Abstract
Background
Myxoid/ round cell liposarcoma (MRCL) is the second most common liposarcoma subtype, accounting for more than one third of liposarcomas and approximately 10% of all soft tissue sarcomas. Although MRCL is a chemosensitive subtype, patients with metastatic disease have a poor outcome. NY-ESO-1 is a cancer-testis antigen (also known as cancer germ cell antigen) that has been successfully targeted in vaccine and also adoptive T cell therapy trials for the treatment of several solid tumors.
Methods
We examined the feasibility of targeting NY-ESO-1 in patients with MRCL by evaluating the prevalence of NY-ESO-1 expression among tumors using immunohistochemistry and qRT-PCR. We also analyzed NY-ESO-1 specific tumor recognition by NY-ESO-1 specific T cells using chromium release assay.
Results
A search of the University of Washington Sarcoma Tissue Bank revealed paraffin embedded tumor samples from 25 patients with MRCL. NY-ESO-1 expression was observed in every MRCL tumor assessed (100%); in 18 (72%), staining was homogenous. In all but 2 cases, staining was sufficiently robust (2+) that such patients would be eligible for clinical trials of NY-ESO-1 directed therapy. Using NY-ESO-1 specific CD8+ T cells, we demonstrate in vitro sensitivity of myxoid liposarcoma cell lines to antigen-specific lysis.
Conclusions
These results establish NY-ESO-1 as an important target antigen for the treatment of patients with MRCL.
Keywords: NY-ESO-1, Sarcoma, Myxoid, immunotherapy, cancer testis antigens
Introduction
Based on its immunogenicity, NY-ESO-1 is considered to be among the most attractive antigens for immunotherapy. It has been targeted in a number of clinical studies including several vaccine trials that have induced serologic, CD4+ and CD8+ T cell responses. Delayed type hypersensitivity responses following NY-ESO-1 vaccination have been associated with long-term survival [1, 2]. Objective clinical responses have been observed in melanoma patients following vaccination against NY-ESO-1, including one complete response [3].
NY-ESO-1 has also been successfully targeted in trials of antigen specific adoptive T cells therapy. For example, transfer of NY-ESO-1 specific CD4+ cells have been effectively used to treat patients with metastatic melanoma [4]. NY-ESO-1 has also been targeted using a class I TCR retrovirally transfected into T cells [5] inducing complete responses in melanoma patients. To date there have been no known grade III or grade IV autoimmune toxicities associated with anti-NY-ESO-1 therapy.
NY-ESO-1, a member of the family of Cancer Testis Antigens (CT antigens), was first discovered through serological analysis in esophageal cancer patients and was subsequently found to induce a strong cytotoxic T-cell response [6-8]. As their name implies, CT Antigens (also sometimes referred to as cancer germ-cell antigens), are expressed on a protein level in various malignant tumors and germ cells of the testis but not other adult tissues.
Soft tissue sarcomas are a heterogeneous group of malignancies of mesenchymal origin with poor prognosis in the metastatic setting and a median overall survival less than one year [9, 10]. Liposarcomas account for approximately 10-20% of soft tissue sarcomas and can be classified into three subtypes each with their own distinct clinical behaviors: pleomorphic, well/de-differentiated and myxoid/ round cell liposarcoma (MRCL). MRCL accounts for 40-50% of liposarcomas and is almost always associated with a chromosomal translocation, most commonly t(12;16)(q13;p11) though a number of less common translocations have also been described [11]. The resultant fusion proteins have an activity that is not well understood [12]. MRCL is a relatively sensitive to front line chemotherapy and trabectedin as second line treatment for metastatic MRCL is promising [10, 13]. However, mortality remains high for patients with metastatic disease, suggesting the need for novel approaches.
