Abstract
Background and Purpose
Hematoma volume is the most potent predictor of outcome in spontaneous intracerebral hemorrhage (ICH), and hematoma expansion after hospital presentation occurs in up to 40% of individuals. Among patients with lobar ICH, the APOE ε2 allele predicts larger hematoma volumes at presentation. We investigated whether the ε2 allele also identifies individuals at increased risk of hematoma expansion.
Methods
We analyzed 510 patients with primary ICH and genetic data available from an ongoing prospective cohort study. Baseline and follow-up CT scans were assessed for ICH location and volume using computer-assisted volumetric methods.
Results
Individuals with lobar ICH who possessed APOE ε2 were at increased risk for hematoma expansion (OR = 2.72 [95% CI 1.19 – 6.23]; p = 0.009). The highest odds of expansion were in patients who qualified for the diagnosis of cerebral amyloid angiopathy-related ICH and carried the APOE ε2 allele (OR = 6.02 [95% CI 1.60 – 22.58]; p = 0.008). There was no effect of ε2 on hematoma expansion in deep ICH and APOE ε4 had no effect on hematoma expansion in lobar or deep ICH.
Conclusions
Possession of APOE ε2 predisposes individuals with lobar ICH to hematoma expansion. This effect is even more pronounced in patients with amyloid angiopathy-related ICH, consistent with the ε2 allele’s role in vascular amyloid deposition and vessel fragility.
Keywords: Intracerebral hemorrhage, Genetics, APOE, Hematoma expansion, Cerebral Amyloid Angiopathy
INTRODUCTION
Apolipoprotein E (APOE) ε2 and ε4 alleles are independent risk factors for spontaneous intracerebral hemorrhage (ICH) in the lobar brain regions1, most likely a reflection of their role in cerebral amyloid angiopathy (CAA).2,3 Previous pathological studies have shown each allele’s unique effect in CAA. The ε4 allele increases the severity of vascular amyloid deposition, while the ε2 allele appears to cause increased vasculopathic changes that lead to vessel rupture.4,5 Consistent with this, a multi-center genetic association study conducted by the International Stroke Genetics Consortium (ISGC) showed that larger ICH volume is associated with the APOE ε2, but not ε4, allele. As a result, APOE ε2 carriers are at disproportionately increased risk of mortality and poor outcome after ICH.6
Hematoma expansion, or ongoing bleeding after initial presentation, is common in acute ICH, with up to 40% of patients showing some degree of growth during hospital admission.7-9 This expansion increases risk of poor functional outcome and death.10,11 Therefore, attenuation of growth is an intriguing therapeutic strategy and the target of several clinical trials.12,13 Thus far, these clinical trials have not led to improved neurologic outcome, perhaps because such therapies need to be targeted to the patients at highest risk for expansion in order to show any benefit. Challenges that remain, however, include the reliable identification of those patients at highest risk of subsequent hematoma expansion as well as a thorough understanding of the pathophysiologic mechanisms by which expansion occurs.
We therefore investigated whether possession of the APOE ε2 allele increases risk of hematoma expansion following lobar ICH. Furthermore, since not all lobar ICH is due to CAA, we hypothesized that the effect of APOE ε2 would be heightened in those with CAA-related ICH. To test these hypotheses, we performed a single-center prospective cohort study of patients with acute ICH.
METHODS
Study design
This is a retrospective analysis of data from an ongoing prospective cohort study performed at Massachusetts General Hospital (MGH), Boston, USA. The study was approved by the Institutional Review Board of MGH and written informed consent was obtained from all participants or their surrogates.
Patient selection
Between January 1999 and December 2010 consecutive patients who presented with acute primary ICH14 were screened and approached for participation in an ongoing genetic study of ICH. For the present analysis, we included patients meeting the following criteria: (1) 55 years or older (in order to minimize the possibility of inadvertently including patients with secondary ICH); (2) self-reported European or European-American ancestry; (3) diagnosis of non-traumatic ICH; (4) APOE genotyping available; and (5) a follow-up Computed Tomography (CT) scan within 48 hours of the baseline CT. Exclusion criteria were defined as: presence of aneurysmal subarachnoid hemorrhage, trauma, brain tumor, hemorrhagic transformation of a cerebral infarct, vascular malformation, or any other known or suspected cause of secondary ICH. Cerebellar hemorrhages were excluded given differences in clinical course, including the routine performance of surgical decompression. (Figure 1: Flowchart)
Figure 1.

