Abstract
The endocannabinoids 2-arachidonoyl glycerol (2-AG) and N-arachidonoyl ethanolamine (anandamide) are principally degraded by monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH), respectively. The recent discovery of O-aryl carbamates such as JZL184 as selective MAGL inhibitors has enabled the functional investigation of 2-AG signaling pathways in vivo. Nonetheless, JZL184 and other reported MAGL inhibitors still display low-level cross-reactivity with FAAH and peripheral carboxylesterases, which can complicate their use in certain biological studies. Here, we report a distinct class of O-hexafluoroisopropyl (HFIP) carbamates that inhibit MAGL in vitro and in vivo with excellent potency and greatly improved selectivity, including showing no detectable cross-reactivity with FAAH. These findings designate HFIP-carbamates as a versatile chemotype for inhibiting MAGL and should encourage the pursuit of other serine hydrolase inhibitors that bear reactive groups resembling the structures of natural substrates.
Endocannabinoids are a class of lipid transmitters that serve as endogenous ligands for the cannabinoid receptors CB1 and CB2, which are also activated by Δ9-tetrahydrocannabinol, the psychoactive ingredient of marijuana. The two major endocannabinoids, 2-arachidonoyl glycerol (2-AG) and N-arachidonoyl ethanolamine (anandamide or AEA), play important roles in a wide range of physiological and pathological processes, including pain, cognition, emotionality, and feeding (Ligresti et al., 2009; Graham et al., 2009; Di Marzo, 2009; De Petrocellis & Di Marzo, 2009; Fowler, 2008; Di Marzo, 2008; Ahn et al., 2008; Guindon et al., 2011). The signaling functions of 2-AG and AEA are terminated by enzymatic hydrolysis in processes principally mediated by the serine hydrolases monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH), respectively (Ahn et al., 2008). Additional 2-AG hydrolases exist, such as ABHD6 and ABHD12, and these enzymes may also regulate specific endocannabinoid pathways in vivo (Blankman et al., 2007; Marrs et al., 2010).
Pharmacologic and/or genetic disruption of MAGL and FAAH has provided evidence that elevations in endocannabinoid signaling can produce an intriguing subset of the behavioral effects of direct CB1 agonists. FAAH(−/−) mice or rodents treated with FAAH inhibitors, for instance, show anti-hyperalgesia and anti-anxiety/depression without exhibiting psychotropic responses or deficits in motor function (Cravatt et al., 2001; Lichtman et al., 2004; Kathuria et al., 2003; Ahn et al., 2009). MAGL inhibitors show a similar, but somewhat broader spectrum of CB1-dependent behavioral effects (Long et al., 2009a), and, at high doses, can lead to desensitization and downregulation of CB1 receptors (Schlosburg et al., 2010). Dual FAAH/MAGL inhibitors, on the other hand, promote cataleptic and drug-dependence behaviors in mice that are more reminiscent of direct CB1 agonists (Long et al., 2009c). These data designate selective FAAH and MAGL inhibitors as useful probes for dissecting the functions of different branches of the endocannabinoid system and as potential therapeutic agents for treating pain and neuropsychiatric disorders. However, the results also underscore the importance of maintaining high levels of selectivity to avoid simultaneous blockade of both FAAH and MAGL.
Lead MAGL inhibitors from the O-aryl carbamate class, such as JZL184, which inactivate MAGL by covalent carbamylation of the enzyme’s serine nucleophile, show good selectivity (> 100-fold) for MAGL over FAAH, ABHD6, and most other serine hydrolases (Long et al., 2009a; Long et al., 2010). However, we have found that the residual, low-level cross-reactivity displayed by JZL184 for FAAH does lead to partial inhibition of this enzyme following high-dosing and chronic treatment regimens (Long et al., 2009a, 2009b; Schlosburg et al., 2010). Thus, a need remains to identify inhibitors that show more complete selectivity for MAGL over FAAH to avoid the potentially confounding effects of dual activation of 2-AG and AEA pathways in vivo. Here, we report the discovery and characterization of a structurally distinct class of carbamate inhibitors bearing a hexafluoroisopropanol leaving group that act as highly selective and in vivo-active inhibitors of MAGL. Competitive activity-based protein profiling (ABPP) and endocannabinoid-specific biomarker assays confirmed that a HFIP carbamate analogue of JZL184, here named KML29, potently and selectively inhibited MAGL in vitro and in vivo with minimal cross-reactivity toward other central and peripheral serine hydrolases, including no detectable activity against FAAH.
