SUMMARY
Toll-like receptor (TLR) stimulation activates macrophages to resist intracellular pathogens. Yet, the intracellular bacterium Listeria monocytogenes (Lm) causes lethal infections in spite of innate immune cell activation. Lm uses direct cell-cell spread to disseminate within its host. Here, we have shown that TLR-activated macrophages killed cell-free Lm but failed to prevent infection by spreading Lm. Instead, TLR signals increased the efficiency of Lm spread from “donor” to “recipient” macrophages. This enhancement required nitric oxide (NO) production by nitric oxide synthase-2 (NOS2). NO increased Lm escape from secondary vacuoles in recipient cells and delayed maturation of phagosomes containing membrane-like particles that mimic Lm-containing pseudopods. NO also promoted Lm spread during systemic in vivo infection, as inhibition of NOS2 with 1400W reduced spread-dependent Lm burdens in mouse livers. These findings reveal a mechanism by which pathogens capable of cell-cell spread can avoid the consequences of innate immune cell activation by TLR stimuli.
INTRODUCTION
Toll-like receptors (TLR) are pattern recognition receptors that promote host defense through the activation of inflammatory responses (Nish and Medzhitov). The recognition of microbial products by TLRs stimulates the activation of macrophage anti-microbial mechanisms, including the production of nitric oxide (NO) by the inducible NO synthetase-2 (NOS2) (Endres et al., 1997; Gregory et al., 1993; MacMicking et al., 1997; Myers et al., 2003; Shiloh et al., 1999). TLR activation thus increases the ability of macrophages to kill phagocytosed bacteria (Myers et al., 2003; Shiloh et al., 1999). Nonetheless, certain intracellular pathogens are able to overcome early TLR-dependent immune cell activation to establish infections in susceptible hosts. The mechanisms by which these pathogens avoid cellular innate immunity remain unclear.
After infecting an initial cell, many cytosolic intracellular pathogens – including Listeria, Shigella, Rickettsia, vaccinia and HIV – move directly from this primary infected “donor” cell to a secondary uninfected “recipient” cell via a process known as cell-cell spread (spread) (Sattentau, 2008). Spread allows pathogens to remain intracellular and thus avoid extracellular defense mechanisms and humoral immune factors (Sattentau, 2008). Spread can be divided into three steps; (1) primary infection of donor cells with escape from the phagosome, polymerization of host actin and initiation of intracytoplasmic pathogen movement, (2) formation of membrane-encapsulated pseudopods that permit transfer of pathogens to uninfected recipient cells, and (3) pseudopod uptake and the establishment of a productive secondary infection in recipient cells. Previous work has identified several pathogen and host factors contributing to the first two steps in spread. Less is known about host factors involved in secondary infection, in part due to the difficulty of quantifying spread and a dearth of high-throughput assays to measure spread.
The Gram-positive bacterium Listeria monocytogenes (Lm) is a frequent contaminant of foods and causes gastroenteritis, meningitis, and abortion in susceptible individuals (Pizarro-Cerda and Cossart, 2009; Wing and Gregory, 2002). During the first step of spread, Lm is phagocytosed into a primary vacuolar compartment or phagosome. Maturation of this phagosome is controlled by host Rab5a and PKC (Pizarro-Cerda and Cossart, 2009; Prada-Delgado et al., 2005). Host γ-interferon inducible lysosomal thiol (GILT) activates a secreted bacterial hemolysin (hly) that promotes vacuolar rupture and bacterial escape into the cytoplasm (Singh et al., 2008). Once in the cytoplasm, Lm produces an actin nucleating protein, ActA, that co-opts host machinery (ARP 2 and 3, VASP, profilin, PI 3-kinases, and ezrin) to promote actin-based movement and the formation of Lm containing pseudopod projections that are engulfed by recipient cells (Pizarro-Cerda and Cossart, 2009; Sidhu et al., 2005; Tilney and Portnoy, 1989; Welch et al., 1998). Lm can grow in the secondary, double-membraned “phagosomes” (Birmingham et al., 2008) or in the cytoplasm after escape from these phagosomes (Camilli et al., 1993; Gedde et al., 2000).
Here, we investigated whether and how cell-cell spread impacted the ability of Lm to avoid the consequences of TLR-mediated activation. These studies employed a flow cytometry-based approach to quantify cell-cell spread, although similar results were obtained using more conventional microscopy-based and plaquing assays. Paradoxically, while we confirmed that TLR stimulation of macrophages inhibited their ability to be infected as primary cells, it enhanced their ability to be infected as secondary cells via cell-cell spread. Strikingly, the mechanism for this enhanced infection was dependent on iNOS-dependent production of NO. NO delayed maturation of vacuoles containing carboxylated beads, which chemically resemble the cell-membrane-encapsulated pseudopods formed by spreading Lm. Consistent with such delayed maturation, macrophages exposed to NO were more susceptible to cytoplasmic infection by spreading Lm. These studies reveal host pathways that are exploited by intracellular pathogens during cell-cell spread in order to avoid the consequences of TLR-induced macrophage activation.
RESULTS
Analysis of macrophage-to-macrophage spread of Listeria monocytogenes (Lm)
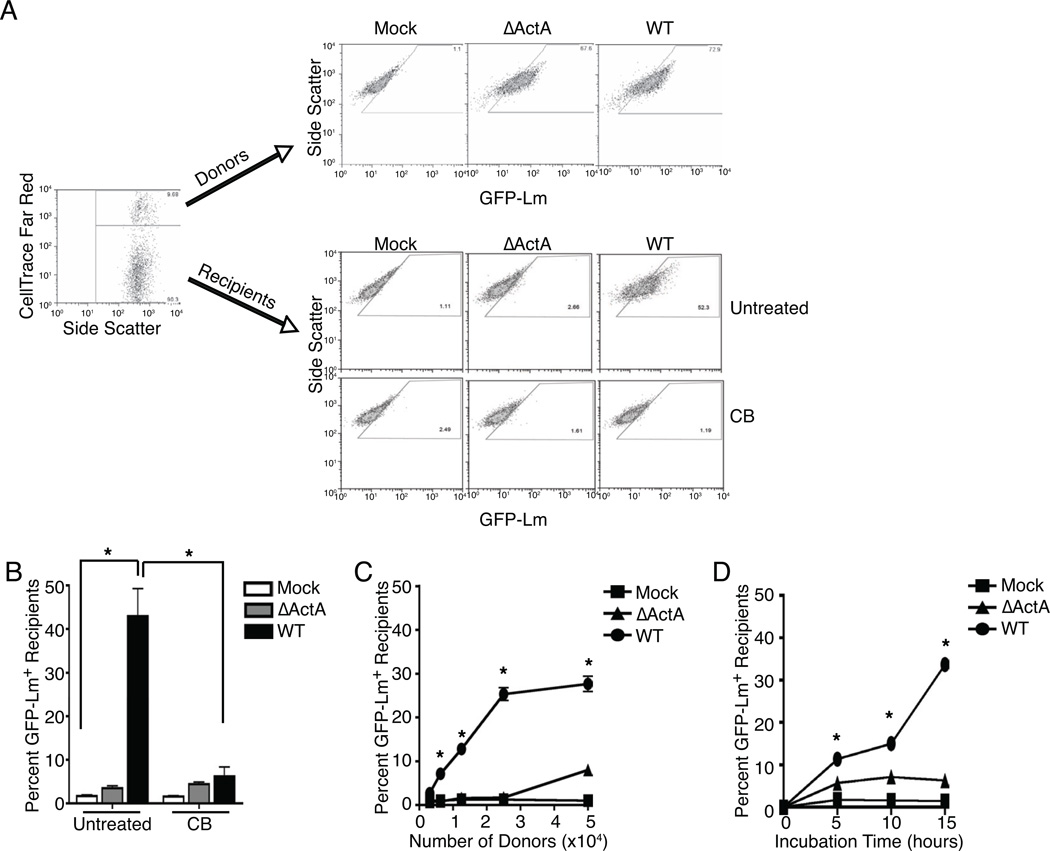
A flow cytometry-based assay was developed to identify host factors that alter Lm spread. In one permutation, fluorescently labeled donor macrophages were infected with green fluorescent protein (GFP)-expressing Lm strains (GFP-Lm; see methods) and mixed with unlabeled, uninfected recipient macrophages to allow detection of the Lm spreading to the recipient cells. Similar results were obtained by labeling donors or recipients with CellTrace Far Red, CmDiI or DiD and by staining for major histocompatibility complex class I glycoproteins (MHC I) after using donors or recipients from either Balb/C (Kd) and C57BL/6 (Kb) or β2 microglobulin deficient mice (no MHC I) and C57BL/6 (Kb) (data not shown). In all cases, gentamicin was used to kill cell-free extracellular bacteria. Gates for quantifying GFP-Lm+ recipients were drawn using uninfected donors mixed with recipients (Figure 1A). A population of recipients became GFP+ when mixed with wild type (WT) GFP-Lm infected donors (Figure 1A, B). Visual inspection by microscopy verified that recipients were infected with GFP-Lm (see below). Intracellular Lm movement and pseudopod formation within the donors were required for spread as recipients did not become GFP+ when mixed with donors infected using ActA-deficient (ΔActA) GFP-Lm, despite equivalent primary infection of WT and ΔActA donors, or when cytocalasin B was used to block actin polymerization (Figure 1A, B). Increasing the number of infected donors or the incubation time increased the percentage of infected recipients (Figure 1C, D). A ratio of 10:1 recipients:donors yielded the greatest infection of recipients by WT GFP-Lm without increasing the background infection of recipients by ΔActA GFP-Lm. An incubation of 15–18 hr had the greatest detection of spread due to an increase in transfer from donors (or recipients) to recipients, an improved ability to detect secondary infection due to the survival and growth of transferred GFP-Lm in recipients, or a combination of these. Together, these studies confirmed that GFP accumulation in recipients is due to spread from primary infected donors and enabled us to assay and quantify Lm spread with optimal conditions and sensitivity.
