Abstract
Background and Purpose
Despite evidence suggesting that vitamin D deficiency may lead to elevated cardiovascular disease risk, results regarding the association of 25(OH)D levels with stroke risk are inconclusive. We aimed to examine this association in a prospective study in women and to summarize all existing data in a meta-analysis.
Methods
We measured 25(OH)D levels among 464 women who developed ischemic stroke and an equal number of controls who were free of stroke through 2006 in the Nurses’ Health Study (NHS). We searched MEDLINE and EMBASE for articles published through March 2011 that prospectively evaluated 25(OH)D levels in relation to stroke.
Results
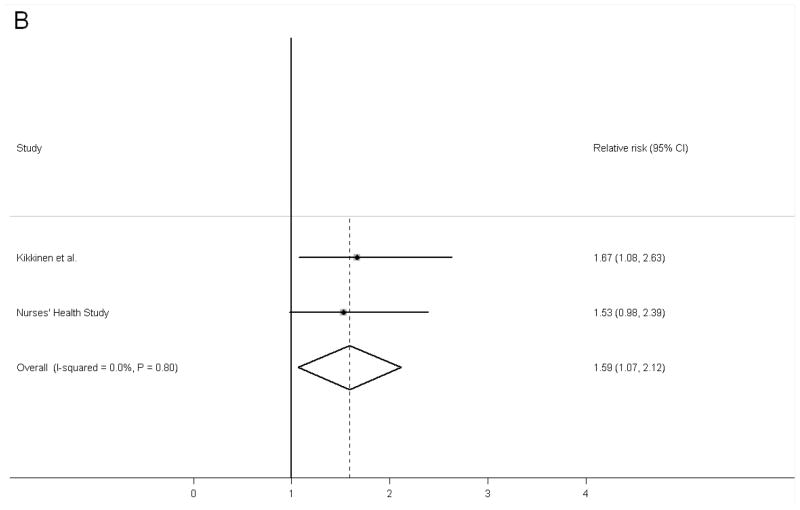
After multivariable adjustment for lifestyle and dietary covariates, lower 25(OH)D levels were associated with an elevated risk of ischemic stroke in the NHS: the odds ratio (95% CI) comparing women in the lowest vs. highest tertiles was 1.49 (1.01, 2.18; Ptrend=0.04). We found 6 other prospective studies that examined 25(OH)D in relation to stroke outcomes. After pooling our results with these prospective studies that included 1,214 stroke cases in total, low 25(OH)D levels were associated with increased risk of developing stroke outcomes in comparison to high levels: the pooled relative risk (95% CI) was 1.52 (1.20, 1.85; I2 = 0.0%, Pheterogeneity=0.63). In two studies that explicitly examined ischemic stroke, this association was 1.59 (1.07, 2.12; I2 = 0.0%, Pheterogeneity=0.80).
Conclusions
These data provide evidence that low vitamin D levels are modestly associated with risk of stroke. Maintaining adequate vitamin D status may lower risk of stroke in women.
Keywords: vitamin D, stroke, meta-analysis
INTRODUCTION
Hypertension and diabetes are among the leading risk factors for stroke and targets for stroke prevention.1 Interestingly, human observational studies strongly suggested beneficial effects of vitamin D, a hormone that primarily regulates calcium metabolism, on lowering the risk of both conditions.2–4 In addition, mechanistic studies have provided mounting evidence supporting direct vascular effects of vitamin D that may lead to a lower risk of stroke.5, 6 Despite the basic science research evidence, epidemiologic studies examining vitamin D status, as measured by 25-hydroxyvitamin D [25(OH)D] levels in blood, in relation to risk of stroke morbidity or mortality have been inconsistent. Some,7–9 but not all,10–12 of these studies documented significant, inverse associations between 25(OH)D levels and stroke risk. The inconsistency of the results may be partially explained by insufficient statistical power, because most previous studies only accumulated a small number of stroke cases. Therefore, we conducted the largest prospective study, thus far, to examine the relationship between plasma 25(OH)D levels and risk of ischemic stroke among women who were initially free of stroke in the Nurses’ Health Study (NHS). We also summarized the existing evidence via a meta-analysis to shed light on this important association.
METHODS
Study populations
The NHS cohort consists of 121,700 female registered nurses aged 30 to 55 years who were residing in 11 U.S. states and enrolled in 1976 through responding to a mailed questionnaire on their medical history and lifestyle practices. In 1989–1990, blood samples were collected from 32,826 participants. Among these participants, we prospectively identified and confirmed 483 incident ischemic stroke cases from the date of blood draw through June 2006. We used a risk-set sampling scheme13 to randomly select one control for each case from the rest of the population who remained free of stroke when the case occurred; the probability of being selected as a controls is proportional to the length of follow-up. We further matched cases and controls for age at blood draw (±1 year), date of blood draw (±3 months), menopausal status (yes, no), use of postmenopausal hormone (current or non-user), race (white or other races), and smoking status (past, current, or non-smoker). After excluding 19 case-control pairs with missing 25(OH)D data, 464 pairs were available for analysis.
