differentiation (71). We and others have found that osteoclast progenitors and B lymphocytes represent separate lineages, and B cells are not normally capable of generating mature osteoclasts (72). However, it has been proposed that during development B cells can express cytokines, which affect osteoclast generation. For example it has been shown that B cells can secrete OPG in certain conditions (73). Because, IL-7 is critical for B cell progression, the lack of IL-7 and the subsequent resultant decrease in B cells could explain the increased osteoclastogenic response observed in IL-7 deficient mice. However, it has also been reported that animals deficient in B cells, through mutations in the immunoglobulin recombination machinery, do not have the bone phenotype observed in IL-7 deficient mice (74). This may be due to the fact that IL-7 regulation of B cell development is earlier than the block that occurs in these recombinase deficient models. Hence, the action of IL-7 in very early progenitors could be responsible for the modulation of osteoclastogenic pathways. Additionally, IL-7 influences the rapid expansion and contraction of early B cells during selection of B cell specificities, and the coupling between osteoclastogenesis and B cells could be in part responsible for the observed phenotypes.
In summary we have shown that mice overexpressing human IL-7 in the osteoblast lineage demonstrated increased trabecular bone volume in vivo and decreased osteoclast formation in vitro. Furthermore, targeted overexpression of IL-7 in osteoblasts rescues the osteopenic bone phenotype of IL-7 KO mice. These results likely reflect a direct inhibitory effect of IL-7 on osteoclastogenesis in vivo.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Ernesto Canalis and Ms. Lauren Kranz (St. Francis Hospital & Medical Center, Hartford, CT) for their help with the embedding and sectioning of calcein labeled femurs.
Authors’ roles: HLA performed flow cytometry, SHM performed quantification of dynamic histomorphometry, HLA, JK and SKL performed cell cultures and in vivo experiments, DJA performed microCT analysis, HLA, JAL and SKL designed the project, discussed and wrote the manuscript.
Footnotes
Conflict of Interest
All authors have no conflicts of interest.
Contributor Information
Hector L. Aguila, Email: Aguila@nso1.uchc.edu.
Se Hwan Mun, Email: Mun@uchc.edu.
Judith Kalinowski, Email: kalinowski@nso.uchc.edu.
Douglas J. Adams, Email: dadams@nso.uchc.edu.
Joseph A. Lorenzo, Email: Lorenzo@nso2.uchc.edu.
Sun-Kyeong Lee, Email: klee@nso2.uchc.edu.
REFERENCES
- 1.Henney C. Interleukin 7: effects on early events in lymphopoiesis. Immunology Today. 1989;10:170–173. doi: 10.1016/0167-5699(89)90175-8. [DOI] [PubMed] [Google Scholar]
- 2.Namen A, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 3.Namen A, Schmierer AE, March CJ, Overell RW, Park LS, Urdal DL, Mochizuki DY. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Experimental Medicine. 1988;167:988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrew D, Aspinall R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Experimental Gerontology. 2002;37:455–463. doi: 10.1016/s0531-5565(01)00213-3. [DOI] [PubMed] [Google Scholar]
- 5.Sakata T, Iwagami S, Tsuruta Y, Teraoka H, Tatsumi Y, Kita Y, Nishkawa H, Aiso S, Hibi T, Ishii H. Constitutive expression of interleukin-7 mRNA and production of IL-7 by a cloned murine thymic stromal cell line. J Leukocyte Biology. 1990;48:205–212. doi: 10.1002/jlb.48.3.205. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe M, Ueno Y, Yajima T, Iwao Y, Tsuchiya M, Ishikawa H, Aiso S, Hibi T, Ishii H. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J Clinical Investigation. 1995;95:2945–2953. doi: 10.1172/JCI118002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiles M, Ruiz P, Imhof BA. Interleukin-7 expression during mouse thymus development. European J Immunology. 1992;22:1037–1042. doi: 10.1002/eji.1830220424. [DOI] [PubMed] [Google Scholar]