Figure 1. Analysis of Lm spread by flow cytometry.
To measure spread, 2.5 × 105 unlabeled. recipients were mixed with 2.5 × 104 CellTrace Far Red+ donors that were mock, ΔActA or WT GFP-Lm infected. 15–18 hr after mixing, cells were collected and analyzed by flow cytometry. (A) Flow cytometry identification of CellTrace Far Red+ donors and unlabeled recipients. In the top panel, donors that were ΔActA or WT GFP-Lm infected, but not mock infected, were GFP-Lm+. In the bottom panel, only recipients incubated with WT GFP-Lm infected donors were GFP-Lm+. Treatment with cytochalasin B (CB) blocked actin polymerization and prevented spread from WT donors into recipients. (B) Quantification of spread into recipients that were mixed with mock, ΔActA or WT infected donors alone (untreated) or given CB, n=5. Spread into recipients was dependent on (C) the donor to recipient ratio, n=5, and (D) the time of incubation, n=3. Histograms are representative of all experiments.
TLR-activation of recipients increases their susceptibility to Lm spread due to NO
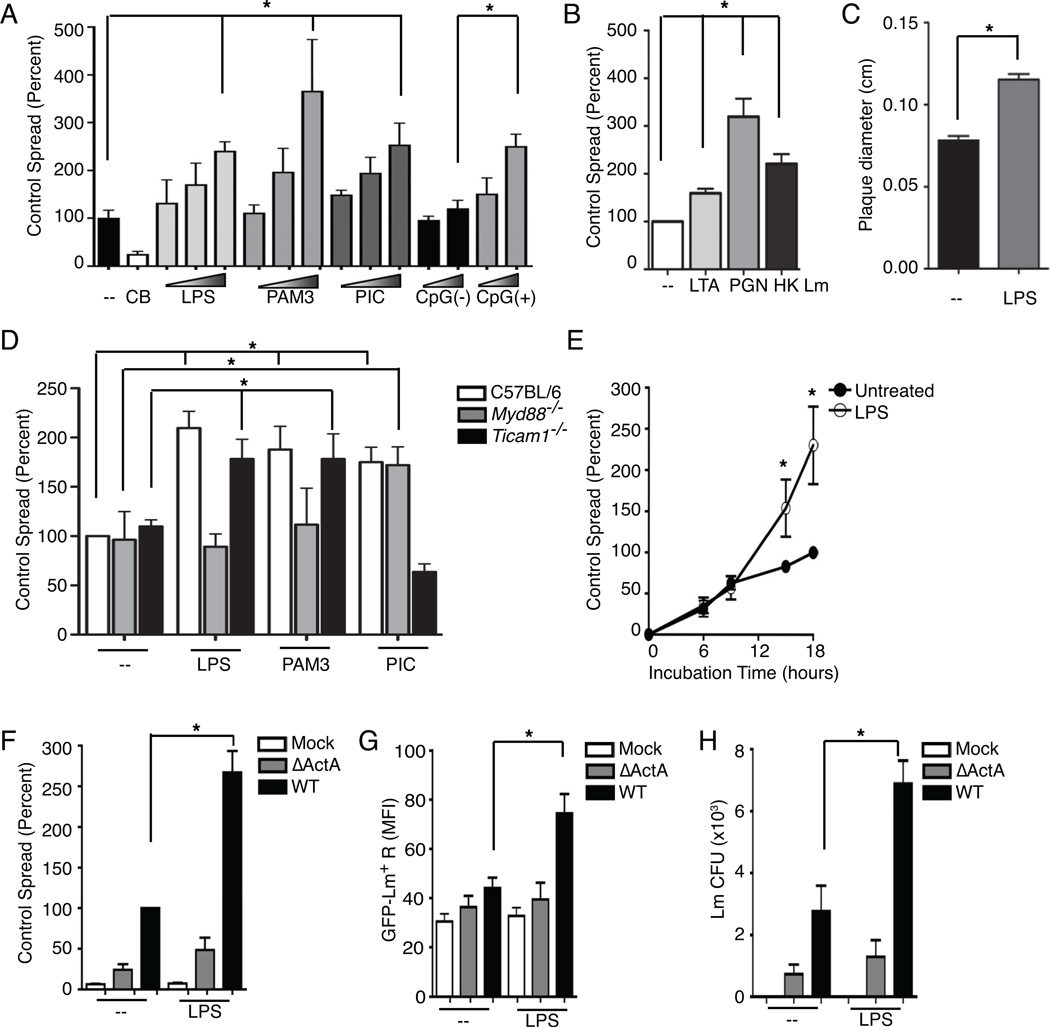
To evaluate the impact of TLR-activation on spread, recipients were pretreated with agonists for TLR4 (lipopolysaccharide-LPS), TLR2 (Pam3CSK), TLR3 and RIG-I (PIC) and TLR9 (CpG[+]). Each agonist induced a dose dependent increase in GFP+ recipients (Figure 2A). Pre-treating recipients with lipoteichoic acid (LTA), peptidoglycan (PGN) or heat-killed Lm (HK Lm) likewise increased the ability of Lm to spread from untreated, WT-Lm infected donors (Figure 2B). LPS treatment also increased spreading of Lm as measured by plaquing assays (Figure 2C) and by microscopy-based quantification of the spreading index (see below), confirming that this increased spread was not an artifact of our flow cyometry-based assay. LPS and Pam3CSK failed to increase spread when using macrophages from MyD88 deficient mice (Myd88−/−) and PIC was ineffective at increasing spread using macrophages from Trif deficient mice (Ticam1−/−), suggesting that the respective canonical TLR signaling pathways were required (Figure 2D). Spread was equally enhanced by pretreatment of recipients alone with LPS or by the addition of LPS when donors and recipients were mixed (not shown). Increased spread was first detected after 8 hr of co-incubation (Figure 2E). LPS stimulation did not increase the percentage of GFP+ recipients when ΔActA infected donors were used (Figure 2F). Both the mean fluorescence intensity (MFI) for GFP-Lm in recipients and the number of Lm colony forming units (CFU) recovered from the cultures increased in parallel with LPS treatment (Figure 2G and 2H) confirming that LPS increased the number of live Lm within recipient macrophages.
Figure 2. TLR stimulation of recipients enhances spread of Lm.
(A–B) Flow cytometry analysis for WT GFP-Lm+ recipients 15–18 hr after mixing 2.5 × 105 recipients with 2.5 × 104 WT GFP-Lm infected donors. (A) Recipients were treated overnight with various concentrations of TLR ligands (LPS at 0.4, 4 and 40 ng/mL, Pam3CSK4 at 10, 100 and 1000 ng/mL, poly(I:C) (PIC) at 40, 200 and 1000 ng/mL, and CpG(−) and CpG(+) at 5 and 20 nm), n=4. TLR stimulation enhanced spread into recipients compared to untreated (Control). (B) Recipients were treated overnight with lipoteichoic acid (LTA), peptidoglycan (PGN) or heat-killed (HK) Lm (MOI=1), washed and incubated with infected donors, n=3. (C) To confirm the enhancement of spread by TLR ligands measured by flow cytometry, confluent monolayers of macrophages were infected at an MOI of 0.01, given LPS, washed and overlayed with agar. Forty-eight hr later, plaque size was measured from images of plates that had been counterstained with neutral red dye, imaged to measure plaque size. The average size of plaques was increased by LPS treatment of macrophages, n=3. (D) Macrophages from C57BL/6, Myd88−/− or Ticam1−/− mice were used as recipients. Recipients were treated as in (A) with LPS (40 ng/mL), Pam3CSK4 (1000 ng/mL) or PIC (1000 ng/mL), washed and incubated with infected donors, n=3. (E) Time course analyses showed that LPS enhancement occurred after 9 hr of co-incubation, n=3. (F–H) 2.5 × 104 Mock, ΔActA or WT GFP-Lm infected donors were added to 2.5 × 105 Control (−) or LPS treated recipients, incubated for 15–18 hr and collect for flow cytomtery. (F) LPS enhanced spread from WT but not ΔActA infected donors, n=7. LPS also increased then numbers of bacteria in recipients as assessed by (G) the mean fluorescence intensity (MFI) for GFP in recipient cells, n=6, and (H) the total Lm CFU in the culture, n=4.
