Abstract
Despite a substantially lower rate of live donor kidney transplantation among Black Americans compared to White Americans, there are few systematic efforts to reduce this racial disparity. This paper describes the rationale and design of a randomized controlled trial aims evaluating the comparative effectiveness of three different educational interventions for increasing live donor kidney transplantation in Black Americans. This trial is a single-site, urn-randomized controlled trial with a planned enrollment of 180 Black Americans awaiting kidney transplantation. Patients are randomized to receive transplant education in one of three education conditions: through group education at their homes (e.g., House Calls), or through group (Group-Based) or individual education (Individual Counseling) in the transplant.. The primary outcome of the trial is the occurrence of a live donor kidney transplant, with secondary outcomes including living donor inquiries and evaluations as well as changes in patient live donor kidney transplantation readiness, willingness, knowledge, and concerns. Sex, gender, dialysis status, and quality of life are evaluated as moderating factors. Findings from this clinical trial have the potential to inform strategies for reducing racial disparities in live donor kidney transplantation. Similar trials have been developed recently to broaden the evaluation of House Calls as an innovative disparity-reducing intervention in kidney transplantation.
Trial Registration: Clinicaltrials.gov NCT00785265
Keywords: randomized trial, kidney transplantation, living donation, transplant education, disparities
1. Introduction / Background
While kidney transplantation is the best treatment option for improved quality of life and longer-term survival for adults in late-stage chronic kidney disease (CKD) [1–3], profound racial disparities persist [4–7]. Black Americans, for instance, experience more barriers in access to kidney transplantation, are more likely to have initiated dialysis at time of referral for transplant evaluation, are less likely to be added to the waiting list for transplantation, wait longer for a deceased donor transplantation after being added to the waiting list, have higher rates of death on the transplant waiting list, and are substantially less likely to receive a live donor kidney transplantation (LDKT) compared to Whites [8–16].
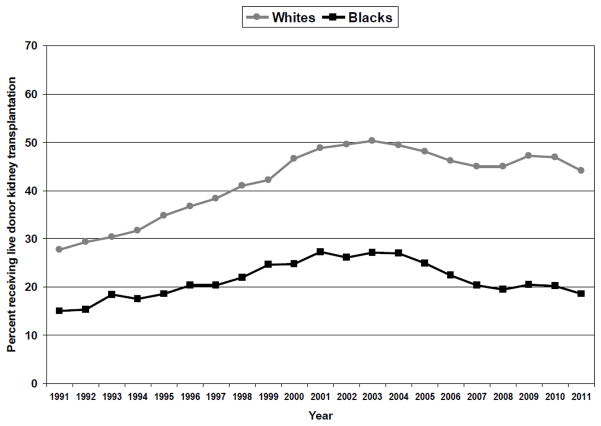
Relative to deceased donor kidney transplantation, LDKT has superior graft and patient survival rates, lower acute rejection rates, avoids or reduces dialysis exposure, pre-empts rapidly deteriorating quality of life, and is more cost effective in the long-term. There were 5,769 LDKTs in the United States in 2011, which represents a 140% increase over the past two decades [17]. However, over the last two decades the percentage of LDKTs has increased by 16% for Whites and by only 3% for Black Americans (Figure 1). In 2011, only 18.5% of all kidney transplants for Black Americans were LDKTs, which is considerably lower than the 44.1% observed for Whites. While educational efforts have helped to expand awareness about the benefits of LDKT among Black Americans [18], there have been very few attempts to systematically examine strategies for increasing LDKT rates in this minority population.
Figure 1.
Percentage receiving live donor kidney transplantation, relative to total number of kidney transplants within race category, 1991–2011
There are several potential barriers to LDKT for Black Americans. Relative to Whites, Black Americans who come forward as potential living donors are more likely to have medical conditions (e.g., diabetes, hypertension, obesity) that disqualify them as donors [19–22], more likely to be disqualified due to blood type incompatibility [22], and more likely to be lost to follow-up once the donor evaluation is initiated [22–24]. Additionally, Black Americans may cope with the need for transplantation and the possibility of LDKT differently than Whites [14,23–26]. Some are more likely to deny the need for kidney transplantation, have religious objections to transplantation, question the survival and quality of life benefits of transplantation, and mistrust the healthcare system because of their experience of discrimination in dialysis units [14,25,27,28]. Furthermore, healthy family members and friends of Black Americans may lack awareness of the benefits of LDKT or risks of kidney donation, may have inaccurate assumptions about their suitability for living donation, and may have high mistrust of the healthcare system [22,24,29–31]. Importantly, Ayanian et al. [32] found that when Black Americans are well informed of their transplant options and the benefits of transplantation, they prefer transplantation at the same rate as Whites. However, only one-third of patients and fewer than half of their family members (spouses, adult children) have discussed LDKT with physicians [33].