The study protocol was approved by the institutional review board of the Brigham and Women’s Hospital and the Human Subjects Committee Review Board of Harvard School of Public Health.
Ascertainment of ischemic stroke
In the NHS cohort, we requested permission of access to medical records of participants who reported having stroke diagnosis in follow-up questionnaires. Study physicians, who were blinded as to the exposure status of these participants, reviewed medical records including results of computerized tomography or magnetic resonance imaging. An ischemic non-fatal stroke diagnosis was confirmed if the medical records demonstrated a neurological deficit with sudden or rapid onset that persistedfor >24 hours or until death in accordance with the criteria of the National Survey of Stroke,14 with an underlying etiology of thrombotic or embolic mechanism. Fatal stroke cases were identified from reports of next of kin or postal authorities, or by searching the National Death Index if stroke was indicated as the cause of death in autopsy reports, hospital records, or death certificates. Fatal ischemic stroke cases were confirmed by reviewing medical or autopsy records, following the same diagnostic criteria as above.
Assessment of exposure and covariates
Plasma samples of each case-control pair were handled identically and analyzed in the same run by the same technicians in a random sequence under identical conditions. Plasma 25(OH)D levels were measured using the LIAISON 25 OH Vitamin D TOTAL Assay (DiaSorin, Stillwater, MN), which is a direct competitive chemiluminescence immunoassay performed on the DiaSorin LIAISON automated analyzer. Quality control samples were dispersed throughout each analytical run. Based on the measurements of these control samples, the average intra-assay coefficient of variation was 13.9% for 25(OH)D. Assessments of covariates were described in Supplementary Material.
Statistical methods
We categorized the cases and controls into tertiles according to the distribution of the 25(OH)D levels among the controls. Conditional logistic regressions, in which matching design is taken into account, were used to examine the association between 25(OH)D and stroke risk. In the multivariable analysis, we controlled for covariates that may confound the association of interest, including body mass index (BMI), physical activity, and dietary factors. P values for linear trend were calculated by entering an ordinal score based on the median value in each tertile of 25(OH)D into the models. In a secondary analysis, we calculated the season-adjusted residuals of 25(OH)D levels by regressing this biomarker on the month at blood draw in a linear regression model. We used the residuals that are statistically independent of season as exposures of interest and repeated the analysis.
All P values were 2-sided, and 95% confidence intervals (CI) were calculated for ORs or RRs. Data were analyzed with the Statistical Analysis Systems software package, version 9.2 (SAS Institute, Inc., Cary, NC).
Meta-analysis
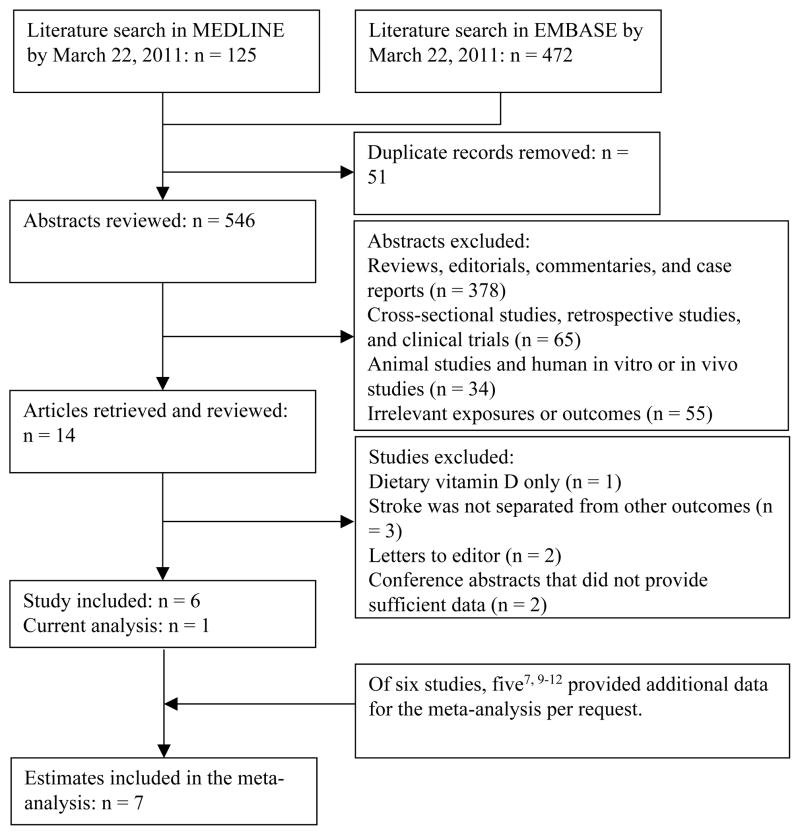
We identified and included 6 studies7–12 that explicitly evaluated 25(OH)D levels in relation to incident stroke or stroke mortality (Figure 1). Study selection and data extraction were described in Supplementary Material. Meta-analyses were performed by using Stata 11.0 (Stata-Corp, College Station TX). We fitted a fixed-effects model to derive summary estimates that were based on the logarithms of RRs and corresponding standard errors in each individual study. Heterogeneity among the results of these studies was evaluated using the Cochran Q test and I2 statistic. To detect any non-linear dose-response relationship, we first used a restricted cubic spline regression model (Stata RC_SPLINE command) with three knots to create spline variables of 25(OH)D levels. We then fitted two