Results
Discovery of HFIP Carbamates as Selective MAGL Inhibitors
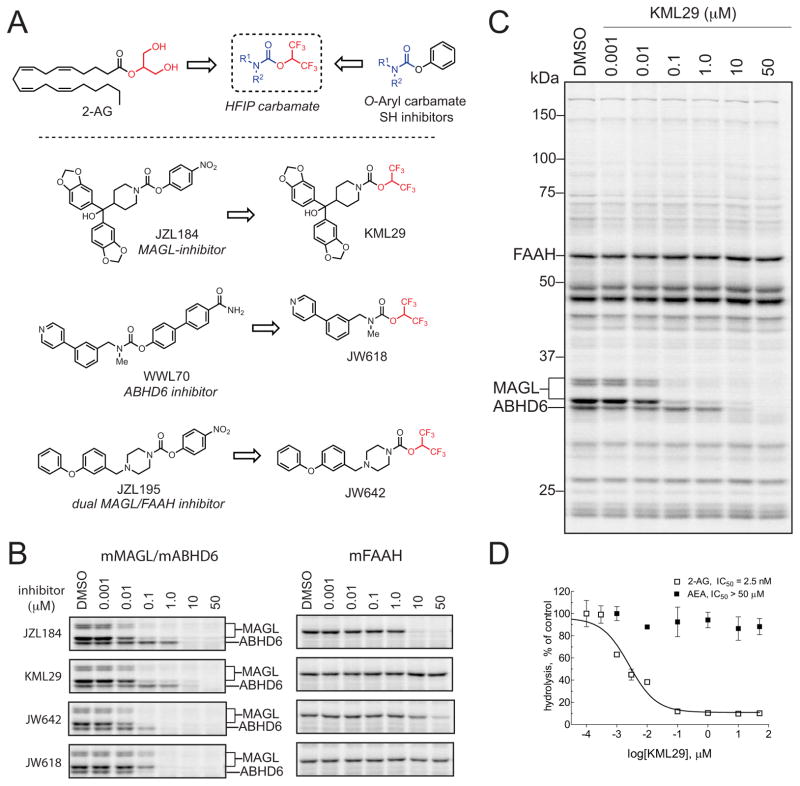
In our search for new classes of inhibitors that might display improved selectivity for MAGL over FAAH, we sought to replace the p-nitrophenol leaving group of JZL184 with another electrophilic moiety that better discriminated between these two endocannabinoid hydrolases. We were particularly attracted to hexafluoroisopropanol as a leaving group, since it possesses a low pKa value (pKa ~ 9.3) and a structure that resembles the glycerol of MAGL’s natural substrates (Figure 1A). It is furthermore known that modification of the methylene proximal to the amide on AEA impairs FAAH hydrolysis (Figure 1A) (Lang et al., 1999). We therefore reasoned that HFIP carbamates might retain excellent activity against MAGL while, at the same time, exhibiting reduced inhibition of FAAH. We synthesized HFIP carbamate analogues for three known endocannabinoid hydrolase inhibitors: the MAGL-selective inhibitor JZL184 (KML29, Figure 1A) (Long et al., 2009a; Long et al., 2010), the ABHD6-selective inhibitor WWL70 (JW618, Figure 1A) (Li et al., 2007), and the dual MAGL/FAAH-inhibitor JZL195 (JW642, Figure 1A) (Long et al., 2009c; Long et al., 2010).
Figure 1. Discovery of HFIP carbamates as potent and selective MAGL inhibitors.
(A) Structures of 2-AG, representative O-aryl carbamate inhibitors of endocannabinoid hydrolases, and HFIP carbamate analogues of these inhibitors, showing the bioisosteric nature of the HFIP group compared to the glycerol head group of MAGL substrate 2-AG.
(B) Competitive ABPP gels comparing the potency and selectivity of JZL184, KML29, JW642 and JW618 against MAGL, ABHD6, and FAAH in mouse brain proteomes. See Figure S1 for full gel profiles of each inhibitor. See also Table 1 for IC50 values calculated from gel-based ABPP experiments. Note that MAGL migrates as two bands in the mouse brain likely due to alternative spliced isoforms (Karlsson et al., 2001).
(C) Full competitive ABPP gel showing KML29 activity against mouse brain serine hydrolase activities, which revealed selective inhibition of MAGL at inhibitor concentrations of 1 μM of less and inhibition of ABHD6 at concentrations > 1 μM. No inhibition of FAAH was detected at any tested concentration of KML29.
(D) Blockade of MAGL and FAAH by KML29 in mouse brain proteomes as measured by hydrolysis of 2-AG and AEA, respectively. Data are presented as means ± SEM of three independent experiments.
Gels are representative images from three independent experiments (n = 3).
We initially assayed each HFIP carbamate for inhibition against MAGL, FAAH, ABHD6, and other mouse brain serine hydrolases using competitive activity-based protein profiling (ABPP) (Leung et al., 2003). In this assay, cell or tissue proteomes are treated with inhibitors followed by the serine hydrolase-directed activity-based probe fluorophosphonate-rhodamine (FP-Rh), and inhibition is recorded as a reduction in FP-Rh probe labeling of individual serine hydrolases following separation by SDS-PAGE and in-gel fluorescence scanning. Competitive ABPP gels of mouse brain membrane proteome revealed that HFIP carbamates exhibit outstanding selectivity for MAGL over FAAH compared to the corresponding O-aryl carbamates (Figure 1B, Table 1 and Figure S1). The HFIP analogue of JZL184, KML29, for instance, showed complete selectivity for MAGL over FAAH across the concentration range tested (0.001–50 μM) and greater than 100-fold selectivity for ABHD6, the only observable off-target in the mouse brain proteome (Figure 1C and Table 1). This improvement in selectivity came without any sacrifice in potency, as KML29 and JZL184 were found to exhibit equivalent IC50 values for MAGL inhibition (Table 1). Similar results were obtained using 2-AG and AEA substrate assays, which confirmed that KML29 is a potent inhibitor of 2-AG hydrolysis, but did not affect AEA hydrolysis at any concentration tested (Figure 1D). JW618, the HFIP analogue of the selective ABHD6 inhibitor WWL70 (Li et al., 2007), acted as a dual MAGL/ABHD6 inhibitor with no detectable crossreactivity with FAAH (Figure 1B and Table 1). Finally, and perhaps most surprisingly, JW642, the HFIP analogue of the low nanomolar dual MAGL/FAAH inhibitor JZL195, inhibited MAGL with > 1000-fold greater potency than FAAH, indicating that simple replacement of the p-nitrophenyl group with the HFIP group was sufficient to convert potent dual FAAH/MAGL inhibitors into agents that displayed high selectivity for MAGL. That the selectivities displayed by KML29 and JW642 for MAGL over ABHD6 were nearly equivalent to those observed for their parent p-nitrophenyl carbamates indicates the HFIP group is accommodated equally well by both of these enzymes, consistent with their shared ability to hydrolyze the substrate analogue 2-AG.
