Abstract
Heterotopic ossification is a debilitating condition that can result from traumatic injury, surgery, or genetic disease. We investigated the cellular origins of heterotopic skeletogenesis in the mouse using lineage tracing and bioassays of heterotopic ossification based on intramuscular transplantation. We identify, characterize and purify a tissue-resident stem/progenitor cell population that exhibits robust osteogenic potential and represents a major cell-of-origin for heterotopic ossification. These progenitors reside in the interstitium of skeletal muscle and other tissues, and are distinct from the endothelium, which does not exhibit osteogenic activity in response to BMP2 stimulation. Intramuscular transplantation together with clonal analysis in culture revealed that these progenitors are multipotent, exhibiting the capacity for both BMP-dependent skeletogenic differentiation and spontaneous adipogenic differentiation. Identifying the cells-of-origin responsible for heterotopic ossification provides a potential therapeutic target to treat, mitigate or prevent this disabling condition.
Keywords: heterotopic ossification, fibrodysplasia ossificans progressiva, FOP, tissue-specific stem cell, skeletal muscle, Cre/loxP, Tie2, osteogenesis, adipogenesis, clonal analysis
Introduction
Heterotopic ossification is a debilitating condition characterized by the formation of bone in skeletal muscle and associated soft tissues(1–3). Soft tissue injury is a proximate trigger for heterotopic ossification, which is prevalent among combat personnel suffering injuries to the extremities and following total hip replacement and other surgical procedures(2,4,5). Available data from animal models indicate that these acquired forms of heterotopic ossification result from excessive bone morphogenetic protein (BMP) signaling in the context of an inflammatory milieu(6–12). Soft tissue injury can lead to increased expression of BMP4(13) and other osteogenic cytokines(11), as well as to induction of the BMP intracellular effectors, Smads 1 and 5(14), providing a potential pathophysiological mechanism for the initiation of heterotopic ossification.
The most severe form of heterotopic ossification is manifested in the rare, autosomal dominant genetic disorder, fibrodysplasia ossificans progressiva (FOP), in which heterotopic bone forms progressively throughout the life of the individual, resulting in devastating effects on health, life expectancy and quality of life(5,15). A connection between BMP signaling and heterotopic ossification was directly shown by the discovery that FOP results from mutations in the highly conserved glycine-serine (GS) regulatory domain of the BMP Type I receptor, Alk2 (also known as ACVR1 and ActR1)(14,16–18), which renders the BMP signaling pathway hypersensitive or independent of BMP ligands(14,18,19). Injury also is a trigger for heterotopic ossification in FOP; however, in this genetically susceptible background, even mild soft tissue trauma can result in pronounced heterotopic skeletogenesis(20).
Despite significant advances in understanding the pathophysiology of heterotopic ossification, the source of osteogenic progenitors has been a matter of considerable debate, dating from the pioneering work of Urist, who first demonstrated the osteo-inductive properties of demineralized bone matrix(21). Further, it remains unclear whether osteoprogenitor cells responsible for heterotopic ossification are restricted to skeletal muscle and associated soft tissues, or whether the muscle environment provides unique and essential growth, survival or differentiation factors required for elaboration of the osteogenic phenotype of more widely distributed progenitor cell populations. Indeed, circulating osteoprogenitors may contribute to heterotopic lesions(22,23), although bone marrow transplantation has yielded conflicting data on the contribution of circulating cells(22,24,25), and lineage analysis in the mouse(26) did not detect a direct cellular contribution of the tested hematopoietic lineages to heterotopic skeletogenesis. Among local, tissue-resident populations, satellite cells—muscle-specific stem cells responsible for skeletal muscle growth and regeneration—have received considerable attention as a possible cell-of-origin primarily because of their muscle-restricted distribution and their capacity for BMP-dependent osteogenic differentiation in culture(27,28). Cre/loxP-based lineage analysis in the mouse, however, demonstrated that satellite cells in vivo do not appreciably contribute to heterotopic skeletal lesions(26,29).
Vascular endothelium has emerged as the leading candidate source of lesional skeletal cells in FOP and mouse models. Human endothelial cells exhibit multipotentiality, including osteogenic activity, under certain experimental settings(30), and recent Cre-loxP lineage analyses reported by our group and others(29,30) are consistent with an endothelial origin of lesional skeletal tissue. This interpretation remains equivocal, however, because of the lack of strict lineage specificity of the Cre driver used. Here we demonstrate that multipotent mesenchymal cells that are resident in the skeletal muscle interstitium are a predominant source of progenitors that drive heterotopic ossification and that the endothelium does not detectably contribute to BMP2-induced skeletogenesis in the mouse. We describe the isolation, characterization and purification to near homogeneity of this progenitor population.
Materials and Methods
Mice and Genotyping
Animal procedures were reviewed and approved by the University of Connecticut’s Institutional Animal Care and Use Committee. Tie2-Cre transgenic mice(31) were kindly provided by Dr. Tom Sato (UT Southwestern). VE-Cadherin-Cre(32) transgenic mice (B6.Cg-Tg(Cdh5-cre)7Mlia/J) and SCID mice (B6.CB17-Prkdcscid/SzJ) were obtained from Jackson Laboratories. R26NG Cre-dependent GFP reporter mice were developed in our laboratory(33). Transgenic and reporter mice were carried on an FVB-enriched background. Experimental mice, which were hemizygous for the Cre transgene and heterozygous for the reporter allele, were genotyped by PCR and/or reporter fluorescence. The following primers were used for genotyping: Tie2-Cre: 5′-CCCTGTGCTCAGACAGAAATGAGA-3′ and 5′-CGCATAACCAGTGAAACAGCATT GC-3′; VE-Cadherin-Cre: 5′-CATTTGGGCCAGCTAAACAT-3′ and 5′-CGGATCATCAGCT ACACCAG-3′; R26NG: 5′-GATCAGCAGCCTCTGTTCCACA-3′ and 5′-CGCTGAACTTGTG GCCGTTTAC-3′. Tissue-specific recombination was visually confirmed at the time of tissue harvest. For most experiments, young adult mice between 8 and 12 weeks old were used, although comparable results were obtained with mice up to 6 months old (latest time tested; unpublished observations), when the musculoskeletal system is fully mature. Littermate controls that lacked the Cre transgene or the reporter allele were used to establish FACS gates for GFP fluorescence.
BMP2-induced Heterotopic Ossification
The BMP2 injection model of heterotopic ossification has been previously described(6,29). Briefly, 47.5μl of growth factor reduced Matrigel (BD Biosciences) was mixed with 2.5μl of 1 mg/ml human recombinant BMP2 (provided by Wyeth Pharmaceuticals) at 4°C, loaded into an ice cold 0.3cc insulin syringe and injected into the mid-belly of the tibialis anterior (TA) muscle of mice under general anesthesia. Tissue was harvested at 8 and 15 days post-injection to assess cellular contributions at chondrogenic and osteogenic stages respectively.
Tissue Preparation and Sectioning
Muscles and lesional tissues were fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS; 150mM NaCl, 16mM Na2HPO4, 4mM NaH2PO4; pH 7.4) for 3–6hr at 4°C, washed with PBS, cryo-protected by overnight incubation at 4°C in 30% sucrose, and embedded in Tissue Tek O.C.T. compound (Sakura), and frozen in dry ice-cooled pentanes. Blocks were cryostat sectioned at 12μm (Leica CM3050S) and sections collected on positively charged glass slides (Superfrost Plus, Fisher Scientific). Sets of five serial sections, each separated by 36–48 μm (transplantations into BMP2-induced lesional tissue and regenerating muscle) or 60–72μm (uninjured muscle for anatomical localization), were collected throughout each sample. Slides were then stored at −80°C prior to processing for immunofluorescence.
