Abstract
Previous studies have found that subjects can increase the velocity of accommodation using visual exercises such as pencil push ups, flippers, Brock strings and the like and myriad papers have shown improvement in accommodation facility (speed) and sufficiency (amplitude) using subjective tests following vision training but few have objectively measured accommodation before and after training in either normal subjects or in patients diagnosed with accommodative infacility (abnormally slow dynamics). Accommodation is driven either directly by blur or indirectly by way of neural crosslinks from the vergence system. Until now, no study has objectively measured both accommodation and accommodative-vergence before and after vision training and the role vergence might play in modifying the speed of accommodation. In the present study, accommodation and accommodative-vergence were measured with a Purkinje Eye Tracker/Optometer before and after normal subjects trained in a flipper-like task in which the stimulus stepped between 0 and 2.5 diopters and back for over 200 cycles. Most subjects increased their speed of accommodation as well as their speed of accommodative vergence. Accommodative vergence led the accommodation response by approximately 77 msec before training and 100 msec after training and the vergence lead was most prominent in subjects with high accommodation and vergence velocities and the vergence leads tended to increase in conjunction with increases in accommodation velocity. We surmise that volitional vergence may help increase accommodation velocity by way of vergence-accommodation cross links.
Keywords: accommodation, accommodative-vergence, blur, eye movements, human
INTRODUCTION
Accommodation facility is a measure of the speed of the accommodative step response and is tested subjectively in the clinic by having patients perform a flipper test in which plus and minus lenses (usually ±1.5 or ±2.0 diopters) are alternated before one or both eyes as the patient views either a far (e.g. 6 meters) or near (e.g. 40 cm) target. The subject indicates when the target is in focus by saying “clear” at which time the examiner switches to the other lens power. A preferred performance based method is to have the subject read a word from an array of words to make sure the target is focused well enough to resolve. This task continues for either a set number of flipper cycles or a fixed time period. Normal subjects can perform 12–20 complete cycles per minute depending on whether the test is done monocularly or binocularly, the power of the lenses used, the distance to the target, how the subject indicates that the target is in focus, how the lens is changed and if the subject is emmotropic or myopic (Radhakrishnan, Allen & Charman. 2007). Patients with a history of headaches, blurred vision and symptoms of asthenopia often have low flipper rates (accommodative infacility), inadequate accommodation (accommodative insufficiency) and vergence infacility or insufficiency (Rouse, 1987; Ciuffreda, 2002; Martinez, Munoz & Ruiz-Cantaro, 2009). Patients with accommodative infacility often receive vision therapy (VT) wherein they practice accommodation with flippers with near and far targets, pencil pushups where they focus on the print on a pencil while smoothly changing the distance and so on. The patients are usually required to practice these skills in the clinic under guidance and at home for a period of 4–6 weeks or longer. The precise regimen varies between clinics but usually involves practice step changes in accommodation without changes in vergence, changes in vergence without changes in accommodation and, lastly, concordant changes in both accommodation and vergence. A number of papers show that VT alleviates asthenopia in most patients (see Rouse, 1987, and, Ciuffreda, 2002 for reviews).
Although there are myriad reports showing improved flipper rates with VT, there are few reports in which objective recordings of accommodation were used to document improvements and there are no reports where vergence was simultaneously recorded. The first objective measurements of accommodation were performed by Liu, Lee, Jang, Ciuffreda, Song, Grisham & Stark, 1979 who measured accommodation before, during and after four to five weeks of vision training. Bobier and Sivak (1983) made similar measurements on four subjects with accommodative infacility and provided them with VT until they achieved normal flipper rates. Radhakrishnan, Allen and Charman (2007) used a PowerRefractor to measure the difference in accommodative facility between emmetropes and myopes and Allen, Charman and Radhakrishnan (2010) used the same method to objectively measure changes in the dynamics of accommodation after three days of VT in normal subjects. None of these studies measured the effect of training on accommodative vergence or the possible role of vergence-accommodation in improvements in accommodative facility.
Bharadwaj, Vedamurthy and Schor (2009) did not examine patients undergoing VT but examined the ability of young subjects (age 18–34) to modify the dynamics of accommodation using a paradigm which was designed to mimic the type of changes in compliance of the ocular lens that occur with age. These researchers pointed out that, even though the lens becomes less compliant during nascent presbyopia, the dynamics of accommodation change much less than might be expected (Heron, Charman & Schor, 2001). In conjunction with the observation that the response of accommodative-vergence per diopter of accommodation (AC/A ratio) increases with aging, which suggests a greater effort to accommodate with age, these authors interpreted these findings to mean that the neural control system for accommodation adapts with age. The training paradigm used by Bharadwaj et al. was inspired by the double-step method used in saccade adaptation studies wherein a target is presented in the periphery and then jumped during the saccade to a new location either just a bit short of the original target or a bit beyond (see Hopp & Fuchs, 2004, for a review). In their implementation, subjects were presented with an initial step in defocus from 0D to 2D. After 350 msec (the average latency period for an accommodation response) the stimulus stepped from 2D to 4D. The notion was that feedback in the system would report a shortfall in the accommodative response and increase the initial dynamics and, in fact, most subjects showed an increase in both accommodation velocity and acceleration following training. In separate sessions the double step called for a reduction in accommodation wherein the target was initially stepped from 0D to 2D and after a gap of 350 msec, stepped back to 1.75D. Most subjects did not adapt well to this stimulus as will be discussed later.
Most models of accommodation assume that accommodation is driven primarily by blur but that vergence also contributes by way of neural cross links between the two systems, so called, vergence-accommodation. Likewise, vergence is driven primarily by disparity but is also driven by accommodation by way of accommodative-vergence crosslinks. While the dynamics of accommodation following VT have been measured objectively (Liu et al., 1979; Bobier & Sivak, 1983; Radhakrishnan et al., 2007; Bharadwaj et al., 2009; Allen et al., 2010) the associated accommodative-vergence has not. The present experiment essentially repeat the adaptation experiment of Bharadwaj et al. (2009) with the addition of recording eye position for both eyes in order to measure accommodative-vergence.
METHODS
Subjects
Twelve young adults (2 male, 10 female) ages 20–27 were used. All of the subjects were recruited from the University’s student population and all but three were students in the School of Optometry. None of the subjects were told the purpose of the study. Subjects were remunerated for their time. Five were emmetropes and the others were myopes with corrections of less than −2.00 D (spherical equivalent). Subject WL had a sizable exophoria (6D at distance and 16D at 40 cm) so her accommodative vergence data were not used. Each subject was briefed on the experiments and signed a written consent form that was approved by the human subjects committee at the University of California. Phenylephrine eye drops (2.5%) were administered prior to recording and we made sure that pupil diameter remained constant throughout the experiment as fluctuations of the pupil would interfere with accurate optometer measurements of accommodation. The 2.5% concentration is below the 10% concentration found to influence accommodation dynamics (Mordi, Tucker & Charman, 1986). A concerted effort was made to prevent the subjects from experiencing fatigue. Trials were self-paced and initiated by the subject with a button press. Subjects were encouraged to take frequent breaks and saline eye drops were administered periodically since subjects tend to blink much less often when they are in the SRI eye tracker.