Table 1. IC50 Values for JZL184 and HFIP Carbamates Against Mouse, Rat and Human Orthologues of MAGL, FAAH and ABHD6.
Serine hydrolase inhibition was measured by competitive ABPP where reductions in FP-Rh labeling of MAGL, ABHD6 and FAAH were quantified following preincubation of proteomic samples with the indicated inhibitor. Brain membrane proteomes were used to measure inhibition of mouse and rat enzymes, while human enzymes were evaluated from proteomes of transiently transfected HEK293T cells. IC50 values are reported as means from three independent experiments.
| Inhibitor IC50 (95% CI), nM | ||||
|---|---|---|---|---|
| JZL184 | KML29 | JW642 | JW618 | |
| Mouse: | ||||
| MAGL | 10* | 15 (11–21) | 7.6 (5.5–10) | 123 (91–160) |
| FAAH | 4,690* | > 50,000 | 31,000 (22,700–44,100) | > 50,000 |
| ABHD6 | 3,270* | 4,870 (4,120–5,760) | 107 (41–285) | 38 (29–50) |
| Rat: | ||||
| MAGL | 262 (188–363) | 43 (36–52) | 14 (13–16) | 385 (329–451) |
| FAAH | 3,570 (2,540–5,020) | > 50,000 | 14,000 (12,300–17,000) | > 50,000 |
| ABHD6 | 2,940 (1,441–6,010) | 1,600 (1,260–2,040) | 50 (32–78) | 13 (8.9–18) |
| Human: | ||||
| MAGL | 3.9 (1.8–8.1) | 5.9 (4.0–9.9) | 3.7 (2.3–5.9) | 6.9 (4.4–11) |
| FAAH | > 50,000 | > 50,000 | 20,600 (13,000–32,600) | > 50,000 |
Previously determined IC50 values (Long et al., 2010).
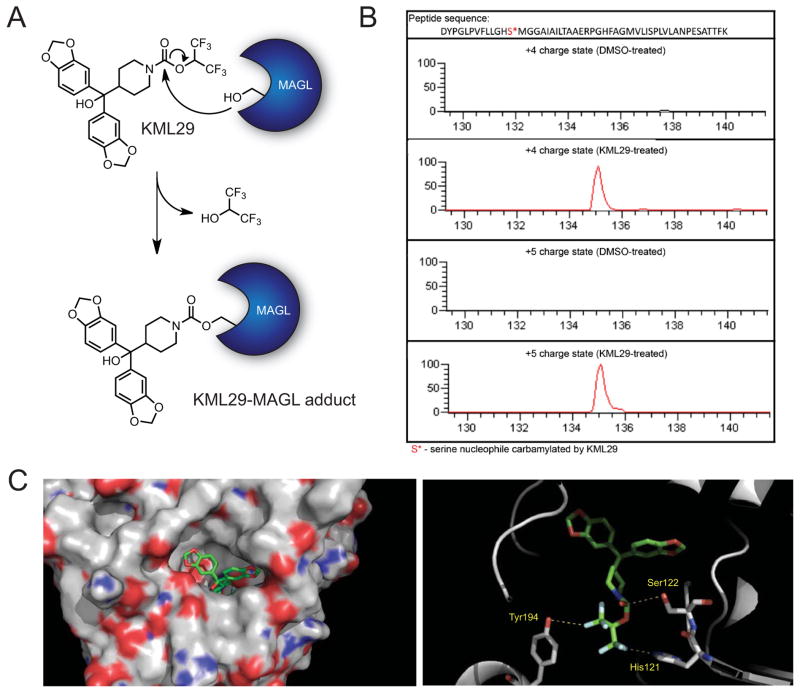
To gain further insights into the mode of inhibition of MAGL by HFIP carbamates, we first determined by mass spectrometry analysis that KML29 reacts with the catalytic serine nucleophile of MAGL to form a stable, carbamylated adduct (Figures 2A and 2B). This covalent mechanism of inhibition mirrors that of the p-nitrophenoxy carbamate JZL184 (Long et al., 2009b). We also modeled the binding of KML29 to the MAGL active site using recently described crystal structures of the human MAGL enzyme (Bertrand et al., 2010). The resulting model provided evidence that the HFIP group of KML29 could bind to MAGL in a similar manner to that predicted for the glycerol moiety of 2-AG (Bertrand et al., 2010) including potential interactions with the conserved active site residues His121 and Tyr194 (Figure 2C).
Figure 2. Characterization of KML29-mediated inactivation of MAGL.
(A) Proposed mechanism of MAGL inactivation by KML29. The catalytic serine (Ser122) of MAGL attacks the activated carbamate of KML29, releasing hexafluoroisopropanol and forming a stable, carbamylated KML29-MAGL covalent adduct.