- 8.Murray R, Suda T, Wrighton N, Lee F, Zlotnik A. IL-7 is a growth and maintenance factor for mature and immature thymocyte subsets. International Immunology. 1989;1:526–531. doi: 10.1093/intimm/1.5.526. [DOI] [PubMed] [Google Scholar]
- 9.Takeda S, Gillis S, Palacios R. In vitro effects of recombinant interleukin 7 on growth and differentiation of bone marrow pro-B and pro-T-lymphocyte clones and fetal thymocyte clone. Proceedings of National Academy of Sciences USA. 1989;86:1634–1638. doi: 10.1073/pnas.86.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin R, Lupton S, Schmierer A, Hjerrild KJ, Jerzy R, Clevenger W, Gillis S, Cosman D, Namen AE. Human interleukin 7: molecular cloning and growth factor activity on human and murine B-lineage cells. Proceedings of National Academy of Sciences USA. 1989;86:302–306. doi: 10.1073/pnas.86.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee G, Namen AE, Gillis S, Kincade PW. Recombinant interleukin-7 supports the growth of normal B lymphocyte precursors. Current Topics of Miocrobial Immunology. 1988;141:16–18. doi: 10.1007/978-3-642-74006-0_3. [DOI] [PubMed] [Google Scholar]
- 13.Conlon P, Morrissey PJ, Nordan RP, Grabstein KH, Prickett KS, Reed SG, Goodwin R, Cosman D, Namen AE. Murine thymocytes proliferate in direct response to interleukin-7. Blood. 1989;74:1368–1373. [PubMed] [Google Scholar]
- 14.Chazen G, Pereira GMB, LeGros G, Gillis S, Shevach EM. Interleukin-7 is a T-cell growth factor. Proceedings of National Academy of Sciences USA. 1989;86:5923–5927. doi: 10.1073/pnas.86.15.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrissey P, Goodwin RG, Nordan RP, Anderson D, Grabstein KH, Cosman D, Sims J, Lupton S, Acres B, Reed SG. Recombinant interleukin 7, pre-B cell growth factor, has costimulatory activity on purified mature T cells. J Experimental Medicine. 1989;169:707–716. doi: 10.1084/jem.169.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park L, Friend DJ, Schmierer AE, Dower SK, Namen AE. Murine interleukin-7 (IL-7) receptor. Characterization on an IL-7-dependent cell line. J Experimental Medicine. 1990;171:1073–1089. doi: 10.1084/jem.171.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlissel M, Durum SD, Muegge K. The interleukin 7 receptor is required for T cell receptor gamma locus accessibility to the V(D)J recombinase. J Experimental Medicine. 2000;191:1045–1050. doi: 10.1084/jem.191.6.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muegge K, Vila MP, Durum SK. Interleukin-7: a cofactor for V(D)J rearrangement of the T cell receptor beta gene. Science. 1993;261:93–95. doi: 10.1126/science.7686307. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Surh CD. Role of interleukin-7 in bone and T-cell homeostasis. Immunological Reviews. 2005;208:169–180. doi: 10.1111/j.0105-2896.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 20.Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989;125:1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi N, Akatsu T, Sasaki T, Nicholson GC, Moseley JM, Martin TJ, Suda T. Induction of calcitonin receptors by 1α, 25dihydroxyvitamin D3 in osteoclast-like multinucleated cells formed from mouse bone marrow cells. Endocrinology. 1988;123:1504–1510. doi: 10.1210/endo-123-3-1504. [DOI] [PubMed] [Google Scholar]
- 22.Wu JY, Purton LE, Rodda SJ, Chen M, Weinstein LS, McMahon AP, Scadden DT, Kronenberg HM. Osteoblastic regulation of B lymphopoiesis is mediated by Gsα-dependent signaling pathways. Proceedings of National Academy of Science, USA. 2008;105:16976–16981. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato I, Sato H, Kudo A. TRANCE together with IL-7 induces pre-B cells to proliferate. European J Immunology. 2003;33:334–341. doi: 10.1002/immu.200310007. [DOI] [PubMed] [Google Scholar]
- 24.Manabe N, Kawaguchi H, Chikuda H, Miyaura C, Inada M, Nagai R, Nabeshima Y-I, Nakamura K, Sinclair AM, Scheuermann RH, Kuro-o M. Connection between B lymphocyte and osteoclast differentiation pathways. J Immunology. 2001;167:2625–2631. doi: 10.4049/jimmunol.167.5.2625. [DOI] [PubMed] [Google Scholar]