We previously described the utility of a socioecological framework that considers the influences of personal values, family systems, extended social networks, the health care system at large, and community or culture on LDKT decision making and behavior (Figure. 1) [34]. Although 30% of living kidney donors are extended family members who do not reside with the recipient and 25% come from the recipient’s extended social network (e.g., friends, co-workers), few systematic efforts exist within transplant programs to include extended family and social network members in LDKT educational efforts. Our socioecological model and the changing landscape of the donor-recipient relationship highlight the need to develop LDKT outreach efforts that incorporate the recipient’s larger family and social network in the educational process, especially for minority patients.
2. Study Design / Methods
2.1. Overview
This study is a single-site, urn-randomized controlled trial with a planned enrollment of 180 Black Americans awaiting kidney transplantation. Patients are randomized to receive transplant education in one of three education conditions: through group education at their homes (e.g., House Calls), or through group (Group-Based) or individual (Individual Counseling) education in the transplant center. The educational content delivered across interventions is standardized, as described below.
The specific aims of the study are to: (1) evaluate the effects of the three interventions on LDKT rates at 2 years post-intervention; (2) determine the effects of the three interventions on LDKT readiness, willingness, knowledge, and concerns at 1 and 6 weeks post-intervention; and (3) examine whether intervention effects are moderated by patient sociodemographic and medical characteristics. The central hypothesis is that the House Calls intervention will yield a higher proportion of patients with LDKT at 2 years post-intervention compared to the Group-Based or Individual Counseling interventions. Also, we are evaluating the secondary hypotheses that patients who receive the House Calls intervention will have more living donor inquiries, have more donors evaluated, and will have more substantial improvements in LDKT readiness and willingness and fewer LDKT concerns than patients in the other two conditions.
The urn randomization strategy [35] is used to maximize the likelihood that the three groups are balanced on patient gender, dialysis status, prior transplant status, and time on the waiting list. This randomization strategy is less vulnerable to selection bias than permuted-block or biased-coin randomization procedures. Using this strategy, if an enrolled patient is assigned to one condition, envelopes representing the other two groups are added to the randomization set. The composition of the randomization envelope set is such that the probability of selection is larger for the intervention condition(s) that has been selected less often at that point in the trial. As sample size increases in the study, the likelihood of selection bias approximates that of complete randomization (i.e., zero) [35,36].
2. 2. Participants
We plan to recruit 180 adults who meet study eligibility criteria. Study participants are: (1) self-identified as Black American, (2) have chronic kidney disease or end-stage renal disease, (3) meet medical eligibility criteria for activation on the kidney transplant waiting list, (4) at least 21 years old, and (5) reside within 2.5 hr driving time from the transplant center. Patients are excluded if they are awaiting combined kidney-liver transplantation, do not speak/comprehend English, or have known or suspected cognitive impairment that would interfere with participation in the intervention or completion of study procedures.
2.3. Recruitment and informed consent
To facilitate recruitment, the transplant nephrologist co-investigators introduce the study via written letter to all patients who appear to meet study eligibility criteria and who have a transplant clinic appointment scheduled within the following month. The nature and purpose of the study, study procedures, and time commitment required are described to patients. A one-page Q & A summary sheet, listing commonly asked questions and answers about the study, accompany the letter. At their next clinic visit, if the patient is interested in taking part in the study, s/he meets with the Principal Investigator or Project Manager to confirm eligibility and, if eligible, to obtain written informed consent.
2.4. Study Timeline
The first patient was enrolled into the study in August 2007 and the final 2-yr study endpoint will be collected for all patients by June 2013.
2.5. Interventions
This study is designed to examine whether an outreach educational intervention will help to increase rates of LDKT. Currently, the standard LDKT educational approach in transplant centers includes provider-patient discussions about the benefits of LDKT and the provision of written brochures and pamphlets, which are done individually or in a group setting in the transplant center. However, there are some important limitations to this educational approach. First, only the patient and those individuals who accompany the patient to clinic are directly informed about LDKT by transplant healthcare providers. Second, if sufficient time has not been set aside to discuss LDKT with patients in the clinic, there may not be sufficient time to assess the patient’s primary concerns about pursuing LDKT (e.g., imposing on others, uncertainty about how to discuss live donation with others, misinformation about donor eligibility criteria, donor outcomes, etc.) or to address these potential barriers directly. Third, it a clinic-based educational approach relies on patients to disseminate living donation information to others, which requires that patients have a high level of health literacy and knowledge about LDKT and living donation. Fourth, clinic appointments are typically scheduled during daytime, week-day hours, thus potentially excluding family members and others who are employed from the educational process. Finally, clinic-based LDKT education may not be delivered in a culturally sensitive manner. Boulware et al. [33] argues that culturally-sensitive family-physician meetings to discuss LDKT are critically important for increasing awareness of LDKT, enhancing patients’ access to LDKT prior to dialysis, identifying medically eligible live donors, and improving post-transplant outcomes.