Cohort flowchart
Cerebral amyloid angiopathy-related ICH
In order to determine the effect of APOE genotype on hematoma expansion in patients meeting criteria for CAA-related ICH, we separately analyzed possible and probable / definite CAA cases according to the Boston criteria.15 Probable / definite CAA was defined as lobar ICH in the presence of confirmed CAA pathology and / or microbleeds confined to the lobar brain region.16,17 Possible CAA consisted of lobar ICH cases with available MRI scans lacking lobar microbleeds. A total of 114 (43%) lobar ICH cases had pathology and / or T2*, susceptibility, or Gradient Echo (GRE) MRI data available for analysis. The presence and location of microbleeds was assessed using previously validated protocols.15,18
Clinical data
Patients (or their families or surrogates) were interviewed for age, sex, time of ictus, medical history, family history (including first-degree relative with ICH), pre-ICH use of warfarin, antiplatelet therapy or statins, alcohol and tobacco use. Hospital charts were reviewed for Glasgow Coma Scale (GCS) score on arrival, mean arterial blood pressure, time to baseline imaging and interscan time. 90-day modified Rankin Scale (mRS) assessments were performed via telephone by trained study staff to assess functional outcome.19
CT analysis
ICH location was assigned based on admission CT by study neurologists blinded to APOE genotype and clinical information. ICH exclusively involving the brainstem, thalamus or basal ganglia was defined as deep or non-lobar ICH. ICH originating at the cortical-subcortical junction was defined as lobar ICH. Hemorrhages involving both territories were defined as mixed ICH and were excluded from analysis (n = 16). Individuals with imaging of insufficient quality for location determination were also excluded from all analyses (n = 16). Differences in ICH location were adjudicated by consensus.
Initial and follow-up ICH and intraventricular hemorrhage (IVH) volumes were measured using Alice (PAREXEL International Corporation) and Analyze 9.0 (Mayo Clinic, Rochester, Minnesota) software using previously described methods.20,21 Significant hematoma expansion was defined as an increase in ICH volume greater than 6 mL or greater than 33 % from the baseline ICH volume.7,9,22
Genotyping
DNA was isolated, quantified and normalized to a concentration of 30 ng/μl. Two SNPs of the APOE locus, rs7412 and rs429358, were independently genotyped using two separate assays.17 The allelic reads from each assay were translated to APOE genotypes (ε3ε3, ε3ε4, ε4ε4, ε3ε2, ε2ε2, and ε2ε4). Genotyping personnel were blinded to clinical and neuroimaging data. All ICH cases were in Hardy-Weinberg equilibrium for APOE genotypes.
Statistical analysis
We tested for association between the APOE ε2 and ε4 alleles and hematoma expansion, analyzing deep and lobar ICH separately using logistic regression. Hematoma expansion was dichotomized (yes / no) for this analysis. Possible and probable / definite CAA-related ICH cases were also analyzed separately. Multivariate models included age, sex, hypertension, warfarin use, antiplatelet therapy, baseline ICH volume, intraventricular extension (yes / no), time to baseline scan, interscan time, number of ε2 alleles (0, 1 or 2) and number of ε4 alleles (0, 1 or 2).
Each patient’s ICH expansion probability (ranging from 0 to 100%) was assigned using individual-level data and regression estimates from the results of logistic regression. These probability values were subsequently plotted (stratified by APOE genotype), to provide a qualitative estimate of the association between APOE variants and ICH expansion. In addition, we performed a time to event analysis using a Cox regression model . A p-value of < 0.05 was considered statistically significant.
RESULTS
Study subjects
During the study period, a total of 1677 patients of 55 years or older presented with primary ICH. After applying clinical and radiological exclusion criteria and genotyping quality control, a total of 409 lobar ICH and 465 deep ICH cases remained. After selecting the cases with a follow-up CT available, a total of 265 lobar ICH and 245 deep ICH cases were available for analysis (Table 1). In addition, we created a table with the cohort characteristics for lobar ICH patients stratified by APOE genotype (Supplementary table 1; please see http://stroke.ahajournals.org). Patients without available follow-up CT more often used warfarin, had greater baseline ICH and IVH volumes, lower GCS scores, worse 90-day mRS assessments and higher mortality rates (all p < 0.05).