Procedure
Targets were projected onto a tangent screen that was 180 cm from the exit pupil of a Badal stimulus optometer mounted in a binocular dual Purkinje eye tracker (SRI) and Scheiner measurement optometer. The exit pupil is conjugate to the pupil of the eye. The characteristics of the SRI system have been described previously (Bharadwaj & Schor, 2005). The Badal lens system produces a barely noticeable size change so for all intents and purposes there is no size cue for changing distance although there is a constant size-distance cue. Prior experiments showed that in the absence of disparity or other distance cues, the subjects were unable to judge the direction of blur to large steps (>2D; Heath, 1956; Fincham & Walton 1957; Ogle 1966; Crane, 1966) and as a result the latencies and velocities of accommodative vergence were extremely variable in magnitude and direction. Since we are primarily interested in dynamics of the step response and not stimuli, we chose to make the direction of the accommodative step stimulus predictable though the onset of the stimulus relative to the button press and the size of the step in defocus or the size of the disparity were not predictable. Because of this predictability, we did not analyze onset latencies, as discussed below. We knew anecdotally that some subjects had difficulty accommodating to targets in the absence of other cues for distance and we always afforded them a few (10–20) steps in accommodation to familiarize them with the eye tracker. Subjects were occasionally given feedback as to how well they were accommodating and were encouraged to focus the targets sharply.
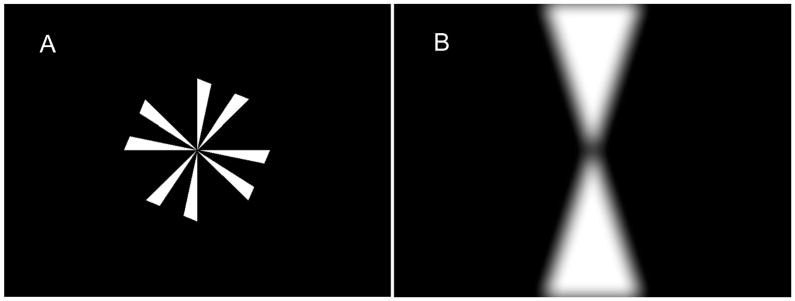
Subjects viewed the targets through irregularly shaped apertures that prevented them from seeing anything other than the projected targets, and from binocularly fusing the apertures, such that there were no cues for distance other than blur (or disparity) and the subject’s recollection of the distance to the target. The target for accommodation was a Maltese cross with wedges removed to increase the spatial frequency and rotated slightly so that two edges were sharp and did not require anti-aliasing (Figure 1a). Subjects were instructed to fixate the center of the cross and were encouraged to make it as clear as possible. The Maltese cross was projected onto a tangent screen with the center of the cross aligned with the subject’s left eye and with the left stimulus optometer. The cross had a diameter of approximately 10 degrees. Unless extraordinary measures are taken (Schor, Lott, Pope & Graham, 1999) movement of the left eye relative to the optometer may result in an artifactual change in optometer output. For that reason, we made sure that movement of the eye did not exceed a half degree. Subjects initially saw the target binocularly. When they were ready, they pressed a button that initiated the trial. The telecentric lens in front of the left eye then stepped to form a virtual image before the Badal lens that stimulated from 0D to either1.5, 2.5 or 3.5 D with an unpredictable delay between the button press and the step. At the same time, the right eye was occluded automatically thereby leaving vergence open loop. The lens system stepped back to 0 D after 3 seconds and the right eye was unoccluded so that the view of the target was again binocular. Accommodation trials were run in blocks of 24 and two blocks were run before and after the training period. Nine of the twelve subjects trained with both the Single-Step and Double-Step paradigms, one subject performed only the Single-Step trials and two subjects performed only the Double-Step trials. The subject who performed both types of trials did so on different days and we varied the order of which paradigm was given first in case the first session affected the second. Four of the subjects performed the Single-Step experiment first and five performed the Double-Step experiment first.
Figure 1.
Targets. A. Modified Maltese cross with a diameter of 10 degrees. B. Low-pass filtered hourglass-shaped target with a width of 6 degrees and a height of 22 degrees.
The accommodation tests were followed by 2 blocks of 20 disparity vergence trials. The target for disparity vergence was an hourglass-shaped figure (Figure 1b) that was low-pass filtered so as to not stimulate accommodation (Tsuetaki & Schor, 1987). The target had a width at its base of 6 degrees and a height of 22 degrees. Crossed horizontal disparities were presented with both eyes unoccluded by equally deflecting the right and left horizontal mirrors of the SRI tracker nasally. The size of the disparity varied pseudo-randomly between 1, 2, 3 and 4 degrees. Because vergence movements were symmetrical the vergence movements took the left eye out of alignment with the optometer, accommodation was not measured during disparity vergence trials.
Following pre-testing, the subjects trained with one of two adaptation paradigms. The first paradigm replicated the adaptation method used by Bharadwaj et al (2009), their “increasing paradigm,” in which the focus stimulator first stepped from 0 to 2D and after 350 msec stepped from 2D to 4D. Bharadwaj et al. did not test to see whether a double step was necessary for adaptation. To test this, a procedure was run in a second session in which subjects trained with single steps in defocus where the focus stimulator jumped from 0 to 2.5D, remained at 2.5D for 3 seconds and returned to 0D. As with testing, the subject viewed the target binocularly at the beginning of each trial, the right eye was occluded when the trial was initiated with the button press and the subject saw the target binocularly again at the end of the trial so as to avoid the change in phoria that might occur had they been kept monocular throughout the training and testing period. Eight blocks of 25 training trials were run for a total of 200 trials. We then repeated the pre-training tests of 48 accommodation trials and 40 disparity vergence trials.