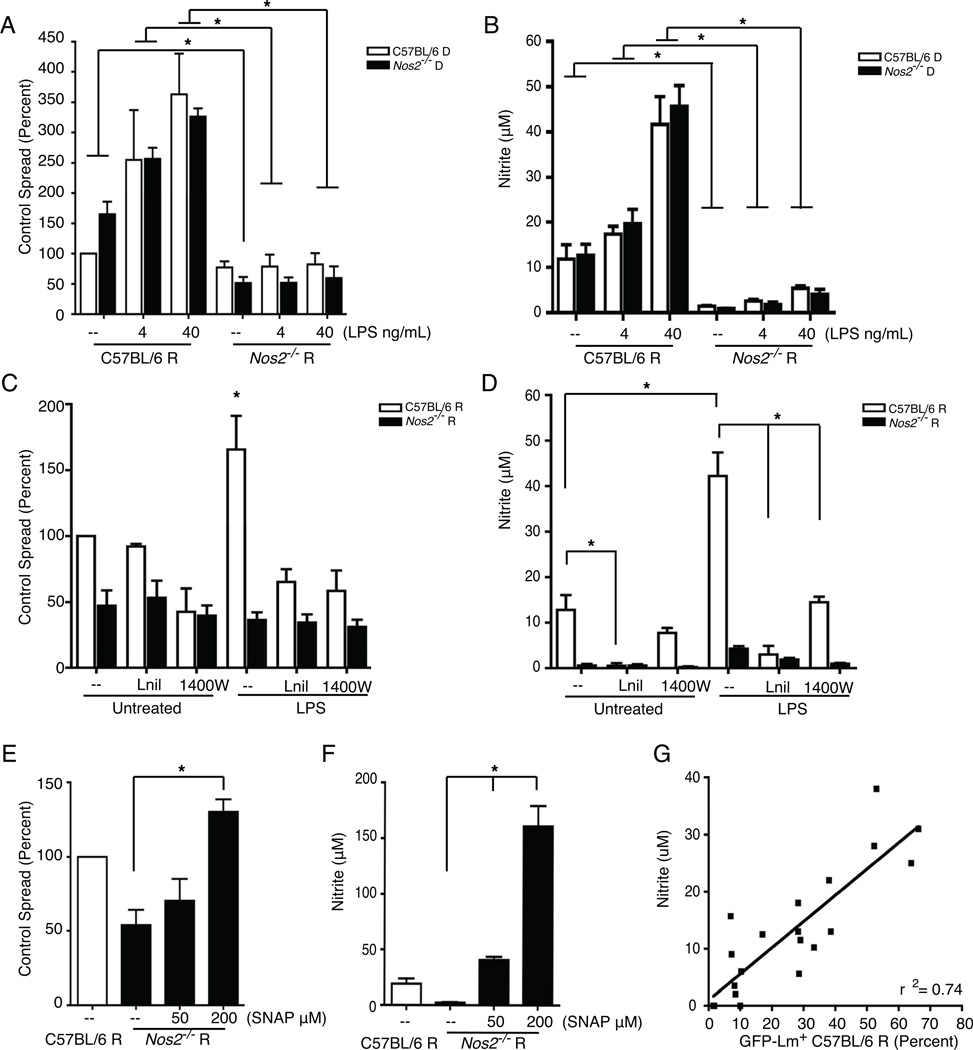
TLR stimulation induced expression of NOS2. We found that basal spread between NOS2-deficient (Nos2) donor and recipient macrophages was significantly (~50%) lower than between WT (C57BL/6) recipients and donors (Figure 3A). Moreover, LPS did not enhance spread from either WT or Nos2−/− donors into Nos2−/− recipients (Figure 3A). Measuring nitrite in the supernatant confirmed that Nos2−/− recipients produced very little NO (Figure 3B). These findings suggested that induced NOS2 expression and NO production in recipients was necessary for the TLR-induced enhancement of Lm spread. The requirement for NOS2 in donor cells was consistent with a modest induction of NOS2 by Lm infection itself (not shown), and suggested that some NO might diffuse from these cells to affect recipients. Indeed, inhibition of NOS2 with either L-nil or 1400W prevented the LPS enhancement of spread between WT cells as measured by our flow cytometry assay (Figure 3C) and reduced nitrite production (Figure 3D). Addition of SNAP, a NO donor, increased spread from WT donors to Nos2−/− recipients (Figure 3E) and increased nitrite in the supernatant (Figure 3F). Overall, we observed a strong correlation between the amount of spread and supernatant nitrite concentrations (Figure 3G).
Figure 3. TLR-induced NO enhances Lm spread.
Flow cytometry analysis for WT GFP-Lm+ recipients and measurement of nitrite in the supernatant 15–18 hr after mixing 2.5 × 105 recipients with 2.5 × 104 WT GFP-Lm infected donors. (A) Spread of WT GFP-Lm from C57BL/6 (n=4) or NOS2-deficient (Nos2−/−, n=3) donors into Nos2−/− recipients was reduced compared to C57BL/6 recipients. Furthermore, the LPS enhancement did not occur in the absence of NOS2. (B) Nitrite concentrations in the supernatants, n=3. The NOS2 inhibitors (Lnil or 1400W) prevented the LPS enhancement of (C) spread (n=4) and (D) nitrite production (n=4) from C57BL/6 donors into C57BL/6 recipients. The NOS2 inhibitors had no effect on the spread or nitrite production from C57BL/6 donors into Nos2−/− recipients. (E) SNAP, a NO donor, increased spread into Nos2−/− recipients from C57BL/6 donors (n=4), and (F) the nitrite concentrations, n=3. (G) Correlation between the percentage of GFP-Lm+ C57BL/6 recipients and the supernatant nitrite, n=20.
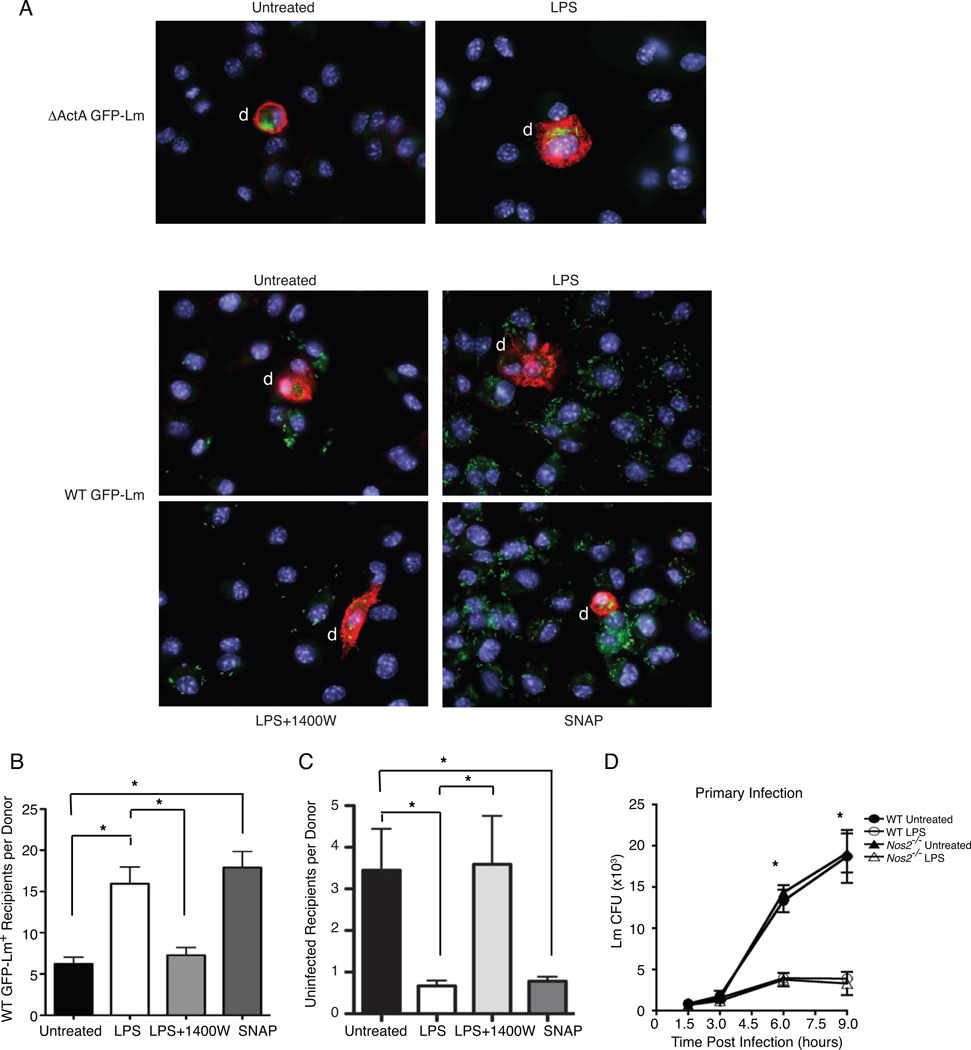
We also used a microscopy-based assay to confirm that nitric oxide production in response to TLR stimulation enhanced Lm spread. CD45.1 donor cells infected with ΔActA or WT GFP-Lm were added to coverslips containing uninfected C57BL/6 (CD45.2) recipients in media with no treatment, LPS, LPS+1400W, or SNAP. At 15 hr after the donor cell addition the number of infected recipient cells per donor cell was approximately three-fold higher when LPS or SNAP was added to the culture, as compared to LPS+1400W or untreated macrophages (Figure 4A, B). This increased number of infected cells per donor in the LPS and SNAP treated samples corresponded with a ~80% reduction in the number of uninfected cells (Figure. 4C), confirming that the difference in numbers of infected cells was due to increased bacterial spread and donor cell infection rather than any change in the cell numbers. In contrast to the enhancement of secondary infection by LPS treatment, treatment of macrophages with LPS prior to primary infection inhibited Lm survival and growth in a NOS2-independent manner (Figure 4D). This inhibition was observed within 6 hr after infection (a time at which very little cell-cell spread is apparent). These data strongly suggest that TLR stimulation suppresses primary infection. By contrast, the spreading data show that TLR-induced NO increases the susceptibility of macrophages to infection by pseudopod-associated Lm.