Table 1. Cohort characteristics.
| Variable | Deep ICH (n, %) | Lobar ICH (n, %) |
|---|---|---|
| Number of subjects | 245 | 265 |
| Age (mean, SD) | 69.9 (13.4) | 75.4 (10.6) |
| Female sex | 110 (0.45) | 133 (0.50) |
| Diabetes mellitus | 59 (0.24) | 48 (0.18) |
| Hypertension | 206 (0.84) | 183 (0.69) |
| Coronary artery disease | 57 (0.23) | 56 (0.21) |
| Atrial fibrillation | 47 (0.19) | 53 (0.20) |
| Hyperlipidemia | 49 (0.20) | 91 (0.34) |
| Previous ICH | 10 (0.04) | 24 (0.09) |
| Pre-ICH AIS / TIA | 39 (0.16) | 37 (0.14) |
| Pre-ICH dementia | 22 (0.09) | 50 (0.19) |
| Warfarin use | 49 (0.20) | 56 (0.21) |
| Antiplatelet therapy | 100 (0.39) | 106 (0.40) |
| Statin use | 62 (0.25) | 77 (0.29) |
| Probable / definite CAA | 0 (0) | 106 (0.40) |
| Possible CAA | 0 (0) | 159 (0.60) |
| GCS (median, IQR) | 14 (11 - 15) | 14 (12 - 15) |
| Time to baseline imaging in hours (median, IQR) |
5.9 (1.8 - 16.3) | 7.4 (1.3 - 21.9) |
| Baseline ICH volume (median, IQR) | 12.6 (1.9 - 28.5) | 18.6 (8.0 - 36.0) |
| Baseline IVH volume (median, IQR)* | 3.9 (1.0 - 21.0) | 2.8 (0.7 - 12.0) |
| Intraventricular extension | 120 (0.49) | 98 (0.37) |
| Interscan time in hours (median, IQR) | 17.6 (7.5 - 26.3) | 18.5 (8.2 - 29.2) |
| Follow-up ICH volume (median, IQR) | 13.7 (4.3 - 38.0) | 26.5 (10.8 - 36.0) |
| Follow-up IVH volume (median, IQR)* | 7.6 (2.5 - 25.0) | 6.5 (1.0 - 17.5) |
| Hematoma expansion (> 33%) | 24 (0.10) | 39 (0.15) |
| IVH expansion (> 2 cc)* | 29 (0.12) | 28 (0.12) |
| Death (90 days) | 81 (0.33) | 87 (0.33) |
| mRS: 0 – 2 (90 days) | 27 (0.11) | 32 (0.12) |
| APOE ε2 (minor allele frequency) | 0.08 | 0.11 |
| APOE ε4 (minor allele frequency) | 0.16 | 0.20 |
Data refers only to ICH cases with intraventricular extension and / or IVH expansion
ICH = Intracerebral Hemorrhage; n = number of patients; % = percentage; SD = standard deviation; AIS = acute ischemic stroke; TIA = transient ischemic attack; CAA = Cerebral Amyloid Angiopathy; GCS = Glasgow Coma Scale; IQR = interquartile range; IVH = intraventricular hemorrhage; mRS = modified Rankin Scale; APOE = Apolipoprotein E
CT images
The median time from last seen normal to baseline CT was 7.4 hours (Interquartile Range [IQR] 1.3 – 21.9) for lobar ICH and 5.9 hours (IQR 1.8 – 16.3) for deep ICH. The median time from baseline CT to follow-up CT was 18.5 hours (IQR 8.2 – 29.2) for lobar ICH and 17.6 hours (IQR 7.5 – 26.3) for deep ICH. For lobar ICH, median baseline hematoma volume was 18.6 mL (IQR 8.0 – 36.0) and follow-up volume was 26.5 mL (IQR 10.8 – 36.0). In deep ICH, median baseline hematoma volume was 12.6 mL (IQR 1.9 – 28.5) and median follow-up volume was 13.7 mL (IQR 4.3 – 38.0). (Table 1)
Hematoma expansion
Lobar ICH
