Abstract
Objectives
Convergent evidence supports limbic, anterior paralimbic, and prefrontal cortex (PFC) abnormalities in emotional processing in bipolar disorder (BD) and suggests that some abnormalities are mood-state-dependent and others persist into euthymia. However, few studies have assessed elevated, depressed, and euthymic mood states while individuals processed emotional stimuli of varying valence to investigate trait- and state-related neural system responses. Here, regional brain responses to positive, negative, and neutral emotional stimuli were assessed in individuals with BD during elevated, depressed, and euthymic mood states.
Methods
One hundred and thirty-four subjects participated in functional magnetic resonance imaging scanning while processing faces depicting happy, fearful, and neutral expressions: 76 with BD (18 in elevated mood states, 19 depressed, 39 euthymic) and 58 healthy comparison (HC) individuals. Analyses were performed for BD trait- and mood-state-related features.
Results
Ventral anterior cingulate cortex (VACC), orbitofrontal cortex (OFC), and ventral striatum responses to happy and neutral faces were decreased in the BD group, compared to the HC group, and were not influenced by mood state. Elevated mood states were associated with decreased right rostral PFC activation to fearful and neutral faces, and depression was associated with increased left OFC activation to fearful faces.
Conclusions
The findings suggest that abnormal VACC, OFC, and ventral striatum responses to happy and neutral stimuli are trait features of BD. Acute mood states may be associated with additional lateralized abnormalities of diminished right rostral PFC responses to fearful and neutral stimuli in elevated states and increased left OFC responses to fearful stimuli in depressed states.
Keywords: bipolar disorder, magnetic resonance imaging, prefrontal cortex, ventral striatum
Over the past several decades, functional neuroimaging research has contributed to significant advances in understanding neural system abnormalities that underlie bipolar disorder (BD). Findings have converged to support dysfunction in a neural system that subserves emotional processing and regulation, which includes anterior paralimbic cortices and their subcortical connections sites, including amygdala and ventral striatum, as well as more rostral and lateral prefrontal cortices (1). Studies that focused on elevated, depressed, or euthymic mood states of the disorder suggest that some abnormalities are present only in individuals experiencing acute mood episodes of the disorder; whereas other abnormalities reflect the BD trait in that they are also present in euthymia. However, there are few studies that concurrently examined individuals in elevated, depressed, and euthymic mood states to clarify mood-state- and trait-related features. Such clarification may provide information important to understanding neural system features associated with vulnerability to, and recovery from, acute mood episodes and may suggest treatments to prevent or treat episodes of a particular valence, as well as markers of treatment response.
Neural system findings associated with acute mood states of BD have primarily been in anterior paralimbic and more rostral and lateral cortices, including ventral anterior cingulate cortex (VACC), orbitofrontal cortex (OFC), rostral PFC (RPFC), and dorsolateral PFC (DLPFC). In comparison to healthy individuals, individuals with BD experiencing elevated mood episodes (including manic, mixed, or hypomanic episodes) have shown alterations in activation in these prefrontal regions at rest and in responses to activation tasks (2–7) that have tended to be decreases in the right hemisphere. These findings are consistent with lesions studies in which disinhibited manic-type states have been observed in association with frontal lesions, particularly when they occur in the right hemisphere (8–10). In individuals with BD experiencing depressive episodes, differences have tended to be in the left hemisphere and have tended to be activation increases (4, 11, 12), which may be elicited especially in response to negative emotional stimuli. For example, in one study in which faces of varying emotional expressions were presented, fearful face processing was associated with increases in left ventrolateral PFC activation (11). Recent functional neuroimaging investigations demonstrating dysfunction in VACC, OFC and RPFC during euthymia suggest dysfunction in these regions may be a trait feature of BD (4, 13–18), which is particularly elicited by emotional challenge and stimuli that may be of positive as well as negative valence (17, 19).
Some studies have suggested that subcortical abnormalities may also be present across mood states of BD. Amygdala increases have been reported in manic, depressed, and euthymic mood states of BD, suggesting amygdala dysfunction may not be dependent on the valence of the mood state (11, 12, 20–33). However, there are some recent studies that suggest subcortical findings may be influenced by mood state, as there are reports of negative findings in euthymia and of abnormally increased amygdala activity that has been particularly associated with the depressed state in BD (17, 20, 34). The ventral striatum is highly implicated in BD because of its role in motivated behaviors, which are characteristically disrupted in acute mood episodes, such as in the disinhibited pursuit of motivationally-charged situations in mania and amotivated behavior in depression. The ventral striatum has less often been a focus in neuroimaging studies of BD although ventral striatal abnormalities have been reported in adults and adolescents with BD (11, 28, 35, 36).