The discovery that over 80% of synovial sarcomas express NY-ESO-1 [14], often homogenously, established synovial sarcoma as a malignancy with one of the highest rates of NY-ESO-1 expression and led to the perception that synovial sarcoma is a model disease for the study of NY-ESO-1 directed therapy. Supporting this concept, a recently published adoptive therapy trial [5] using retrovirally transfected NY-ESO-1 specific TCR, documented 4 partial responses out of 6 synovial sarcoma patients treated.
Here we report that another soft tissue sarcoma subtype, MRCL, ubiquitously expresses the cancer testis antigen NY-ESO-1, most often homogenously, raising the possibility of NY-ESO-1 directed therapy for this challenging disease with limited treatment options in the metastatic setting.
Methods
Tumor Samples
Both paraffin embedded and flash frozen human MRCL samples were obtained through the University of Washington sarcoma tumor bank (IRB approved protocol #21369).
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissues. MAb E978 was used to detect NY-ESO-1 as previously described [15]. For all assays, appropriate positive (normal testis with preserved spermiogenesis) and negative controls (omission of primary antibody and replacement with phosphate buffer saline, pH 7.4) were included.
Tissue sections were deparaffinized in xylene and rehydrated in a series of graded alcohols. Endogenous peroxidase was blocked by incubating slides for 30 minutes at room temperature in 99.7% methanol containing 0.3% hydrogen peroxide. Slides were washed with Tris-buffered saline solution and blocked in 2% bovine serum albumin (BSA) at room temperature for 5 minutes to prevent unspecific protein interactions. A heat-based antigen retrieval technique using a commercial vegetable steamer was used by heating slides in a buffer solution (Table1) for 30 minutes at approximately 96°C. Tissue slides were incubated with primary antibodies overnight at 4°C in a wet chamber. Primary antibody detection was performed by use of a Novolink polymer detection kit (Leica Microsystems Inc., Bannockburn, IL) in accord with the manufacturer’s instructions. 3,3-Diaminobenzidine (DAB) served as a chromogen and counterstains were done with Harris hematoxylin. Finally, slides were dehydrated in a series of graded ethanols and cover slipped.
| Patient | Cytogenetics | Site of primary tumor |
Primary or Metastatic Specimen |
Outcome | NY-ESO- 1 Staining |
qRT-PCR confirmed |
|---|---|---|---|---|---|---|
| 1 | cytogenetics unavailable | right thigh | Primary | Metastatic Disease, deceased | 3+ | Not available |
| 2 | cytogenetics unavailable | right hip | Primary | Metastatic Disease | 3+ | Not available |
| 3 | cytogenetics unavailable | left buttock | Primary | Local recurrence, currently disease free |
3+ | Not available |
| 4 | cytogenetics unavailable | right thigh | Metastatic | Metastatic Disease | 2+ | Yes |
| 5 | cytogenetics unavailable | left popliteal fossa | Primary | Metastatic Disease, deceased | 4+ | Not available |
| 6 | t(12;16) with other complex cytogenetics |
right groin | Primary | Metastatic Disease, deceased | 4+ | Not available |
| 7 | t(12;16)(q13;p11) | left popliteal fossa | Primary | Disease free | 1+ | Not available |
| 8 | t(12;16) with other complex cytogenetics |
left thigh | Primary | Metastatic Disease, deceased | 3+ | Not available |
| 9 | t(10;16;12)(q26;p11;q13) | left calf | Primary | Disease free | 2+ | Not available |
| 10 | cytogenetics unavailable | right thigh | Primary | Disease free | 4+ | Not available |
| 11 | t(12;16)(q13;p11) | right thigh | Primary | Local recurrence, currently disease free |
2+ | Not available |
| 12 | t(12;16)(q13;p11) | right thigh | Primary | Isolated recurrence, currently disease free |
4+ | Not available |
| 13 | t(12;16)(q13;p11) | left popliteal fossa | Primary | Disease free | 3+ | Yes |