Out of 265 lobar ICH patients, hematoma expansion was present in 39 (15%). In order to determine factors associated with hematoma expansion in lobar ICH, we first performed a univariate analysis (Supplementary table 2; please see http://stroke.ahajournals.org). Hematoma expansion was more frequent in patients with a history of hypertension (Odds Ratio [OR] = 3.55; 95% Confidence Interval [95% CI] 1.25 – 10.11; p = 0.018), patients on warfarin (OR = 3.42 [95% CI 1.86 – 6.30]; p < 0.001) and those who carried an APOE ε2 allele (OR = 2.65 [95% CI 1.15 – 6.11]; p = 0.010). In multivariate analysis the discovered effects for warfarin and APOE ε2 remained significant (warfarin use: OR = 4.44 [95% CI 1.19 – 17.63]; p = 0.034, APOE ε2: OR = 2.72 [95% CI 1.19 – 6.23]; p = 0.009) after controlling for age, sex, hypertension, warfarin, antiplatelet therapy, baseline ICH volume and APOE ε4 status (Table 2). In addition, we performed a subgroup analysis of patients presenting within 6 hours of symptom onset (Supplementary table 3; please see http://stroke.ahajournals.org). Figure 2 shows the predicted probability of lobar hematoma expansion stratified by APOE genotype. We found no evidence for an association between APOE ε4 and hematoma expansion (OR = 0.71 [95% CI 0.30 – 1.64]; p = 0.42) (Table 2).
Table 2. Multivariate analysis of hematoma expansion in lobar ICH.
| Variable* | OR (95% CI) | p – value |
|---|---|---|
| Age | 1.00 (0.96 - 1.05) | 0.80 |
| Sex | 0.62 (0.25 - 1.53) | 0.30 |
| Hypertension | 1.32 (0.54 - 3.29) | 0.54 |
| Warfarin | 4.44 (1.19 - 17.63) | 0.034 |
| Antiplatelet therapy | 0.62 (0.25 - 1.53) | 0.32 |
| Time to imaging: | ||
| < 6 hours | Ref. | Ref. |
| 6 – 12 hours | 0.58 (0.18 - 1.86) | 0.36 |
| < 24 hours | 0.81 (0.30 - 2.19) | 0.69 |
| Interscan time: | ||
| < 6 hours | Ref. | Ref. |
| 6 – 24 hours | 0.47 (0.17 - 1.26) | 0.13 |
| > 24 hours | 0.60 (0.24 - 1.52) | 0.28 |
| Baseline ICH volume | ||
| < 30 cc | Ref. | Ref. |
| 30 – 60 cc | 1.81 (0.60 - 5.55) | 0.28 |
| > 60 cc | 2.63 (0.44 - 14.28) | 0.29 |
| Intraventricular extension | 0.44 (0.18 - 1.09) | 0.079 |
| APOE ε2 | 2.72 (1.19 - 6.23) | 0.009 |
| APOE ε4 | 0.71 (0.30 - 1.64) | 0.42 |
Analysis adjusted for: age, sex, hypertension, warfarin use, antiplatelet therapy, baseline ICH volume, intraventricular extension, interscan time
OR = Odds Ratio; 95% CI = 95% Confidence Interval; Ref. = reference; APOE = Apolipoprotein E
Figure 2.
Hematoma expansion in lobar ICH
In order to account for variation in time to repeat CT, and hence time to detection of expansion, we performed a time-to-event analysis. Both APOE ε2 (Hazard Ratio [HR] 2.26 [95% CI 1.30 – 4.00]; p = 0.005) and warfarin (HR 3.21 [95% CI 1.11 – 9.27]; p = 0.031) remained independent predictors of hematoma expansion in lobar ICH after adjusting for potential confounders.