Calibration
The right and left eye position trackers were calibrated separately for each eye using targets that were placed at optical infinity. The targets for the right and left eyes were separated by 6 cm, the average interpupillary distance. The five targets were presented at −10, −5, 0, 5 and 10 degrees. The optometer could not be calibrated in a like manner because there is no way to be sure that the subjects fully accommodated to the demands of the stimulus. Instead, we tested each subject on a Stigmatoscope- Haploscope under stimulus conditions that were as similar as possible to the SRI stimulus optometer, the chief difference being that the target viewed in the SRI was projected onto a screen and the same target for the stigmatoscope was printed on a card so that proximal cues could have been different in the two cases. The accommodative response measured on the stigmascope was used to calibrate the output of the SRI’s optometer. For example, if the subject had a stimulus-response slope of 0.9 on the stigmatoscope then we assumed their response to the stimuli on the SRI would be the same, so instead of calibrating the output voltages of the optometer to the size of the stimulus (1.5, 2.5 and 3.5 D), we converted the output voltages to 90% of those values. In retrospect, a more accurate calibration may have resulted by using real targets placed at several different distances to elicit maximal accommodation in both the stigmatoscope and eye tracker because accommodation is more accurate when proximal and disparity cues are available (Gwiazda, Thorn, Bauer & Held, 1993; Horwood & Riddell, 2008). Thus we more than likely overestimated accommodation amplitudes and velocities. Because pre-training measurements were compared to post-training measurements it is relatively unimportant if the calibrations were perfect for these comparisons. Imprecise calibrations could affect a comparison of accommodation speeds between subjects, however. This caveat would not apply to vergence measurements since those calibrations were precise.
Data Analysis
Vergence eye movements and accommodation responses were recorded at 200 Hz and analyzed by a custom interactive Matlab program (Maxwell, Tong & Schor, 2010). The raw eye position and accommodation signals from the two eyes were first smoothed by a 10-point sliding average filter. Vergence was calculated as left-eye position minus right-eye position and conjugate eye position was calculated as the average of left-eye and right-eye positions. The vergence and accommodation data were differentiated with a central-difference algorithm spanning five samples and subsequently smoothed by a 10-point sliding average filter to obtain velocity (deg/sec) profiles. Degrees were converted to meter angles (MA) which are easily compared to diopters (D) since both express distance in reciprocal meters, for example, a target at 1.0 meter requires 1.0 D of accommodation and 1.0 MA of vergence. 1.0 MA is equal to approximately 3.4 degrees of vergence for a nominal 6 cm interpupillary distance. The program first aligned all of the traces for a given stimulus size (usually 16 for accommodation trials) by peak velocity. The trials were aligned by peak accommodation velocity for averaging accommodation and by peak vergence velocity for averaging vergence. Occasionally, when there were multiple peaks of velocity in an individual trace, the peak selected by the program was not the peak that aligned best with the mean trace as a whole in which case the operator manually adjusted the temporal alignment of the individual trace with respect to the mean trace. Once the individual traces were optimally aligned with the mean trace (and each other), the program found the response onset, offset, peak velocity and time to peak velocity for each trial and also for the mean trial. The onset of convergence was defined as the point where the convergence velocity of five successive points first exceeded 2 degrees/sec and accommodation onset was defined as the point where the velocity first exceeded 0.5 diopters/sec. The offset of the convergence response was defined by the point at which the convergence velocities of five successive points were less than 5% of the peak velocity. The onset and offset latencies were somewhat difficult to ascertain in the individual trials but not for the average traces. Vergence and accommodation amplitudes were calculated as the amplitude at offset minus the amplitude at onset. It is important to note, therefore, that amplitudes are not the maximal amplitudes but the amplitude at which the velocities fell below 5% of the peak velocity, unless otherwise specified.
RESULTS
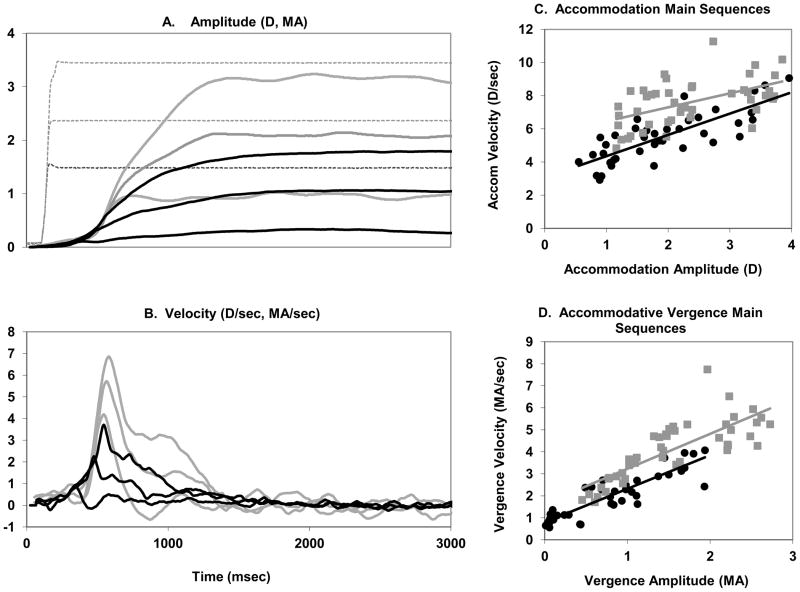
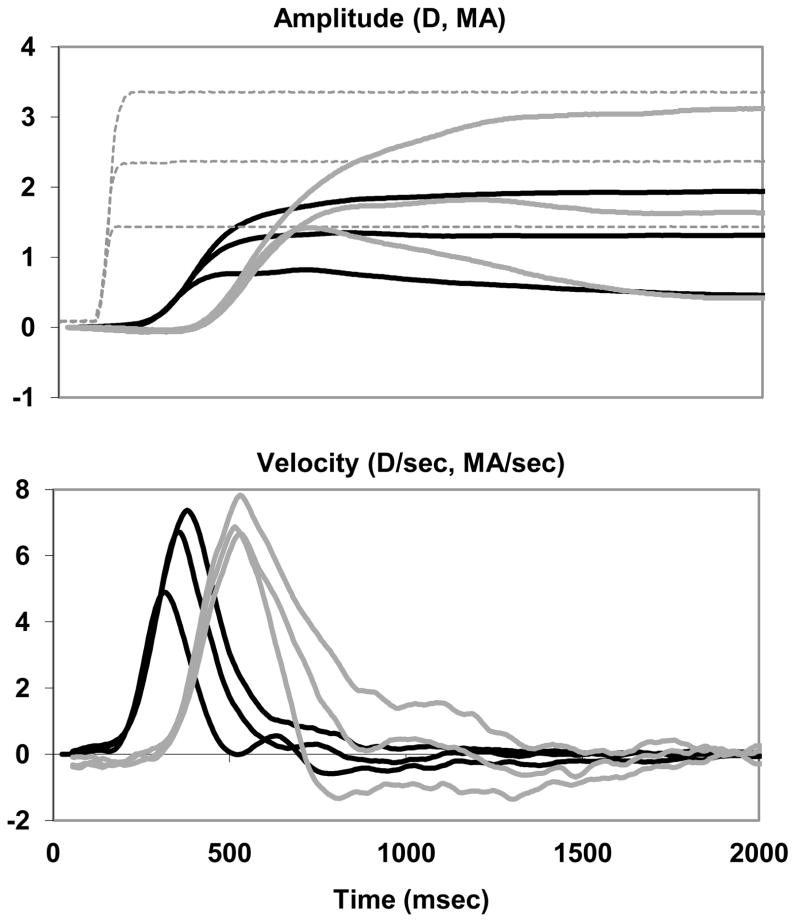
Figure 2 illustrates the accommodation and accommodative vergence responses to three step amplitudes (1.5D, 2.5D, 3.5D) of subject SS to the double step paradigm. Each trace represents the average of approximately 16 individual traces aligned by peak accommodation velocity for the accommodation traces and by peak vergence velocity for accommodative-vergence traces. The traces were temporally aligned using the mean time to peak for accommodation and vergence relative to stimulus onset so that the relative timing of the two traces was maintained. The peak velocity of the accommodation and accommodative vergence responses increased with response amplitude. In our experience, when disparity, blur and other cues for distance are available, accommodation and accommodative vergence increase smoothly and the velocities have one clear peak. When the blur cue is well isolated, on the other hand, accommodation and vergence do not increase smoothly and may have several peaks. The second part of Figure 2(c, d) show the main sequences (velocity vs. amplitude) for accommodation and vergence with linear regression lines superimposed. The main sequences were plotted for each subject in each condition and linear equations were derived. The linear equations were then used to calculate the velocity at a response amplitude of 2.5 D for accommodation and 2.5 MA for accommodative vergence. This analysis allowed us to use one pair of numbers to represent each subject in subsequent plots.