Fluorescence Activated Cell Sorting and Analysis
Total hindlimb muscle, kidney, and lung were carefully dissected, rinsed in PBS and minced for 6–8 min with scissors. Mechanically digested tissue was transferred to a conical tube containing 35 U/ml Collagenase Type II (Gibco) and 1U/ml Dispase (Gibco) in high glucose Dulbecco’s Modified Eagle Medium (DMEM, Gibco), and incubated in a 37°C water bath for 60–85 min with gentle trituration every 15 min to maximize digestion. Enzymatic digestion was terminated with the addition of 20% FBS (characterized, Hyclone) in DMEM and passed serially through 100μm and 70μm cell strainers (BD Biosciences). Digests were centrifuged at 500 X g for 10 min, washed with PBS, and resuspended in 10% FBS in PBS for antibody staining. For lung and kidney preparations, erythrocytes were lysed with 0.8% NH4Cl prior to the PBS wash. Antibody concentrations and sources are listed in the Supplemental Table.
Cells were incubated with fluorescently-conjugated antibodies for 30 min at 4°C. The cells were washed with PBS, collected by centrifugation, resuspended in 0.5–2% FBS and placed on ice until sorting or analysis. Just prior to sorting, the samples were filtered through a 30μm cell strainer (BD Biosciences) and propidium iodide was added at a final concentration of 1μg/ml to assess viability. To test for non -specific staining, Fluorescence-Minus-One (FMO) controls with the appropriate isotype control antibody were performed. For the GFP FMOs, cells from wild type or R26NG/+ mice were used. Strict sorting gates were set to minimize cross contamination between populations and target populations were sorted into ice-cold 5ml FACS tubes containing 10% FBS in PBS. Sorting and analysis was done on a FACSAriaII (BD Biosciences) equipped with 407, 488, and 633 lasers and a FACSAriaI equipped with 355, 405, 488, 561, and 633 lasers.
Cell Transplantation
Immunodeficient SCID mice were used as hosts to avoid immunological rejection of donor cells, which were of a mixed genetic background. In pilot experiments, we confirmed that SCID mice exhibit a robust osteogenic response following intramuscular BMP2 injection, with no obvious difference in timing or extent of the response relative to wild type mice. For each experiment, FACS-sorted populations from one (muscle, lung) or two (kidney) mice were washed with ice-cold PBS and viable cell numbers were determined by Trypan Blue staining. Cells were collected by centrifugation and resuspended in either 50μl of Matrigel containing 2.5μg BMP2 or in Matrigel alone. The cell suspension was loaded into an ice-cold 0.3cc insulin syringe and implanted into the mid-belly of the TA muscle of host mice under general anesthesia. In a typical experiment, 2 X 104 muscle-derived cells were injected, although a range of cell concentrations of muscle-derived cells (4.5 x 103 to 3 x 104 per 50μl injection volume) were tested for osteogenic activity. For direct comparisons between FACS fractions, equivalent numbers of cells were injected. Fewer cells were available for analysis of kidney- and lung-derived cells, and numbers used are noted in the text. At 10.5 days post-injection, a stage at which both chondrogenic and osteogenic lesional cells are present, the TA muscle was fixed and processed for cryosectioning as described above. For cell transplantation into injured muscle, cells were resuspended in 50μl PBS and implanted into the TA muscle 2.5 days after cardiotoxin-induced injury. Muscle was fixed 14 days post-injury, when regeneration is essentially complete, and GFP+ cells in representative sections were scored for engraftment into skeletal muscle fibers.
To quantify the contribution of transplanted cells to skeletal anlagen, sections were surveyed for the presence of GFP+ cells, and sections containing at least one GFP+ cell were chosen at random for analysis. At least four images were examined for each BMP2-induced lesion, and GFP+ cells were scored for expression of Sox9, Osterix, Perilipin or CD31. The total number of GFP+ cells assessed varied widely between FACS fractions (n=352–3552; Table 1), reflecting large differences in levels of engraftment. The range in number of GFP+ cells scored across all experiment for each FACS fraction is as follows: CD31-CD45-PDGFRα+Sca-1+ (n=816–1442); CD31+ (n=313–588); CD31-CD45-PDGFRα-Sca-1- (n=12–260). Numbers in Table 1 represent the percentages of all GFP+ cells that were skeletogenic (Sox9+ or Osterix+), adipogenic (Perilipin+) or endothelial (CD31+). Values (± SEM) represent the mean of two (GFP+CD31+) or three (GFP+PDGFRα+Sca-1+; GFP+ PDGFRα-Sca-1-) independent experiments. A one-way ANOVA test was used to test for statistical differences in developmental outcomes between FACS fractions (Table 1).
Table 1.
Fates adopted by GFP+ donor-derived cells in BMP2-induced lesions1
| Donor Fraction | n2 | Chondrogenic/ Osteogenic | Endothelium | Adipogenic | Unidentified3 |
|---|---|---|---|---|---|
| CD31− CD45− PDGFRα+ Sca-1+ |
3552 | 58.6 ± 4.2* | 0.1 ± 0.1 | 14.1 ± 5.9** | 27.2 |
| CD31+ | 901 | 0.9 ± 0.4 | 90.3 ± 0.5* | 0.0 | 8.8 |
| CD31− CD45− PDGFRα− Sca-1− |
352 | 1.0 ± 0.6 | 1.0 ± 0.8 | 0.6 ± 0.6 | 97.4 |
Numbers represent the % of GFP+ cells that adopted a particular fate, ± SEM, and are the mean of a minimum of two independent experiments.
Total number of GFP+ cells scored for each FACS fraction.
Calculated by subtracting the % of cells scored as either skeletogenic or adipogenic from 100%.
A one-way ANOVA test was used to test for statistical differences in developmental outcomes between donor fractions.
p < 0.01,
p < 0.05.
Cell Culture and Clonal Analysis
Single cells were FACS sorted directly into individual wells of Matrigel coated 96-well plates containing 20% FBS (characterized, Hyclone), 10% Horse Serum (Invitrogen), 2.5ng/ml human recombinant bFGF (Invitrogen), 50U/ml Penicillin, and 50μg/ml Streptomycin (Pen/Strep, Gibco) in DMEM and the plates incubated at 37°C in a humidified atmosphere at 5% CO2. Individual wells were carefully inspected by light or fluorescence microscopy over the next 24hr and rare wells containing more than one cell were excluded from further analysis.
Cells were fed on day 4 or 5, and then every 3 days thereafter, by replacing half the volume of medium with fresh medium. On day 14, when the majority of wells had reached 80–90% confluency, half of the wells were given a complete change of growth medium and half were switched to osteogenic induction medium (300ng/ml BMP2, 5% FBS, Pen/Strep, DMEM). The medium was changed every 3 days for the remaining 9 days. Wells were rinsed in PBS, fixed for 10 min in 4% PFA in PBS at room temperature, and rinsed and stored in PBS until staining.
Adipogenesis was quantified after 10–14 days in growth medium. Cells were scored as adipogenic if they contained intracellular multilocular fat droplets, stained with Oil Red O, or expressed the adipocyte marker, Perilipin. Clones in 23-day cultures were scored as osteogenic if they stained for alkaline phosphatase activity or contained Osterix+ cells. Data in Fig. 5M represents the mean of three independent experiments ± SEM. The Chi Square Test was used to test for statistical significance.