Deep ICH
Hematoma expansion occurred in 24 out of 245 (10%) deep ICH patients. We did not identify associations between either of the APOE alleles, ε2 (OR 1.41 [95% CI 0.67 – 3.01]; p = 0.37) or ε4 (OR 0.96 [95% CI 0.51 – 1.81]; p = 0.89), and hematoma expansion, despite statistical power of 0.89 to identify an effect size of OR = 1.50 at the p = 0.05 level. Only warfarin predicted hematoma expansion in deep ICH (OR 2.08 [95% CI 1.20 – 3.61 ] ; p = 0.009 ) . (Supplementary table 2; please see http://stroke.ahajournals.org)
Expansion in CAA-related ICH
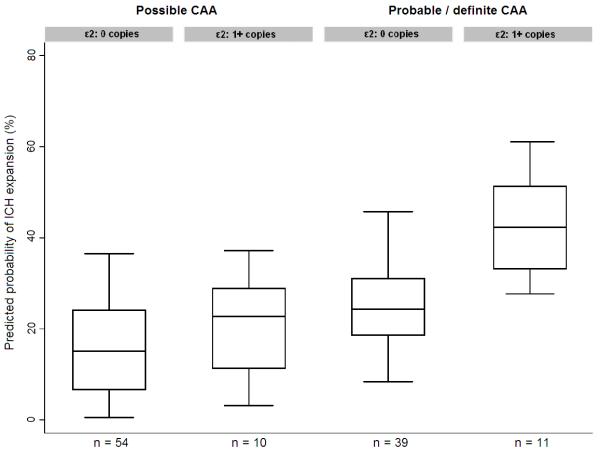
Out of 265 lobar ICH patients, 114 had MRI (n = 104) and / or CAA pathology (i.e. post-mortem examination or cortical biopsy, n = 49) data available. 50 out of these 114 (44%) patients qualified for the diagnosis of probable / definite CAA. Among these 50 individuals we found an association between APOE ε2 and hematoma expansion (OR = 6.02 [95% CI 1.60 – 22.58], p = 0.008) compared to individuals with possible CAA without APOE ε2 allele (Table 3). Figure 3 shows the predicted probability of hematoma expansion stratified by APOE genotype and CAA diagnosis.
Table 3. Multivariate analysis of hematoma expansion in probable / definite CAA in subjects with MRI.
| Variable* | n | OR (95% CI) | p – value |
|---|---|---|---|
|
Possible CAA (Lobar ICH, no lobar MB) |
|||
| APOE ε2: 0 copies | 54 | Ref. | Ref. |
| APOE ε2: 1+ copies | 10 | 1.40 (0.40 – 5.00) | 0.60 |
|
Probable / definite CAA (Lobar ICH, 1+ lobar MB, ± CAA pathology ) |
|||
| APOE ε2: 0 copies | 39 | 2.13 (0.64 – 7.10) | 0.23 |
| APOE ε2: 1+ copies | 11 |
6.02 (1.60 – 22.58) |
0.008 |
Analysis adjusted for: age, sex, hypertension, warfarin, antiplatelet therapy, baseline ICH volume, intraventricular extension, interscan time
OR = Odds Ratio; 95% CI = 95% Confidence Interval; CAA = Cerebral Amyloid Angiopathy; MB = microbleeds; APOE = Apolipoprotein E; Ref. = referenc
Figure 3.
Hematoma expansion in probable / definite CAA-related ICH (in subjects with MRI or pathology)
DISCUSSION
Our results demonstrate that patients over 55 years of age with lobar ICH who carry the APOE ε2 allele are at an increased risk of hematoma expansion. This effect is particularly pronounced in patients meeting criteria for probable or definite CAA-related ICH. There was no effect of APOE ε2 on hematoma expansion in deep ICH and APOE ε4 had no effect on hematoma expansion in lobar or deep ICH. Consistent with the results of prior studies9,23, use of warfarin also predicted subsequent hematoma expansion, regardless of hemorrhage location or CAA diagnosis.