| 14 | t(12;16) with other complex cytogenetics |
right tibia | Primary | Disease free | 4+ | Not available |
| 15 | t(12;16)(q13;p11) | right thigh | Primary | Disease free | 4+ | Not available |
| 16 | t(12;22)(q13;q12) | right great toe | Primary | Disease free | 2+ | Not available |
| 17 | cytogenetics unavailable | left leg | Metastatic | Metastatic Disease | 4+ | Not available |
| 18 | t(12;16) with other complex cytogenetics |
right thigh | Primary | Disease free | 1+ | Not available |
| 19 | t(12;16)(q13;p11) | right pelvis | Primary | Metastatic Disease | 4+ | Not available |
| 20 | normal cytogenetics | left thigh | Metastatic | Metastatic Disease | 3+ | Not available |
| 21 | t(12;16) with other complex cytogenetics |
left axilla | Primary | Disease free | 4+ | Yes |
| 22 | t(12;16)(q13;p11) | left gluteal | Primary | Metastatic Disease | 3+ | Not available |
| 23 | t(15;17)(q22;q23) | left thigh | Primary | Disease free | 3+ | Yes |
| 24 | t(12;16)(q13;p11) | right thigh | Primary | Disease free | 3+ | Yes |
| 25 | t(12;16) with other complex cytogenetics |
left ankle/fibula | Primary | Metastatic Disease | 2+ | Yes |
Scoring of staining is based on percentage of cells staining positive for NY-ESO-1: Focal <5%; 1+ 5-25%; 2+ 25-50%; 3+ 50-75%; 4+ >75%
Slides were examined under a light microscope by two pathologist including an experienced bone and soft tissue pathologist. Characteristic morphological features of myxoid liposarcoma were confirmed, including myxoid stroma, primitive mesenchymal cells, lipoblasts and an arborizing capillary vasculature. Cases with staining present in less than 5% of cells were considered focally positive. 1+ was considered 5-25%. 2+ was 25-50%. 3+ was considered 50-75%. Tumors with staining >75% were considered 4+.
qRT-PCR
RNA was extracted from frozen tumor samples using Trizol (invitrogen) and from cell lines using RNeasy kit (Qiagen). Due to varying tissue collection conditions, RNA quality was recorded prior to analysis, using either a gel or bioanalyzer. Samples with poor quality RNA were not analyzed further. One non-myxoid liposarcoma sample was invading into the spermatic cord and was thus not included in the analysis because of concern for false positive.
RNA samples were converted to cDNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche). Results were analyzed using GAPDH as a house keeping gene and were calculated relative to testis using the standard curve method. NY-ESO-1 primers were TGCTTGAGTTCTACCTGCCA and TATGTTGCCGGACACAGTGAA [16]. GAPDH primers were GAAGGTGAAGGTCGGAGTC and GAAGATGGTGATGGGATTTC [17]. In all MRCL tumors, NY-ESO-1 expression was also confirmed and quantitated using primers from SA Biosciences. Amplification was performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, Ca) on an ABI 7900HT (Applied Biosystems, Foster City, Ca).
Antigen Specific T cells
NY-ESO-1 specific effectors were generated from a HLA -A*0201+ synovial sarcoma patient who was leukapheresed under established protocols (Fred Hutchinson Cancer Research Center, protocol #1246). PBMC derived dendritic cells [18] were pulsed with the NY-ESO-1 peptide SLLMWITQC. PBMC were depleted of CD25+ T cells using CliniMACS CD25 MicroBeads (Miltenyi Biotech, Auburn, CA) according to manufacturer’s instructions and were stimulated using IL-21 as previously described [19]. NY-ESO-1+ cells were sorted using NY-ESO-1 tetramer then cloned with limited dilution and expanded using a Rapid Expansion Protocol [20]. MART-1 specific T cell clones were used as control effector cells.
Cell Lines
The human myxoid liposarcoma cell lines 402 and 1765 (gifts of Pierre Aman) have been previously described [21]. They were maintained in RPMI 1640 containing 25 mM HEPES, 2 mM L-glutamine, 50 U/ml penicillin, 50 mg/ml streptomycin (Invitrogen Life Technologies, Carlsbad, CA), and 10% FBS.