Delivering personalized education in the family’s home and including others in this informative process may be an effective strategy for overcoming the relative limitations of clinic-based education. The House Calls concept, in which healthcare providers travel to the patient’s home, is not new or innovative. House Calls were the primary mode of healthcare delivery by physicians through the early part of the 20th century [37]. However, its utilization declined precipitously as transportation systems changed, new diagnostic and therapeutic technologies emerged, physicians became more specialized, liability concerns arose, and payments systems changed to reward efficient office encounters [37–39] When physician House Calls are made today, they tend to be within the context of geriatric or palliative care [40]. Due, in part, to the increasing demands of geriatric care in the United States, favorable changes in Medicare reimbursement rates, and improved curricula in medical education, House Calls have increased in both absolute number and in proportion over the past two decades [37,40].
Making House Calls to discuss LDKT has several intuitive advantages over clinic-based models of care. These advantages include the inclusion of family members and friends who otherwise could not attend a transplant clinic appointment, direct contact with potential living donors in the patient’s social network, enhanced patient satisfaction with the delivery of transplant-related care, convenience of patients with CKD who are increasingly older and who have multiple co-morbidities, and the secondary benefits of educating others about chronic kidney disease, its prevention, and its treatment. For Black Americans in particular, House Calls may represent a way to attenuate mistrust of the healthcare system and involve the larger social network in transplant-related discussions. Indeed, in a preliminary investigation [30], we found that a House Calls educational approach yielded statistically more living donor inquiries, evaluations, and LDKTs compared to a clinic-based education approach. Of particular importance was the house call’s effectiveness at reaching Black American patients and their extended support system [41]. In the three years prior to study initiation, only 13% of Black Americans received LDKTs, which is consistent with the 14% rate we observed in the clinic-based group. In contrast, 45% of Black American patients who received the house calls intervention underwent LDKT, which represents a nearly four-fold increase from the three years prior to study implementation.
2. 5.1. Common elements of the interventions
As part of their evaluation for kidney transplantation, all patients referred to our program review the LDKT option with a nurse coordinator, social worker, nephrologist, and surgeon. In addition, they receive a hardcover Transplant Education binder, which contains printed information about the benefits of LDKT, the process for living donor evaluation, and information about kidney paired donation. They are strongly encouraged to consider LDKT and to talk to family members and friends about living donation. Patients are given the name and contact information of our living donor nurse coordinator and they are advised to distribute her business cards to individuals within their social network. Additionally, they are referred to our transplant center website for a more in-depth review of LDKT and a podcast on how to talk to others about living kidney donation.
The three conditions to which patients are randomized in this study represent active interventions designed to provide more in-depth discussions about LDKT, beyond what patients receive as part of standard care practice in our center. The three conditions have some common elements: the patient attends only one LDKT educational session, the session is led by one or two health educators trained in living donation and transplantation, and the duration of the educational session is 60 to 90 minutes. Additionally, the core educational content delivered by the health educator(s) orally is identical (Table 1) and the same information packets are distributed to all study patients and, for those receiving the House Calls or Group-Based intervention, their invited guests. The information packet contains several brochures that provide detailed information about the living donation process, common concerns and misperceptions, and donation resources; the Living Kidney Donation: What You Need to Know DVD produced and distributed by the American Society of Transplant Surgeons; The Living Gift booklet published by the Missouri Kidney Program; informational brochures from the Explore Transplant clinical trial (NCT01048437); pamphlets emphasizing the number of Black Americans in need of transplantation (e.g., Linkages to Life: Organ Tissue and Bone Marrow Donation Awareness Program of The Links, Inc.); and information about our transplant center (e.g., copy of our quarterly newsletter, contact information). In all instances, we attempt to deliver the intervention within six weeks of the patient completing the baseline assessment.
Table 1.
Core transplant educational content discussed during all interventions
| Different types of organ donation | Living kidney donation – eligibility criteria, evaluation components, surgical and medical outcomes, psychological outcomes, medical and non-medical risks |
| Current deceased organ donation rates | |
| Living kidney donation rates | |
| Differences in donation rates by race/ethnicity | |
| Transplant evaluation process | Paired exchange programs (kidney paired donation, list exchange) |
| Different types of transplantation | |
| National waiting list (total patients, by race) | Possible indirect costs |
| Regional waiting list (total patients, by race) | National Living Donor Assistance Center |
| Median waiting times on regional list | Common transplant recipient and living donor concerns |
| Geographic disparities in waiting times | |
| Benefits of transplantation before dialysis | Unique considerations for Black American living donors |
| Benefits of live donor kidney transplantation | |
| National and regional transplant outcomes | Helpful resources for transplant patients |
| Helpful resources for living donors |
2. 5.2. House Calls intervention
Patients assigned to the House Calls condition receive an LDKT educational session in their home, involving any family members and friends s/he chooses to invite to the session. There are instances when the session is held in another setting (e.g., another residence, local library or church meeting room) to accommodate a particularly large number of invited guests. To generate the list of invited guests, the patient is encouraged to identify immediate and extended family members, close friends, co-workers, and important others, such as church leaders and fellow parishioners who might be willing to attend the educational session. We encourage patients to not limit their guest list to those they feel might consider or be eligible for living donation, since a goal is to increase knowledge and awareness among as many individuals as possible within the patient’s social network. Once the list is finalized, the House Calls session is scheduled and the patient is given invitations to distribute to those on the guest list. The invitation is a brochure that informs guests about the nature and purpose of the educational session, the patient’s participation in a research study, and the content to be discussed during the session. Additionally, it states that invited guests will not be asked to participate in or complete any study procedures. The patients and all guests who attend the session are paid $10 for their time and to offset any travel expenses to the patient’s home.