(B) Extracted ion chromatograms for +4 (m/z = 1408.2310) and +5 (m/z = 1126.7862) charge states corresponding to the KML29-modified adduct of the active site tryptic peptide from DMSO-treated and KML29-treated recombinant, purified human MAGL.
(C) Proposed docking mode of KML29 (green) to MAGL (grey) illustrated in views of the whole protein (left image) and the active site (right image). When the catalytic Ser122 is positioned for nucleophilic attack at the carbonyl of KML29, His121 and Tyr194, which are predicted to interact with 2-AG’s head group (Bertrand et al., 2010), show potentially favorable interactions with the KML29’s HFIP leaving group. RosettaLigand 3.3 (http://www.ncbi.nlm.nih.gov/pubmed/19041878) was used to perform the docking and the MAGL structure used for docking was 3JWE from the protein data bank (Bertrand et al., 2010).
Together, these data demonstrate that HFIP carbamates can serve as potent and selective inhibitors of MAGL and possibly other 2-AG hydrolases.
KML29 Selectively Inhibits MAGL in Mice
Having demonstrated that KML29 is a potent and selective MAGL inhibitor in vitro, we next evaluated the activity of this compound in vivo. C57Bl/6 mice were treated with a dose-range (1–40 mg kg−1, p.o.) of KML29 or JZL184, or vehicle, and, after 4 h, were sacrificed and their tissues removed and analyzed by competitive ABPP to assess serine hydrolase activities (Figure 3A). KML29 and JZL184 showed comparable dose-dependent blockade of MAGL activity in brain, with partial inhibition observed at 5 mg kg−1 and maximal inhibition achieved by 20 mg kg−1. Consistent with the in vitro selectivity profile for each inhibitor, JZL184 was found to partially inhibit FAAH at the highest dose tested (40 mg kg−1), whereas KML29 exhibited complete selectivity over FAAH at all doses tested. Similar profiles were generated for KML29 when administered to mice intraperitoneally (Figure S2). We have previously noted that the limited solubility of JZL184 necessitates sonication in the chosen vehicle prior to administration. Prolonged sonication and mild heating were also required for the dissolution of KML29 in vehicles for p.o. (PEG) or i.p. [saline:emulphor:ethanol (18:1:1)] routes of administration (see Supplemental Data for details).
Figure 3. In vivo characterization of KML29 activity in mice.
(A) Competitive ABPP gel of FP-Rh labeling of brain serine hydrolase activities from mice treated with JZL184 or KML29 at the indicated dose (1–40 mg kg−1, p.o.) for 4 h.
(B) Brain lipid profile for 2-AG, AA, AEA, PEA, OEA across the indicated dose-range of KML29 (p.o.). 2-AG and AEA hydrolytic activity of brain tissue isolated from KML29 treated mice (far right graph).
(C) Competitive ABPP gels of serine hydrolase activities in liver and lung tissues from mice treated with either JZL184 or KML29 (1–40 mg kg−1, p.o.) for 4 h. Red boxes mark various CES enzymes that show differential sensitivity to JZL184 versus KML29. Also see Figure S2 for in vitro inhibition of CESs by JZL184 and KML29 in lung proteomes and ABPP gels from spleen proteomes isolated from mice treated with JZL184 and KML29.
Data are presented as means ± SEM, n = 3 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for vehicle-treated versus inhibitor-treated mice.
Consistent with the dose-dependent inhibition of MAGL observed by competitive ABPP, KML29 produced significant elevations in brain 2-AG at doses as low as 5 mg kg−1, and these increases peaked at ~10-fold over vehicle controls at the 20 and 40 mg kg−1 doses (Figure 3B). As previously reported for other MAGL inhibitors (Long et al., 2009a, 2009b) and MAGL(−/−) mice (Schlosburg et al., 2010), we observed, concomitant with 2-AG elevations, significant reductions in brain arachidonic acid (AA). In contrast, brain levels for the endogenous FAAH substrates AEA, N-palmitoylethanolamine (PEA) and N-oleoylethanolamine (OEA) were unaltered by KML29 treatment at all tested doses (Figure 3B). Finally, we measured residual MAGL and FAAH activity in brain proteomes from KML29-treated mice using substrate assays (Figure 3B), which confirmed the dose-dependent inhibition of MAGL and the lack of any effect on FAAH.
We also evaluated the duration of action of KML29 in vivo by assessing MAGL activity at various time points following a single, oral dose of the inhibitor (20 mg kg−1) (Figure S2). As has been reported previously for JZL184 (Long et al., 2009a), KML29 caused maximal inhibition of MAGL in the brain by 1 h post-administration, the earliest time point tested. This inhibition was sustained until 12–24 h, at which times MAGL activity began to return to control levels. Importantly, KML29 maintained complete selectivity for MAGL over FAAH for the duration of the time course experiment. Brain lipid profiles for 2-AG, AA and AEA corroborated these gel profiles, revealing maximal elevations of 2-AG and reductions of AA at 1 h followed by a gradual return toward basal levels over the 24 h time course. AEA levels, on the other hand, were unaltered throughout the 24 h period.