The T2 cell line is a TAP-deficient T–B cell hybrid expressing the HLA-A2 allele which was used (unpulsed) as a negative control and pulsed with target peptide as a positive control.
Chromium Release Assay Using Recombinant Vaccinia – HLA-A2 Transfected Targets
Because the target cells (402 and 1765) did not express the HLA-A*0201, we used recombinant vaccinia to endow target cells with the presenting HLA restricting element according to established methods[22]. NY-ESO-1+ MRCL tumor lines, 402 and 1765 were transfected with the HLA-A*0201 gene using a vaccinia vector at a multiplicity of infection (MOI) 2.5, found to be optimal in titration experiments. As a control against cell lysis resulting from the vaccinia infection, cell lines were also treated with wild type Vaccinia virus. For the chromium release assay, cell lines were labeled with 100 μCi 51Cr and cocultured with effector cells for 4-6 hours at 37°C plus 5% CO2.
Results
MRCL samples expressed NY-ESO-1 in 100% of cases; homogenous expression was observed in over 70% of patients
We stained 25 MRCL tumors for NY-ESO-1, all were positive for NY-ESO-1 expression. In two cases, only 1+ staining was observed (5-25% positive for staining). In the remaining 23 cases at least 2+ staining was observed (2+ staining has been the cut off for clinical trial eligibility in some trials of NY-ESO-1 directed therapy [5]). In 6 of 25 cases, flash frozen tissue was available for RNA extraction and qRT-PCR analysis (see supplemental table 1). In all of these cases, NY-ESO-1 expression was confirmed (Table 1).
In 18/25 patients (72%), staining was homogenous (3+ or 4+), ie. present in more than 50% of the tumor. Nine of these patients had 4+ staining. In all but 3 cases, the primary tumor was stained from a resection specimen, however, in the 3 cases that were from metastatic disease staining appeared to have at least similar pattern of expression (4+ in cases, 2+ in one case). At least eleven patients subsequently developed metastatic disease (a number of patients were lost to follow up) but we were unable to observe a clear correlation between disease out come and staining intensity.
Importantly, although most patients had the classic t(12;16)(q13;p11) translocation there were four patients with different cytogenetics: t(10;16;12), t(12;22), t(15;17) as well as one patient with normal cytogenetics that was repeated and confirmed (growth of normal host cells could not be excluded). All of these patients had NY-ESO-1 staining regardless of karyotype.
To determine if NY-ESO-1 expression was limited to the MRCL subtype, we tested seven non-myxoid liposarcoma specimens by IHC and frozen samples from three (additional) patients by qRT-PCR. These ten tumors included 2 pleomorphic liposarcomas, four well-differentiated and four de-differentiated tumors. None of these tumors expressed NY-ESO-1. While these negative results are not definitive, they do suggest that the high prevalence of NY-ESO-1 expression seen in MRCL tumors is not shared amongst the other liposarcoma subtypes.
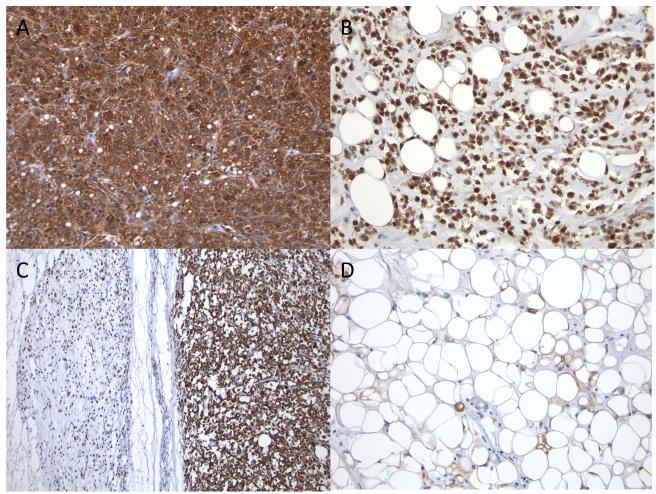
Of note, although both myxoid and round cell areas of the tumor tended to stain positive for NY-ESO-1, a more uniform and intense appearance of the staining was observed in the round cell component. Also of interest was that the well-differentiated elements of some tumors stained less homogenously (1+ to 2+) than the more typical Myxoid and round cell components within the same tumor. (Figure 1).