The isolated effect of the APOE ε2 allele on hematoma expansion is in line with the results of previous studies, which suggest separate effects of ε2 and ε4 on vasculopathic CAA vessels. In these histopathological series, it appears that the ε4 allele increases the severity of vascular amyloid deposition, while the ε2 allele appears to cause vasculopathic changes that are presumably more directly tied to vessel rupture.4,5 Consistent with this finding, the ISGC recently reported the association between APOE ε2 and larger hematoma volumes in patients with lobar ICH, an effect not seen for APOE ε4, supporting the hypothesis that the APOE ε2 allele plays a unique role in the severity of vasculopathic changes in CAA.6
The association between APOE ε2 and hematoma expansion is consistent with a model in which an initial ruptured vessel leads to cascading injury of nearby diseased small vessels.24 Patients with increased severity of vascular amyloid without extensive secondary vessel wall breakdown (as might be expected in APOE ε4 carriers) appear at no higher risk of expansion, while those in whom the vascular walls have greater breakdown are at higher risk. Such patients (those with the APOE ε2 allele) are more likely to have a severely damaged vessel near the rupture site, which might then rupture in response to injury and lead to more additional bleeding. The fact that increased copies of the ε2 allele increases risk is consistent with this model. However, hematoma expansion occurring late in the course of disease might suggest that other mechanisms are also playing a role in the pathophysiology of hematoma expansion.
In previous work we noted differential effects of APOE ε2 and ε4 in relation to initial ICH volume, which may well reflect the same process; an initial rupture would normally be contained in a small space, unless there is an adequately damaged vessel nearby to rupture itself in response to injury.6 This raises the intriguing possibility of a threshold effect of amyloid-related vessel breakdown, whereby a small hematoma requires a sufficiently diseased nearby vessel to expand, although further studies are necessary to confirm this.
There is an urgent need for biomarkers that can be used to select patients and guide interventions that restrict hematoma expansion.25 To date, clinical trials aimed at restricting expansion have not led to improved neurologic outcome12,13; it may be necessary to target patients at highest risk for expansion in order to show benefit. One radiographic finding, the CT angiography (CTA) ‘spot sign’ has proven to be the strongest predictor of hematoma expansion and clinical outcome in primary ICH patients.20-22,26 It may be that APOE genotype, or other markers of underlying vascular disease, can provide additional biomarkers with which to improve prediction in the acute setting.
This study is limited by its size, lack of replication and the fact that follow-up CT scans were not performed with standardized timing. Thus, it is important to note that our ability to identify the precise time course over which expansion occurs is limited. This is a fundamental shortcoming for observational studies, where CT timing is dependent upon the clinical care providers. Furthermore, follow-up CT scans were disproportionately not available for those with the largest hematomas and early death. Although not replicated, our findings are consistent with previous research investigating the relationship between APOE and ICH. A last potential limitation of our study is the use of the Boston criteria to classify possible, probable and definite CAA-related ICH. Because not all patients had pathology available (from either post-mortem examination or cortical biopsy), this classification was partly based on MRI findings only. Overall, this hypothesis-generating analysis raises intriguing questions regarding the pathophysiology of hematoma expansion which warrant further investigations.
In conclusion, we demonstrate that the APOE ε2 allele is associated with hematoma expansion in patients with lobar ICH. This effect is particularly prominent in the subset of patients who meet criteria for probable / definite CAA. Although future studies are required to replicate these results, our findings are consistent with the accumulating body of data on the role of APOE genotype in ICH.
Supplementary Material
Acknowledgements
We would like to thank Tammy Gills, B.Sc., and Marcy MacDonald, Ph.D., for technical assistance in genotyping APOE variants.
Sources of Funding
All funding entities had no involvement in study design, data collection, analysis, and interpretation, writing of the manuscript and in the decision to submit for publication. The study was funded by NIH-NINDS grants R01NS059727, 5K23NS059774, the Keane Stroke Genetics Research Fund and the Edward and Maybeth Sonn Research Fund. Dr. Brouwers was supported by the NIH-NINDS SPOTRIAS fellowship grant P50NS051343. Dr. Biffi was supported in part by the American Heart Association / Bugher Foundation Centers for Stroke Prevention Research (0775010N).
Footnotes
Disclosures
H.B. Brouwers, None; A. Biffi, None; A.M. Ayres, None; K. Schwab, None; L. Cortellini, None; J.M. Romero, Imaging Committee DIAS trial / advisory board Lundbeck pharmaceuticals; N.S. Rost, None; A. Viswanathan, None; S.M. Greenberg, Research Grant NIH, Honoraria: Medtronic, Pfizer, Consultant / advisory board: Hoffman-La Roche, Janssen Alzheimer Immunotherapy, Bristol-Myers Squibb Company; J. Rosand, Research Grant NIH and American Heart Association; J.N. Goldstein, Research Grant National Institute of Neurological Disorders and Stroke, Consultant / advisory board CSL Behring.