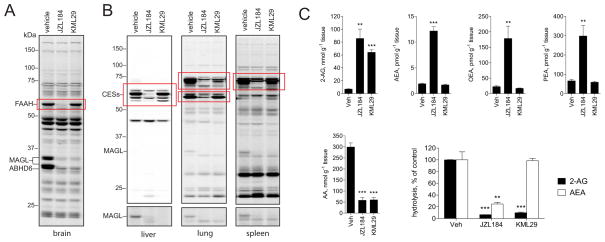
In addition to showing low-level cross-reactivity with FAAH, JZL184 also inhibits a handful of carboxylesterase (CES) enzymes in peripheral tissues (Long et al., 2009b). To evaluate whether KML29 also showed improved selectivity against CESs, we performed competitive ABPP experiments in liver, lung, and spleen proteomes. In vitro profiles confirmed the superior selectivity of KML29, which showed much less cross-reactivity with CESs in mouse lung proteomes compared to JZL184 (Figure S2). We also evaluated the peripheral activity and selectivity of JZL184 and KML29 in vivo by analyzing whole tissue homogenates from inhibitor-treated mice by gel-based ABPP (Figure 3C). Consistent with our previous work, JZL184 inhibited MAGL at low doses (1 mg kg−1), but also displayed significant reactivity with several CESs in the liver, lung and spleen (Long et al., 2009b). KML29 produced near-complete blockade of MAGL in these peripheral tissues at the lowest dose tested (1 mg kg−1), and, in contrast to JZL184, did not show any detectable CES cross-reactivity until higher doses (20–40 mg/kg), where the blockade of a single ~70 kDa enzyme was detected. This enzyme likely corresponds to carboxylesterase 1 (ES1), which is found abundantly in plasma (Krishnasamy et al., 1998) and exhibits promiscuous reactivity with many carbamates, including JZL184 (Long et al., 2009b; Alexander & Cravatt, 2005; Bachovchin et al., 2010; Chang et al., 2011).
The superior selectivity of KML29 over JZL184 was perhaps most strikingly demonstrated in a chronic dosing study, where the competitive ABPP results were compared for brain and peripheral tissues from mice treated for six days with each inhibitor (40 mg kg−1, p.o.). Under this dosing regime, JZL184 not only inhibited MAGL, but also FAAH in brain (Figure 4A), as has been reported previously (Schlosburg et al., 2010), as well as several CES enzymes in peripheral tissues (Figure 4B and Figure S3). In contrast, chronic dosing with KML29 selectively inhibited MAGL without any effect on FAAH (Figure 4A) and minimal cross-reactivity with CES enzymes (Figure 4B). The selectivity of KML29 over JZL184 in chronic dosing experiments was further demonstrated by measuring MAGL and FAAH substrate levels in inhibitor-treated mice. KML29 was found to selectively raise 2-AG, but not NAE levels, while JZL184 caused increases in both 2-AG and NAEs (Figure 4C). Brain endocannabinoid hydrolytic activities were also measured ex vivo from chronically treated mice revealing that KML29 selectively blocked 2-AG, but not AEA-hydrolysis, while JZL184 inhibited both of these activities (Figure 4C).
Figure 4. Characterization of serine hydrolase activities and endocannabinoid metabolism in mice treated chronically with KML29 and JZL184.
(A) Competitive ABPP gels of serine hydrolase activities in brain from mice chronically treated with vehicle, JZL184, or KML29 (40 mg kg−1 day−1, p.o., 6 days).
(B) Competitive ABPP gels of serine hydrolase activities in liver, lung and spleen from mice chronically treated with vehicle, JZL184, or KML29. Red boxes mark various off-targets that show differential sensitivity to JZL184 versus KML29.
(C) Brain lipid profiles and endocannabinoid hydrolytic activities measured from mice chronically treated with vehicle, JZL184, or KML29.
KML29 Selectively Inhibits Rat and Human Orthologues of MAGL
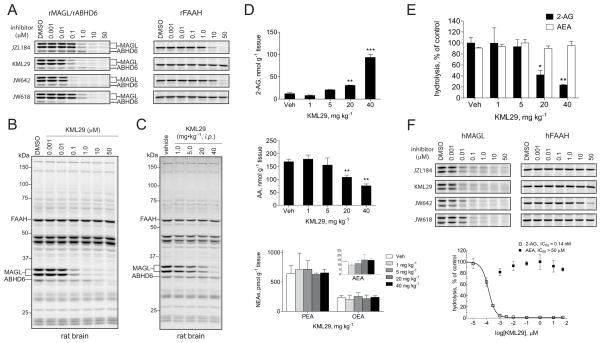
As has been reported previously (Long et al., 2009b), JZL184 exhibits equivalent potency for mouse and human MAGL, but substantially lower activity against rat MAGL (Figure 5 and Table 1). Although the basis for JZL184’s reduced potency against rat MAGL remains unknown, we wondered whether KML29 and other HFIP carbamates might show improved activity for this enzyme. Indeed, competitive ABPP analysis of rat brain homogenates revealed that HFIP carbamates retain much of their potency and selectivity for rat MAGL (and rat ABHD6, in the case of JW618) (Figure 5A and Table 1). KML29, for instance, displayed an IC50 value of 43 nM for inhibiting rat MAGL (Table 1), which was within approximately three-fold of its potency for mouse MAGL (IC50 = 15 nM), and maintained good selectivity relative to rat FAAH (no detectable cross-reactivity) and ABHD6 (> 20-fold selectivity) (Table 1). We did not detect any additional off-target reactivity with other rat brain serine hydrolases for KML29 (Figure 5B) or for JW618 and JW642 (Figure S3).
Figure 5. Inhibition of rat and human MAGL enzymes by KML29.