Figure 2.
Accommodation (gray lines) and accommodative vergence (black lines) amplitude (A)and velocity (B) responses to three stimulus amplitudes (1.5D, 2.5D 3.5D) for subject SS (Double Step paradigm). Each trace is the average of approximately 16 trials aligned by peak accommodation velocity or peak vergence velocity. Dotted line: stimulus. C, D. Main sequences for subject SS (Double Step Paradigm) pre-training (black diamonds) and post-training (gray squares) along with lines fit by linear regression.
The Effect of Training on Accommodation and Accommodative Vergence Velocity
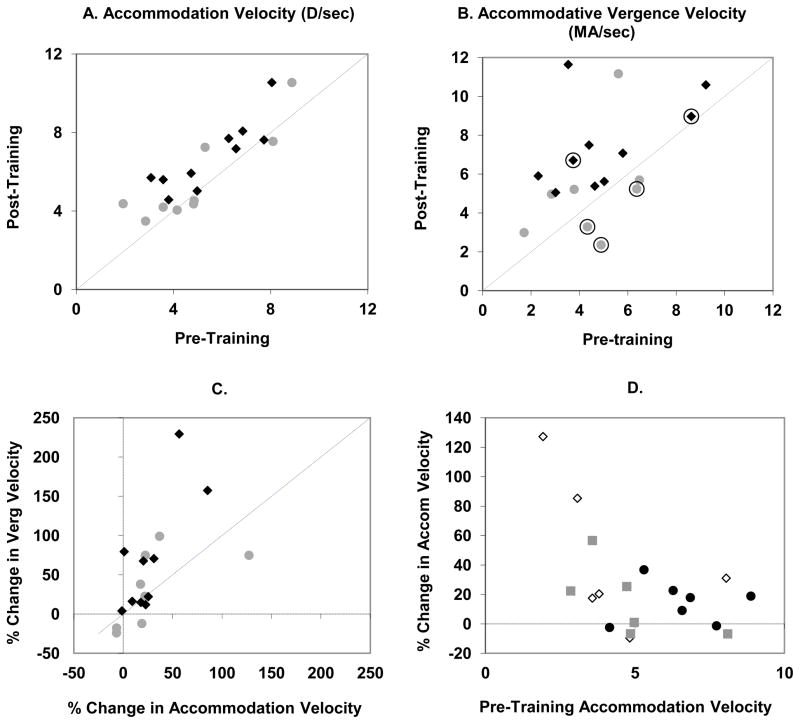
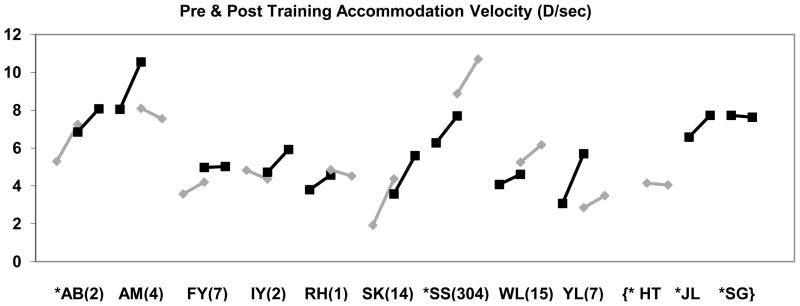
Most of the subjects increased the velocities of accommodation and accommodative vergence regardless of whether the training was with Single Steps or Double Steps. The average accommodation velocity for an accommodation response of 2.5D increased from 5.1 D/sec (s.d. = 1.9) pre-training to 6.2 D/sec (s.d.= 2.0) post-training, an increase of 24% (s.d.= 32). Figure 3a shows post-training accommodation velocity relative to pre-training velocity and Figure 3b shows post-training accommodative vergence velocity relative to pre-training accommodative vergence velocity. Any values above the unity (dotted) line represent an increase in velocity. The data are shown separately for the Single Step paradigm (gray circles) and Double Step paradigm (black diamonds). Of the 12 subjects and 21 Single and Double-Step training sessions, 5 resulted in a decrease in accommodation velocity. If these 5 sessions are excluded from the means, then the mean accommodation velocity increased from 5.0 D/sec (s.d.= 2.0) to 6.4 D/sec (s.d. = 2.16). Figure 3c indicates that the percent increase in accommodative vergence velocity tended to exceed the increase in accommodation velocity. The average pre-training accommodative vergence velocity for a 2.5 MA response was 4.8 MA/sec (s.d. = 2.0) and the post-training vergence velocity was 6.4 MA/sec (s.d. = 2.7) which constitutes a change of 33% over all the subjects. If only the sessions that showed an increase in accommodation velocity are averaged then there was an increase in accommodative vergence velocity of 52% from 4.5 MA/sec (s.d. = 2.0) to 6.8 MA/s (s.d. = 2.7). The experiment was not designed to do a thorough statistical analysis of potential differences between Single Step and Double Step training and as a result there are not enough data to perform meaningful statistics to definitively answer the question of whether Double-Step trials are more effective than Single Step trials. A complete understanding of the effect of the paradigm would require not only a comparison of Single Step to Double Step training but also the order of presentation (Single of Double step as the first or second session), the number of days between sessions and the prior experience of the subject. Figure 3a shows that most of the trials in which adaptation did not occur were Single Step trials. Figure 4 makes the potential influence of the order of presentation more clear. The data for Single Step and Double Step trials are shown in the order in which the sessions were given for each subject. Each pair of symbols show the mean pre-training and post-training peak accommodation velocity values for each subject joined by a line for Single Step (gray diamonds) and Double Step (black squares) training. It is evident in Figure 4 that many of the subjects, AB, RH, SK, for example, increased their accommodation velocity and accommodative vergence velocity in the first session and maintained the elevated velocity through to the beginning of the next session. Many subjects further increased their velocities in the second session. Approximately 10 months elapsed between the Double Step and Single Step sessions for subject SS yet her pre-training accommodation velocity was still elevated from the first session.