log-linear dose-response regression models (Stata GLST command); in the first model we only included a continuous 25(OH)D variable, and in the second model we included both linear and spline terms of 25(OH)D levels. Lastly, we used the likelihood ratio test to examine the significance of any non-linearity by comparing the model with the linear term only to the model with both the linear and the cubic spline terms. For this dose-response analysis, we requested data on levels of 25(OH)D and person-time (if Cox regression was used) for each category for six studies.8–12 Five studies7, 9–12 responded to our requests and provided these data. For one study that did not respond to our request,8 we multiplied number of subjects by average follow-up time to estimate the person-years. Begg funnel plots and Egger tests were used to assess potential publication bias.15, 16
Figure 1.
Literature search and study selection.
RESULTS
The Nurses’ Health Study
Table 1 shows the baseline characteristics of cases and controls. The cases and controls had similar baseline levels of 25(OH)D. Among controls, 41.6% would be considered vitamin D deficient, with a 25(OH)D levels <50 nmol/L,17 compared with 45.3% of cases. We also examined the association of 25(OH)D with age, BMI, physical activity, systolic blood pressure, dietary vitamin D intake, race, and season of blood draw among controls. As expected, 25(OH)D levels were significantly (P<0.05) correlated with BMI [Partial Pearson correlation coefficient (r)=−0.23], physical activity (r=0.20), dietary vitamin D intake (r=0.19). In addition, white participants had higher 25(OH)D levels than African Americans [mean 25(OH)D levels were 56.9 vs. 44.7 nmol/L, respectively], and 25(OH)D levels were higher for women living in the Southern states (mean: 59.7 nmol/L) than those who lived in the Northern states (mean: 55.5 nmol/L) or when blood samples were collected during Summer months (mean: 59.8 nmol/L) in comparison to other seasons (mean: 55.8 nmol/L). Plasma 25(OH)D levels were not correlated with age (r=0.01) or systolic blood pressure (r=0.02).
TABLE 1.
Baseline characteristics of ischemic stroke cases and controls, the Nurses’ Health Study.
| Characteristics* | Cases (n = 464) | Controls (n = 464) | P value† |
|---|---|---|---|
| Demography and lifestyle | |||
| Age (year)‡ | 60.8±5.9 | 60.8±6.0 | 0.93 |
| European ancestry (%)‡ | 97.6 | 97.6 | >0.99 |
| Body mass index (kg/m2) | 26.0±5.1 | 25.4±4.8 | 0.09 |
| Physical activity (MET-hr/week) | 15.1±19.6 | 16.2±18.4 | 0.41 |
| Smoking status (%)‡,§ | 0.08 | ||
| Never smoked | 41.9 | 41.6 | |
| Past smoker | 40.4 | 42.0 | |
| Current smoker | 17.7 | 16.4 | |
| Menopausal status (%)‡ | >0.99 | ||
| Pre-menopause | 6.3 | 6.3 | |
| Post-menopause, current hormone users | 42.2 | 42.0 | |
| Post-menopause, not current hormone users | 46.8 | 47.2 | |
| Post-menopause, hormone use status unknown | 4.7 | 4.5 | |
| Aspirin use (%)§ | 0.02 | ||
| <1 tablet/week | 39.6 | 30.2 | |
| 1–2 tablets/week | 25.3 | 30.4 | |
| 3–6 tablets/week | 11.6 | 16.6 | |
| 7–14 tablets/week | 17.6 | 16.8 | |
| 15+ tablets/week | 6.0 | 6.1 | |
| Use of multivitamin (%) | 37.9 | 34.3 | 0.25 |
| History of hypertension (%) | 47.8 | 34.3 | <0.001 |
| History of high cholesterol (%) | 47.6 | 46.3 | 0.69 |
| History of heart disease (%) | 15.3 | 14.0 | 0.58 |
| History of diabetes (%) | 12.9 | 6.3 | 0.0005 |
| Diet | |||
| Vitamin D (IU/day) | 353.8±208.7 | 347.1±211.0 | 0.63 |
| Calcium (mg/day) | 1021.0±414.1 | 1042.3±378.0 | 0.41 |
| Glycemic load | 101.3±16.5 | 100.9±16.4 | 0.73 |
| Polyunsaturated to saturated fat ratio | 0.57±0.15 | 0.58±0.17 | 0.31 |
| Trans fat (g/day) | 1.7±0.4 | 1.7±0.5 | 0.77 |
| Alcohol (g/day) | 6.7±11.0 | 6.0±9.7 | 0.32 |
| 25(OH)D (nmol/L) | 55.0±25.5 | 56.8±22.7 | 0.24 |
Abbreviations: MET-hr, Metabolic Equivalent Task-hour; 25(OH)D, 25-hydroxyvitamin D.