Figure 1.
Round cell components often had intense and uniform staining (A) but myxoid components were still frequently homogenously stained (B). In tumors with both myxoid and round cell components, both segments stained homogenously but sometimes more intensely in the round cell compartment (C). In some of the lesser staining tumors, well differentiated areas of mature appearing fat were sometimes present (D).
MRCL Cell Lines Expressing NY-ESO-1 can be recognized and specifically lysed by NY-ESO-1 specific effectors
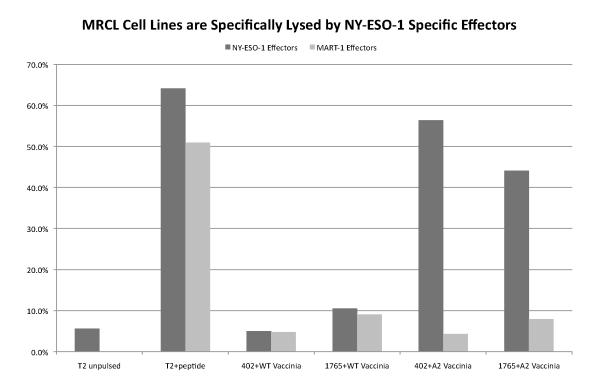
The MRCL cell lines 402 and 1765 were analyzed by qPCR and found to express NY-ESO-1 mRNA transcripts at levels even higher than testis normalized to GAPDH (2.5- and 3.5-fold higher). Both cell lines under went class I typing at the Puget Sound Blood Center and were found to be negative for HLA-A*02. Since HLA-A2-restricted NY-ESO-1 specific CTL were used to evaluate antigen recognition, 402 and 1765 were pre-infected with a recombinant vaccinia virus expressing HLA-A*0201 (Vac-A2). When treated with Vac-A2, cells were lysed at > 30% after 4 hours with an E:T ratio of 20:1. Control MART-1-specific effectors were unable to kill either cell line. Similarly, MRCL cell lines transfected with wild-type vaccinia virus were not sensitized to lysis by NY-ESO-1 specific effectors (Figure 2).
Figure 2.
NY-ESO-1 Effectors recognize and specifically lyse MRCL cell lines at an effector to target ratio of 20:1 following transfection with vaccinia virus expressing HLA-A*0201. MART-1 effectors and WT vaccinia virus were used as controls.
Discussion
NY-ESO-1 is widely considered an attractive target for immunotherapy. Complete responses have been seen in melanoma trials targeting NY-ESO-1 using both vaccines as well as adoptively transferred T cells. The discovery that 80% of synovial sarcomas express NY-ESO-1 was rapidly translated into a clinical trial; the NCI surgery branch treated six synovial sarcoma patients using T cells transfected with a retrovirus expressing the NY-ESO-1 TCR. Partial responses were observed in 4 of 6 patients [3-5].
Here we report another soft-tissue sarcoma subtype that demonstrates a pattern of NY-ESO-1 expression that is even more prevalent than synovial sarcoma. Although other reports have included NY-ESO-1 expression in liposarcomas including MRCL generally, this is the first study to specifically examine NY-ESO-1 protein expression in MRCL [23-28]. Based on our analysis of 25 samples, MRCL appears to express NY-ESO-1 with a frequency that is unmatched by any other malignancy studied to date. Furthermore, a high proportion (9 of 25) had 4+ (>75%) staining and an additional 9 of 25 patients had 3+ (>50%) staining. Interestingly, patients with the histopathologic phenotype of MRCL, possessing a variety of chromosomal translocations were included in this analysis and all expressed NY-ESO-1. Furthermore, we demonstrated that MRCL cell lines are capable of presenting in vitro NY-ESO-1 peptide such that it can be recognized by NY-ESO-1 specific effectors initiating cell mediated lysis of tumor cells.