Figure 3.
Effect of training on the velocities of accommodation (A) and accommodative vergence (B) for the Single Step (gray circles) and Double Step (black diamonds) paradigms. Each symbol represents the pre-training and post-training velocities for one subject calculated for 2.5D or 2.5 MA. The vergence velocities circled in B are sessions in which there was a decrease in accommodation velocity, i.e. those below the unity line in A. C. Comparison of the % increase in velocity between accommodation and accommodative vergence for Single Step (gray circles) and Double Step (black diamonds) paradigms. D. The relationship between pre-training accommodation velocity and the increase in velocity after training for naïve subjects (open diamonds), subjects in their second session (gray squares) and experienced subjects (black circles).
Figure 4.
Comparison of the first and second sessions. Each pair of points joined by a line represents the pre-training accommodation velocity followed by the post-training accommodation velocity for each subject for Double-Step trials (black squares) and Single Step trials (gray diamonds). The data are presented in the order in which the subject performed the experiment with the earliest session on the left. The subject’s initials are below their data and the number in parentheses indicates the number of days between session 1 and session 2. The three subjects on the right side of the plot performed only one of the two paradigms.
Somewhat evident in Figure 4 but made more explicit in Figure 3d is the observation that subjects who had relatively high accommodation velocities in the pre-training trials tended to adapt less than subjects who started out with low velocities as also observed by Bharadwaj et al. (2009). The data in Figure 3d are divided into three groups: The first (open diamonds) had received experience during their initial orientation only. The second group (gray squares) had already performed one of the two paradigms and the third group (black circles; subjects AB, HT, JL, SG and SS) had served as subjects in a prior experiment (Maxwell et al., 2010), were very familiar with the experimental apparatus and had performed hundreds of accommodation and disparity vergence trials before starting the present experiment. These subjects tended to have the highest pre-training velocities and the smallest increase in accommodation velocity.
Relative Latencies
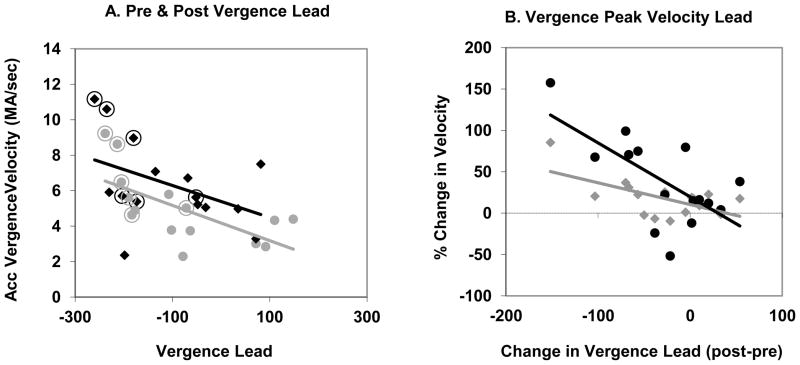
Onset latencies (i.e., the time between stimulus and response onsets) are often used to describe adaptive changes in oculomotor behavior. In the present case, onset latency was not considered a good measure of adaptation because the direction of the step stimulus was entirely predictable. In fact, the onset latency of both accommodation and accommodative vergence not only shortened considerably with training but in a few cases the response actually preceded the onset of the stimulus even though the size of the stimulus and the delay between the button press and the step in defocus were randomly varied. For this reason, we did not measure onset latency with respect to the stimulus but instead measured the difference in time between vergence onset and accommodation onset as well as the difference in time to peak vergence velocity and peak accommodation velocity. It has been shown in prior experiments (Maxwell et al., 2010; Schor et al., 1999; Judge & Cumming, 1986; Wilson, 1973) that accommodative vergence onset paradoxically precedes accommodation onset and may be due to a difference in the two plants. In the present case, vergence onset led accommodation onset by an average of 111 msec before training and by 135 msec after training for the subjects as a group though a t-test showed that these differences were not significant. Likewise, vergence peak velocity led accommodation peak velocity by a mean of 70 msec before training and 99 msec after. Again, these differences were not statistically significant. It should be noted that vergence did not lead accommodation for all subjects as might be supposed if the vergence lead were only the result of different plant dynamics. For example, there is no apparent vergence onset lead for the subject shown in Figure 2 and the slight vergence lead that is seen in time to peak velocity for this subject might well be because the peak velocities were much smaller for vergence than for accommodation. For other subjects such as SG (Figure 5a) peak vergence velocity clearly led peak accommodation velocity. SG was unusual in that she alone had very clear, clean single peaks of both accommodation and vergence velocity. It is probably relevant that SG was a very experienced subject who participated not only in a prior experiment (Maxwell et al., 2010) but, was a subject in a number of pilot experiments. Subjects with greater vergence peak velocity leads tended to have higher accommodative vergence velocities in both pre-training and post-training trials (Figure 6a) and an increase in vergence lead was associated with an increase in vergence and accommodation velocity after training (Figure 6b). The more experienced subjects (circled symbols in Figure 6a) tended to have greater vergence leads and correspondingly greater velocities. We will offer possible reasons for this in the discussion section.
Figure 5.
Accommodation (gray lines) and accommodative vergence (black lines) for subject SG (Double Step paradigm) showing the vergence lead. Dotted lines: stimulus amplitude.
Figure 6.
A. The relationship between vergence lead (time to peak vergence velocity – time to peak accommodation velocity) and peak accommodative vergence velocity pre-training (gray circles) and post-training (black diamonds). Each symbol represents one subject for either the Double-Step or Single-Step session. The data points for the well-experienced subjects are circled. B. The change in accommodation (post-training as a percentage of pre-training; gray diamonds) and accommodative-vergence velocity (black circles) as a function of the change in vergence lead (post-training – pre-training).