For continuous variables, values were expressed as mean±SD; for categorical variables, % was used.
P values were based on Student’s t test for continuous variables or Pearson χ2 test for categorical variables.
Matching factors.
Based on non-missing data.
In conditional logistic regression accounting for matching factors, women in the lowest 25(OH)D tertile had a non-significantly higher risk of ischemic stroke (Table 2). After adjustment for BMI and physical activity, the association was attenuated to 1.24 (0.89, 1.74; Ptrend=0.20) comparing extreme tertiles. However, when we adjusted for other lifestyle risk factors and history of chronic conditions, the association was strengthened to some extent: the OR for stroke (95% CI) was 1.42 (0.99, 2.04; Ptrend=0.06) for the women in the lowest tertile compared with those in the highest. Finally, after additional adjustment for dietary risk factors, including use of multivitamin, intakes of calcium and trans fat, polyunsaturated fat to saturated fat ratio, and glycemic load, the association was further strengthened, with an OR (95% CI) of 1.49 (1.01, 2.18; Ptrend=0.04), comparing extreme tertiles. Further adjustment of estimated glomerular filtration rate (eGFR) or C-reactive protein (CRP) did not materially change the association; the ORs (95% CI) were 1.49 (1.02, 2.19; Ptrend=0.04) and 1.46 (1.00, 2.14; Ptrend=0.05), respectively. When we used alternate cut-points that were largely consistent with the only prior study of ischemic stroke,8 we observe similar associations. For example, in comparison to participants whose 25(OH)D levels >47 nmol/L (62.3% of total participants), women who had 25(OH)D levels ≤31 nmol/L (13.6% of total participants) had a multivariable OR (95% CI) of 1.53 (0.98, 2.39; Ptrend=0.09) for stroke. When we used season-adjusted residuals of 25(OH)D levels as the exposure, we found a similar, inverse association, suggesting that our observations were not subject to seasonal variations of 25(OH)D levels: the multivariable-adjusted ORs (95% CI) were 1.41 (0.99, 2.02) and 1.48 (1.02, 2.17; Ptrend=0.04) for the 2nd and 1st tertiles of 25(OH)D residuals, respectively.
TABLE 2.
Odds ratio (95% CI) of ischemic stroke for 25(OH)D levels at baseline, the Nurses’ Health Study.
| Plasma levels of 25(OH)D (nmol/L) at baseline
|
||||
|---|---|---|---|---|
| Tertile 3 (highest) | Tertile 2 | Tertile 1 (lowest) | Ptrend | |
| Median (Range) | 77.6 (65.5, 264.3) | 55.0 (45.8, 65.4) | 35.0 (9.2, 45.7) | - |
| Case/control n. | 133/156 | 160/154 | 171/154 | - |
| Model 1* | 1.0 | 1.20 (0.88, 1.64) | 1.31 (0.95, 1.82) | 0.10 |
| Model 2† | 1.0 | 1.16 (0.84, 1.59) | 1.24 (0.89, 1.74) | 0.20 |
| Model 3‡ | 1.0 | 1.25 (0.89, 1.76) | 1.42 (0.99, 2.04) | 0.06 |
| Model 4¶ | 1.0 | 1.26 (0.89, 1.79) | 1.49 (1.01, 2.18) | 0.04 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D.
Model 1 was conditioned on all matching factors, i.e., age at blood draw (year), menopausal status (yes, no), use of postmenopausal hormone (current or non-user), White (yes, no), and smoking status (past, current, or non-smoker).
Based on model 1, model 2 was further adjusted for body mass index (kg/m2) and physical activity (MET-hr/week, in tertiles).
Based on model 2, model 3 was further adjusted for use of aspirin (<1 tablet/week, 1–2 tablets/week, 3–6 tablets/week, 7–14 tablets/week, and 15+tablets/week), hypertension (yes, no), high cholesterol (yes, no), history of heart disease or diabetes (yes, no), and alcohol consumption (gram/day, in tertiles).