MRCL is generally associated with a characteristic fusion protein, however it is not clear what role the mutation plays in oncogenesis. Most cases contain t(12;16) (q13;p11) producing the FUS-CHOP fusion protein although a notable minority contain the t(12;22)(q13;12) translocation associated EWSR1-CHOP (containing EWSR1, the Ewings sarcoma (EWS) breakpoint region 1). Both FUS and EWS (along with TAF15) are in the FET family (also known as the TET family) of RNA binding proteins [29]. However, although the RNA binding profiles of the FET family proteins are remarkably similar to one another [30], Ewing’s sarcomas, which typically have translocations of EWS [31, 32] do not generally express NY-ESO-1 [33].
There is evidence to suggest that murine adipocyte derived mesenchymal stem cells transfected with a FUS-CHOP gene develop an MRCL phenotype; however the translocation alone was insufficient to induce a MRCL tumor like phenotype using human adipocyte derived mesenchymal stem cells transfected with the FUS-CHOP suggesting the need for additional genetic “hits” [34-37]. Similar to MRCL, in synovial sarcoma models the presence of SYT-SSX alone appears insufficient on its own to cause oncogenesis [38]. Mesenchymal stem cells have also been postulated as a potential cell of origin in synovial sarcoma [39].
There has never been a study of NY-ESO-1 specific serologic response in MRCL patients [28, 40]. An analysis of the serologic response to a number of CT antigens including NY-ESO-1 was reported from 54 sarcoma patients including 5 synovial sarcoma patients and an MRCL patient. Serology was negative except for two patients (one with pleomorphic sarcoma and another with fibrosarcoma) [41]. Serology was also analyzed in the study by Ayyoub et al, which included one patient with liposarcoma, though histologic subtype was not mentioned [28].
Expression of CT antigens has been correlated with outcomes in a number of malignancies [42-44]. Although the numbers in this study would be underpowered to perform an adequate analysis assessing a difference in outcome pattern between strong and weak NY-ESO-1 expression, we are assessing the feasibility of this approach in our patient population. We are also assessing ways to apply this knowledge to preclinical models such as MRCL xenografts in order to advance NY-ESO-1 directed immunotherapy for sarcoma patients [45].
No other malignancy, including synovial sarcoma, has been described having NY-ESO-1 expression in 100% of cases or with such a high proportion homogenous expression. We believe that like synovial sarcoma, these results will establish MRCL as a model disease for the study of NY-ESO-1 directed therapy.
Supplementary Material
Acknowledgments
Grant Support: This work is supported by the Bob and Eileen Gilman Family Sarcoma Research Program as well as the Walker Immunotherapy Research Fellowship and the SARC Career Development Award. The University of Washington Tissue Bank is supported by RO1 CA65537-16.
Funding:
Seth Pollack, MD is a recipient of the SARC Career Development Award as well as the Walker Immunotherapy Research Fellowship.
Marie Bleakley, MD, PhD, is the Damon Runyon-Richard Lumsden Foundation Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (CI-57-11), and in part by K23CA154532-01 from the National Cancer Institute. The content is soley the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Eve Rodler is supported by Abbott Labs.
Janet F. Eary is supported the RO1 CA65537-16.
Robin L. Jones is supported by the Bob and Eileen Gilman Family Sarcoma Research Program.
Cassian Yee is a recipient of a Burroughs Wellcome Fund Clinical Scientist Awards in Translational Research.