(A) Competitive ABPP gels comparing the potency and selectivity of JZL184, KML29, JW642 and JW618 against MAGL, ABHD6 and FAAH in rat brain proteomes. See Figure S3 for full gel profiles of each inhibitor. See also Table 1 for IC50 values calculated from gel-based ABPP experiments.
(B) Full competitive ABPP gel showing KML29 activity against rat brain serine hydrolase activities, which revealed selective inhibition of MAGL at inhibitor concentrations of 1 μM or less and inhibition of ABHD6 at concentrations > 1 μM. No inhibition of FAAH was detected at any tested concentration of KML29.
(C) Competitive ABPP gel of brain serine hydrolase activities from rats treated with KML29 (1–40 mg kg−1, i.p.) for 4 h. Also see Figure S3 for gel profiles of liver, lung and spleen from rats treated with KML29.
(D) Brain lipid profile of 2-AG, AA, AEA, PEA, OEA from rats treated with KML29 (1–40 mg kg−1, i.p.).
(E) Brain MAGL and FAAH activity from rats treated with KML29 (1–40 mg kg−1, i.p.) as measured by 2-AG and AEA hydrolysis, respectively.
(F) Activity of JZL184, KML29, JW642, and JW618 against recombinant human MAGL and FAAH by gel-based competitive ABPP (upper gel panels) and substrate hydrolysis (lower graph) assays. See also Table 1 for IC50 values calculated from gel-based ABPP experiments.
Data are presented as means ± SEM, n = 3 rats per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for vehicle-treated versus inhibitor-treated rats.
We next tested whether KML29 could inhibit rat MAGL in vivo. Wistar rats were treated with KML29 (1–40 mg kg−1, i.p.) or vehicle. After 4 h, each rat was sacrificed and their tissues collected for competitive ABPP and lipid analyses. Gel-based ABPP revealed clear dose-dependent inhibition of MAGL in rat brain without any detectable cross-reactivity with FAAH or ABHD6 (Figure 5C). KML29 was slightly less potent in rats than mice, producing > 90% inhibition of brain MAGL at 40 mg kg−1. Analysis of peripheral tissues revealed complete MAGL inhibition at 5 mg kg−1 in lung and spleen and 20 mg kg−1 in liver (Figure S3). As was observed in mice, only one off-target was detected for KML29 in peripheral tissues of rat by competitive ABPP, a 70 kDa serine hydrolase that is likely the rat orthologue of ES1.
Analysis of brain lipids corroborated our competitive ABPP results, revealing approximately 10-fold elevations in 2-AG without alteration of AEA, PEA, or OEA (Figure 5D). We also measured brain 2-AG hydrolytic activity, which was diminished by 50% at 20 mg kg−1 and 80% at 40 mg kg−1 doses of KML29 (Figure 5E). Since ABHD6 and ABHD12 can also hydrolyze 2-AG (Blankman et al., 2007), it is plausible that the remaining 20% 2-AG hydrolytic activity in rat brain is due to these enzymes, which were not inhibited by KML29 in vivo. Brain AEA hydrolytic activity, on the other hand, was not affected at any dose of KML29 (Figure 5E). Finally, brain AA levels were found to decrease proportionally with the elevations in 2-AG caused by KML29 treatment (Figure 5D). Together, these data indicate that KML29 offers a selective, in vivo-active probe for investigating MAGL function in rats, an objective that has not been straightforward to achieve with past inhibitors.
Endocannabinoid hydrolase inhibitors have therapeutic potential for treating a range of human disorders. Rodent studies have revealed, however, that dual inhibitors of FAAH and MAGL produce psychotropic effects that resemble the activity of direct cannabinoid agonists (Long et al., 2009c). It is therefore important that inhibitors of MAGL maintain excellent selectivity for the human form of this enzyme (hMAGL) compared to human FAAH (hFAAH). By performing competitive ABPP assays with cell lysates from HEK293T cells transiently transfected with hMAGL or hFAAH cDNAs, we found that KML29 inhibited hMAGL with an IC50 value of 5.9 nM, while showing no-detectable cross-reactivity with hFAAH (Figure 5F). Other HFIP carbamates also showed excellent potency for hMAGL and maintained high selectivity over hFAAH (Figure 5F). We should further note that JZL184 itself displayed striking selectivity for hMAGL, exhibiting no detectable cross-reactivity with FAAH up to 50 μM (Figure 5F). Finally, we also measured the inhibition of hMAGL and hFAAH by KML29 using substrate hydrolysis assays, which revealed subnanomolar inhibition of 2-AG hydrolysis (IC50 = 0.14 nM) and undiminished AEA hydrolysis up to 50 μM (Figure 5F). The lower IC50 value calculated by substrate compared to competitive ABPP assays may reflect titration of MAGL protein in the latter format, which used higher concentrations of protein for analysis (0.01 versus 0.1 mg MAGL-transfected cell proteome/mL, respectively).