Figure 5.
Clonal analysis of GFP+CD31-CD45-PDGFRα+Sca-1+ progenitors in culture. (A) Single cells were sorted directly into individual wells of 96 well plates and evaluated for adipogenic and osteogenic differentiation, with or without the addition of BMP2 on day 14 of culture. (B, C) Typical example of a live colony maintained in growth medium for 12d. Insets are magnified views of the boxed areas. Adipocytes with multilocular lipid deposits are apparent (insets). Panel B, Hoffman modulation contrast microscopy. (D) 14d culture stained with Oil Red O to definitively identify lipid droplets. (E–H) Typical examples of 23d cultures maintained in growth medium and stained for the osteogenic markers, ALP (E) or Osterix (F, G), or for the adipogenic marker, Perilipin (H). 89% of colonies were negative for ALP activity, and no cells with nuclear localized Osterix immunoreactivity were observed. Perilipin+ adipocytes were abundant in these cultures. Fibroblastic-like cells that stain for SMA are also present in these cultures (data not shown). (I–L) Examples of 23d cultures that were switched from growth medium to BMP2-containing osteogenic medium on day 14. Most colonies were characterized by intense ALP staining (I) or nuclear localized Osterix expression (J, K; arrows). Perilipin+ adipogenic cells persisted under osteogenesis-inducing conditions (L). Scale bars represent 100μm (B, D, E, I), or 50μm (F, J, H, L). (M) Quantification of adipogenic and osteogenic differentiation in clonal cultures. Adipogenic differentiation was assessed between 10 and 14 days of culture, and colonies were scored as positive if they contained cells with large lipid droplets, stained with Oil Red O, or expressed Perilipin (representative examples of positive criteria are shown in panels B, D, H, and I. Colonies were scored as osteogenic if they contained cells that stained positively for either ALP activity or nuclear-localized Osterix expression. Osterix was not detected in growth media cultures. Error bars represent ± SEM. Data is from three independent experiments. *P< 0.001 (Chi-Square Test).
Immunofluorescence and Histochemistry
Antibody information can be found in the Supplemental Table. For Osterix, Perilipin, UCP1 and CD31 staining of lesional tissue, sections were rehydrated in PBS, permeabilized in 0.1% Triton X-100 in PBS, blocked in 1% BSA (Sigma), 10% goat serum (Sigma), and 0.1% Tween 20 in PBS (blocking buffer), and stained with primary antibody in PBS containing 10% goat serum and 1% BSA (dilution buffer) overnight at 4°C. Sections were then washed in PBS, stained with Alexa Fluor conjugated secondary antibody in dilution buffer at room temperature for 1.5–2hr, washed in PBS, counterstained with DAPI and coverslipped using Fluoro-Gel (Electron Microscopy Sciences). For Sox9 staining, sections were permeabilized with 0.2% TritionX-100 in PBS, and blocked and stained with primary antibody as above. After washing in PBS, sections were incubated with a biotinylated anti-rabbit antibody in dilution buffer for 2hr at room temperature, washed in PBS, and incubated with Alexa Fluor-conjugated streptavidin in dilution buffer for 30 min at room temperature. Sections were stained with DAPI and coverslipped as above. For CD31, PDGFRα, Sca-1, SMA, NG2, and Laminin detection, a comparable procedure to that of Osterix staining was used except that permeabilization was done in 0.005% – 0.01% Triton X-100 in PBS and donkey serum (Millipore) was substituted for goat serum in the blocking and dilution buffers. Controls lacking primary antibody were conducted simultaneously on adjacent sections.
For Osterix, Perilipin and SMA staining of cultured cells, wells were washed with PBS, permeabilized in 0.25% Triton X-100 in PBS, blocked in blocking buffer and stained with primary antibody in dilution buffer overnight at 4°C. Cells were then washed with PBS, stained with Alexa Fluor-conjugated secondary antibody in dilution buffer at room temperature for 1hr, washed in PBS, counterstained with DAPI and stored in PBS for microscopic analysis. Controls for non-specific staining lacked primary antibody. ALP activity was detected using a commercial kit according to the manufacturer’s recommendations (Sigma). To detect neutral lipids wells were rinsed with 60% isopropanol, stained with Oil Red O in 60% isopropanol, rinsed with 60% isopropanol and distilled H2O, and stored in PBS for microscopic analysis.
Imaging
Stained tissues or cells were imaged on a Nikon E600 upright microscope equipped with a Spot RT3 camera and Spot Advanced image capture software (Diagnostic Instruments) or on a Nikon TE2000-U inverted microscope equipped with a Qimaging Retiga EXi camera and Openlab imaging software (Improvision). A Nikon A1R four-laser spectral confocal microscope (excitation wavelengths of 405, 488, 561, and 640nm) was used for immunofluorescence microscopy, and images were acquired using Nikon Elements software. Image processing was performed using Nikon Elements software, the GNU Image Manipulation Program (GIMP), and ImageJ. Only minor linear adjustments were made to image brightness and contrast. Images were assembled in PowerPoint.
Gene Expression Analysis
FACS-sorted cell fractions were collected in ice-cold 10% FBS in PBS, pelleted by centrifugation and washed in PBS. Total RNA was isolated and first strand cDNA was reverse transcribed with the Applied Biosystems Cells to CT kit in accordance with the manufacturer’s protocol. Due to low cell numbers, unbiased preamplification was performed per the manufacturers instructions. 5μl of the diluted preamplification product was used in the PCR reaction and quantitative PCR was performed using the ABI 7900HT real-time PCR machine with the following cycling conditions: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of amplification (95°C for 15s and 60°C for 1 min). Data were acquired and analyzed using the ABI Sequence Detection Software (SDS) 2.4. The following TaqMan gene amplification assays were used: GAPDH: Mm99999915_g1; Tie2: Mm00443254_m1; and Von Willebrand Factor: Mm00550376_m1.
Results
Tie2+ Progenitors Are a Major Contributor to Heterotopic Ossification
Recent lineage tracing studies have implicated the vascular endothelium in heterotopic ossification(29,30), as cells expressing the Angiopoietin receptor, Tie2, robustly contribute to all stages of BMP-induced skeletogenic lesions(29,30). We re-established this finding using a new, highly sensitive Cre-dependent GFP reporter (R26NG)(33), and cell-specific markers for definitive identification of lesional cells. To induced heterotopic ossification, 2.5μg of BMP2 was mixed with Growth Factor Reduced Matrigel as previously described(6,29), and injected into the tibialis anterior (TA) muscle of Tie2-Cre;R26NG/+ adult mice. The contribution of GFP-labeled cells to heterotopic skeletogenesis was assayed at 8 and 15 days post-injection, stages when chondrogenic and osteogenic cells, respectively, predominate in heterotopic lesions (Fig. 1A–C). Matrigel is a semi-solid at 37°C, providing an effective means of delivering a localized source of BMP2 to skeletal muscle. As documented previously(6,29), Matrigel alone does not elicit an osteogenic response. GFP+ cells robustly contributed to heterotopic cartilage and bone, as revealed by immunofluorescence for the transcription factors, Sox9 and Osterix (Fig. 1D–G, L–O), which label all stages of cartilage and osteogenic differentiation, respectively(34,35).
Figure 1.