References
- 1.Davis ID, et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci U S A. 2004;101(29):10697–702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholaou T, et al. Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res. 2009;15(6):2166–73. doi: 10.1158/1078-0432.CCR-08-2484. [DOI] [PubMed] [Google Scholar]
- 3.Jager E, et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci U S A. 2006;103(39):14453–8. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunder NN, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358(25):2698–703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins PF, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–24. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YT, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94(5):1914–8. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254(5038):1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 8.Jager E, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187(2):265–70. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoro A, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13(7):1537–45. doi: 10.1200/JCO.1995.13.7.1537. [DOI] [PubMed] [Google Scholar]
- 10.Jones RL, et al. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer. 2005;41(18):2853–60. doi: 10.1016/j.ejca.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Conyers R, Young S, Thomas DM. Liposarcoma: molecular genetics and therapeutics. Sarcoma. 2011;2011:483154. doi: 10.1155/2011/483154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aman P, et al. Rearrangement of the transcription factor gene CHOP in myxoid liposarcomas with t(12;16)(q13;p11) Genes Chromosomes Cancer. 1992;5(4):278–85. doi: 10.1002/gcc.2870050403. [DOI] [PubMed] [Google Scholar]
- 13.Grosso F, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8(7):595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 14.Jungbluth AA, et al. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer. 2001;94(2):252–6. doi: 10.1002/ijc.1451. [DOI] [PubMed] [Google Scholar]
- 15.Jungbluth AA, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92(6):856–60. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 16.Vaughan HA, et al. Immunohistochemical and molecular analysis of human melanomas for expression of the human cancer-testis antigens NY-ESO-1 and LAGE-1. Clin Cancer Res. 2004;10(24):8396–404. doi: 10.1158/1078-0432.CCR-04-0809. [DOI] [PubMed] [Google Scholar]
- 17.Pattyn F, et al. RTPrimerDB: the real-time PCR primer and probe database. Nucleic Acids Res. 2003;31(1):122–3. doi: 10.1093/nar/gkg011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bender A, et al. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196(2):121–35. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111(1):229–35. doi: 10.1182/blood-2007-05-089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128(2):189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 21.Crozat A, et al. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature. 1993;363(6430):640–4. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- 22.Bleakley M, et al. Leukemia-associated minor histocompatibility antigen discovery using T-cell clones isolated by in vitro stimulation of naive CD8+ T cells. Blood. 2010;115(23):4923–33. doi: 10.1182/blood-2009-12-260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal NH, et al. Identification of cancer-testis genes expressed by melanoma and soft tissue sarcoma using bioinformatics. Cancer Immun. 2005;5:2. [PubMed] [Google Scholar]
- 24.Skubitz KM, et al. Identification of heterogeneity among soft tissue sarcomas by gene expression profiles from different tumors. J Transl Med. 2008;6:23. doi: 10.1186/1479-5876-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skubitz KM, et al. Differential gene expression in liposarcoma, lipoma, and adipose tissue. Cancer Invest. 2005;23(2):105–18. doi: 10.1081/cnv-50432. [DOI] [PubMed] [Google Scholar]
- 26.Segal NH, et al. Classification and subtype prediction of adult soft tissue sarcoma by functional genomics. Am J Pathol. 2003;163(2):691–700. doi: 10.1016/S0002-9440(10)63696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer S, et al. Gene expression profiling of liposarcoma identifies distinct biological types/subtypes and potential therapeutic targets in welldifferentiated and dedifferentiated liposarcoma. Cancer Res. 2007;67(14):6626–36. doi: 10.1158/0008-5472.CAN-07-0584. [DOI] [PubMed] [Google Scholar]