- 25.Weitzmann M, Roggia C, Toraldo G, Weitzmann L, Pacifici R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clinical Investigation. 2002;110:1643–1650. doi: 10.1172/JCI15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grabstein K, Waldschmidt TJ, Finkelman FD, Hess BW, Alpert AR, Boiani NE, Namen AE, Morrissey PJ. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Experimental Medicine. 1993;178:257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Era T, Nishikawa S, Sudo T, Wang FH, Ogawa M, Kunisada T, Hayashi S. How B-precursor cells are driven to cycle. Immunological Reviews. 1994;137:35–51. doi: 10.1111/j.1600-065x.1994.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Kalinowski JF, Jacquin C, Adams DJ, Gronowicz G, Lorenzo JA. Interleukin-7 influences osteoclast function in vivo but is not a critical factor in ovariectomy-induced bone loss. J Bone Mineral Research. 2006;21:695–702. doi: 10.1359/jbmr.060117. [DOI] [PubMed] [Google Scholar]
- 29.Miyaura C, Onoe Y, Inada M, Maki K, Ikuta K, Ito M, Suda T. Increased B-lymphopoiesis by interleukin 7 induces bone loss in mice with intact ovarian function: Similarity to estrogen deficiency. Proceedings of National Academy of Sciences USA. 1997;94:9360–9365. doi: 10.1073/pnas.94.17.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weitzmann M, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood. 2000;96:1873–1878. [PubMed] [Google Scholar]
- 31.Toraldo G, Roggia C, Qian W-P, Pacific R, Weitzmann MN. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor κB ligand and tumor necrosis factor α from T cells. Proceedings of National Academy of Sciences USA. 2002;100:125–130. doi: 10.1073/pnas.0136772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan M, Shepherd R, Leavey JK, Gao Y, Grassi F, Schnell FJ, Qian WP, Kersh GJ, Weitzmann MN, Pacifici R. An IL-7-dependent rebound in thymic T cell output contributes to the bone loss induced by estrogen deficiency. Proceedings National Academy Sciences USA. 2005;102:16735–16740. doi: 10.1073/pnas.0505168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H, Nishikawa SI. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proceedings of National Academy of Sciences USA. 1993;90:9125. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatia S, Tygrett LT, Grabstein KH, Waldschmidt TJ. The effect of in vivo IL-7 deprivation on T cell maturation. J Experimental Medicine. 1995;181:1399–1409. doi: 10.1084/jem.181.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrissey P, Conlon P, Charrier K, Braddy S, Alpert A, Williams D, Namen AE, Mochizuki D. Administration of IL-7 to normal mice. J Immunology. 1991;147:561–568. [PubMed] [Google Scholar]
- 36.Fraser C, Thacker JD, Hogge DE, Fatur-Saunders D, Takei F, Humphries RK. Alterations in lymphopoiesis after hematopoietic reconstitution with IL-7 virus-infected bone marrow. J Immunology. 1993;151:2409–2418. [PubMed] [Google Scholar]
- 37.Mertsching E, Grawunder U, Meyer V, Rolink T, Ceredig R. Phenotypic and functional analysis of B lymphopoiesis in interleukin-7-transgenic mice: Expansion of pro/pre-B cell number and persistence of B lymphocyte development in lymph nodes and spleen. European J Immunology. 1996;26:28–33. doi: 10.1002/eji.1830260105. [DOI] [PubMed] [Google Scholar]
- 38.Valenzona H, Pointer R, Ceredig R, Osmond DG. Prelymphomatous B cell hyperplasia in the bone marrow of interleukin-7 transgenic mice: precursor B cell dynamics, microenvironmental organization and osteolysis. Experimental Hematology. 1996;24:1521–1529. [PubMed] [Google Scholar]
- 39.Mertsching E, Meyer V, Linares J, Lombard-Platet S, Ceredig R. Interleukin-7, a non-redundant potent cytokine whose over-expression massively perturbs B-lymphopoiesis. International Reviews of Immunology. 1998;16:285–308. doi: 10.3109/08830189809042998. [DOI] [PubMed] [Google Scholar]
- 40.Fisher A, Burdet C, Bunce C, Merkenschlager M, Ceredig R. Lymphoproliferative disorders in IL-7 transgenic mice: expansion of immature B cells which retain macrophage potential. International Immunology. 1995;7:415–423. doi: 10.1093/intimm/7.3.415. [DOI] [PubMed] [Google Scholar]