Tie2+ cells from a non-endothelial origin contribute to BMP2-induced heterotopic ossification. (A) Lateral view of a mouse hindlimb showing a heterotopic lesion (dashed oval) in the TA muscle 15d after BMP2 administration. (B, C) H&E stained cryostat sections of typical lesions 8d and 15d after BMP2 injection. (B) 8d lesions are primarily comprised of cartilage (C) and surrounding fibroproliferative cells (FP). M, muscle fibers. (C) By 15d, cartilage largely has been replaced by bone (B). Marrow cavities are evident (□). (D–G, L–O) Confocal immunofluorescence images of cartilage and bone stage lesions from Tie2-Cre;R26NG/+ TA muscle. Boxed areas in (D) and (L) are shown at higher magnification in (E, F) and (M–O), respectively. Many GFP+ chondrocytes (Sox9+) and osteogenic cells (Osterix+) are evident (examples shown at white arrows). Examples of GFP+ endothelium are shown at yellow arrows. (H–K, P–S) Sections of representative cartilage and bone stage lesions from VE-Cadherin-Cre;R26NG/+ TA muscle. Boxed areas in (H) and (P) are shown at higher magnification in (I, J) and (Q–S), respectively. CD31+ lesional vasculature is GFP+ (yellow arrows) whereas heterotopic cartilage and bone are GFP-. Images are representative of a minimum of three independent lesions from three mice at each stage. Scale bars are 40μm.
Tie2+ Progenitors of Heterotopic Ossification Are Not of Endothelial Origin
Although endothelium is the predominant cell type labeled in skeletal muscle tissue of Tie2-Cre;R26NG/+ mice (Supplemental Figs. S1A, C, D, and S2), the contribution of endothelial-derived cells to heterotopic skeletogenesis remains equivocal because Tie2 is expressed in a number of non-endothelial cell types (Supplemental Fig. S1D)(31,36–38). In addition, Tie2 expression was detected in the BMP-induced lesional cells themselves(29,30), raising the possibility that Cre-dependent labeling resulted from de novo Tie2-Cre expression rather than reflecting a lineage relationship with Tie2+ muscle-resident populations. For these reasons, we re-assessed the contribution of endothelial cells to heterotopic ossification using VE-Cadherin-Cre transgenic mice, which provide an alternative means of efficiently labeling endothelium of all types of vascular elements(32). Using the pan-endothelial marker, CD31, we estimated by immunofluorescence that essentially 100% of endothelial cells of the skeletal muscle vasculature were GFP+ in VE-Cadherin-Cre;R26NG/+ mice (Supplemental Fig. S1B). Vascular elements of heterotopic lesions also were uniformly GFP+ (Fig. 1H–K, P–S). However, GFP+ cells did not contribute to heterotopic cartilage or bone, as identified by Sox9 and Osterix expression, respectively (Fig. 1H–K, P–S), strongly arguing against an endothelial origin of BMP2-induced heterotopic cartilage and bone.
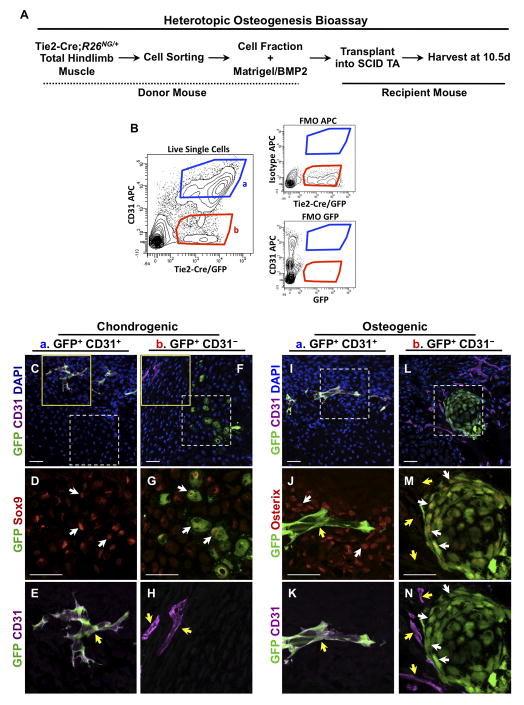
The capacity of endothelial cells to participate in heterotopic skeletogenesis was further assessed in cell transplantation experiments. Total mononuclear cells from hindlimb musculature of adult Tie2-Cre;R26NG/+ mice were fractionated by FACS for the expression of GFP and CD31, and cellular subfractions mixed with BMP2/Matrigel and tested for osteogenic activity following intramuscular injection into the mid-belly of the TA muscle of immunodeficient (SCID) mice (Fig. 2A, B). Tissue was harvested 10.5d post-injection, when lesions are comprised of both chondrogenic and osteogenic cells. Approximately 95% of CD31+ cells were also GFP+, demonstrating highly efficient Tie2-Cre-dependent endothelial cell labeling (Supplemental Figs. S1A, C, and S2). Transplanted GFP+CD31+ cells did not contribute to heterotopic cartilage or bone, but did participate in lesional angiogenesis, as evidenced by the presence of GFP+ cells that expressed CD31, and incorporation into histologically identifiable vascular elements (Fig. 2A–C, E, I–K). ~90% of GFP+CD31+ cells in lesions were identified as endothelium (Table 1). In contrast, the GFP+CD31-, non-endothelial, fraction consistently incorporated into chondrogenic and osteogenic lesions, with the majority of GFP+ lesional cells expressing Sox9 and Osterix, respectively (Fig. 2F–H, L–N). These data demonstrate that Tie2+ progenitor cells with osteogenic potential reside in native, uninduced muscle tissue and are not derived from the endothelium.
Figure 2.
Non-endothelial progenitors participate in heterotopic ossification following intramuscular transplantation. (A) Schematic of the experimental design. (B) After sorting for live, mononuclear cells (Supplemental Fig. S2), GFP+ cells from Tie2-Cre;R26NG/+ total hindlimb muscles were fractionated by flow cytometry into CD31+ (endothelial cells) and CD31- populations and tested for osteogenic activity. Strict sorting gates were applied that minimized cross population contamination and fluorescence-minus-one controls (FMOs) were conducted to assess labeling specificity. ~2 X 104 cells in 50μl were injected in a typical experiment. (C–E, I–K) Confocal images of cryostat sections of heterotopic lesions 10.5d after transplantation of GFP+/CD31+ cells. Solid and dashed boxed areas are shown at higher magnification in (E) and (D, J, K), respectively. GFP+/CD31+ cells contribute to CD31+ lesional vasculature (yellow arrows), but not to Sox9+ cartilage or Osterix+ bone of heterotopic lesions (white arrows). (F–H, L–N) GFP+/CD31- cells contribute to chondrogenic and osteogenic cells of the skeletal anlagen (white arrows) but do not contribute to endothelium (yellow arrows). Solid and dashed boxed areas are shown at higher magnification in (H) and (G, M, N), respectively. Images are representative of a minimum of two independent lesions at each stage. Scale bars are 40μm.
Prospective Isolation of Tie2+ Skeletogenic Progenitors From Skeletal Muscle
We established FACS fractionation methods to enrich for the Tie2+ osteoprogenitor population, which constituted a sub-fraction of the GFP+CD31- cells isolated from Tie2-Cre;R26NG/+ muscle. Circulating fibrocyte-like cells, which express the pan-hematopoietic marker, CD45, and exhibit osteogenic activity following subcutaneous implantation, were observed in early inflammatory and fibroproliferative regions of FOP lesions(25). Since Tie2 is expressed by hematopoietic stem cells(38), and the majority of hematopoietic cells should be labeled in Tie2-Cre mice, we tested both CD45+ and CD45-subfractions of the GFP+CD31-population (Supplemental Figs. S1D and S2) for BMP2-dependent osteogenic activity following cell transplantation. Only the CD45- fraction appreciably participated in heterotopic skeletogenesis; GFP+CD31-CD45+ cells engrafted poorly, giving rise to occasional cells of unknown identity scattered among mesenchymal cells of the lesions (data not shown).