Figure 4. NO is required for enhanced Lm spread but not primary infection.
(A–B) Microscopy for ΔActA (top) or WT (bottom) GFP-Lm CD45.1- recipients 15 hr after plating 5 × 105 recipients with 2.5 × 104 WT GFP-Lm infected CD45.1+ donors on coverslips and treating with gentamicin alone (untreated), LPS, LPS+1400W or SNAP. Pictures of CD45.1+ donors (red) were analyzed for the number of unlabeled recipients that were infected with WT GFP-Lm (green) or uninfected. (A) Representative images of infection foci seen after each treatment. (B) The number of WT GFP-Lm+ recipient per donor (n=41–47 per treatment) was calculated for each image and the total for each treatment was graphed. Microscopy confirms the flow cytometry results indicating that LPS and SNAP enhance spread and the requirement for NO in enhanced spread, n=2. (C) The number of uninfected recipient per donor (n=41–47 per treatment) was calculated for each image and the total for each treatment was graphed to verify that treatments did not viability or total cell numbers, n=2. (D) The role of LPS was tested in primary Lm infection by plating macrophages on coverslips overnight alone or with LPS. Cells were infected the next day at an MOI of 1, washed at 1 hr and treated with gentamicin to prevent growth of bacteria in the media. The number of colony forming units (CFU) was determined by plating lysates from coverslips collected at 1.5, 3, 6 and 9 hpi. For primary infection, LPS pretreatment suppressed the growth of Lm compared to untreated. Nos2−/− mice showed comparable infection to WT cells, n=3.
NO delays phagolysosome fusion to promote Lm survival in recipients
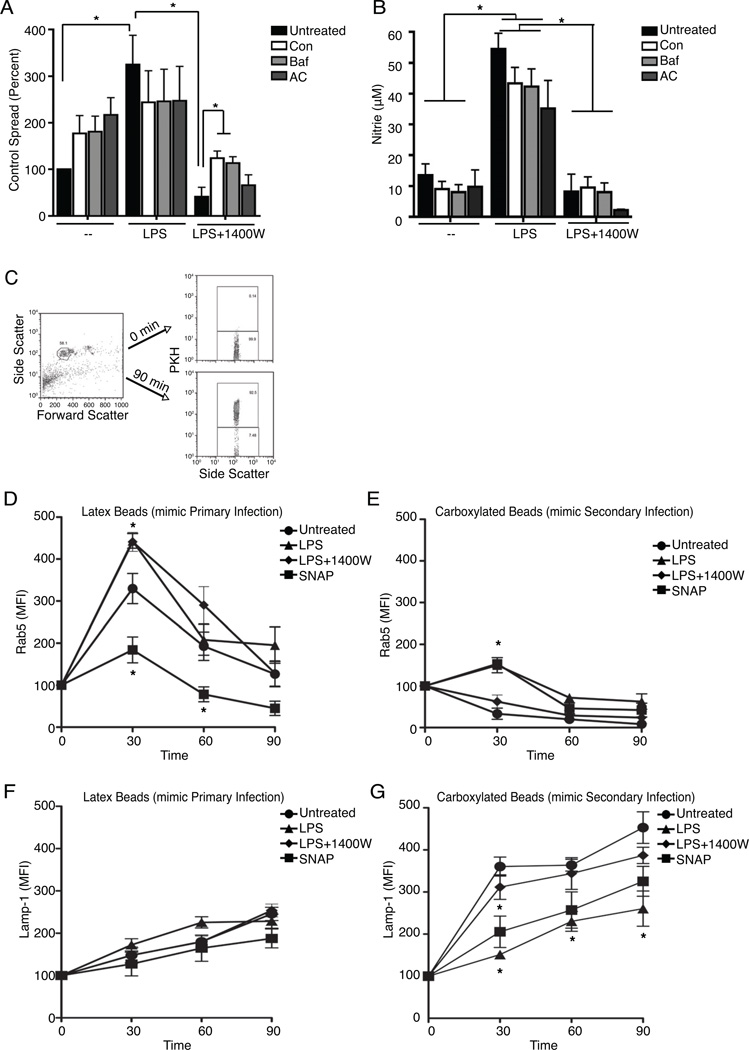
NO did not enhance spread by promoting Lm growth in the cytoplasm, by increasing pseudopod formation by donors, pseudopod phagocytosis by recipients, nor by directly activating the Lm hemolysin (hly) or phospholipases (plcA or plcB) (Supplemental 1). However, we found that NO only enhanced spread into cells with the ability to kill Lm during primary infection (Supplemental 2). This finding suggested that NO increased spread by protecting Lm from bactericidal mechanisms in the recipient cells. Two known bactericidal factors, cathepsin D and the NADPH oxidase, were protective against secondary infection, but not primary infection. However, neither was responsible for NO-enhanced spread (Supplemental 2). We thus hypothesized that NO might alter the kinetics of phagosome fusion with lysosomes in the recipient cells. Indeed, inhibiting phagolysosome fusion by preventing the acidification of phagosomes with ammonium chloride (AC) or by directly inhibiting the vacuolar H+-ATPase using Concanamycin (Con) or Bafilomycin (Baf) increased recipient cell infection (Figure 5A). Moreover, no further enhancement was achieved by combining LPS treatment with these agents, and both Con and Baf treatments significantly enhanced secondary infection when LPS-induced NO production was blocked with 1400W (Figure 5A). Importantly, AC, Con, and Baf treatments did not affect nitrite production (Figure 5B). These data suggested that NO regulates spreading Lm survival by decreasing phagolysosome fusion.
Figure 5. NO -dependent delay in phagolysosome fusion increases Lm escape from secondary vacuoles thereby enhancing spread in vitro and in vivo.
(A–B) Flow cytometry analysis for WT GFP-Lm+ recipients and measurement of nitrite in the supernatant 15–18 hr after mixing 2.5 × 105 recipients with 2.5 × 104 WT GFP-Lm infected donors. (A) Inhibiting phagosomal acidification with Concanamycin (Con), Bafilomycin (Baf) or ammonium chloride (AC) did not change untreated or LPS enhanced secondary infection but did prevent the inhibition of spread by LPS+1400W, n=6. (B) Inhibiting phagosomal acidification did not impact nitrite production, n=6. (C–G) Flow cytometry analysis was performed to determine the rate of digestion of beads phagocytosed by PKH-membrane labeled macrophages. Synchronized uptake of latex beads (mimic free Lm for Primary Infection) or carboxylated beads (mimic pseudopod-contained Lm for Secondary Infection) by PKH-membrane labeled macrophages that were untreated, pre-treated overnight with LPS or LPS+1400W, or given SNAP 30 min prior to starting the assay. Lysates were collected and stained for the early endosomal marker Rab-5 or the lysosomal marker Lamp-1 at 0, 30, 60 or 90 min after phagocytosis. (C) Flow cytometry plots depicting how beads were identified by Forward and Side scatter. Phagocytosed beads were identified by their staining for macrophage PKH-membrane dye. (D–E) Colocalization of phagocytosed beads with the early endosomal marker Rab-5. (D) Mean fluorescence intensity (MFI) for Rab-5 staining on phagocytosed PKH+ latex beads that mimic Primary Infection was not affected by LPS, but was reduced by SNAP treatment, n=3. (E) The MFI for Rab-5 on carboxylated beads that mimic Secondary Infection was increased by LPS or SNAP treatment that induce NO, n=3. (F–G) Colocalization of phagocytoses beads with the lysosomal marker Lamp-1. (F) MFI for Lamp-1 on phagocytosed PKH+ latex beads (Primary Infection) was not affected by NO, n=4. (G) NO induced by LPS or SNAP reduced the colocalization of phagocytosed carboxylated beads (Secondary Infection) with the lysosome as measured by the MFI for Lamp-1, n=4.