2. 5.3. Group-Based intervention
Patients assigned to the Group-Based condition attend an LDKT educational session involving 3 to 5 study patients and their invited guests. The session is held in a conference room in the transplant center. The session is scheduled at a time that is convenient for patients, most often during evening or weekend hours. Similar to the House Calls intervention, guest lists are generated with assistance from a research team member and the invitational brochures are subsequently distributed to family members and friends by the patients themselves. The patient and all guests are paid $10 for their attendance and to offset any travel and parking expenses to attend the session at the transplant center.
2.5.4. Individual Counseling intervention
Patients assigned to the Individual Counseling condition receive a one-on-one session with a transplant health educator. Family members and friends are not included in this session. The session is held in a private office within the transplant center, and is typically scheduled on the same day the patient has other clinic appointments.
Table 2 highlights the hypothesized benefits and limitations of the three interventions.
Table 2.
Hypothesized benefits and limitations of the House Calls (HC), Group-Based (GB), and Individual Counseling (IC) educational interventions
| HC | GB | IC | |
|---|---|---|---|
| BENEFITS | |||
| Natural environment for new learning to occur | x | ||
| Time-efficient and cost-effective for patients and guests | x | ||
| Amplifies the transplant program’s commitment to the patient | x | ||
| Attenuates feelings of mistrust | x | ||
| Convenience of educational setting enhances guest attendance | x | ||
| Information is disseminated simultaneously to the patient and members of their support system | x | x | |
| Does not rely solely on the patient to inform others about living donation | x | x | |
| Inclusion of other patients and invited guests enhances the likelihood of additional LDKT questions and concerns being raised and discussed | x | ||
| Greater sense of community in knowing there are other patients and family members in similar circumstances | x | ||
| Patients feel more at ease and comfortable openly discussing their primary concerns and worries about pursuing LDKT | x | ||
| Health educator can more effectively identify misperceptions or inaccuracies in the patient’s understanding of LDKT or living donation | x | ||
| Time-efficient when combined with other scheduled appointments | x | ||
| LIMITATIONS | |||
| Patients feel uneasy about having health educators in their home | x | ||
| Patients feel compelled in a group setting to share more details about their personal health information than they would otherwise | x | ||
| Patients with a small support network may be concerned that this will adversely impact their standing on the transplant waiting list | x | x | |
| Patients and/or guests may not ask questions or share their concerns about LDKT or living donation in the presence of others | x | x | |
| Inconvenience of educational setting limits guest attendance | x | ||
| Relies entirely on the patient to educate others about LDKT and living donation without assistance from transplant educators | x | ||
| Patients do not hear directly about the issues that are important to potential donors in their social network | x | ||
2. 5.5. Health educators and cultural sensitivity
Health educators for this trial are selected on the basis of their educational background, strong interpersonal skills, and desire to work in the field of healthcare disparities. The ethnic and racial background of the health educators is mixed and includes those who are Black, White, and Asian. Our prior research showed that health educator race was not associated with primary outcomes [30], so in our selection of health educators we emphasized the ability to understand, communicate with, and effectively interact with people across cultures.
Several strategies are used to promote cultural awareness and sensitivity. For study staff, a goal is to enhance understanding and to gain a more informed view of Black American culture. For study patients, a goal is to inform them of racial disparities in ESRD care and kidney transplantation and to provide them with strategies for attenuating these disparities on an individual level. First, study staff review published articles on racial inequities in healthcare and on the incidence of CKD and ESRD in Black Americans, racial disparities in access to renal care and kidney transplantation, and transplant-related outcomes. Second, study staff members are educated about the historical maltreatment of Black Americans in medical research [42] and the protection of human research participants. Third, various aspects of the study interventions use culturally congruent venues (e.g., patient’s home) and include the patient’s larger support system, both strategies shown to be critical to Black Americans’ healthcare behaviors. Fourth, we use educational materials (print, video) that profile Black American patients and living donors, and all educational sessions include a discussion of racial disparities in transplantation.
2. 6. Assessment Protocol and Timing
2.6.1. Live donor kidney transplantation (LDKT)
The primary outcome of the trial is the occurrence (yes, no) of LDKT within two years of receiving a study intervention.
2.6.2. Living donor inquiry and evaluation
As secondary outcomes, we are tracking whether an enrolled patient has any living donor inquiries (yes, no) or evaluations (yes, no), as well as the absolute number of donor inquiries and evaluations at the two-year endpoint. A donor inquiry is operationalized as a request by a family member, friend, or acquaintance for more information about living donation. This request for information may be directed toward the living donor nurse coordinator, transplant nephrologist, or social workers during an office visit or by telephone, email or letter. A donor evaluation is defined as initiation and completion of preliminary blood compatibility testing and/or telephone health screening conducted by the living donor nurse coordinator.