We enriched for the osteoprogenitor fraction based on expression of PDGFRα and Sca-1, cell surface markers commonly expressed by mesenchymal progenitors. Using either PDGFRα(39) or Sca-1(12) expression, previous studies have prospectively isolated osteoprogenitors from skeletal muscle that were represented within heterogeneous cell populations. All four combinations of PDGFRα and Sca-1 marker expression were observed among GFP+CD31-CD45- cells, with the PDGFRα+Sca-1+ fraction representing ~90% of all GFP+CD31-CD45- cells (Figs. 3A and 4A; Supplemental Fig. S2), and ~0.5% of total live mononuclear cells. ~99% of GFP+CD31-CD45-PDGFRα+ cells were also positive for Sca-1. Expression of the endothelial marker, von Willebrand Factor, was not detected by qRT-PCR in the GFP+CD31-CD45-PDGFRα+Sca-1+ cell fraction, consistent with the lack of endothelial cell representation in this population.
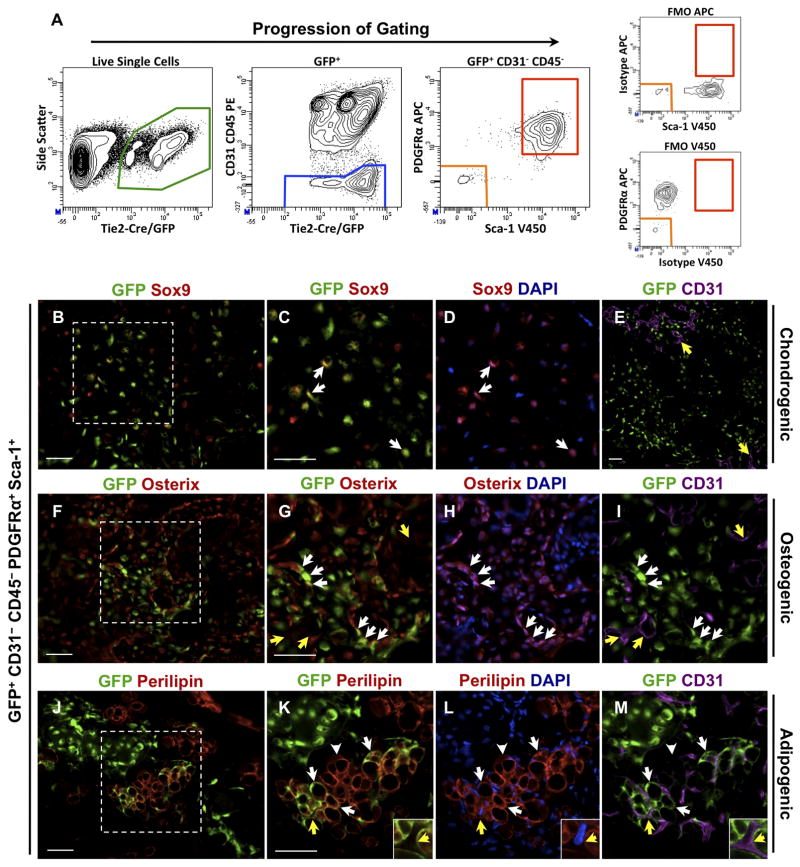
Figure 3.
Identification of GFP+CD31-CD45-PDGFRα+Sca-1+ skeletogenic progenitors from uninjured skeletal muscle. (A) After sorting for live, mononuclear cells (Supplemental Fig. S2), GFP+ cells from Tie2-Cre;R26NG/+ total hindlimb muscles were fractionated by flow cytometry. Strict gates were set to minimize cross contamination between populations, and FMO controls were conducted to assess labeling specificity. (B–I) GFP+CD31-CD45-PDGFRα+Sca-1+ cells were tested for osteogenic activity as in Fig. 2. Boxed areas in (B) and (F) are shown at higher magnification in (C, D) and (G–I), respectively. The contribution of GFP+ cells to chondrocytes and osteoblasts/osteocytes of induced lesions was readily apparent (white arrows), based on Sox9 and Osterix staining, respectively. Endothelium was GFP- (yellow arrows in E, G, I). (J–M) Donor-derived Perilipin+ adipocytes were consistently observed (white arrows). Adipocytes typically were unilocular and did not stain with the brown fat marker UCP1, indicating a white fat phenotype. GFP- host-derived adipocytes were also apparent (arrowheads in K–M). Insets in (K–M) are high magnification views of the area noted by the yellow arrows, showing the distinction between GFP+ adipocytes and GFP-CD31+ endothelium. Images are representative of a minimum of four independent lesions for each stage. Scale bars are 50μm.
Figure 4.
Cell surface antigen expression of GFP+CD31-CD45-PDGFRα+Sca-1+ progenitors derived from Tie2-Cre;R26NG/+ skeletal muscle. (A–C) Among GFP+CD31-CD45- cells, ~96% of Sca-1+ cells also express PDGFRα+, whereas ~99% of PDGFRα+ cells express Sca-1. Therefore, expression of other surface markers was assessed by replacing the anti-PDGFRα+ APC antibody (A, B) or the anti-Sca-1 V450 antibody (C) with antibodies to other markers, as shown. Quadrants were established based on FMO controls. Percentages in each quadrant represent the proportion of total GFP+CD31-CD45- cells.
Cells negative for both PDGFRα and Sca-1 did not detectably contribute to heterotopic cartilage or bone, and did not incorporate into histologically identifiable structures (Table 1). In contrast, in four separate experiments, GFP+CD31-CD45-PDGFRα+Sca-1+ cells robustly contributed to definitive cartilage and bone (Fig. 3B–I; Table 1). Analysis of confocal stacks of donor-derived Sox9+ and Osterix+ lesional cells did not reveal the presence of bi-nucleated cells, indicating that the skeletogenic fate of donor cells was not the result of cell fusion. Of note, the majority of donor cells stained positively for either Sox9 or Osterix 10.5 days after intramuscular transplantation, and donor-derived endothelial cells were not observed (Table 1). GFP+CD31-CD45-PDGFRα+Sca-1+ cells injected without the addition of BMP2 did not adopt cartilage or bone fates (Supplemental Fig. S3). Expression of the endogenous Tie2 gene was detected by qRT-PCR in this population, demonstrating the fidelity of Tie2-Cre transgene expression. Hereafter, the GFP+CD31-CD45-PDGFRα+Sca-1+ BMP2-responsive cell population will be referred to as Tie2+PDGFRα+Sca-1+ progenitors.
To further characterize the Tie2+PDGFRα+Sca-1+ progenitor population, we used FACS to assay a panel of cell surface markers, with an emphasis on those previously used to characterize osteogenic and adipogenic progenitors(12,23,39–42) (Fig. 4). Progenitors were uniformly positive for CD29 (β1-integrin) and CD34, and negative for CD11b (Integrin αM), CXCR4, and c-kit, indicating a highly enriched progenitor population. A minority of progenitor cells were weakly positive for CD44 and CD73.