Based on model 3, model 4 was further adjusted for use of multivitamin (yes, no) and intakes of calcium and trans fat, polyunsaturated fat to saturated fat ratio, and glycemic load (all in tertiles).
We further conducted several secondary analyses to explore the association of 25(OH)D in certain subgroups or with certain stroke subtypes. First, we examined interaction between plasma 25(OH)D levels and risk factors of stroke or factors that influence 25(OH)D levels, including age, BMI, physical activity, use of hormone therapy, season of blood draw, latitude of participants’ residence, eGFR, and CRP (Supplementary Table 1). We found a marginally significant interaction by current use of hormone therapy: comparing low vs. high tertiles of 25(OH)D, the OR (95% CI) was 1.06 (0.62, 1.84; Ptrend=0.80) for nonusers, whereas this OR (95% CI) was 2.16 (1.17, 4.00; Ptrend=0.01) among users, the majority (>75%) of whom used oral hormone therapy. Second, we examined the association of 25(OH)D with several subtypes of ischemic stroke: large artery infarction (n=142), lacunar infarction (n=173), or other types of ischemic infarction (n=149). The ORs (95% CI) comparing extreme tertiles of 25(OH)D levels were 1.78 (0.77, 4.10; Ptrend = 0.20) for large artery infarction, 2.47 (1.15, 5.32; Ptrend=0.02) for lacunar infarction, and 1.21 (0.59, 2.51; Ptrend=0.59) for all other types of ischemic stroke. Lastly, we used cutpoints that were used to define vitamin D deficiency/adequacy18 to categorize the study participants and repeated the analysis. Although similar trend was observed, the association was somewhat attenuated: in comparison to women whose 25(OH)D levels ≥75 nmol/L, the ORs (95% CI) were 0.96 (0.65, 1.43) for levels in 50–74 nmol/L, 1.11 (0.72, 1.70) for levels in 30–49 nmol/L, and 1.39 (0.80, 2.42) for levels <30 nmol/L.
Meta-analysis
The characteristics of the 6 other prospective cohort studies that evaluated 25(OH)D levels in relation to various stroke outcomes are shown in Supplementary Table 2. Most studies included both men and women, except Bolland et al’s study11 that included women only. Serum 25(OH)D levels were measured in all studies, whereas study outcomes varied among these studies: two studies examined fatal strokes only and four other studies evaluated total strokes. Only Kilkkinen et al’s study8 examined ischemic stroke and hemorrhagic stroke separately. Cox proportional hazard regression was used to model the association of interest in all but Pilz et al’s study,7 in which logistic regression was used to model the association. The number of stroke cases ranged from 42 to 293 in these studies. In total, including our study, there were 1,214 stroke cases included in the current meta-analysis.
When all data were pooled together, the combined RR (95% CI) of stroke comparing low vs. high vitamin D status was 1.52 (1.20, 1.85) (Figure 2). We did not observe evidence for heterogeneity of the results; the I2 statistics was 0.0% and the Pheterogeneity was 0.63. When we pooled the results in the NHS cohort with Kilkkinen et al’s results specifically for ischemic stroke, the pooled RR (95% CI) was 1.59 (1.07, 2.12). Similarly, no evidence of heterogeneity was found between the results of these two studies. Of note, in this analysis for ischemic stroke we used RR comparing ≤ 31 nmol/L vs. >47 nmol/L in the NHS study to be consistent with the cutpoints used in the Kilkkinen et al’s study.8 The Begg funnel plot and Egger test did not suggest evidence of publication bias (Supplementary Figure 1).
Figure 2.
Pooled fixed-effects relative risk (95% CI) of stroke comparing high 25(OH)D levels with low 25(OH)D levels. A, total stroke; B, ischemic stroke. Bars indicate 95% CIs and P values are P for heterogeneity.
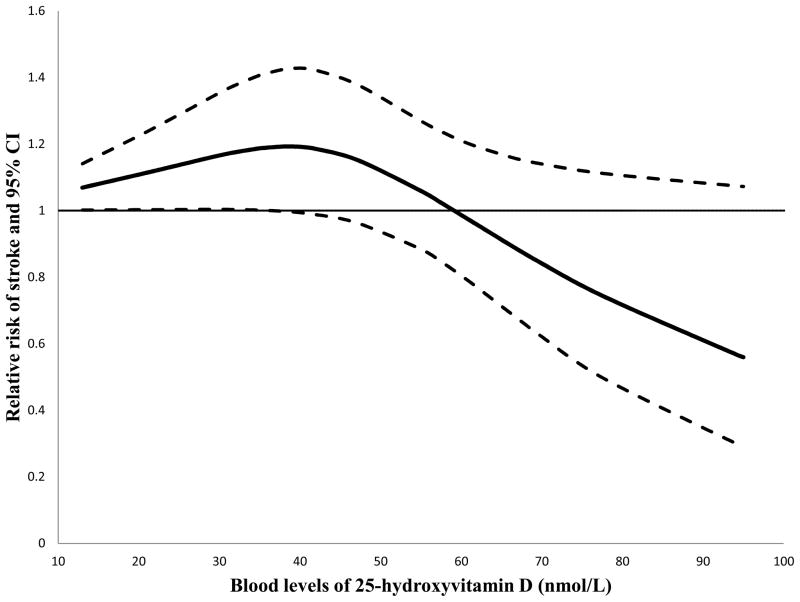
In the cubic spline regression model, we detected a potentially non-linear dose-response relationship; the P for non-linearity was 0.02. This dose-response relationship is shown in Figure 3. There was a clear threshold effect in that the risk of stroke did not start decreasing until the 25(OH)D levels exceeded certain levels around 40 nmol/L.