The effects of NO on fusion of phagosomes with lysosomes were thus quantified by allowing macrophages whose plasma membrane had been labeled with the PKH dye to phagocytose either latex or carboxylated beads to mimic uptake of free Lm or pseudopod-associated Lm (Erwig et al., 2006), respectively (Figure 5C). PKH+ beads (phagosome-associated) were isolated from the cells and co-stained to detect the presence of markers for early endosomes (Rab5) or lysosomes (Lamp-1) as previously described (Hmama et al., 2004). This assay revealed rapid accumulation of Rab5 with phagocytosed latex beads, followed by a steady decrease in Rab5 co-staining (Figure 5D). The kinetics of reduced Rab5 staining was hastened in LPS-treated cells but this was not affected by 1400W treatment and thus independent of NO (Figure 5D). Compared to phagosomes containing latex beads, phagosomes containing carboxylated beads had substantially lower Rab5 staining at all timepoints analyzed (Figure 5E). This suggested a more rapid maturation of vacuoles with carboxylated beads into Rab5-negative late endosomes. Indeed, the phagosomes with carboxylated beads also more rapidly co-stained with Lamp-1 (compare Figure 5F, G), indicating more rapid fusion with lysosomes. Thus, in the untreated macrophages, phagosomes containing the pseudopod-like carboxylated beads matured more rapidly than those containing free-bacteria-like latex beads. This finding suggests that secondary phagosomes may normally be more inhospitable to Lm than primary phagosomes. However, the presence of NO from LPS or SNAP treatment significantly delayed the maturation of the carboxylated bead phagosomes – as indicated by the increased early Rab5 co-staining (Figure 5E) and reduced Lamp-1 co-staining (Figure 5G). These effects were independent of altered protein expression, since total cellular Rab-5 amounts were unchanged while total cellular Lamp-1 was increased by LPS or SNAP treatment (data not shown). Hence, these data argue that NO selectively delays maturation of phagosomes and the formation of phagolysosomes upon engulfment of pseudopod-like particles.
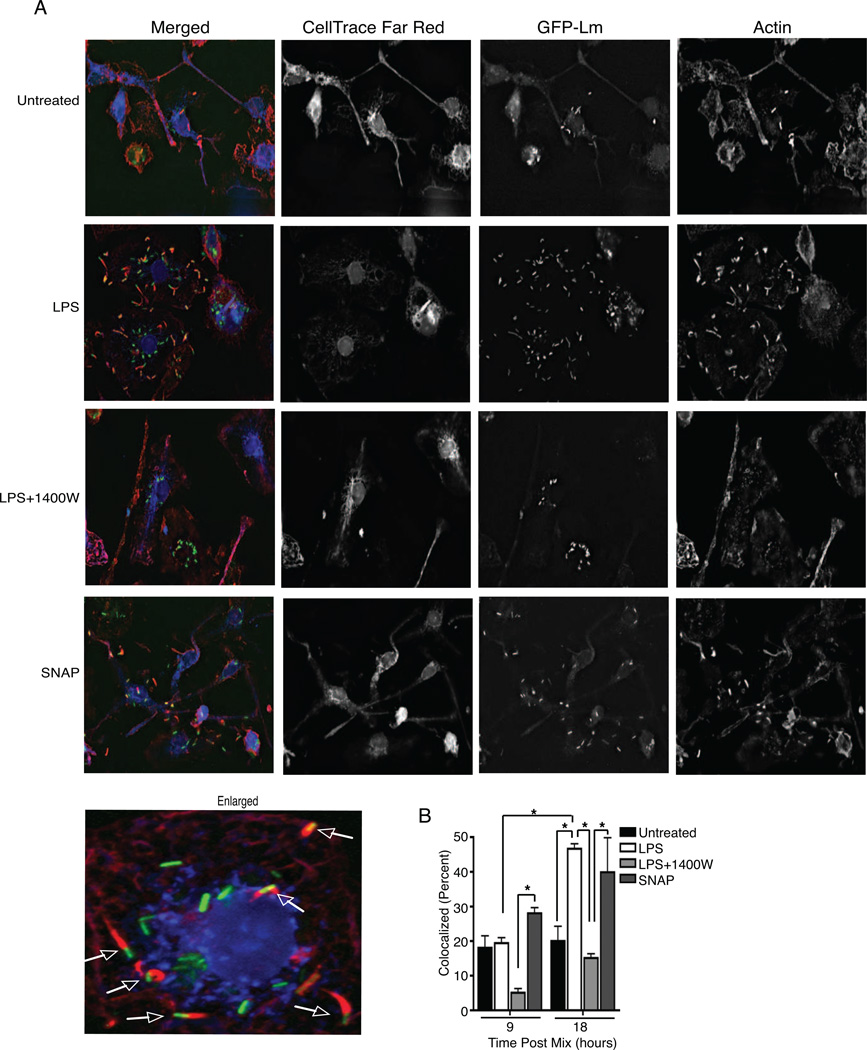
Delayed phagolysosome formation presumably enhances survival of pseudopod-encapsulated Lm in the recipient cells, possibly by allowing greater intraphagosomal survival and/or greater bacterial escape from secondary vacuoles. Our flow cytometry, and microscopy data above showed that NO increased the number of Lm-infected cells during spread, but these assays did not address whether recipient cells contained more cytosolic Lm. We thus used the established method of quantifying the co-localization of Lm with actin to estimate the proportion of Lm escaping secondary vacuoles and localizing to the cytosol of labeled recipient macrophages in the presence and absence of NO (Figure 6A). LPS treatment significantly increased the proportion of actin-colocalized Lm in the recipient cells at 18 hrs post infection (hpi), compared to both the untreated or LPS+1400W cells (Figure 6B). Moreover, treatment with 1400W reduced actin co-localization below that seen with no treatment, and treatment with the NO donor SNAP was sufficient to increase the proportion of actin associated bacteria in the recipient cells (Figure 6B). These data confirm that the ability of NO to increase Lm spread is associated with an enhancement of bacterial escape into the cytoplasm of recipient cells.
Figure 6. NO increases cytosolic Lm in secondary cells.
To measure WT GFP-Lm Secondary Infection, 2.5 × 105 unlabeled. recipients were mixed with 2.5 × 104 CellTrace Far Red+ donors that were WT GFP-Lm infected and plated on coverslips with no treatment, LPS, LPS+1400W or SNAP. 9 or 18 hr after mixing, coverslips were collected, fixed and stained for actin and mounted to slides. Images were obtained from deidentified slides and the percentage of Lm that were colocalized with actin in recipient cells was determined. (A) Representative images of GFP-Lm (green) colocalization with actin (red) in the cytoplasm of CellTrace Far Red+ recipient (blue) macrophages that were untreated or given LPS, LPS+1400W, or SNAP. Arrows in the enlarged picture indicate colocalization. (B) Graph of the analysis of images described in (A). The proportion of GFP-Lm colocalizing with actin was increased by LPS and SNAP compared to untreated or LPS+1400W at 18 hr post mixing with infected donors, n=3.
Inhibiting NO in vivo prevents Lm spread
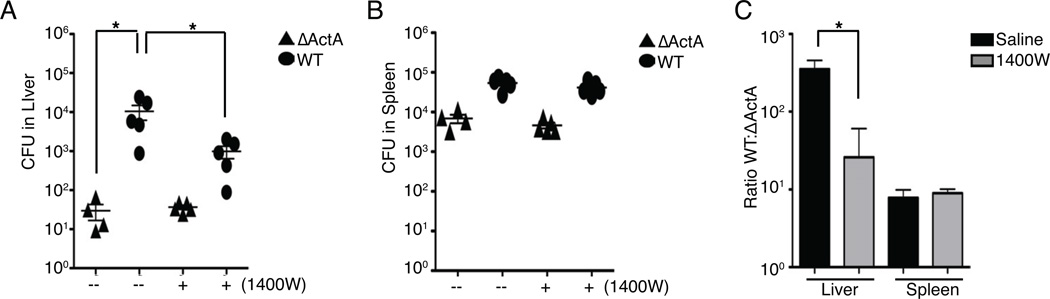
The importance of Lm spread for host infection was previously demonstrated by the observation that ΔActA-Lm are 1000 times less virulent than WT-Lm (Camilli et al., 1993). To confirm that NO promoted spread in vivo, C57BL/6 mice were treated with saline or 1400W and infected with either WT or ΔActA Lm. At 48 hpi treatment with 1400W significantly reduced the CFU in the liver of WT Lm infected mice (Figure 7A). Treatment with 1400W did not affect bacterial burdens in the spleens (Figure 7B), where ActA expression has minimal effects on Lm burdens (Camilli et al., 1993). Bacterial spread in the infected mice was further evaluated by comparing the ratio of WT to ActA CFU. This analysis showed that 1400W treatment significantly reduced the advantage that ActA expression gives Lm in the livers of infected mice (Figure 7C), but had no effect in mouse spleens. Take together, these data support our in vitro finding that NO is important for regulating the spread of Lm.
Figure 7. NO contributes to enhanced bacterial spread in vivo.
(A–C) C57BL/6 mice were treated with saline or 1400W and infected with either WT or ΔActA Lm. Mice were harvested 48 hpi. (A) CFU in the liver of mice shows that there is a decrease in WT CFU when mice were treated with 1400W. (B) CFU in the spleen. (C) The ratio of WT to ΔActA CFU was calculated as a measure of Lm spread for the liver and spleen of saline or 1400W treated mice. Enhanced spread was seen in the liver compared to the spleen. Treatment with 1400W significantly reduced Lm spread in the liver. Data points represent each mouse, means±SEM. from all mice. n=5 mice per experiment, performed twice.