Tie2+PDGFRα+Sca-1+ Progenitors Are Multipotent
Although the majority of transplanted cells adopted skeletogenic fates, we routinely observed abundant adipocytes of donor origin, as revealed by Oil Red O staining and immunofluorescence for the lipid-binding protein Perilipin (Fig. 3J–M; Table 1). Donor-derived adipocytes had characteristics of white fat, including large, unilocular lipid accumulation and absence of staining for the brown fat defining marker, UCP1 (data not shown). GFP- host-derived adipocytes were also present in these lesions (Fig. 3J–M). The presence of donor-and host-derived adipocytes was not dependent on BMP2 (Supplemental Fig. S3). Others have shown that progenitor cells sharing related marker profiles exhibit robust adipogenic potential following intramuscular transplantation, but only under the adipogenesis-promoting conditions of intramuscular glycerol injection(39,40), or treatment with BMP7 prior to transplantation(41). Elaboration of the adipogenic phenotype in our experiments was not dependent on these conditions. Whether this apparent difference reflects the biological properties of distinct cell populations, or reflects differences in experiment design remains to be determined. Surprisingly, although intramuscular glycerol injection(39,40) into Tie2-Cre;R26NG/+ mice resulted in adipocyte accumulation, adipocytes were not GFP+ (data not shown), indicating that Tie2+PDGFRα+Sca-1+ progenitors in their native cellular context do not respond to these adipogenic signals.
Clonal analysis of the Tie2+PDGFRα+Sca-1+ population was undertaken to distinguish whether this cellular fraction consists of multipotent progenitors or is a mixture of distinct adipogenic and chondrogenic/osteogenic subpopulations. Mononuclear cells from Tie2-Cre;R26NG/+ hindlimb muscles were fractionated by FACS and single GFP+ cells with the marker profile CD31-CD45-PDGFRα+Sca-1+ were collected directly into wells of 96-well plates containing growth medium (20% FBS, 10% horse serum, 2.5ng/ml bFGF, DMEM, Pen/Strep). Collectively, in three separate experiments, a total of 345 colonies were produced from 944 total wells, representing a cloning efficiency of 36.5%. By 10–14 days of culture, without the addition of adipogenesis-inducing agents, 95% of the 317 colonies assayed contained adipogenic cells, as determined by their morphology in living cultures (multilocular lipid-containing cells), staining with Oil Red O (Fig. 5A–D, M) or Perilipin expression. When maintained in growth medium for 23 days, colonies were comprised primarily of Perilipin+ adipocytes (Fig. 5H) and Smooth muscle actin+ (SMA+) cells (data not shown); SMA reactivity is consistent with a smooth muscle or fibroblast-like fate, as reported recently for a highly related PDGFRα+Sca-1+ population(39,40). Osterix+ cells were not observed and only 3 of 27 colonies contained alkaline phosphatase+ (ALP+) cells (Fig. 5E–G). Typically, ALP staining was weak and observed in a small minority of cells of the colony. In contrast, addition of osteogenic medium (300ng/ml BMP2, 5% FBS, DMEM, Pen/Strep) on day 14 resulted in robust induction of ALP or nuclear-localized Osterix expression in 84% of the colonies (31 of 37 colonies) by day 23; colonies at this endpoint were comprised primarily of osteogenic cells and Perilipin+ adipocytes (Fig. 5M, I–L). Thus, colonies derived from single, FACS-sorted, progenitors exhibited both BMP2-dependent osteogenic differentiation and BMP2-independent adipogenic and smooth muscle/fibroblastic differentiation.
Tie2+PDGFRα+Sca-1+ Progenitors Reside in the Skeletal Muscle Interstitium
Confocal microscopy of the Soleus muscle from Tie2-Cre;R26NG/+ mice was used to investigate the anatomical location of Tie2+PDGFRα+Sca-1+ progenitors (Fig. 6). Similar results were obtained with the TA and EDL muscles (data not shown). GFP+ cells expressing both Sca-1 and PDGFRα were readily apparent in the muscle interstitium (Fig. 6A). PDGFRα+Sca-1+ cells were often associated with the microvasculature, but were distinct from endothelial cells, as expected (Fig. 6B, C). These progenitors were negative for the pericyte marker, NG2 (Fig. 6D; Supplemental Fig. S4), and did not share a basal lamina with the adjacent endothelium (Fig. 6C)—a defining anatomical feature of pericytes—indicating that they are distinct from pericytes. Tie2+PDGFRα+Sca-1+ progenitors did not exhibit myogenic potential in culture or following transplantation into uninjured or injured muscle (Supplemental Fig. S5), clearly distinguishing these progenitors from PICs, highly myogenic Pw1+ interstitial muscle-resident progenitors(43). Based on marker expression and myogenic activity, mesoangioblasts, muscle SP cells and muscle-derived stem cells also are distinct from the multipotent progenitors described here(44–47).
Figure 6.
GFP+CD31-CD45-PDGFRα+Sca-1+ progenitors reside in the skeletal muscle interstitium of Tie2-Cre;R26NG/+ mice. The Soleus muscle is shown. Results with the EDL and TA muscles were comparable. (A) Confocal image of a muscle cross section that was immunostained for Sca-1 and PDGFRα. Dotted lines represent muscle fiber borders. A GFP+ cell that expresses both PDGFRα and Sca-1 is visible in this view (white arrow and lower right insets). Note its close association with the adjacent GFP+Sca-1+ endothelial cell (yellow arrow; also see panel B). Two GFP- cells that express PDGFRα+ and are weakly positive for Sca-1 are also present (purple arrows). Lower left insets: cell at smaller purple arrows. Levels for the red channel were increased for the left inset to visualize the faint Sca-1 staining. (B) GFP+PDGFRα+ cells (white arrows and insets) were often observed in close proximity to CD31+ endothelium (yellow arrow and insets). Note that ~99% of GFP+PDGFRα+ cells were also positive for Sca-1 (Fig. 4A). (C) GFP+PDGFRα+ cells (white arrows and insets) do not share a basal lamina (Laminin+) with endothelial cells (yellow arrows and insets), indicating that these progenitor cells are distinct from pericytes. GFP+PDGFRα+ cells reside in interstitial spaces between muscle fibers, each of which is circumscribed by a Laminin+ basal lamina. (D) GFP+PDGFRα+ cells (white arrows and inset) do not express the pericyte marker, NG2 (blue arrows). Images are representative of sections taken from a minimum of two Soleus muscles isolated from two independent mice. Scale bars are 20μm.
We also observed GFP-PDGFRα+Sca-1+ cells in histological sections (Fig. 6A), and quantification by FACS revealed that ~43% of all CD31-CD45-PDGFRα+Sca-1+ progenitors were GFP- (Supplemental Fig. S6A). Inefficient Tie-2-Cre-mediated recombination probably accounts for this GFP- population, as GFP+ and GFP- progenitors show related marker profiles (Supplemental Figs. S3 and S6B–D), express comparable levels of Tie2 by qRT-PCR (~50% compared to endothelium), and exhibit similar developmental potential in clonal cultures (Fig. 5; data not shown). GFP+ cells, however, show less heterogeneity in levels of surface marker expression—particularly in regards to levels of Sca-1 and CD34 expression—suggesting that PDGFRα+Sca-1+ progenitors represent a subpopulation of all GFP-PDGFRα+Sca-1+ cells.