Figure 3.
Dose-response relationship between blood levels of 25(OH)D and risk of stroke. The solid line represents point estimates of the association between 25(OH)D levels and stroke risk, and the dotted lines are 95% CIs. The horizontal line is the reference line.
We further conducted several sensitivity analyses to examine the robustness of our observations. First, when we used a random-effects model, we observed essentially the same results. Second, when we excluded Drechsler et al and Pilz et al’s studies that included dialysis patients or patients referred to coronary angiography, respectively, the results did not change substantially: the pooled RR (95% CI) was 1.51 (1.18, 1.83). Lastly, when we examined the dose-response relationship among prospective cohort studies that used Cox regression to model the association,8–12 a marginally significant P value for non-linearity of 0.06 was documented, probably because of low power for this stratified analysis.
DISCUSSION
In this prospective study among women without prior history of stroke, with multivariable adjustment for lifestyle and dietary factors, we documented a modest association between low plasma 25(OH)D levels and an increased risk of incident ischemic stroke. Associations appeared most robust for lacunar infarction. This observation was confirmed by a meta-analysis pooling our data with six other prospective studies that overall demonstrated the same inverse associations between blood 25(OH)D levels and various stroke outcomes. A potentially non-linear dose-response relationship between 25(OH)D levels and stroke risk was further suggested by the current investigation.
Independent of the effects of vitamin D on calcium metabolism, emerging evidence suggests several mechanisms through which vitamin D modulates cardiovascular health. For example, vitamin D receptor-null mice, have significantly increased expression of renin and plasma angiotensin, whereas injection of 1,25-dihydroxyvitamin D to wild type mice suppressed renin production, showing significant effects of vitamin D on this pathway.5 Independent of the tightly–controlled circulating 1,25-dihydroxyvitamin D levels, intracellularly-produced hormone may directly regulate the function and behaviors of vascular smooth muscles cells, endothelial cells, macrophages, and adipocytes.19–22 In addition, local production of 1,25-dihydroxyvitamin D appears to be regulated in part by levels of 25(OH)D,20 suggesting that the autocrine or paracrine effects of vitamin D may depend on the availability of 25(OH)D as well. Consistently, animal and human experiments provide data supporting the effects of vitamin D treatment/supplementation on increasing endothelium-dependent vascular relaxation,23, 24 inhibiting vascular smooth muscle cell growth,25 improving insulin resistance and beta-cell dysfunction,26, 27 inhibiting production of inflammatory cytokines,27–29 and regulating the reaction of monocytes to environmental stressors.30 These mechanisms may also likely underlie the stronger association observed among current users of hormone replacement therapy; the effects low 25(OH)D status may be further exaggerated by the adverse effects of oral hormone therapy on inflammation, thromboembolism, and coagulation.31
Despite the basic science evidence, only limited prospective data existed regarding the relationship between vitamin D status and risk of stroke. In addition, most of these studies did not document significant findings probably because most of these earlier studies employed small sample sizes resulting in limited statistical power for detecting a significant association. However, when we pooled our findings with all previous prospective data, we found a significant association suggesting that low vitamin D status may lead to an increased risk of stroke. Moreover, we did not observe significant heterogeneity of individual study associations; six of seven studies suggested an inverse association. With respect to the associations for subtypes of stroke, only Kilkkinen et al examined this association for ischemic stroke and hemorrhagic stroke separately.8 They found a significant inverse association between baseline 25(OH)D levels and risk of fatal ischemic stroke (n = 175), although the inverse association for fatal hemorrhagic stroke (n = 43) was not significant.8 When we categorized our participants according to cutpoints of 25(OH)D levels that corresponded to those in this Finnish study, we observed similar associations for combined fatal and nonfatal ischemic stroke. Thus far, the current study was the only investigation that further examined 25(OH)D levels in relation to subtypes of ischemic stroke, and we found a stronger association for lacunar infarction. The effects of vitamin D on hypertension and diabetes may explain this stronger association because these diseases were believed to be major risk factors for lacunar stroke,32 although a recent meta-analysis suggested that these two conditions were equally associated with lacunar and nonlacunar strokes.33 Apparently, more studies are warranted to derive a solid estimate on the effects of vitamin D on subtypes of stroke.