Subject codes: [30] CT and MRI, [43] Acute Cerebral Hemorrhage, [55], Genetics of Stroke, [62] Intracerebral Hemorrhage, [109] Clinical genetics
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- (1).Biffi A, Sonni A, Anderson CD, Kissela B, Jagiella JM, Schmidt H, et al. International Stroke Genetics Consortium Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68:934–943. doi: 10.1002/ana.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- (3).Pezzini A, Padovani A. Cerebral amyloid angiopathy-related hemorrhages. Neurol Sci. 2008;29(Suppl 2):S260–3. doi: 10.1007/s10072-008-0957-7. [DOI] [PubMed] [Google Scholar]
- (4).Greenberg SM, Vonsattel JP, Segal AZ, Chiu RI, Clatworthy AE, Liao A, et al. Association of apolipoprotein E epsilon2 and vasculopathy in cerebral amyloid angiopathy. Neurology. 1998;50:961–965. doi: 10.1212/wnl.50.4.961. [DOI] [PubMed] [Google Scholar]
- (5).McCarron MO, Nicoll JA, Stewart J, Ironside JW, Mann DM, Love S, et al. The apolipoprotein E epsilon2 allele and the pathological features in cerebral amyloid angiopathy-related hemorrhage. J Neuropathol Exp Neurol. 1999;58:711–718. doi: 10.1097/00005072-199907000-00005. [DOI] [PubMed] [Google Scholar]
- (6).Biffi A, Anderson CD, Jagiella JM, Schmidt H, Kissela B, Hansen BM, et al. on behalf of the International Stroke Genetics Consortium APOE genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: a genetic association study. Lancet Neurol. 2011;10:702–709. doi: 10.1016/S1474-4422(11)70148-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- (8).Mayer SA. Ultra-early hemostatic therapy for intracerebral hemorrhage. Stroke. 2003;34:224–229. doi: 10.1161/01.str.0000046458.67968.e4. [DOI] [PubMed] [Google Scholar]
- (9).Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–1064. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- (10).Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE, VISTA Collaboration Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–1244. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- (12).Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. FAST Trial Investigators Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- (13).Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, et al. INTERACT Investigators Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7:391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- (14).Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- (15).Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- (16).Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke. 1997;28:1418–1422. doi: 10.1161/01.str.28.7.1418. [DOI] [PubMed] [Google Scholar]
- (17).Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- (18).Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Microbleed Study Group Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Janssen PM, Visser NA, Dorhout Mees SM, Klijn CJ, Algra A, Rinkel GJ. Comparison of telephone and face-to-face assessment of the modified Rankin Scale. Cerebrovasc Dis. 2010;29:137–139. doi: 10.1159/000262309. [DOI] [PubMed] [Google Scholar]
- (20).Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. 2009;40:2994–3000. doi: 10.1161/STROKEAHA.109.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Oleinik A, Brouwers HB, et al. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke. 2010;41:54–60. doi: 10.1161/STROKEAHA.109.565382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Goldstein JN, Fazen LE, Snider R, Schwab K, Greenberg SM, Smith EE, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889–894. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
- (23).Cucchiara B, Messe S, Sansing L, Kasner S, Lyden P, CHANT Investigators Hematoma growth in oral anticoagulant related intracerebral hemorrhage. Stroke. 2008;39:2993–2996. doi: 10.1161/STROKEAHA.108.520668. [DOI] [PubMed] [Google Scholar]
- (24).Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536–550. doi: 10.1097/00005072-197107000-00015. [DOI] [PubMed] [Google Scholar]
- (25).Montaner J. Genetics of intracerebral haemorrhage: a tsunami effect of APOE ε2 genotype on brain bleeding size? Lancet Neurol. 2011;10:673–675. doi: 10.1016/S1474-4422(11)70157-0. [DOI] [PubMed] [Google Scholar]
- (26).Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.