Several questionnaires are administered at Baseline (prior to randomization and intervention) and then again at 1- and 6-weeks following the intervention. Participants are paid $20 for the completion of each assessment.
2.6.3. LDKT readiness stage
Using the stages of change assessment model [43], we ask patients to identify whether they are in Pre-contemplation (“I am not thinking about or considering LDKT.”), Contemplation (“I am now beginning to think about or consider LDKT.”), Preparation (“I have thought about LDKT and I am seriously considering this possibility.”), Action (“I have thought about LDKT, and I have talked to someone who is willing to be evaluated as a possible living donor.”), or Maintenance (“I have thought about LDKT and I have someone who has been evaluated and approved to be a living donor.”) in thinking about LDKT.
2.6.4. LDKT willingness
Patients complete a 1-item questionnaire (1 = “not at all willing” to 7 = “extremely willing”) asking them to rate their willingness to talk to family members and/or friends about living kidney donation.
2.6.5. Living donation knowledge
A 16-item true-false questionnaire assesses what patients know about LDKT and living kidney donation (e.g., “Only a blood relative is able to be a living kidney donor.” “A living kidney donor must have his/her own health insurance to cover the costs of surgery.”). Scores range from 0 to 15, with higher scores reflective of more LDKT knowledge.
2. 6.6. Concerns about LDKT
A 21-item questionnaire measures patients’ concerns about pursuing LDKT (e.g., “I am concerned that the donor would no longer be able to do activities that they enjoy.” “I am worried that I might do something to ‘waste’ the kidney that someone donates to me – for example, by not living healthy or not taking my medications.”). Responses to each question are given on a Likert-type scale (1 = not at all concerned to 5 = extremely concerned), with higher scores indicating more concern.
In addition to the foregoing, we closely track participation rates, attrition rates, reasons for attrition, no-shows and cancellations, barriers to attending intervention sessions, and intervention protocol adherence. Covariates are measured in multiple domains, including sociodemographics (age, sex, education, marital status), patient medical status (primary disease, glomerular filtration rate, creatinine clearance, number of co-morbid medical conditions, dialysis status, dialysis type and duration), time on waiting list, and quality of life (QOL). The SF-36v2 [44] is used to assess general health-related quality of life and it is administered only at the time of the Baseline assessment. Also, we recognize that patients may decide to pursue LDKT based on information acquired outside of the study interventions. Therefore, we ask patients at each assessment point to indicate all sources of LDKT information they have encountered to date, using a checklist created for this study. This allows us to evaluate the degree to which other sources of information may have contributed to decision-making processes regarding pursuing LDKT. Finally, we gather the number of guests per patient who attend the House Calls and Group-Based intervention sessions, as well as the overall satisfaction ratings for the LDKT interventions from patients and, as appropriate, their guests.
2. 7. Data management and statistical analyses
Standard medical record data collection forms are completed by a research assistant and entered into an SPSS database. Questionnaire data are collected by telephone by a trained research assistant and entered directly into the SPSS database. Monthly study recruitment and enrollment reports, scheduled and missed assessments, and interventions completed are generated by the Project Manager and reviewed with the Principal Investigator.
The primary outcome for the study will be analyzed using an intent-to-treat strategy in which each patient is included in their initial randomization condition regardless of adherence or attrition. Baseline analyses will include descriptions of the baseline sociodemographic and medical characteristics of the patients. The primary outcome will be summarized within each intervention group using point estimates of the proportion of participants who received LDKT, and the corresponding 95% confidence interval (CI). Two-sided 97.3% CIs will also be calculated for the difference between each of the interventions to assess their relative effectiveness. Chi-square test (2-sided significance level of 0.05) will also be performed. The secondary outcomes of the study will be analyzed in several ways. The proportion of patients with living donor inquiries and evaluations will be summarized within each intervention group using point estimates of the proportion of participants with live donor inquiries and live donor evaluations, and the corresponding 95% confidence intervals (CIs). Two-sided 97.3% CIs will also be calculated for the difference between each of the interventions to assess their relative effectiveness on these variables, with subsequent Chi-square tests (2-sided significance level of 0.05). Finally, multivariate analyses of variance will be used to examine changes in LDKT knowledge, concerns, and willingness, using data collected at Baseline and at the two follow-ups (1 week, 6 weeks). Each multivariate model will comprise two factors: the intervention factor (3 levels) and the longitudinal factor (3 time points), and their interaction. Analyses will be conducted on overall scores for each of these measures. In these models, interactions between intervention condition and baseline characteristics (sociodemographic, medical, quality of life) will be examined to determine whether intervention effects are consistent across different subgroups. Stratified analyses will be conducted on these characteristics if interaction effects are significant.