Tie2+PDGFRα+Sca-1+ Progenitors Are Not Restricted to Skeletal Muscle
Using the same FACS fractionation protocol as above, we prospectively isolated highly similar progenitor cells with the marker profile, GFP+CD31-CD45-PDGFRα+Sca-1+, from both lung and kidney of Tie2-Cre;R26NG/+ mice (Supplemental Fig. S7). In a typical analysis, the GFP+CD31-CD45-PDGFRα+Sca-1+ fraction from lung and kidney represented 0.13% and 0.04% of total live mononuclear cells, respectively, compared to 0.5% of skeletal muscle-derived cells. Approximately 500 lung-derived, GFP+PDGFRα+Sca-1+ cells were isolated per mouse, which was insufficient for detectable engraftment (unpublished observations). Engraftment was observed, however, after transplantation of 3.7 X 103 kidney-derived progenitors, which represented the total yield from four kidneys. Donor-derived, GFP+ progenitors contributed to Sox9+ chondrocytes, Osterix+ osteogenic cells, and Perilipin+ adipocytes of heterotopic lesions (Supplemental Fig. S7). Adipogenic progenitors with related marker profiles were recently isolated from white adipose tissue(42,48). Collectively, these data support the notion that a unique signaling environment or susceptibility to injury, rather than a unique progenitor population, explains the prevalence of heterotopic ossification in skeletal muscle and associated soft tissues.
Discussion
Development of heterotopic bone shares striking molecular and histological features with normal endochondral bone development. Heterotopic ossification is distinguished, however, by the requirement for an injury/inflammatory trigger(7,26,29,49,50) and by distinct progenitors from which the ectopic skeleton is constructed(29,30). In this latter regard, vascular endothelium has emerged as a leading candidate source of lesional cells for acquired and hereditary heterotopic ossification(29,30). Thus, human umbilical vein endothelial cells that are treated with BMP4 or TGFβ2 or are engineered to over-express a mutant form of the ACVR1 receptor found in FOP adopted a mesenchymal stem cell-like phenotype, which included the capacity for osteogenic differentiation(30). Further, heterotopic lesions in FOP patients(30) and mice(29,30) express endothelial markers, and previous lineage analyses in the mouse were consistent with an endothelial origin of heterotopic cartilage and bone(29,30). We showed, however, that mouse endothelial cells in their native in vivo context do not participate in heterotopic ossification when exposed to BMP2 levels that elicit a robust osteogenic response. In addition, FACS-purified endothelial cells maintain their endothelial identity following intramuscular transplantation and contribute to lesional angiogenesis but not to BMP2-induced cartilage or bone. Whether the stability of the endothelial phenotype observed here reflects differences in potency of mouse and human endothelial cells will require further investigation.
We identified and characterized a population of Tie2+PDGFRα+Sca-1+ multipotent mesenchymal progenitors that reside in the skeletal muscle interstitium and represent a significant cell-of-origin for heterotopic ossification in the mouse. In lineage tracing experiments with Tie2-Cre transgenic mice(29); present study), heterotopic cartilage and bone was comprised of approximately equivalent numbers of labeled and unlabeled cells, either implicating two or more major osteoprogenitor populations, or resulting from inefficient Cre-mediated recombination of the PDGFRα+Sca-1+ population. The similarities in cell surface marker expression, developmental potential, and anatomical location of GFP+ and GFP-fractions is consistent with the latter possibility. These data support the view that Tie2+PDGFRα+Sca-1+ interstitial cells are the predominant BMP-responsive mesenchymal progenitor of heterotopic skeletogenesis.
The normal physiological functions of Tie2+PDGFRα+Sca-1+ progenitors in skeletal muscle and other tissues are unknown, although roles in regulating muscle differentiation(40) and energy metabolism(41) have been suggested for related cell types. We suggest that in the setting of soft tissue injury/inflammation, elevated local levels of osteogenic cytokines(11,51), or dysregulated BMP signaling as in FOP(14,19,50,52), these progenitors become programmed for osteogenesis, leading to heterotopic ossification. We and others showed that Tie2+ progenitors contribute to all stages of heterotopic ossification, including the precartilage mesenchyme(29,30); present study), suggesting that recruitment of Tie2+ progenitors into the skeletogenic pathway represents an early key event in the induction of heterotopic bone. In this regard, the identification and prospective isolation of highly enriched progenitors reported here will facilitate the characterization of initiating events in heterotopic ossification and the role of the inflammatory environment. It is noteworthy that chronic muscle disease often is accompanied by infiltration of adipose and fibrotic tissue(53), two additional fate choices adopted by Tie2+PDGFRα+Sca-1+ progenitors in certain experimental settings (also see refs.(39,40)). Whether there is a common cellular basis to the accumulation of multiple non-myogenic cell types in skeletal muscle is currently under investigation.
Supplementary Material
Acknowledgments
Funding: This work was supported by grants from the NIH and DOD to DJG.
We thank Dr. Tom Sato (UT Southwestern) for Tie2-Cre transgenic mice, and Wyeth Pharmaceuticals for human recombinant BMP2. We also thank Youfen Sun for assistance with mouse colony maintenance, members of the Goldhamer lab for helpful comments during the course of this work, and FACS Core Facility scientists, Dr. Carol Norris (University of Connecticut, Storrs) and Diane Gran (University of Connecticut Health Center), for their expert technical assistance and training. MNW participated in the Advanced Multi-colour Flow Cytometry Course at the University of British Columbia, which was funded by the Stem Cell Network of Canada. This work was supported by grants from the NIH (AR057371) and DOD to DJG.
Footnotes
Disclosures
None of the authors have financial relationships with any entities that influenced or can be perceived as influencing the objectivity or integrity of the work reported in this manuscript.
Authors’ roles: DJG directed the study and takes responsibility for the integrity of the data analysis. DJG and MNW designed the experiments, analyzed the data and prepared the manuscript. MNW performed the FACS analysis, cell transplantations, clonal analysis, immunostaining and confocal imaging. AB and CAC prepared tissue sections, conducted immunostaining experiments, assisted with other experimental procedures and contributed to data analysis. All authors discussed the results and their implications and commented on the manuscript. MNW, AB, CAC and DJG approved the final version of the manuscript.