- 41.Salopek D, Grčević D, Katavić V, Kovačić N, Lukić IK, Marušić A. Increased bone resorption and osteopenia are a part of the lymphoproliferative phenotype of mice with systemic over-expression of interleukin-7 gene driven by MHC class II promoter. Immunology Letters. 2008;121:134–139. doi: 10.1016/j.imlet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 42.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SEG, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Experimental Medicine. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeurer M, Lotze MT. Interleukin-7 (IL-7) knockout mice: Implications for lymphopoiesis and organ-specific immunity. International Reviews of Immunology. 1998;16:309–322. doi: 10.3109/08830189809042999. [DOI] [PubMed] [Google Scholar]
- 44.Rich B. Autocrine expression of interleukin-7 rescues lymphoid expansion in interleukin-7 deficient mice. Immunology Today. 1997;92:374–380. doi: 10.1046/j.1365-2567.1997.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maki K, Sunaga S, Komagata Y, Kodaira Y, Mabuchi A, Karasuyama H, Yokomuro K, Miyazaki J, Ikuta K. Interleukin-7 receptor-deficient mice lack γδT cells. Proceedings of National Academy of Sciences USA. 1996;93:7172–7177. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pechon J, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Zigler SF, Williams DE, Ware CB, Meyer JD, Davidson BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Experimental Medicine. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, Kalinowski JF, Jastrzebski SL, Puddington L, Lorenzo JA. Interleukin-7 is a direct inhibitor of in vitro osteoclastogenesis. Endocrinology. 2003;144:3524–3531. doi: 10.1210/en.2002-221057. [DOI] [PubMed] [Google Scholar]
- 48.Brunton L, Lupton SD. Human interleukin 7: Molecular cloning and growth factor activity on human and murine B-lineage cells. Proceedings of National Academy of Sciences USA. 1989;86:302–306. doi: 10.1073/pnas.86.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chirgwin J, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 50.Alonso S, Minty A, Bourlet Y, Buckingham M. Comparison of the three actin-coding sequences in the mouse: evolutionary relationships between the actin genes of warm-blooded vertebrates. J Molecular Evolution. 1986;23:11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- 51.Parfitt A, Drezner MK, Vlorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry:stardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Mineral Research. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi N, Yamana H, Yoshiki S, Roodman GD, Mundy GR, Jones SJ, Boyde A, Suda T. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988;122:1373–1382. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- 53.Lee S-K, Goldring SR, Lorenzo JA. Expression of the calcitonin receptor in bone marrow cell cultures and in bone: A specific marker of the differentiated osteoclast that is regulated by calcitonin. Endocrinology. 1995;136:4572–4581. doi: 10.1210/endo.136.10.7664679. [DOI] [PubMed] [Google Scholar]
- 54.Akatsu T, Tamura T, Takahashi N, Udagawa N, Tanaka S, Sasaki T, Yamaguchi A, Nagata N, Suda T. Preparation and characterization of a mouse osteoclast-like multinucleated cell population. Journal of Bone and Mineral Research. 1992;7:1297–1306. doi: 10.1002/jbmr.5650071109. [DOI] [PubMed] [Google Scholar]
- 55.Akatsu T, Takahashi N, Debari K, Morita I, Nagata N, Takatani O, Suda T. Prostaglandins promote osteoclast-like cell formation by a mechanism involving cyclic adenosine 3’, 5’-monophosphate in mouse bone marrow cell cultures. J Bone and Mineral Research. 1989;4:29–35. doi: 10.1002/jbmr.5650040106. [DOI] [PubMed] [Google Scholar]
- 56.Shuto T, Kukita T, Hirata M, Jimi E, Koga T. Dexamethasone stimulates osteoclast-like cell formation by inhibiting granulocyte-macrophage colony-stimulating factor production in mouse bone marrow cultures. Endocrinology. 1994;134:1121–1126. doi: 10.1210/endo.134.3.8119150. [DOI] [PubMed] [Google Scholar]