DISCUSSION
Our studies reveal a mechanism by which innate immune activation increases host susceptibility to infection by Listeria monocytogenes (Lm). Lm and several other pathogens co-opt host actin polymerization to induce direct cell-cell spread from infected “donor” cells to neighboring uninfected “recipients.” Cell-cell spread by Lm requires ActA, a secreted bacterial factor that promotes host actin polymerization (Domann et al., 1992; Kocks et al., 1992). Using quantitative assays we found that TLR-stimulation significantly increased productive Lm infection of recipient cells via cell-cell spread. The increased infection required ActA and macrophage nitric oxide (NO) production by NOS2. NO acted to increase both the percentage of infected recipients and the number of bacteria per recipient cell but did not enhance formation of pseudopods or transfer and uptake of bacteria. Rather, NO delayed phagolysosome fusion when vacuoles contained particles that resemble host membranes and increased Lm survival and growth in the recipient cells. This delay in maturation of secondary vacuoles presumably increased the ability of Lm to avoid host bactericidal factors such as cathepsin D and the NADPH oxidase. This process is crucial during systemic Lm infection, where NO production selectively increased liver burdens of WT but not ΔActA Lm. Together, these data reveal how cell-cell spread enables Lm to exploit innate immune activation during in vitro and in vivo infection of phagocytes and other bactericidal cells, such as hepatocytes (Wing and Gregory, 2002). This mechanism may also permit subversion of innate immune activation by other bacterial and viral pathogens capable of cell-cell spread.
The ability of TLR-stimulation and NO production to enhance host susceptibility to Lm was limited to infection via cell-cell spread. Multiple lines of evidence indicate that infection via cell-cell spread is mechanistically distinct from direct infection. For example, electron microscopy shows that spreading Lm are initially contained by a double membrane vacuole, while Lm taken up from the extracellular environment are contained by a single membrane vacuole (Tilney and Portnoy, 1989). In addition, the secreted Lm enzyme PlcB is selectively required for escape from secondary but not primary vacuoles (Camilli et al., 1993; Poussin and Goldfine, 2005). The unique features of infection via cell-cell spread likely explain why NO uniquely impacts Lm infection via this route, thus we considered whether NO might act by enhancing the function of virulence factors such as PlcB. However, we failed to see any effect of NO on PlcB activity in Lm supernatants using a colorimetric phospholipase assay. Conversely, NO directly inhibited hemolytic activity of Hly in Lm supernatants. Hly function is required for Lm escape from both primary and secondary vacuoles (Alberti-Segui et al., 2007; Burrack et al., 2009; Gedde et al., 2000; Portnoy et al., 1988). Thus, the observed inhibition of Hly activity in vitro might be expected to correspondingly inhibit both primary and secondary Lm infection in NO-producing macrophages. The fact that we did failed to observe such inhibition suggests that: (i) nitric oxide does not directly interact with Hly during infection of macrophages, (ii) the inhibition of Hly (and possibly escape) by nitric oxide measured in vitro is below the threshold required for biologically relevance, (iii) the potential anti-bacterial effects of impairing Hly function are counteracted by other effects of NO, such as altering the rate of phagolysosome fusion, or (iv) the ability of NO to directly inhibit hemolytic activity in vitro might be counteracted by other host cell factors, such as the γ-interferon inducible lysosomal thiol (GILT) (Singh et al., 2008). NO reportedly can suppress primary vacuolar escape by another intracellular bacterial pathogen, Francisella tularensis (Tancred et al., 2011), although the suppression mechanism is in this case pathogen-specific. Thus, it is conceivable that NO prevents Lm invasion of the cytoplasm during direct infection and the Lm growth we observed occurs in vacuoles, as previously shown (Birmingham et al., 2008). However, Lm clearly accessed the cytosol following infection of NO-producing cells via cell-cell spread. Hence, our findings argue against the notion that NO increases bacterial spread by increasing bacterial virulence factor function and instead point to unique effects of NO on the maturation of secondary vacuoles containing membrane-encapsulated bacteria.
Why do secondary vacuoles traffic differently than those formed around extracellular bacteria? We hypothesize that Lm-containing pseudopods mimic apoptotic bodies and thus co-opt the process of “efferocytosis.” Under normal circumstances, efferocytosis is non-inflammatory and non-immunogenic and vacuoles containing phagocytosed apoptotic cells very rapidly fuse with lysosomes (Erwig and Henson, 2008; Erwig et al., 2006). As we show here, the production of NO delays this maturation. Perhaps NO acts to signify an inflammatory environment in which the selectively delay in digestion of dying (infected) cells permits enhanced processing and presentation of microbe antigens. It remains unclear precisely how NO selectively attenuates secondary vacuole maturation. TLR stimulation alters phagosome maturation by raising phagosomal pH and lowering degradative capacity (Yates et al., 2007), but it is unclear if NO is responsible for these effects. NO does directly and reversibly inhibit the vacuolar H+-ATPase (Forgac, 1999; Swallow et al., 1991; Tojo et al., 1994), which could delay maturation of secondary vacuoles. However, blocking of the vacuolar H+-ATPase with Con or Baf did not reverse the effects of NO. Hence, we speculate that NO acts by another mechanism, such as direct modification of Rab proteins or indirect activation of protein kinase G (Akaike et al., 2010; Francis et al., 2010).
TLR-stimulation of macrophages or stimulation with the “classical” combination of LPS+IFNγ dramatically curtails direct Lm infection (Mackaness, 1964; Portnoy et al., 1989; Myers et al., 2003; Shiloh et al., 1999). A prior microscopy-based study suggested that this impairment in J774 macrophages was due to the ability of NO to limit Lm escape from primary vacuoles (Myers et al., 2003). However, our data suggested that any suppressive effect of NO on Lm escape from primary vacuoles is insufficient to prevent normal Lm intracellular replication. Moreover, others found that NOS2 is dispensable for killing of Lm by IFN-γ-activated macrophages and Nos2−/− mice are only modestly more susceptible to systemic Lm infection (Edelson and Unanue, 2002; Endres et al., 1997; Gregory et al., 1993; Shiloh et al., 1999). In one study, NOS2 expression even appeared to impair bacterial killing (Edelson and Unanue, 2002). Given the ability of IFN-γ to strongly induce NO production, it was thus surprising that macrophages treated with IFN-γ were not susceptible to enhanced Lm spread (and even resisted spread a bit). This result indicated that IFN-γ administered alone to macrophages can trump the pro-bacterial effects of NO production. Presumably this trumping is due to induction of other anti-microbial effectors by IFNγ, such as the p65 GTPases that modulate vacuolar traffic and thus killing of Lm in both primary and secondary vacuoles (Kim et al., 2011). By contrast, when IFNγ was added with LPS it failed to prevent NO-dependent increases in recipient cell infection with Lm via cell-cell spread. This result indicates that the effects of LPS stimulation trump those of IFNγ during cell-cell spread. We speculate that TLR signals (or signals induced by TLR-stimulated factors such as IFN-αβ, IL-6, or TNF-α) impair the induction of p65 GTPases or other potentially protective responses by IFNγ. Prior studies from our lab and others support this model in that they show the ability of IFN-β to suppress macrophage gene induction in response to IFN-γ (Rayamajhi et al, 2010).
The contribution of NO to host protection can be critical, ancillary, deleterious, or imperceptible (Nathan, 1997). We showed here that NO production in response to TLR-activation can benefit Lm by enhancing pathogen spread to increase bacterial burdens during systemic infection. NO production also appears to increase susceptibility in models of Shigella infection (MacMicking et al., 1997), and could contribute to the enhanced susceptibility of mice exposed to zymosan or influenza to infection by Lm (Jamieson et al., 2010; Young et al., 2009). Excessive NO production might also contribute to the increased incidence of Listeriosis in patients with solid organ transplants, liver disease, diabetes and malignancy (Bennion et al., 2008; Fernandez-Sabe et al., 2009). NADPH oxidase and Cathepsin D were previously reported to inhibit Lm infection, but under conditions where it was unclear if inhibition occurred at the level of primary or secondary infection (del Cerro-Vadillo et al., 2006; Myers et al., 2003; Shiloh et al., 1999). Our findings here revealed selective anti-bacterial effects of these factors during secondary infection and thus indicate that macrophages do possess mechanisms for counteracting cell-cell spread. Enhancing NADPH oxidase and Cathepsin D activity may thus have selective efficacy in therapy of infections by microbes capable of cell-cell spread. Future efforts to determine when, where, and how innate immune activation increases host susceptibility to pathogens may reveal additional targets for therapeutic inhibition during infectious and inflammatory diseases.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6 mice for in vitro experiments were from Jackson Laboratories. ΔNOS2 mice were from Jackson (B6.129P2-Nos2tm1Lau/J) or Taconic (B6.129S2-Nos2tm1MrI N12) Mice from Jackson Laboratories were used for in vivo experiments. ΔMyd88 mice were provided by Dr. Tina Leslie at National Jewish Health (Suram et al.; Takeuchi et al., 1999). Trif-deficient mice were a gift from Dr. Pippa Marrack at National Jewish Health. The Institutional Animal Care and Use Committee at National Jewish Health approved all animal experimental procedures.