2. 8. Sample size justification
Considering the primary outcome of interest in the proposed study (i.e., LDKT at the two-year endpoint), we estimated that recruiting 60 patients per intervention group would permit adequate power to compare the proportion of patients with LDKTs at study conclusion. Based on our previous study [30] and current LDKT rates at BIDMC, we estimated a proportional difference of 0.18 between the House Calls and Group-Based interventions and a proportional difference of 0.26 between the House Calls and Individual Counseling interventions. Sample sizes of 102 (34 per group) achieves 80% power to detect proportional differences between the three intervention groups, using a chi-square test with continuity correction and at a significance level of 0.05. An enrollment of 180 patients (60 per group) achieves excellent power (>95%) to detect statistical significance. Based on historical trends, we have anticipated a 20% attrition rate before the two-year endpoint due to death, removal from the waiting list, and transfer of care to another transplant center.
3. Discussion
As with any clinical trial, we considered several issues in designing this study. First, we decided to enroll patients regardless of their baseline LDKT readiness stage. An argument can be made for enrolling only those patients who may be in Pre-contemplation or Contemplation, as they may have the most to gain from more LDKT education. However, our previous studies suggest that even those in Preparation or Action stages could benefit from LDKT education because patients often consider only one living donor possibility. Often, this potential donor comes forward spontaneously and approaches the patient directly. The patient may not have had any discussion with others or any active consideration of the potential risks and benefits of pursuing LDKT. Consequently, if this donor is not medically eligible or decides against donation, the patient does not have others in the queue and may not have the knowledge to initiate a new discussion with family members or friends. For this reason, we found it beneficial to include patients at all stages of LDKT readiness.
Second, we considered assessing the LDKT knowledge and concerns of guests attending the HC or GB intervention to examine the effectiveness of the intervention for these individuals. However, our decision not to do this was most influenced by our prior research experiences and ethical considerations. In our preliminary work [30], guests were invited to participate in the research by completing several questionnaires, although we did not limit participation in the House Calls session if someone chose not to be part of the research. This became a difficult situation to manage in the patient’s home because it required extensive amounts of time and it raised some ethical concerns regarding coercion and the preservation of confidentiality. In light of this experience and considering the primary objective of this trial, we decided not to introduce the assessment of guests into the current study design.
As we and others have described elsewhere [31,45], some have questioned whether a proactive LDKT educational approach that involves interventions in the home and includes other family member/friends could be considered a subtle (or overt) form of coercion. For instance, inviting family members and friends to the home or clinic for LDKT education may place some guests in a difficult position – either accept the invitation and then be confronted with the possibility of being asked by the patient (or other family members) at some later time to consider living donation, or decline the invitation and be perceived by the patient (or other family members) as uncaring or otherwise not interested in being a potential living donor. While we understand and recognize the issue of coercion, we argue that educating patients and families, especially underserved minorities, about LDKT as part of a community outreach program is ethically justifiable [14,31,33].
We do several things during the educational sessions to reduce coercion. First, we discuss the coercion issue openly with patients at the time of study enrollment and encourage them to respect and understand the many issues that others must consider in attending and making a decision about whether to pursue living donor evaluation. For family members and friends, our health educators emphasize that the intent of the education is to provide people with information so that they can both understand the patient’s transplant options and make informed decisions about how they can be most helpful. We specifically encourage guests to not make decisions about living donation at this session, and not to publicly identify others who they feel should step forward for donor evaluation. Finally, satisfaction data collected from participants in our previous study suggested that patients and their guests did not view the process as coercive, but rather as informative and facilitative [30]. Overall, we carefully evaluated the risks relative to the potential benefits and believe that the potential benefits for both patients and those who are actively involved in their healthcare outweigh the potential risks.
Many Black Americans with kidney failure are disadvantaged along the kidney transplant pathway, especially as it pertains to LDKT [5]. Research has also found that Black American patients and their family members desire more open communication about this transplant option [33]. While most transplant programs provide patients with LDKT information, research has shown how this falls short of addressing the specific concerns and issues that serve as barriers to LDKT for patients. Our preliminary work has demonstrated that delivering a LDKT educational program in the patient’s home (House Calls) is superior to clinic-based education in its ability to reach more potential living donors, reduce modifiable barriers to donation, increase patients’ willingness to pursue living donation, and, most importantly, increase actual LDKT rates. Findings from this larger trial have the potential to further inform the transplant community about LDKT educational strategies and their effectiveness in increasing LDKT rates in Black Americans.
Acknowledgments
The project described is supported by Award Number R01DK085185 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
We thank the following individuals for their contributions to the project: Timothy Antonellis, Tracy Brann, Noelle Dimitri, Shonda Ettienne, Elizabeth Gray-Chrzan, Ariel Hodara, Richard McCartney, Nancy Salonpuro, Stacey Senat, and Linda Walsh.