- 28.Ayyoub M, et al. The frequent expression of cancer/testis antigens provides opportunities for immunotherapeutic targeting of sarcoma. Cancer Immun. 2004;4:7. [PubMed] [Google Scholar]
- 29.Andersson MK, et al. The multifunctional FUS, EWS and TAF15 protooncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 2008;9:37. doi: 10.1186/1471-2121-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoell JI, et al. RNA targets of wild-type and mutant FET family proteins. Nat Struct Mol Biol. 2011 doi: 10.1038/nsmb.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delattre O, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359(6391):162–5. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- 32.Brody RI, et al. Molecular analysis of the fusion of EWS to an orphan nuclear receptor gene in extraskeletal myxoid chondrosarcoma. Am J Pathol. 1997;150(3):1049–58. [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs JF, et al. Cancer-germline gene expression in pediatric solid tumors using quantitative real-time PCR. Int J Cancer. 2007;120(1):67–74. doi: 10.1002/ijc.22118. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez R, et al. FUS-CHOP fusion protein expression coupled to p53 deficiency induces liposarcoma in mouse but not in human adipose-derived mesenchymal stem/stromal cells. Stem Cells. 2011;29(2):179–92. doi: 10.1002/stem.571. [DOI] [PubMed] [Google Scholar]
- 35.Riggi N, et al. Expression of the FUS-CHOP fusion protein in primary mesenchymal progenitor cells gives rise to a model of myxoid liposarcoma. Cancer Res. 2006;66(14):7016–23. doi: 10.1158/0008-5472.CAN-05-3979. [DOI] [PubMed] [Google Scholar]
- 36.Panagopoulos I, et al. Characterization of the CHOP breakpoints and fusion transcripts in myxoid liposarcomas with the 12;16 translocation. Cancer Res. 1994;54(24):6500–3. [PubMed] [Google Scholar]
- 37.Panagopoulos I, et al. Characteristic sequence motifs at the breakpoints of the hybrid genes FUS/CHOP, EWS/CHOP and FUS/ERG in myxoid liposarcoma and acute myeloid leukemia. Oncogene. 1997;15(11):1357–62. doi: 10.1038/sj.onc.1201281. [DOI] [PubMed] [Google Scholar]
- 38.Nagai M, et al. Analysis of transforming activity of human synovial sarcomaassociated chimeric protein SYT-SSX1 bound to chromatin remodeling factor hBRM/hSNF2 alpha. Proc Natl Acad Sci U S A. 2001;98(7):3843–8. doi: 10.1073/pnas.061036798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cironi L, et al. Epigenetic features of human mesenchymal stem cells determine their permissiveness for induction of relevant transcriptional changes by SYT-SSX1. PLoS One. 2009;4(11):e7904. doi: 10.1371/journal.pone.0007904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antonescu CR, et al. MAGE antigen expression in monophasic and biphasic synovial sarcoma. Hum Pathol. 2002;33(2):225–9. doi: 10.1053/hupa.2002.31295. [DOI] [PubMed] [Google Scholar]
- 41.Lee SY, et al. Immunomic analysis of human sarcoma. Proc Natl Acad Sci U S A. 2003;100(5):2651–6. doi: 10.1073/pnas.0437972100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou C, et al. Cancer-testis antigens expressed in osteosarcoma identified by gene microarray correlate with a poor patient prognosis. Cancer. 2011 doi: 10.1002/cncr.26486. [DOI] [PubMed] [Google Scholar]
- 43.Cuffel C, et al. Pattern and clinical significance of cancer-testis gene expression in head and neck squamous cell carcinoma. Int J Cancer. 2011;128(11):2625–34. doi: 10.1002/ijc.25607. [DOI] [PubMed] [Google Scholar]
- 44.Lendvai N, et al. Cellular immune responses against CT7 (MAGE-C1) and humoral responses against other cancer-testis antigens in multiple myeloma patients. Cancer Immun. 2010;10:4. [PMC free article] [PubMed] [Google Scholar]
- 45.Frapolli R, et al. Novel models of myxoid liposarcoma xenografts mimicking the biological and pharmacologic features of human tumors. Clin Cancer Res. 2010;16(20):4958–67. doi: 10.1158/1078-0432.CCR-10-0317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.