Reagents
Cells were labeled with CellTrace Far Red, CMDiI and DiD (Invitrogen) at 0.5, 5, 5 µM according to manufacturer’s instructions. Briefly, for CellTrace Far Red, cells were lifted using ice cold dPBS, centrifuged and resuspended at 5×106/mL. CellTrace Far Red was added to final concentration of 0.5µM and cells werer incubated at 37C for 5 minutes. An equal volume of bone marrow media was added to stop reaction. Cells were washed twice with media before being used in assays. For CMDiI and DiD labeling, the appropriate volume of dye was added to macrophages growing in 15cm dish in bone marrow media for 5–15 minutes in incubator. Media was removed, cells were washed twice with warm dPBS and cells were lifted using ice-cold dPBS and used for assays. Phallodin (1:300) was from also from Invitrogen. LPS (#201 from E.coli 0111:D4) was from List Biological Laboratories. Pam3CSK, CpG (−) and CpG(+) oligomers (ODN 1826, (+) 5’-tccatgacgttcctgacgtt-3 and (−) 5’- tcc atg agc ttc ctg agc tt -3’) were from InVivoGen. Cytochalasin B (CB, 3 µM) and concanamycin (Con, 10 ng/mL) were from Enzo. Bafilomycin (Baf, 0.5 µM) was from Alexis. PKH, poly(I:C) (PIC), erythromycin (erm), ammonium chloride (AC, 10µM), lipoteichoic acid (LTA, 10 µg/mL), peptidoglycan (PGN, 50 ng/mL) and Nozyme were from Sigma. S-Nitroso-N-acetyl-DL-penicillamine (SNAP), 1400W (10nM) and Lnil (70nM) were from Caymen. Gentamicin (gent) was from Hyclone. Tryptic Soy Broth (TSB) and bactoagar were from BD. Biotinylated anti-Lamp-1 (1:100) and streptavidin-Alexa 780 (1:300) were from Ebioscience. Anti-Rab-5 (1:100) was from Santa Cruz.
Cell Culture
Bone marrow derived macrophages (BMM) were lifted with ice-cold dPBS and plated 6–7 days after culturing marrow from mice on 150mm plastic dishes in media (DMEM+ 20% Bovine Growth Serum (Hyclone), 10% L929-conditioned media, 2mM L-glutamine, 1mM sodium pyruvate and 55µM β-ME) at 37°C and 7.5% CO2.
Bacteria
WT, ΔActA and Δhly strains of Lm (10403S background) were from Dr. Dan Portnoy (UC Berkeley). Each strain was engineered to express GFP by transfection with expression plasmid pNF8, which contains GFP and a selectable erm resistance gene (Fortineau N, 2000). Colonies from fresh LB agar plates were grown to log phase in TSB with 15 µg/mL erm at 37°C with shaking. Lm were collected, washed and resuspended in dPBS. CFU was calculated by measuring the absorbance of the supernatant at 600nm using the formula OD 0.3 = 5×108 CFU/mL, Heat killed (HK) Lm was prepared by incubating log phase Lm at 67°C for 3 h.
Primary Lm Infection and Growth Assay
Cells were plated overnight on glass coverslips in a 60mm petri dish either alone or with treatment. Macrophages were infected at an MOI of 1 for 1 h, washed twice with dPBS and media with 50µg/mL gent was added. For some experiments, 200µM SNAP was added at 1.5 hpi. Three coverslips per group were collected at 1.5, 3, 6 and 9 hpi. Cells were lysed in 5mL H20, plated onto 10cm LB agar plates and grown overnight at 37°C.
Secondary Lm Infection Assay
To quantify Lm secondary infection, spread assays were performed by mixing uninfected recipients with infected donors on 96-round bottom plates or glass coverslips. Recipients (2.5×105/assay) were either plated overnight with stimuli or collected immediately prior to use. Donors were infected at an MOI of 10–20 for 1 hr, washed twice with dPBS and media with 50µg/mL gent was added. Donors were collected 1–2 hr after gent treatment using Nozyme, centrifuged and mixed with recipients in media with erm (15µg/mL) and gent (10–50µg/mL) at a ratio of 1:10 for donor:recipient and collected at 15–18 h post mix unless otherwise stated. Duplicates were used for each condition. Cytochalasin B, SNAP, 1400W and Lnil were added when cells were mixed and were present for the duration of the spread assay.
For analysis by FACS, cells in 96-well plates were centrifuged, washed once with dPBS, centrifuged and treated with Nozyme, centrifuged and the cell pellet was resuspended in dPBS and then fixed with 2–4% buffered formaldehyde. 10,000 events were captured for each sample. Data were analyzed by gating on recipient cells and determining the percentage of GFP+ recipients for all samples using the gate drawn from the untreated, control recipients mixed with mock-infected donors. The percent GFP+ recipients seen when WT GFP-Lm infected donors were used with varied from 18 to 70% depending on the experiment. Due to this variability, the % Control Spread was calculated for each experiment by dividing the %GFP+ recipients for each treatment by the %GFP+ recipients for the control X 100.
For confirmation of the flow cytometry results, CD45.1 C57BL/6 congenic macrophages were infected with Lm at an MOI of 10. After 1 hr, donors were washed, incubated with 50µg/ml gent and 15µg/ml erm for an additional 1 hr, lifted with Nozyme and mixed with C57BL/6 recipient macrophages on coverslips at a 1:20 ratio. After 15 hr, coverslips were rinsed and surface stained with anti mouse CD45.1 Cy5 (ebioscience) before being fixed with 4% paraformaldehyde. After fixation, coverslips were stained with Alexa Fluor 546 Phalloidin (Invitrogen) and DAPI and mounted to slides. Coverslips were imaged using a 40× air objective on a Leica DM RXA microscope. For each of two independent experiments, 25–35 image fields containing at least one, but no more than two donors, were obtained for each sample condition using Slidebook software. Images were collected and analyzed in a blinded manner. Imaging of the phalloidin channel was used to identify and confirm the perimeter of infected cells. However, we found that our ability to visualize bacteria residing in differing Z-planes of the image was improved when slides were examined without visible phalloiding (as in Fig. 4). For the blind analysis, the total number of (CD45.1+) donor and (CD45.1−) recipient cells per image field was counted together with the number of infected cells and the number of uninfected cells. After de-coding of images, the ratio of infected recipients per donor was used to compare spreading efficiency between the treatments. Essentially identical data were obtained if we instead compared the ratio of uninfected cells per donor. Analysis of the two experiments separately or when pooled and gave identical results.
Plaque Assays
For plaquing, confluent monolayers of macrophages in 12 well plates were infected at an MOI of 0.01 with log phase WT Lm similar to the previously described method (Sun et al., 1990). One h later, cells were washed twice with PBS and gent (10µg/mL) was added along with nothing or 4ng/mL LPS. Cells were washed three times 1 h later and 0.7% agarose was added to each well. Plates were collected 48 hr post infection and counterstained with neutral red dye. Plates were imaged using a HP desktop scanner. Plaques from de-identified pictures of each well were measured using Adobe Photoshop.
Quantification of Lm in cytoplasm of infected recipients
GFP-Lm infected donors were added to CellTrace Far Red+ recipients on glass coverslips. Coverslips were harvested, fixed with 4% paraformaldehyde and 3% sucrose in dPBS, permeabilized with 0.2% Triton X and stained with phallodin 544 and Hoechst before being mounted onto slides. Z-stack images were collected with a Zeiss 200M-Marianas system microscope (3i) with a 63× oil objective and Sedat filters using Slidebook software. 30–80 recipients per condition were analyzed for GFP-Lm colocalization with actin using deconvoluted projection images viewed in Adobe Photoshop. After de-coding of images, the number of Lm colocalized with actin for each treatment was graphed.
Mouse Infections
Female mice between (6–8 weeks old) were infected (tail vein) with 0.5–2 × 104 CFU of log-phase mouse passaged WT or ΔActA Lm. Mice received i.p injection of saline or 1400W (10mg/kg) at 0 and 24 hpi. Blood, spleen and liver were harvested at 48 hpi. CFU were determined by dilution plating as previously described (Rayamajhi et al., 2010).
Quantification of Phagolysosome Fusion
PKH labeled macrophages were plated (6 well plates) and treated overnight with nothing, LPS or LPS+1400W. Cells were pretreated with 200 µM SNAP for 30 minutes. Latex or carboxylated beads (8 × 106 per well, 4µm in diameter, Invitrogen) were bound to cells at 4°C for 45 minutes. Cells were washed 4 times with dPBS and pre-warmed media was added. Cells were collect at 0, 30, 60 and 90 minutes and lysates were collected as previously described (Hmama et al., 2004) and stained using Fixation and Permeabilization buffers (ebioscience).
Greiss Reaction
100µL of supernatant was mixed with 100µL of 1% sulfanilamide, 0.1% naphthylethylene diamine and 2.5% H3PO4. Absorbance was measured at 540nm. Concentration was determined based on a standard curve using NaNO2.
Flow Cytometry
Cells were analyzed by flow cytometry using the FACScan running on CellQuest™ software (BD Biosciences) and plots were rendered using FlowJo software (Tree Star, Inc).
Statistics
Statistical analysis and p-value calculations were conducted using the Prism 4 statistical program (GraphPad Software). One-way ANOVA followed by Tukey tests were performed. Data represent means ± SEM. of n individual experiments. Samples with * indicate a p-value less than 0.05.
HIGHLIGHTS.