Discussion
The development of selective, in vivo-active chemical probes is often the catalyst for groundbreaking discoveries in mammalian biology (Moellering & Cravatt, 2012; Edwards et al., 2011). A historical look at endocannabinoid research bears this out when considering the mechanistic insights that have been gained following the development of selective receptor agonists and antagonists, as well as inhibitors of endocannabinoid hydrolases (Ligresti et al., 2009; Graham et al., 2009; Di Marzo, 2009; De Petrocellis & Di Marzo, 2009; Fowler, 2008; Di Marzo, 2008; Ahn et al., 2008; Guindon et al., 2011). Selective FAAH inhibitors have existed for at least a decade (Boger et al., 2000; Kathuria et al., 2003), whereas selective MAGL inhibitors, such as JZL184 (Long et al., 2009a; Long et al., 2010), have emerged only in the last few years. Even within this short time period, MAGL inhibitors have already played a key part in the discovery of MAGL’s role in processes such as pain (Long et al., 2009a; Kinsey et al., 2009), neuropsychiatric disorders (Sciolino et al., 2011; Busquets-Garcia et al., 2011), cancer (Nomura et al., 2011a; Nomura et al., 2010) and neuroinflammation (Nomura et al., 2011b). Notwithstanding the impact that JZL184 has had on our understanding of MAGL function in mammalian biology, several limitations and concerns have constrained the utility of this agent and have encouraged us to develop inhibitors with improved specificity. Of primary concern was the low-level cross-reactivity that JZL184 displays for FAAH, which becomes problematic in studies that require high doses or chronic administration of the inhibitor (Long et al., 2009a, 2009b; Schlosburg et al., 2010). Because simultaneous inhibition of MAGL and FAAH elicits a unique subset of behaviors distinct from those caused by inhibition of either FAAH or MAGL alone (Long et al., 2009a), it is imperative that FAAH cross-reactivity is avoided to allow for accurate assignment of MAGL-mediated biological effects. JZL184 also inactivates a handful of CES enzymes (Long et al., 2009b), which complicates the use of this inhibitor to investigate peripheral functions of MAGL. With these considerations in mind, we report herein a new class of MAGL inhibitors, the HFIP carbamates, which exhibit vastly improved selectivity for MAGL in both the brain and peripheral tissues.
We selected HFIP carbamates because they possess a leaving group with a pKa value that is comparable to that of p-nitrophenol, the leaving group of JZL184. We also envisioned that the hexafluoroisopropoxy group would serve as an activated isostere of the 2-AG head group and, because of its increased size, might impart additional selectivity for FAAH, which is particularly sensitive to head-group sterics (Lang et al., 1999). These hypotheses were confirmed through the generation of KML29, an HFIP analogue of JZL184 that maintained the original inhibitor’s potency for MAGL while showing greatly improved selectivity over FAAH and CES enzymes. Even incorporation of the HFIP group into a potent FAAH inhibitor like JZL195 converted this agent into an MAGL/ABHD6 selective inhibitor JW642 that showed no observable FAAH reactivity up to 10 μM. It should be emphasized that, in general, the MAGL/ABHD6 selectivity ratios were not altered by incorporation of the HFIP group, suggesting that ABHD6 selectivity is driven primarily by the amine portion of the tested carbamates. Our competitive ABPP assays also did not uncover any additional off-targets for HFIP carbamates across the serine hydrolase class, although deeper profiling by mass spectrometry methods (Jessani et al., 2005) will be required to more fully explore the proteome-wide selectivity of these agents. These results, taken together, designate HFIP carbamates as a useful scaffold for inhibiting the 2-AG hydrolases MAGL and ABHD6.
We found that KML29 selectively inactivated MAGL in vivo without any detectable FAAH inhibition across the entire tested dose range. This selectivity was reflected not only in measurements of enzyme activity, but also in brain lipid profiles, where KML29 produced ~10-fold elevations in 2-AG (and reductions in AA) without alteration in FAAH substrates, AEA, OEA or PEA. KML29 proved active in both mouse and rat, thus offering a more versatile chemical probe than JZL184 for exploring a range of rodent behavioral models. The superior selectivity of KML29 relative to JZL184 was also confirmed in vivo using both acute and chronic dosing regimes (Figures 3 and 4). Finally, we found that HFIP carbamates also display excellent potency and specificity for the human orthologue of MAGL, suggesting that they could offer a useful starting point for the development of therapeutic agents. On this note, we were gratified to identify doses where KML29 produced partial inhibition of brain MAGL (as reflected in both enzyme activity and brain lipid measurements; see Figures 3 and 5), since complete blockade of this enzyme is known to produce tolerance and desensitization of CB1 receptors in mice (Schlosburg et al., 2010).
Significance
Monoacylglycerol lipase (MAGL), through catalyzing the hydrolysis of 2-AG to arachidonic acid, serves as a key metabolic hub connecting the endocannabinoid and eicosanoid signaling networks. Small-molecule inhibitors that selectively target MAGL are critical tools for unraveling the functions and crosstalk between these two lipid transmitters systems, and hold potential for treating a wide spectrum of disorders, including anxiety, pain, inflammation, and cancer. We report herein a new class of MAGL inhibitors, O-hexafluoroisopropyl (HFIP) carbamates, that display excellent potency and in vivo activity and, in comparison to previously described O-aryl carbamates, greatly enhanced selectivity over FAAH and others serine hydrolases. That HFIP carbamates appear to achieve their potency and selectivity for MAGL (and ABHD6) at least in part due to the bioisosteric nature of their leaving group leads us to speculate that this type of substrate-mimetic strategy for covalent inhibitor design could be generalized to develop useful probes for many additional serine hydrolases.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grants DA017259 (B.F.C.), DA009789 (B.F.C.), DA032541 (MJN) and the Skaggs Institute for Chemical Biology.