Disparity Vergence
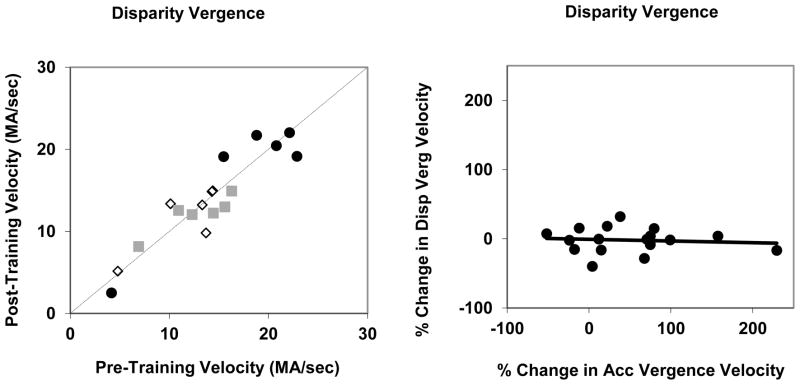
Disparity vergence velocity, on average, was approximately twice as high as accommodative vergence velocity for corresponding vergence amplitudes. The mean main sequence slope for disparity vergence was 3.13 MA/sec per MA and the mean slope for accommodative vergence was 1.44. This conformed to our prior finding (Maxwell et al., 2010) that disparity vergence velocity was much greater than accommodative vergence given the same amplitude response. The training of accommodation and the resulting increase in accommodative vergence velocity had no effect on disparity vergence velocity (Figure 7). Disparity vergence had a mean velocity of 4.2 MA/sec for both pre-training and post-training trials when all trials are considered and velocities of 4.7 for pre-training and 4.6 MA/sec for post-training when the means are calculated for only those sessions in which the post-training accommodative velocity was greater than the pre-training velocity. The change in accommodation velocity and accommodative vergence velocity had no significant correlation with the velocity of disparity vergence (Figure 7b).
Figure 7.
A. Disparity vergence velocity pre-training and post-training for naïve subjects (open diamonds), subjects in their second session (gray squares) and subjects with more than two sessions of experience (black circles). B. The % change in disparity vergence pre-training to post-training as a function of the % change in accommodation velocity.
DISCUSSION
The most experienced subjects had both higher pre-training accommodation and accommodative-vergence velocities, and training had the least effect on subjects who started out with relatively high velocities. Somewhat surprisingly, the most experienced subjects also had the highest velocities of disparity vergence even though disparity vergence received no specific practice outside of the 40 pre-training and 40 post-training test trials. Vergence onset preceded accommodation onset due possibly to the relative speeds of the vergence and accommodation plants (Wilson, 1973; Schor et al., 1999; Maxwell et al., 2010). The vergence leads were greater for the more experienced subjects and vergence leads tended to increase in the sessions in which accommodation velocity increased. The accommodative vergence velocity that was measured with vergence open loop in response to steps in defocus tended to increase more with training than did accommodation velocity. SG was our most experienced subject, having performed accommodation and vergence movements in a number of pilot experiments. She was the only subject in a prior study of accommodation and vergence dynamics (Maxwell et al., 2010) whose accommodative vergence velocities were higher than her disparity vergence velocities. In both studies, she was the only subject who consistently had one clean peak in accommodative vergence velocity (Figure 5) and we speculated in the prior paper that she may have been using voluntary vergence-accommodation to help drive accommodation.
The fact that accommodative vergence occurred earlier than the accommodation response (Figures 5 & 6) was not unexpected based on our prior results (Maxwell et al., 2010) and the results of others (Wilson, 1973; Schor et al., 1999). The stimulation experiments of Judge and Cumming (1986) partly explain the paradox of accommodation-driven vergence occurring sooner than accommodation in that the electrical stimulation of midbrain near-response neurons that drive both systems resulted in shorter latencies for vergence presumably because the vergence plant is faster than the accommodation plant. Figure 6 also shows that after training, the vergence lead increased for the subjects who had increased their accommodation and vergence velocities. This means that either the vergence peak occurred with a shorter latency after training or the accommodation peak velocity occurred later. We cannot tell which from our data given that the direction of the step in defocus was predictable meaning that the onsets of both responses decreased. If the vergence lead were due to the plant alone, we are at a loss to explain how the lead of accommodative vergence could change with training and why not all subjects showed similar vergence leads. Taken together, these observations suggest that the increase in accommodation velocity measured in the current study, and perhaps with vision training in patients, may be at least partly mediated by vergence-accommodation evoked by volition or perceived distance from defocus cues (McLin & Schor 1988). There are no prior studies in which both accommodation and vergence dynamics were recorded together before and after vision training and verification of this finding would be welcome.
A potential problem for clinical and laboratory tests of accommodation and accommodative vergence velocity is that cues for distance are in conflict. Normally, disparity, blur and proximal cues (the perceived, or imagined, distance to the target) all signal the same distance. Generally, disparity gives the most reliable estimate and is used to drive both vergence and accommodation (Gwiazda, Thorn, Bauer & Held, 1993; Horwood & Riddell, 2008). With monocular flipper tests, where there is no disparity cue for vergence, the blur cue is at odds with proximal vergence because blur changes while the size-distance cue does not. With binocular flipper tests, both the disparity and size-distance cues for vergence signal that target distance is not changing and so these cues are directly at odds with the blur cue. In addition the accommodative vergence evoked by the accommodative response must be compensated for (nulled) with disparity vergence during the binocular flipper stimulus. These are likely reasons why binocular flipper rates are typically lower than monocular flipper rates. In the accommodation measurements in the present experiment, we went to great lengths to isolate the step in blur from other cues to distance in order to measure accommodation and accommodative-vergence in isolation but the isolation of cues is, of course, unnatural (although modern technology, such as 3-D video displays are giving us more experience with mismatched cues for distance). It is possible that much of the improvement in accommodative velocity was simply the subjects learning how to accommodate in an impoverished visual environment with volitional vergence-accommodation. If this were true then we would hypothesize that accommodative velocity increases would be increased more with monocular flipper training than binocular training where accommodative vergence would be inhibited. It is equally possible that patients who score poorly on flipper tests simply find it difficult to accommodate when the blur cue is in conflict with other cues to proximity. This is not to trivialize the use of VT in any way since it has been shown in myriad studies to be effective in eliminating or ameliorating asthenopia, but, based on the present experiment, much of the increase in flipper rate may have to do with prediction of the stimulus and with learning to accommodate in an unusual visual situation.