TLR-stimulation prevents primary Listeria infection but enhances cell-cell spread
Spread is enhanced due to nitric oxide dependent delay in phagolysosome fusion
NOS2 inhibition reduces Listeria CFU and spread in livers of infected mice
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants HL68864, HL81151 and GM61031 for PMH and AI065638 and AI055701 for LLL from the National Institutes of Health, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akaike T, Fujii S, Sawa T, Ihara H. Cell signaling mediated by nitrated cyclic guanine nucleotide. Nitric Oxide. 2010;23:166–174. doi: 10.1016/j.niox.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Alberti-Segui C, Goeden KR, Higgins DE. Differential function of Listeria monocytogenes listeriolysin O and phospholipases C in vacuolar dissolution following cell-to-cell spread. Cell Microbiol. 2007;9:179–195. doi: 10.1111/j.1462-5822.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- Bennion JR, Sorvillo F, Wise ME, Krishna S, Mascola L. Decreasing listeriosis mortality in the United States, 1990–2005. Clin Infect Dis. 2008;47:867–874. doi: 10.1086/591131. [DOI] [PubMed] [Google Scholar]
- Birmingham CL, Canadien V, Kaniuk NA, Steinberg BE, Higgins DE, Brumell JH. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature. 2008;451:350–354. doi: 10.1038/nature06479. [DOI] [PubMed] [Google Scholar]
- Burrack LS, Harper JW, Higgins DE. Perturbation of vacuolar maturation promotes listeriolysin O-independent vacuolar escape during Listeria monocytogenes infection of human cells. Cell Microbiol. 2009;11:1382–1398. doi: 10.1111/j.1462-5822.2009.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli A, Tilney LG, Portnoy DA. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Cerro-Vadillo E, Madrazo-Toca F, Carrasco-Marin E, Fernandez-Prieto L, Beck C, Leyva-Cobian F, Saftig P, Alvarez-Dominguez C. Cutting edge: a novel nonoxidative phagosomal mechanism exerted by cathepsin-D controls Listeria monocytogenes intracellular growth. J Immunol. 2006;176:1321–1325. doi: 10.4049/jimmunol.176.3.1321. [DOI] [PubMed] [Google Scholar]
- Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W, Leimeister-Wachter M, Wuenscher M, Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. Embo J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- Endres R, Luz A, Schulze H, Neubauer H, Futterer A, Holland SM, Wagner H, Pfeffer K. Listeriosis in p47(phox−/−) and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity. 1997;7:419–432. doi: 10.1016/s1074-7613(00)80363-5. [DOI] [PubMed] [Google Scholar]
- Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15:243–250. doi: 10.1038/sj.cdd.4402184. [DOI] [PubMed] [Google Scholar]
- Erwig LP, McPhilips KA, Wynes MW, Ivetic A, Ridley AJ, Henson PM. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc Natl Acad Sci U S A. 2006;103:12825–12830. doi: 10.1073/pnas.0605331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Sabe N, Cervera C, Lopez-Medrano F, Llano M, Saez E, Len O, Fortun J, Blanes M, Laporta R, Torre-Cisneros J, et al. Risk factors, clinical features, and outcomes of listeriosis in solid-organ transplant recipients: a matched case-control study. Clin Infect Dis. 2009;49:1153–1159. doi: 10.1086/605637. [DOI] [PubMed] [Google Scholar]
- Forgac M. The vacuolar H+-ATPase of clathrin-coated vesicles is reversibly inhibited by S-nitrosoglutathione. J Biol Chem. 1999;274:1301–1305. doi: 10.1074/jbc.274.3.1301. [DOI] [PubMed] [Google Scholar]
- Fortineau N, T-CP, Gaillot O, Pellegrini E, Berche P, Gaillard JL. Optimization of green fluorescent protein expression vectors for in vitro and in vivo detection of Listeria monocytogenes. Research in Microbiology. 2000;151:353–360. doi: 10.1016/s0923-2508(00)00158-3. [DOI] [PubMed] [Google Scholar]
- Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedde MM, Higgins DE, Tilney LG, Portnoy DA. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68:999–1003. doi: 10.1128/iai.68.2.999-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SH, Wing EJ, Hoffman RA, Simmons RL. Reactive nitrogen intermediates suppress the primary immunologic response to Listeria. J Immunol. 1993;150:2901–2909. [PubMed] [Google Scholar]
- Hmama Z, Sendide K, Talal A, Garcia R, Dobos K, Reiner NE. Quantitative analysis of phagolysosome fusion in intact cells: inhibition by mycobacterial lipoarabinomannan and rescue by an 1alpha,25-dihydroxyvitamin D3-phosphoinositide 3-kinase pathway. J Cell Sci. 2004;117:2131–2140. doi: 10.1242/jcs.01072. [DOI] [PubMed] [Google Scholar]
- Jamieson AM, Yu S, Annicelli CH, Medzhitov R. Influenza virus-induced glucocorticoids compromise innate host defense against a secondary bacterial infection. Cell Host Microbe. 2010;7:103–114. doi: 10.1016/j.chom.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332:717–721. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- Mackaness GB. The Immunological Basis of Acquired Cellular Resistance. J Exp Med. 1964;120:105–120. [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Myers JT, Tsang AW, Swanson JA. Localized reactive oxygen and nitrogen intermediates inhibit escape of Listeria monocytogenes from vacuoles in activated macrophages. J Immunol. 2003;171:5447–5453. doi: 10.4049/jimmunol.171.10.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nish S, Medzhitov R. Host defense pathways: role of redundancy and compensation in infectious disease phenotypes. Immunity. 34:629–636. doi: 10.1016/j.immuni.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerda J, Cossart P. Listeria monocytogenes membrane trafficking and lifestyle: the exception or the rule? Annu Rev Cell Dev Biol. 2009;25:649–670. doi: 10.1146/annurev.cellbio.042308.113331. [DOI] [PubMed] [Google Scholar]
- Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy DA, Schreiber RD, Connelly P, Tilney LG. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J Exp Med. 1989;170:2141–2146. doi: 10.1084/jem.170.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poussin MA, Goldfine H. Involvement of Listeria monocytogenes phosphatidylinositol-specific phospholipase C and host protein kinase C in permeabilization of the macrophage phagosome. Infect Immun. 2005;73:4410–4413. doi: 10.1128/IAI.73.7.4410-4413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada-Delgado A, Carrasco-Marin E, Pena-Macarro C, Del Cerro-Vadillo E, Fresno-Escudero M, Leyva-Cobian F, Alvarez-Dominguez C. Inhibition of Rab5a exchange activity is a key step for Listeria monocytogenes survival. Traffic. 2005;6:252–265. doi: 10.1111/j.1600-0854.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- Rayamajhi M, Humann J, Penheiter K, Andreasen K, Lenz LL. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. J Exp Med. 2010;207:327–337. doi: 10.1084/jem.20091746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- Sidhu G, Li W, Laryngakis N, Bishai E, Balla T, Southwick F. Phosphoinositide 3-kinase is required for intracellular Listeria monocytogenes actin-based motility and filopod formation. J Biol Chem. 2005;280:11379–11386. doi: 10.1074/jbc.M414533200. [DOI] [PubMed] [Google Scholar]
- Singh R, Jamieson A, Cresswell P. GILT is a critical host factor for Listeria monocytogenes infection. Nature. 2008;455:1244–1247. doi: 10.1038/nature07344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AN, Camilli A, Portnoy DA. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1990;58:3770–3778. doi: 10.1128/iai.58.11.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suram S, Gangelhoff TA, Taylor PR, Rosas M, Brown GD, Bonventre JV, Akira S, Uematsu S, Williams DL, Murphy RC, Leslie CC. Pathways regulating cytosolic phospholipase A2 activation and eicosanoid production in macrophages by Candida albicans. J Biol Chem. 2011;285:30676–30685. doi: 10.1074/jbc.M110.143800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow CJ, Grinstein S, Sudsbury RA, Rotstein OD. Nitric oxide derived from L-arginine impairs cytoplasmic pH regulation by vacuolar-type H+ ATPases in peritoneal macrophages. J Exp Med. 1991;174:1009–1021. doi: 10.1084/jem.174.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Tancred L, Telepnev MV, Golovliov I, Andersson B, Andersson H, Lindgren H, Sjostedt A. Administration of a nitric oxide donor inhibits mglA expression by intracellular Francisella tularensis and counteracts phagosomal escape and subversion of TNF-alpha secretion. J Med Microbiol. 2011;60:1570–1583. doi: 10.1099/jmm.0.032870-0. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo A, Guzman NJ, Garg LC, Tisher CC, Madsen KM. Nitric oxide inhibits bafilomycin-sensitive H(+)-ATPase activity in rat cortical collecting duct. Am J Physiol. 1994;267:F509–F515. doi: 10.1152/ajprenal.1994.267.4.F509. [DOI] [PubMed] [Google Scholar]
- Welch MD, Rosenblatt J, Skoble J, Portnoy DA, Mitchison TJ. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science. 1998;281:105–108. doi: 10.1126/science.281.5373.105. [DOI] [PubMed] [Google Scholar]
- Wing EJ, Gregory SH. Listeria monocytogenes: clinical and experimental update. J Infect Dis. 2002;185(Suppl 1):S18–S24. doi: 10.1086/338465. [DOI] [PubMed] [Google Scholar]
- Yates RM, Hermetter A, Taylor GA, Russell DG. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic. 2007;8:241–250. doi: 10.1111/j.1600-0854.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- Young SH, Antonini JM, Roberts JR. Preexposure to repeated low doses of zymosan increases the susceptibility to pulmonary infection in rats. Exp Lung Res. 2009;35:570–590. doi: 10.1080/01902140902763201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.