References
- 1.Cushner FD, Morwessel RM. Myositis ossificans traumatica. Orthop Rev. 1992;21 (11):1319–26. [PubMed] [Google Scholar]
- 2.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89(3):476–86. doi: 10.2106/JBJS.F.00412. [DOI] [PubMed] [Google Scholar]
- 3.Shore EM, Kaplan FS. Insights from a rare genetic disorder of extra-skeletal bone formation, fibrodysplasia ossificans progressiva (FOP) Bone. 2008;43(3):427–33. doi: 10.1016/j.bone.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski D. Heterotopic ossification in the residual limbs of traumatic and combat-related amputees. J Am Acad Orthop Surg. 2006;14(10 Suppl):S191–7. doi: 10.5435/00124635-200600001-00042. [DOI] [PubMed] [Google Scholar]
- 5.Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005;37(3):129–36. doi: 10.1080/16501970510027628. [DOI] [PubMed] [Google Scholar]
- 6.Glaser DL, Economides AN, Wang L, Liu X, Kimble RD, Fandl JP, Wilson JM, Stahl N, Kaplan FS, Shore EM. In vivo somatic cell gene transfer of an engineered Noggin mutein prevents BMP4-induced heterotopic ossification. J Bone Joint Surg Am. 2003;85-A(12):2332–42. doi: 10.2106/00004623-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Kan L, Hu M, Gomes WA, Kessler JA. Transgenic mice overexpressing BMP4 develop a fibrodysplasia ossificans progressiva (FOP)-like phenotype. Am J Pathol. 2004;165(4):1107–15. doi: 10.1016/S0002-9440(10)63372-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connor JP. Animal models of heterotopic ossification. Clin Orthop. 1998;346:71–80. [PubMed] [Google Scholar]
- 9.Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop. 1998;346:26–37. [PubMed] [Google Scholar]
- 10.Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280(5368):1455–7. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 11.Jackson WM, Aragon AB, Onodera J, Koehler SM, Ji Y, Bulken-Hoover JD, Vogler JA, Tuan RS, Nesti LJ. Cytokine expression in muscle following traumatic injury. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29 (10):1613–20. doi: 10.1002/jor.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leblanc E, Trensz F, Haroun S, Drouin G, Bergeron E, Penton CM, Montanaro F, Roux S, Faucheux N, Grenier G. BMP-9-induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment. J Bone Miner Res. 2011;26(6):1166–77. doi: 10.1002/jbmr.311. [DOI] [PubMed] [Google Scholar]
- 13.Yaoita H, Orimo H, Shirai Y, Shimada T. Expression of bone morphogenetic proteins and rat distal-less homolog genes following rat femoral fracture. J Bone Miner Metab. 2000;18(2):63–70. doi: 10.1007/s007740050013. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, Noguchi Y, Iwakiri K, Kondo T, Kurose J, Endo K, Awakura T, Fukushi J, Nakashima Y, Chiyonobu T, Kawara A, Nishida Y, Wada I, Akita M, Komori T, Nakayama K, Nanba A, Maruki Y, Yoda T, Tomoda H, Yu PB, Shore EM, Kaplan FS, Miyazono K, Matsuoka M, Ikebuchi K, Ohtake A, Oda H, Jimi E, Owan I, Okazaki Y, Katagiri T. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem. 2009;284(11):7149–56. doi: 10.1074/jbc.M801681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan FS, Glaser DL, Hebela N, Shore EM. Heterotopic ossification. J Am Acad Orthop Surg. 2004;12(2):116–25. doi: 10.5435/00124635-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Lin GT, Chang HW, Liu CS, Huang PJ, Wang HC, Cheng YM. De novo 617G-A nucleotide mutation in the ACVR1 gene in a Taiwanese patient with fibrodysplasia ossificans progressiva. J Hum Genet. 2006;51(12):1083–6. doi: 10.1007/s10038-006-0069-2. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima M, Haga N, Takikawa K, Manabe N, Nishimura G, Ikegawa S. The ACVR1 617G>A mutation is also recurrent in three Japanese patients with fibrodysplasia ossificans progressiva. J Hum Genet. 2007;52(5):473–5. doi: 10.1007/s10038-007-0128-3. [DOI] [PubMed] [Google Scholar]
- 18.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38 (5):525–7. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 19.Billings PC, Fiori JL, Bentwood JL, O’Connell MP, Jiao X, Nussbaum B, Caron RJ, Shore EM, Kaplan FS. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP) J Bone Miner Res. 2008;23(3):305–13. doi: 10.1359/JBMR.071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan FS, Shen Q, Lounev V, Seemann P, Groppe J, Katagiri T, Pignolo RJ, Shore EM. Skeletal metamorphosis in fibrodysplasia ossificans progressiva (FOP) J Bone Miner Metab. 2008;26(6):521–30. doi: 10.1007/s00774-008-0879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urist MR. Bone: formation by autoinduction. Science. 1965;150(698):893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 22.Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Bone marrow-derived osteoblast progenitor cells in circulating blood contribute to ectopic bone formation in mice. Biochem Biophys Res Commun. 2007;354(2):453–8. doi: 10.1016/j.bbrc.2006.12.226. [DOI] [PubMed] [Google Scholar]
- 23.Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26(1):223–34. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan FS, Glaser DL, Shore EM, Pignolo RJ, Xu M, Zhang Y, Senitzer D, Forman SJ, Emerson SG. Hematopoietic stem-cell contribution to ectopic skeletogenesis. J Bone Joint Surg Am. 2007;89(2):347–57. doi: 10.2106/JBJS.F.00472. [DOI] [PubMed] [Google Scholar]
- 25.Suda RK, Billings PC, Egan KP, Kim JH, McCarrick-Walmsley R, Glaser DL, Porter DL, Shore EM, Pignolo RJ. Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells. 2009;27(9):2209–19. doi: 10.1002/stem.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kan L, Liu Y, McGuire TL, Berger DM, Awatramani RB, Dymecki SM, Kessler JA. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009;27(1):150–6. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. Journal of Cell Biology. 1994;127(6 Pt 1):1755–66. doi: 10.1083/jcb.127.6.1755. [published erratum appears in J Cell Biol. 1995. Feb;128(4):following 713] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wada MR, Inagawa-Ogashiwa M, Shimizu S, Yasumoto S, Hashimoto N. Generation of different fates from multipotent muscle stem cells. Development. 2002;129(12):2987–95. doi: 10.1242/dev.129.12.2987. [DOI] [PubMed] [Google Scholar]
- 29.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91(3):652–63. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16(12):1400–6. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230(2):230–42. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 32.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235(3):759–67. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Shook NA, Kanisicak O, Yamamoto S, Wosczyna MN, Camp JR, Goldhamer DJ. A multifunctional reporter mouse line for Cre-and FLP-dependent lineage analysis. Genesis. 2009;47(2):107–14. doi: 10.1002/dvg.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 35.Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage. 2001;9(Suppl A):S69–75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- 36.Sato TN, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A. 1993;90(20):9355–8. doi: 10.1073/pnas.90.20.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8(3):211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Iwama A, Hamaguchi I, Hashiyama M, Murayama Y, Yasunaga K, Suda T. Molecular cloning and characterization of mouse TIE and TEK receptor tyrosine kinase genes and their expression in hematopoietic stem cells. Biochem Biophys Res Commun. 1993;195(1):301–9. doi: 10.1006/bbrc.1993.2045. [DOI] [PubMed] [Google Scholar]
- 39.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12(2):143–52. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 40.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–63. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, Schrier D, Falb D, Kirkland JL, Wagers AJ, Tseng YH. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011;108(1):143–8. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135(2):240–9. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nature cell biology. 2010;12(3):257–66. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 44.De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, Ponzetto C, Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147(4):869–78. doi: 10.1083/jcb.147.4.869. [see comments] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401(6751):390–4. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 46.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851–64. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uezumi A, Ojima K, Fukada S, Ikemoto M, Masuda S, Miyagoe-Suzuki Y, Takeda S. Functional heterogeneity of side population cells in skeletal muscle. Biochem Biophys Res Commun. 2006;341(3):864–73. doi: 10.1016/j.bbrc.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 48.Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27 (10):2563–70. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby RE, Kitterman JA, Groppe J, Shore EM. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22 (1):191–205. doi: 10.1016/j.berh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008;14(12):1363–9. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shafritz AB, Shore EM, Gannon FH, Zasloff MA, Taub R, Muenke M, Kaplan FS. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med. 1996;335(8):555–61. doi: 10.1056/NEJM199608223350804. [see comments] [DOI] [PubMed] [Google Scholar]
- 52.Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Koster B, Pauli RM, Reardon W, Zaidi SA, Zasloff M, Morhart R, Mundlos S, Groppe J, Shore EM. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30(3):379–90. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol. 2009;71:37–57. doi: 10.1146/annurev.physiol.010908.163216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.