Prior adaptation studies
Bharadwaj et al. (2009) examined the ability of the accommodation system to increase the neural drive to the plant in order to maintain the velocity of accommodation as the lens loses compliance (Heron, Charman & Schor, 2001; Mordi & Ciuffreda, 2004). Bharadwaj et al. used a double step adaptation protocol that was inspired by the double step paradigm typically used to modify saccade amplitude (Hopp & Fuchs, 2004) and vergence dynamics (Munoz, Semmlow, Yuan & Alvarez, 1999; Takagi, Oyamada, Abe, 2001). With saccadic adaptation, a target is presented in the periphery, a computer algorithm detects the onset of the saccade and jumps the target either forward or backward during the saccade when vision is suppressed. Because saccades are essentially ballistic and do not use continuous visual feedback to guide their movements, they are kept accurate by an adaptive mechanism that checks the errors at the end of saccades and modifies the size of the saccades to land within a tolerable distance to the target. Bharadwaj et al. (2009) also used a double-step paradigm and even though, unlike saccades, accommodation is continuously sampled. The assumption of those authors was that the double step paradigm would be effective if the second step were presented during the latency period of the accommodation response. Unfortunately, Bharadwaj et al. did not test to see if the double steps were necessary. The result of our Single Step experiment demonstrates that vergence velocity can be increased by practice and may be as effective as the double step although we did not have enough data to test this definitively. We would have needed many more trials where the Single Step or Double Steps alternated between the first and second sessions in otherwise naïve subjects. The fact that many subjects adapted to the Single Step paradigm supports the idea that the increase in dynamics was the result of a higher level practice effect and not a low-level parametric adjustment as is seen with adaptation of the vestibulo-ocular reflex or the adaptation of eye alignment (Maxwell & Schor, 2006). That is, the subjects were learning how to accommodate in an impoverished visual environment where blur was the only cue but where the direction of the stimulus was predictable and with occasional feedback from the experimenters telling them when they were doing well and when they were doing poorly.
As is often the case with saccade adaptation, Bharadwaj et al. attempted to increase accommodation velocity in one paradigm and decrease it in another. Since accommodation is continuously sampled, accommodation amplitude would not be expected to change with training, as it does with saccade adaptation, but the dynamics of the response might and their study showed that both velocity and acceleration could be increased by many of their subjects. There was no evidence that subjects could reliably decrease the dynamics of their accommodation, however, and only one subject was able to significantly decrease his dynamics in the decreasing paradigm and increase it in the increasing paradigm. A possible explanation for the poor results in the decreasing experiment is that Bharadwaj et al. used the same pre-training data for both the increasing paradigm and the decreasing paradigm with the increasing paradigm usually performed first and the decreasing paradigm on a subsequent date. Our Figure 4 shows that many subjects retained the increased dynamics up to weeks later (10 months later for subject SS). If this were also true in the aforementioned study then the measured change in accommodation velocity from pre-training to post-training could have been significantly underestimated. For example, if a subject had increased his velocity in the first session from 10 to 12 D/sec and decreased his velocity from 12 to 10 D/sec in the decreasing paradigm it would have appeared as though no adaptation occurred in the second experiment.
There are several other studies in which accommodation was measured objectively before and after vision training although accommodative vergence was not measured. Two of these studies used patients with asthenopia (Liu et al., 1979; Bobier & Sivak, 1983), two used normal subjects (Radhakrishnan et al., 2007; Allen et al., 2010) and one measured the change in accommodation over a one week period with no explicit vision training (Randle & Murphy, 1974).
In the earliest of these studies (Liu et al., 1979), three subjects diagnosed with accommodative infacility were trained with pencil pushups and ±1.5 D flippers and showed a modest decrease in the time constant and latency of accommodation following training. Peak velocities were not calculated but would have increased with VT assuming no change in accommodative amplitude. The authors stated that controls were performed using subjects with low flipper rates but who did not receive VT training and with normal subjects who did receive vision training. The authors claimed that the accommodation dynamics of the control subjects did not change, though, unfortunately, no data were presented. This statement is at odds with the present data, the study of Bharadwaj et al. (2009) and with the report of Allen et al. (2010) all of whom found increases in accommodation velocity with vision training in normal subjects.
Bobier and Sivak (1983) tested four subjects with subjective infacility before and after VT along with one control who received no training. Subjects were tested subjectively with ± 2 D flippers and objectively with a photorefractor. Test subjects received training until their flipper rates were better than 12 cycles per minute which took about two weeks for the two subjects whose data were shown. Velocities and time constants were not calculated but, for the two subjects whose data were shown, it appears that nearly all of the improvement resulted from shorter latencies and not from decreases in the time to make the movement. It should be noted that because the subjects alternately fixated targets at two fixed distances, that the direction and size of the accommodation change was predictable and proximal cues for depth may have been available as well.
In the most thorough objective test of vision training (Allen et al., 2010), asymptomatic subjects with normal flipper rates were tested before and after 3 days of VT along with a control group. Eighteen subjects received VT over a three-day period and 18 subjects received no training. The test group consisted of equal numbers of emmetropes and myopes. Accommodative facility for the treatment group was measured subjectively with flippers and objectively with a PowerRefractor the day before (day 1) and the day after (day 5) the 3 day training period. The control group, which received no VT, was tested only subjectively on days 1 and 5 and did not show an increase in accommodative facility which was not surprising given their limited exposure to the test stimulus. The facility rates were higher for emmetropes than myopes and the velocity of accommodation was greater for near targets than for far targets. For emmetropes the velocity increased with training from 6.5 D/sec to 9.8 D/sec after training at distance and decreased from 9.9 to 8.9 D/sec for the near distance. For myopes, accommodation velocity increased from 6.1 to 7.8 for the distant target and from 7.3 to 11.3 for the near target. Our data was the most similar to their group of emmetropes at distance since our subjects were tested with targets between 0 and 3.5D, 7 of our 12 subjects were emmetropes and the non-emmetropes averaged −0.8D of correction (range −2.0 to +0.25).
Randle and Murphy (1974) also tested normal subjects but their subjects received no explicit vision training. They were tested on an SRI optometer every three hours (except for a sleep period) over a period of 7 days. The stimuli used for testing were periodic square waves and sine waves with peak-to-peak amplitudes of 4D. Their subjects, therefore, received training similar to ours except in 120 second increments every 3 hours spread out over 7 days. All of their subjects but one (the subject who started out with the highest accommodation velocities at the start of the project) increased their accommodation velocities over the test period until they nearly reached the same velocity of the subject who started out with high velocities and did not adapt. All of their subjects increased the gain and decreased the phase lag of accommodation, presumably, due to the predictability of the targets. We also found, as did Bharadwaj et al. (2009) that subjects who had relatively high baseline velocities tended to adapt less to the training.