Footnotes
Supplemental Information included three figures and Supplemental Experimental Procedures and can be found with this article online at _____.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, et al. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JP, Cravatt BF. Mechanism of carbamate inactivation of FAAH: Implications for the design of covalent inhibitors and in vivo functional probes for enzymes. Chem Biol. 2005;12:1179–1187. doi: 10.1016/j.chembiol.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachovchin DA, Ji T, Li W, Simon GM, Blankman JL, Adibekian A, Hoover H, Niessen S, Cravatt BF. Superfamily-wide portrait of serine hydrolase inhibition achieved by library-versus-library screening. Proc Natl Acad Sci USA. 2010;107:20941–20946. doi: 10.1073/pnas.1011663107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand T, Augé F, Houtmann J, Rak A, Vallée F, Mikol V, Berne PF, Michot N, Cheuret D, Hoornaert C, et al. Structural basis for human monoglyceride lipase inhibition. J Mol Biol. 2010;396:663–673. doi: 10.1016/j.jmb.2009.11.060. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger DL, Sato H, Lerner AE, Hedrick MP, Fecik RA, Miyauchi H, Wilkie GD, Austin BJ, Patricelli MP, Cravatt BF. Exceptionally potent inhibitors of fatty acid amide hydrolase: The enzyme responsible for degradation of endogenous oleamide and anandamide. Proc Natl Acad Sci USA. 2000;97:5044–5049. doi: 10.1073/pnas.97.10.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita As. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiat. 2011;70:479–486. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Chang JW, Nomura DK, Cravatt BF. A Potent and selective inhibitor of KIAA1363/AADACL1 that impairs prostate cancer pathogenesis. Chem Biol. 2011;18:476–484. doi: 10.1016/j.chembiol.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. An introduction to the endocannabinoid system: from the early to the latest concepts. Best Pract Res Clin Endocrinol Metab. 2009;23:1–15. doi: 10.1016/j.beem.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Targeting the endocannabinoid system: To enhance or reduce? Nat Rev Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Edwards AM, Isserlin R, Bader GD, Frye SV, Willson TM, Yu FH. Too many roads not taken. Nature. 2011;470:163–165. doi: 10.1038/470163a. [DOI] [PubMed] [Google Scholar]
- Fowler CJ. “The tools of the trade”—an overview of the pharmacology of the endocannabinoid system. Curr Pharm Des. 2008;14:2254–2265. doi: 10.2174/138161208785740126. [DOI] [PubMed] [Google Scholar]
- Graham ES, Ashton JC, Glass M. Cannabinoid receptors: A brief history and “what’s hot”. Front Biosci. 2009;14:944–957. doi: 10.2741/3288. [DOI] [PubMed] [Google Scholar]
- Guindon J, Guijarro A, Piomelli D, Hohmann AG. Peripheral antinociceptive effects of inhibitors of monoacylglycerol lipase in a rat model of inflammatory pain. Brit J Pharmacol. 2011;163:1464–1478. doi: 10.1111/j.1476-5381.2010.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessani N, Niessen S, Wei BQ, Nicolau M, Humphrey M, Ji Y, Han W, Noh DY, Yates JR, 3rd, Jeffrey SS, et al. A streamlined platform for high-content functional proteomics of primary human specimens. Nat Methods. 2005;2:691–697. doi: 10.1038/nmeth778. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Reue K, Xia Y-R, Lusis AJ, Langin D, Tornqvist H, Holm C. Exon–intron organization and chromosomal localization of the mouse monoglyceride lipase gene. Gene. 2001;272:11–18. doi: 10.1016/s0378-1119(01)00559-5. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther. 2009;330:902–910. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang W, Qin C, Lin S, Khanolkar AD, Goutopoulos A, Fan P, Abouzid K, Meng Z, Biegel D, Makriyannis A. Substrate specificity and stereoselectivity of rat brain microsomal anandamide amidohydrolase. J Med Chem. 1999;42:896–902. doi: 10.1021/jm980461j. [DOI] [PubMed] [Google Scholar]
- Leung D, Hardouin C, Boger DL, Cravatt BF. Discovering potent and selective reversible inhibitors of enzymes in complex proteomes. Nat Biotech. 2003;21:687–691. doi: 10.1038/nbt826. [DOI] [PubMed] [Google Scholar]
- Li W, Blankman JL, Cravatt BF. A Functional proteomic strategy to discover inhibitors for uncharacterized hydrolases. J Am Chem Soc. 2007;129:9594–9595. doi: 10.1021/ja073650c. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Petrosino S, Di Marzo V. From endocannabinoid profiling to ‘endocannabinoid therapeutics’. Curr Opin Chem Biol. 2009;13:321–331. doi: 10.1016/j.cbpa.2009.04.615. [DOI] [PubMed] [Google Scholar]
- Long JZ, Jin X, Adibekian A, Li W, Cravatt BF. Characterization of tunable piperidine and piperazine carbamates as inhibitors of endocannabinoid hydrolases. J Med Chem. 2010;53:1830–1842. doi: 10.1021/jm9016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009a;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009b;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA. 2009c;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, Bodor AL, Muccioli GG, Hu SS, Woodruff G, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering Raymond E, Cravatt Benjamin F. How chemoproteomics can enable drug discovery and development. Chem Biol. 2012;19:11–22. doi: 10.1016/j.chembiol.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Lombardi DP, Chang JW, Niessen S, Ward AM, Long JZ, Hoover HH, Cravatt BF. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol. 2011a;18:846–856. doi: 10.1016/j.chembiol.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MCG, Ward AM, Hahn YK, Lichtman AH, Conti B, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011b;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, Hohmann AG. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res. 2011;64:226–234. doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.