Footnotes
There are no conflicts of interest to report. Dr. Egbuna was a faculty member and transplant nephrologist at Beth Israel Deaconess Medical Center and Harvard Medical School during the development and initial implementation of the study. He is now employed as Clinical Research Medical Director for Amgen Inc., although Amgen has not been involved in any way with the study reported in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kovacs AZ, Molnar MZ, Szeifert L, Ambrus C, Molnar-Varga M, Szentkiralyi A, et al. Sleep disorders, depressive symptoms and health-related quality of life – a cross-sectional comparison between kidney transplant recipients and waitlisted patients on maintenance dialysis. Nephrol Dial Transplant. 2011;26:1058–65. doi: 10.1093/ndt/gfq476. [DOI] [PubMed] [Google Scholar]
- 2.McDonald SP, Russ GR. Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991–2001. Nephrol Dial Transplant. 2002;17:2212–9. doi: 10.1093/ndt/17.12.2212. [DOI] [PubMed] [Google Scholar]
- 3.Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11:917–22. doi: 10.1681/ASN.V115917. [DOI] [PubMed] [Google Scholar]
- 4.Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000;343:1545–52. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 5.Ladin K, Rodrigue JR, Hanto DW. Framing disparities along the continuum of care from chronic kidney disease to transplantation: barriers and interventions. Am J Transplant. 2009;9:669–74. doi: 10.1111/j.1600-6143.2009.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powe NR, Melamed ML. Racial disparities in the optimal delivery of chronic kidney disease care. Med Clin North Am. 2005;89:475–88. doi: 10.1016/j.mcna.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005;68:914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 8.Epstein AM, Ayanian JZ, Keogh JH, et al. Racial disparities in access to renal transplantation–clinically appropriate or due to underuse or overuse? N Engl J Med. 2000;343:1537–44. doi: 10.1056/NEJM200011233432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young CJ, Kew C. Health disparities in transplantation: focus on the complexity and challenge of renal transplantation in African Americans. Med Clin North Am. 2005;89:1003–31. doi: 10.1016/j.mcna.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Fan PY, Ashby VB, Fuller DS, Boulware LE, Kao A, Norman SP, et al. Access and outcomes among minority transplant patients, 1999–2008, with a focus on determinants of kidney graft survival. Am J Transplant. 2010;10:1090–107. doi: 10.1111/j.1600-6143.2009.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Axelrod DA, McCullough KP, Brewer ED, Becker BN, Segev DL, Rao PS. Kidney and pancreas transplantation in the United States, 1999–2008: the changing face of living donation. Am J Transplant. 2010;10:987–1002. doi: 10.1111/j.1600-6143.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 12.Burrows NR, Li Y, Williams DE. Racial and ethnic differences in trends of end-stage renal disease: United States, 1995–2005. Adv Chronic Kidney Dis. 2008;15:147–52. doi: 10.1053/j.ackd.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Ayanian JZ, Cleary PD, Keogh JH, Noonan SJ, David-Kasdan JA, Epstein AM. Physicians’ beliefs about racial differences in referral for renal transplantation. Am J Kidney Dis. 2004;43:350–7. doi: 10.1053/j.ajkd.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Navaneethan SD, Singh S. A systematic review of barriers in access to renal transplantation among African Americans in the United States. Clin Transplant. 2006;20:769–75. doi: 10.1111/j.1399-0012.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 15.Young CJ, Gaston RS. African Americans and renal transplantation: disproportionate need, limited access, and impaired outcomes. Am J Med Sci. 2002;323:94–9. doi: 10.1097/00000441-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med. 2002;137:479–86. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]
- 17.United States Department of Health and Human Services, Health Resources and Services Administration. [Website accessed, March 8, 2012.];Scientific Registry of Transplant Recipients. www.optn.transplant.hrsa.gov.