Our investigation is subject to a few limitations. First, our study population consisted of registered nurses with mostly European ancestry. It is unknown whether our current findings can be generalized to other races that, in general, have lower 25(OH)D levels than Caucasians.13 This restricted generalizability pertains to the meta-analysis results as well because the vast majority of participants in the contributing studies were also Caucasian. In addition, 25(OH)D deficiency (<50 nmol/L) was relatively common (>40%) and dietary calcium was high (>1000 mg/day) in our study population. These characteristics further restrict the generalizability of the results. Second, we only measured baseline 25(OH)D levels, and a single measure may not represent long-term levels. However, in a validation study among 71 NHS participants who provided three blood samples within 2–3 years, 25(OH)D levels at each time point were highly correlated, with an intraclass correlation (ICC) of 0.72.34 In addition, in the current study among 102 controls who provided a second blood sample approximately 10 years after baseline blood draw, an ICC of 0.48 was observed. These data suggest that a single measure of blood 25(OH)D is a reasonable proxy for vitamin D exposure, with correlations over time that are similar to those for cholesterol and blood pressure.35 Further, measurement error in the 25(OH)D assay, although relatively modest (CV=13.9%), may introduce additional random variation that likely attenuates the true association. Third, although the ability to adjust carefully for a wide array of lifestyle and dietary covariates in this study is a strength, residual confounding, in particular due to adiposity, cannot be fully excluded in this observational study. Uncontrolled and residual confounding in studies included in the meta-analysis cannot be excluded, either. The results can be biased by confounding toward either direction. Fourth, although we did not find statistical heterogeneity of associations in our meta-analysis, these prospective studies are heterogeneous in several aspects such as various stroke outcomes (stroke mortality and total, ischemic, and hemorrhagic strokes), diverse methods for identifying and confirming stroke outcomes, different assays for 25(OH)D measurements, varying baseline health status of the study populations (general population vs. patients with specific diseases or conditions), various covariates adjusted in each individual study, and dramatically different levels of 25(OH)D and comparison categories across studies. Large individual-level data are still needed to minimize this between-study heterogeneity and to model any does-response relationship precisely. Likewise, detection of interactions between vitamin D and other stroke risk factors also requires such data. Fifth, since vitamin D deficiency is quite common among prevalent cerebrovascular patients and a prior history of stroke is a strong risk factor of recurrent stroke,1 previous investigations on total stroke or stroke mortality7, 10–12 that did not exclude baseline stroke patients may be subject to confounding bias, which can lead to erroneously strong associations. Lastly, some key factors involved in the biology of 25(OH)D, such as 1,25-dihydroxvvitamin D, parathyroid hormone, phosphate, and fibroblast growth factor 23, were not measured in the current analysis. Therefore, we were unable to examine the role of these molecules in the association between 25(OH)D and risk of ischemic stroke. In terms of strengths, the current study used incident ischemic stroke confirmed by medical records, and such homogeneity of outcome may help detect biologically meaningful associations. Other strengths of the current study include large sample size, long follow-up period, and rich data allowing comprehensive analysis.
CONCLUSIONS
In conclusion, we found a modest association between low 25(OH)D levels and risk of incident ischemic stroke among U.S. women without a prior history of stroke, and this observation was consistent with pooled results based on all prior prospective studies. Further studies are warranted to explore this association among other races and for stroke subtypes. In particular, large-scale clinical trials are needed to shed light on the effects of achieving optimal vitamin D status in the primary prevention of stroke. Given that U.S. populations have a high prevalence of both vitamin D insufficiency and stroke, solid evidence is especially needed to guide public health recommendations to lower the burden of both conditions.
Supplementary Material
Acknowledgments
We are indebted to Drs. Stefan Pilz, Heidi T. May, Jeffrey L. Anderson, Jukka Marniemi, Mark J. Bolland, and Christiane Drechsler for their contributions to the meta-analysis. Dr. Sun has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding sources: This study was supported by research grants CA87969, CA4449, HL34594, and HL088521 from the National Institutes of Health. Dr. Sun was supported by a career development award K99HL098459 from the National Heart, Lung, and Blood Institute. Dr. Hu is a recipient of an American Heart Association Established Investigator Award.
Footnotes
Financial disclosure: Dr. JoAnn Manson received funding from the National Institutes of Health (National Cancer Institute, National Heart, Lung and Blood Institute, and other NIH institutes and agencies) to conduct the VITamin D and OmegA-3 Trial (VITAL) – a large-scale randomized trial of vitamin D and omega-3 fatty acids in the prevention of cancer and cardiovascular disease. Other authors have no conflicts of interest to disclose.