- 57.Menaa C, Kurihara N, Roodman GD. CFU-GM-derived cells form osteoclasts at a very high efficiency. Biochemical Biophysical Research Communication. 2000;267:943–946. doi: 10.1006/bbrc.1999.2042. [DOI] [PubMed] [Google Scholar]
- 58.Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone and Mineral Research. 2002;17:15–25. doi: 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- 59.Vogt T, Link A, Perrin J, Finke D, Luther SA. Novel function for interleukin-7 in dendritic cell development. Blood. 2009;113:3961–3968. doi: 10.1182/blood-2008-08-176321. [DOI] [PubMed] [Google Scholar]
- 60.Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman R, Emerson SG. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 61.Tokoyoda K, Egawa T, Sugiyama T, Choi B, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 62.John V, Hock JM, Short LL, Glasebrook AL, Galvin RJ. A role for CD8+ T lymphocytes in osteoclast differentiation in vitro. Endocrinology. 1996;137:2457–2463. doi: 10.1210/endo.137.6.8641199. [DOI] [PubMed] [Google Scholar]
- 63.Choi Y, Woo KM, Ko SH, Lee YJ, Park SJ, Kim HM, Kwon BS. Osteoclastogenesis is enhanced by activated B cells but suppressed by activated CD8(+) T cells. European J Immunology. 2001;31:2197–2188. doi: 10.1002/1521-4141(200107)31:7<2179::aid-immu2179>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 64.Grcevic D, Lee SK, Marusic A, Lorenzo JA. Depletion of CD4 and CD8 T lymphocytes in mice in vivo enhances 1,25-dihydroxyvitamin D3-stimulated osteoclast-like cell formation in vitro by a mechanism that is dependent on prostaglandin synthesis. J Immunology. 2000;165:4231–4238. doi: 10.4049/jimmunol.165.8.4231. [DOI] [PubMed] [Google Scholar]
- 65.Miller J, Allman D. Linking age-related defects in B lymphopoiesis to the aging of hematopoietic stem cells. Seminars in Immunology. 2005;17:321–329. doi: 10.1016/j.smim.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 66.Merchant M, Garvy BA, Riley RL. B220 bone marrow progenitor cells from New Zealand black autoimmune mice exhibit an age-associated decline in pre-B and B-cell generation. Blood. 1995;85:1850–1857. [PubMed] [Google Scholar]
- 67.Stephan R, Reilly CR, Witte PL. Impaired ability of bone marrow stromal cells to support B-lymphopoiesis with age. Blood. 1998;91:75–88. [PubMed] [Google Scholar]
- 68.Medina K, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nature Immunology. 2001;8:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 69.Igarashi H, Kouro T, Yokota T, Comp PC, Kincade PW. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proceedings of National Academy of Science, USA. 2001;98:15131–15136. doi: 10.1073/pnas.011513098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacome C, Lee SK, Lorenzo J, Aguila HL. Identification and characterization of a common progenitor for osteoclasts, macrophages and dendritic cells in murine bone marrow. J Bone and Mineral Research. 2010;25:S302. doi: 10.1002/jbmr.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horowitz MC, Fretz JA, Lorenzo JA. How B cells influence bone biology in health and disease. Bone. 2010;47:472–479. doi: 10.1016/j.bone.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacquin C, Gran DE, Lee SK, Lorenzo, Aguila HL. Identification of multiple osteoclast precursor populations in murine bone marrow. J Bone and Mineral Research. 2006;21:67–77. doi: 10.1359/JBMR.051007. [DOI] [PubMed] [Google Scholar]
- 73.Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, Weitzmann MN. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109:3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horowitz M, Xi Y, Pflugh DL, Hesslein DGT, Schatz DG, Lorenzo JA, Bothwell ALM. Pax5 deficient mice exhibit early onset osteoporosis with increased osteoclast progenitors. J Immunology. 2004;173:6583–6591. doi: 10.4049/jimmunol.173.11.6583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.