- 18.Lunsford SL, Shilling LM, Chavin KD, Martin MS, Miles LG, Norman ML, et al. Racial differences in the living kidney donation experience and implications for education. Prog Transplant. 2007;17:234–40. doi: 10.1177/152692480701700312. [DOI] [PubMed] [Google Scholar]
- 19.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17:143–52. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Differences in prevalence of obesity among black, white, and Hispanic adults - United States, 2006–2008. MMWR Morb Mortal Wkly Rep. 2009;58:740–4. [PubMed] [Google Scholar]
- 21.Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) Am J Hypertens. 2004;17:963–70. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Lunsford SL, Simpson KS, Chavin KD, Menching KJ, Miles LG, Shilling LM, et al. Racial disparities in living kidney donation: Is there a lack of willing donors or an excess of medically unsuitable candidates? Transplantation. 2006;82:876–81. doi: 10.1097/01.tp.0000232693.69773.42. [DOI] [PubMed] [Google Scholar]
- 23.Bratton C, Chavin K, Baliga P. Racial disparities in organ donation and why. Curr Opin Organ Transplant. 2011;16:243–9. doi: 10.1097/MOT.0b013e3283447b1c. [DOI] [PubMed] [Google Scholar]
- 24.Shilling LM, Norman ML, Chavin KD, Hildebrand LG, Lunsford SL, Martin MS, et al. Healthcare professionals’ perceptions of the barriers to living donor kidney transplantation among African Americans. J Natl Med Assoc. 2006;98:834–40. [PMC free article] [PubMed] [Google Scholar]
- 25.Lunsford SL, Simpson KS, Chavin KD, Hildebrand LG, Miles LG, Shilling LM, et al. Racial differences in coping with the need for kidney transplantation and willingness to ask for live organ donation. Am J Kidney Dis. 2006;47:324–31. doi: 10.1053/j.ajkd.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Klassen AC, Hall AG, Saksvig B, Curbow B, Klassen DK. Relationship between patients’ perceptions of disadvantage and discrimination and listing for kidney transplantation. Am J Public Health. 2002;92:811–7. doi: 10.2105/ajph.92.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon EJ. Patients’ decisions for treatment of end-stage renal disease and their implications for access to transplantation. Soc Sci Med. 2001;53:971–87. doi: 10.1016/s0277-9536(00)00397-x. [DOI] [PubMed] [Google Scholar]
- 28.Waterman AD, Stanley SL, Covelli T, Hazel E, Hong BA, Brennan DC. Living donation decision making: recipients’ concerns and educational needs. Prog Transplant. 2006;16:17–23. doi: 10.1177/152692480601600105. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigue JR, Cornell DL, Kaplan B, Howard RJ. Patients’ willingness to talk to others about living kidney donation. Prog Transplant. 2008;18:25–31. doi: 10.1177/152692480801800107. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigue JR, Cornell DL, Lin JK, Kaplan B, Howard RJ. Increasing live donor kidney transplantation: a randomized controlled trial of a home-based educational intervention. Am J Transplant. 2007;7:394–401. doi: 10.1111/j.1600-6143.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- 31.Waterman AD, Stanley S, Rodrigue JR. Ethically and effectively promoting live donor kidney transplantation. In: Alvaro EM, Siegel JT, editors. Understanding organ donation: applied behavioral science perspectives. Oxford, UK: Wiley-Blackwell; 2010. pp. 292–312. [Google Scholar]
- 32.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM. The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med. 1999;341:1661–9. doi: 10.1056/NEJM199911253412206. [DOI] [PubMed] [Google Scholar]
- 33.Boulware LE, Meoni LA, Fink NE, Parekh RS, Kao WH, Klag MJ, et al. Preferences, knowledge, communication and patient-physician discussion of living kidney transplantation in African American families. Am J Transplant. 2005;5:1503–12. doi: 10.1111/j.1600-6143.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- 34.Waterman AD, Rodrigue JR, Purnell TS, Ladin K, Boulware LE. Addressing racial and ethnic disparities in live donor kidney transplantation: priorities for research and intervention. Semin Nephrol. 2010;30:90–8. doi: 10.1016/j.semnephrol.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials. 1988;9:345–64. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]
- 36.Schouten HJ. Adaptive biased urn randomization in small strata when blinding is impossible. Biometrics. 1995;51:1529–35. [PubMed] [Google Scholar]
- 37.Kao H, Conant R, Soriano T, McCormick W. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19–34. doi: 10.1016/j.cger.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Starr P. The social transformation of American medicine. New York: Basic Books; 1982. [Google Scholar]; Keenan JM, Hepburn KW. The role of physicians in home health care. Clin Geriatr Med. 1991;7:665–75. [PubMed] [Google Scholar]
- 39.Landers SH, Gunn PW, Flocke SA, Graham AV, Kikano GE, Moore SM, et al. Trends in house calls to Medicare beneficiaries. JAMA. 2005;294:2435–6. doi: 10.1001/jama.294.19.2435-b. [DOI] [PubMed] [Google Scholar]
- 40.Committee on the Future Health Care Workforce for Older Americans, Institute of Medicine. Retooling for an aging America: building the health care workforce. Washington (DC): The National Academies Press; 2008. [PubMed] [Google Scholar]
- 41.Rodrigue JR, Cornell DL, Kaplan B, Howard RJ. A randomized trial of a home-based educational approach to increase live donor kidney transplantation: effects in blacks and whites. Am J Kidney Dis. 2008;51:663–70. doi: 10.1053/j.ajkd.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 42.Washington H. Medical apartheid: the dark history of medical experimentation on Black Americans from colonial times to the present. NY: Anchor Books; 2006. [Google Scholar]
- 43.Prochaska J, DiClemente C, Norcross J. In search of how people change: Applications to addictive behaviors. Am Psychol. 1992;47:1102–14. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- 44.Ware JE, Kosinki M, Dewey JE. How to score version 2 of the SF-36® health survey (standard & acute forms) Lincoln, RI: QualityMetric; 2000. [Google Scholar]
- 45.Massey EK, Hilhorst MT, Nette RW, Smak Gregoor PJH, van den Dorpel MA, van Kooij AC, et al. Justification for a home-based education programme for kidney patients and their social network prior to initiation of renal replacement therapy. J Med Ethics. 2011;37:677–81. doi: 10.1136/jme.2011.042366. [DOI] [PubMed] [Google Scholar]