References
- 1.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 2.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, et al. Systematic review: Vitamin d and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin d and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxyvitamin d concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33:2021–2023. doi: 10.2337/dc10-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-dihydroxyvitamin d(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassuk SS, Manson JE. Does vitamin d protect against cardiovascular disease? J Cardiovasc Transl Res. 2009;2:245–250. doi: 10.1007/s12265-009-9111-z. [DOI] [PubMed] [Google Scholar]
- 7.Pilz S, Dobnig H, Fischer JE, Wellnitz B, Seelhorst U, Boehm BO, et al. Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke. 2008;39:2611–2613. doi: 10.1161/STROKEAHA.107.513655. [DOI] [PubMed] [Google Scholar]
- 8.Kilkkinen A, Knekt P, Aro A, Rissanen H, Marniemi J, Heliovaara M, et al. Vitamin d status and the risk of cardiovascular disease death. Am J Epidemiol. 2009;170:1032–1039. doi: 10.1093/aje/kwp227. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of vitamin d deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. American Journal of Cardiology. 2010;106:963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, et al. Vitamin d deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31:2253–2261. doi: 10.1093/eurheartj/ehq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolland MJ, Bacon CJ, Horne AM, Mason BH, Ames RW, Wang TK, et al. Vitamin d insufficiency and health outcomes over 5 y in older women. Am J Clin Nutr. 2010;91:82–89. doi: 10.3945/ajcn.2009.28424. [DOI] [PubMed] [Google Scholar]
- 12.Marniemi J, Alanen E, Impivaara O, Seppanen R, Hakala P, Rajala T, et al. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005;15:188–197. doi: 10.1016/j.numecd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Prentice RL, Breslow NE. Retrospective studies and failure time models. Biometrika. 1978;65:153–158. [Google Scholar]
- 14.Walker AE, Robins M, Weinfeld FD. The national survey of stroke. Clinical findings. Stroke. 1981;12:I13–44. [PubMed] [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holick MF. Vitamin d deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 18.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal. 2006;6:40–57. [Google Scholar]
- 19.Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, et al. 25-hydroxyvitamin d3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 20.Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, et al. Synthesis of 1,25-dihydroxyvitamin d(3) by human endothelial cells is regulated by inflammatory cytokines: A novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 21.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Byrne ME, Chang E, Jiang Y, Donkin SS, Buhman KK, et al. 1alpha,25-dihydroxyvitamin d hydroxylase in adipocytes. J Steroid Biochem Mol Biol. 2008;112:122–126. doi: 10.1016/j.jsbmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borges AC, Feres T, Vianna LM, Paiva TB. Effect of cholecalciferol treatment on the relaxant responses of spontaneously hypertensive rat arteries to acetylcholine. Hypertension. 1999;34:897–901. doi: 10.1161/01.hyp.34.4.897. [DOI] [PubMed] [Google Scholar]
- 24.Borges AC, Feres T, Vianna LM, Paiva TB. Recovery of impaired k+ channels in mesenteric arteries from spontaneously hypertensive rats by prolonged treatment with cholecalciferol. Br J Pharmacol. 1999;127:772–778. doi: 10.1038/sj.bjp.0702581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-dihydroxyvitamin d3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13:954–959. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 26.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis d is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 27.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin d supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 28.Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin d supplementation. J Neuroimmunol. 2003;134:128–132. doi: 10.1016/s0165-5728(02)00396-x. [DOI] [PubMed] [Google Scholar]
- 29.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin d supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 30.Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, et al. Vitamin d3 down-regulates monocyte tlr expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 31.Modena MG, Sismondi P, Mueck AO, Kuttenn F, Lignieres B, Verhaeghe J, et al. New evidence regarding hormone replacement therapies is urgently required transdermal postmenopausal hormone therapy differs from oral hormone therapy in risks and benefits. Maturitas. 2005;52:1–10. doi: 10.1016/j.maturitas.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Arboix A, Marti-Vilalta JL. Lacunar stroke. Expert Rev Neurother. 2009;9:179–196. doi: 10.1586/14737175.9.2.179. [DOI] [PubMed] [Google Scholar]
- 33.Jackson CA, Hutchison A, Dennis MS, Wardlaw JM, Lindgren A, Norrving B, et al. Differing risk factor profiles of ischemic stroke subtypes: Evidence for a distinct lacunar arteriopathy? Stroke. 2010;41:624–629. doi: 10.1161/STROKEAHA.109.558809. [DOI] [PubMed] [Google Scholar]
- 34.Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, et al. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the nurses’ health studies. Cancer Epidemiol Biomarkers Prev. 2010;19:938–946. doi: 10.1158/1055-9965.EPI-09-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willett WC. Nutritional epidemiology. New York, NY: Oxford University Press; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.