Accommodation and accommodative vergence were measured objectively
Velocities were measured before and after short-term vision training
Accommodation and accommodative vergence velocities increased together
Accommodative vergence lead accommodation by about 100 msec
Vergence leads were larger in experienced subjects who also had higher velocities
Acknowledgments
This work was supported by National Eye Institute Grant EY017678
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen PM, Charman WN, Radhakrishnan H. Changes in dynamics of accommodation after accommodative facility training in myopes and emmetropes. Vision Res. 50(10):947–955. doi: 10.1016/j.visres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Bharadwaj SR, Schor CM. Acceleration characteristics of human ocular accommodation. Vision Res. 2005;45(1):17–28. doi: 10.1016/j.visres.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Bharadwaj SR, Vedamurthy I, Schor CM. Short-term adaptive modification of dynamic ocular accommodation. Invest Ophthalmol Vis Sci. 2009;50(7):3520–3528. doi: 10.1167/iovs.08-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobier WR, Sivak JG. Orthoptic treatment of subjects showing slow accommodative responses. Am J Optom Physiol Opt. 1983;60(8):678–687. doi: 10.1097/00006324-198308000-00006. [DOI] [PubMed] [Google Scholar]
- Cacho Martinez P, Garcia Munoz A, Ruiz-Cantero MT. Treatment of accommodative and nonstrabismic binocular dysfunctions: a systematic review. Optometry. 2009;80(12):702–716. doi: 10.1016/j.optm.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Ciuffreda KJ. The scientific basis for and efficacy of optometric vision therapy in nonstrabismic accommodative and vergence disorders. Optometry. 2002;73(12):735–762. [PubMed] [Google Scholar]
- Crane HD. A theoretical analysis of the visual accommodation system in humans. NASA CR-606. NASA Contract Rep NASA CR. 1966:1–77. [PubMed] [Google Scholar]
- Fincham EF, Walton J. The reciprocal actions of accommodation and convergence. J Physiol. 1957;137(3):488–508. doi: 10.1113/jphysiol.1957.sp005829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Held R. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci. 2005;82(4):273–278. doi: 10.1097/01.opx.0000159363.07082.7d. [DOI] [PubMed] [Google Scholar]
- Heath GG. Components of accommodation. Am J Optom Arch Am Acad Optom. 1956;33(11):569–579. doi: 10.1097/00006324-195611000-00001. [DOI] [PubMed] [Google Scholar]
- Heron G, Charman WN, Schor CM. Age changes in the interactions between the accommodation and vergence systems. Optom Vis Sci. 2001;78(10):754–762. doi: 10.1097/00006324-200110000-00015. [DOI] [PubMed] [Google Scholar]
- Hopp JJ, Fuchs AF. The characteristics and neuronal substrate of saccadic eye movement plasticity. Prog Neurobiol. 2004;72(1):27–53. doi: 10.1016/j.pneurobio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Horwood AM, Riddell PM. The use of cues to convergence and accommodation in naive, uninstructed participants. Vision Res. 2008;48(15):1613–1624. doi: 10.1016/j.visres.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Cumming BG. Neurons in the monkey midbrain with activity related to vergence eye movement and accommodation. Journal of Neurophysiology. 1986;55:915–930. doi: 10.1152/jn.1986.55.5.915. [DOI] [PubMed] [Google Scholar]
- Liu JS, Lee M, Jang J, Ciuffreda KJ, Wong JH, Grisham D, Stark L. Objective assessment of accommodation orthoptics. I. Dynamic insufficiency. Am J Optom Physiol Opt. 1979;56(5):285–294. doi: 10.1097/00006324-197905000-00002. [DOI] [PubMed] [Google Scholar]
- Maxwell J, Tong J, Schor CM. The first and second order dynamics of accommodative convergence and disparity convergence. Vision Res. 50(17):1728–1739. doi: 10.1016/j.visres.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin LN, Jr, Schor CM. Voluntary effort as a stimulus to accommodation and vergence. Invest Ophthalmol Vis Sci. 1988;29(11):1739–1746. [PubMed] [Google Scholar]
- Mordi JA, Ciuffreda KJ. Dynamic aspects of accommodation: age and presbyopia. Vision Res. 2004;44(6):591–601. doi: 10.1016/j.visres.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Mordi J, Tucker J, Charman WN. Effects of 0.1% cyclopentolate or 10% Phenylephrine on pupil diameter and accommodation. Ophthalmic Physiol Opt. 1986;6:221–227. [PubMed] [Google Scholar]
- Munoz P, Semmlow JL, Yuan W, Alvarez TL. Short term modification of disparity vergence eye movements. Vision Res. 1999;39(9):1695–1705. doi: 10.1016/s0042-6989(98)00206-5. [DOI] [PubMed] [Google Scholar]
- Ogle KN. The accommodative convergence-accommodation ratio and its relation to the correction of refractive error. Trans Am Acad Ophthalmol Otolaryngol. 1966;70(3):322–339. [PubMed] [Google Scholar]
- Radhakrishnan H, Allen PM, Charman WN. Dynamics of accommodative facility in myopes. Invest Ophthalmol Vis Sci. 2007;48(9):4375–4382. doi: 10.1167/iovs.07-0269. [DOI] [PubMed] [Google Scholar]
- Randle RJ, Murphy MR. The dynamic response of visual accommodation over a seven-day period. Am J Optom Physiol Opt. 1974;51(8):530–544. doi: 10.1097/00006324-197408000-00002. [DOI] [PubMed] [Google Scholar]
- Rouse MW. Management of binocular anomalies: efficacy of vision therapy in the treatment of accommodative deficiencies. Am J Optom Physiol Opt. 1987;64(6):415–420. [PubMed] [Google Scholar]
- Schor CM, Lott LA, Pope D, Graham AD. Saccades reduce latency and increase velocity of ocular accommodation. Vision Res. 1999;39(22):3769–3795. doi: 10.1016/s0042-6989(99)00094-2. [DOI] [PubMed] [Google Scholar]
- Takagi M, Oyamada H, Abe H, Zee DS, Hasebe H, Miki A, Usui T, Hasegawa S, Bando T. Adaptive changes in dynamic properties of human disparity-induced vergence. Invest Ophthalmol Vis Sci. 2001;42(7):1479–1486. [PubMed] [Google Scholar]
- Tsuetaki TK, Schor CM. Clinical method for measuring adaptation of tonic accommodation and vergence accommodation. Am J Optom Physiol Opt. 1987;64(6):437–449. doi: 10.1097/00006324-198706000-00009. [DOI] [PubMed] [Google Scholar]
- Wilson D. A centre for accommodative vergence motor control. Vision Res. 1973;13(12):2491–2503. doi: 10.1016/0042-6989(73)90246-0. [DOI] [PubMed] [Google Scholar]