There are few neuroimaging studies that include individuals with BD in elevated, depressed, and euthymic mood states. A functional magnetic resonance imaging (fMRI) study in which individuals performed a color-word Stroop task showed decreased right OFC responses in association with elevated mood states, increased left OFC responses in association with depression, as well as an area of decreased left medial RPFC that was present across mood states (4). There were some consistencies between these findings and those of another fMRI study of the three mood states of BD during emotional processing. In that study, 12 manic, 12 depressed, and 18 euthymic individuals with BD, and 18 healthy individuals, were scanned while processing blocks of face stimuli depicting happy, fearful, or neutral expressions. Compared with healthy subjects, activation was reduced in OFC in individuals with BD across mood states and activation was also decreased in right DLPFC. No influence of the valence of the facial expression of the stimuli was detected (37). However, these findings were identified in an analysis that focused on specific regions of interest, and ventromedial PFC and RPFC regions were not assessed.
The aim of the current study was to use an event-related fMRI design performed during implicit processing of positive, negative, and neutral faces by individuals with BD in elevated, depressed, and euthymic mood states to identify trait- and state-related abnormalities in neural system patterns of responses to the stimuli. We hypothesized ventral PFC decreases in responses would be present across mood states of BD, consistent with those decreases being trait features of the disorder. We further hypothesized that additional right PFC decreases would be associated with elevated mood states and that left PFC increases would be associated with depression. We anticipated amygdala and ventral striatal abnormalities (1, 24). We also explored wholebrain to assess whether there might be differences in other regions not hypothesized.
Participants and methods
Subjects
The BD group included 76 participants (mean age 32.4 ± 11.9 years, 61% females) recruited from the medical centers of the Yale School of Medicine and Veterans Affairs Connecticut Healthcare System, and the surrounding communities. Diagnoses, presence of rapid-cycling, and mood states at scanning were determined by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) version 2.0 (38). At the time of scanning, 18 BD subjects were experiencing an elevated (manic/mixed/hypomanic) mood episode (mean age 31.4 ± 12.5 years, 56% females), 19 were experiencing a depressive episode (mean age 34.2 ± 10.8 years, 74% females), and 39 were euthymic (mean age 32.1 ± 12.3 years; 56% females). The healthy comparison (HC) group was comprised of 58 participants (mean age 30.0 ± 9.9 years, 59% females) matched for handedness and without personal history of a DSM-IV Axis I Disorder or a first-degree relative with a major mood, psychotic, anxiety, or substance use disorder assessed by the Family History Screen for Epidemiologic Studies (39). Exclusion criteria for all subjects included loss of consciousness for five or more minutes, history of neurological diseases, or major medical disorders that could affect central nervous system functioning.
Forty-four BD participants (58%) had a history of rapid-cycling. Nineteen (25%) BD participants were unmedicated at the time of scanning [3 (16%) depressed, 8 (44%) elevated, and 8 (21%) euthymic]. Medications prescribed to the other BD subjects included lithium salts [7 (37%) depressed, 1 (6%) elevated, and 10 (26%) euthymic], anticonvulsants [9 (47%) depressed, 6 (33%) elevated, and 17 (44%) euthymic], atypical antipsychotics [8 (42%) depressed, 5 (28%) elevated, and 16 (41%) euthymic], and antidepressants [9 (47%) depressed, 4 (22%) elevated, and 16 (41%) euthymic].
This research was approved by the Institutional Review Boards of the Yale School of Medicine and Department of Veterans Affairs and was performed in accordance with the Helsinki Declaration of 1975. All subjects provided written informed consent prior to participation.
Image acquisition and emotional face paradigm
FMRI data were acquired using a 3-Tesla Siemens Trio MR scanner (Siemens Erlangen, Germany) with a single-shot echo planar imaging (EPI) sequence. Thirty-two axial-oblique slices aligned with the anterior commissure-posterior commissure plane were obtained: repetition time (TR) = 2000 msec, echo time (TE) = 25 msec, matrix = 64 × 64, field of view = 240 × 240 mm2, flip angle = 80°, slice thickness = 3 mm without gap.
The event-related emotional face gender-labeling paradigm was conducted as described previously (17, 27, 40). In brief, subjects viewed faces from Ekman series depicting happy, fearful, and neutral expressions (41). Each face stimulus was displayed for 2 sec, during which participants were instructed to make a determination if the face was of a female or male and respond by pressing one of two corresponding buttons on a button box. In the baseline condition, subjects were fixated on a cross-hair for durations of 4 sec, 8 sec, or 12 sec. Within each 4 min 50 sec run, subjects viewed 10 face stimuli (five females and five males) depicting each emotional expression, for 30 emotional face stimuli in each run. The fMRI paradigm consisted of four runs counterbalanced for face identity, gender, emotional expression, and inter-stimulus interval.
FMRI data processing and data analyses
The Statistical Parametric Mapping software package 5 (SPM5) (http://www.fil.ion.ucl.ac.uk/spm) was used for fMRI data processing and model estimation. Images were corrected for slice timing and movement and co-registered to the standard template from Montreal Neurological Institute (MNI). Images were resampled to 3 mm × 3 mm × 3 mm during normalization and subsequently spatially smoothed with a Gaussian filter of 8-mm full-width-at-half-maximum (FWHM). At the first level analysis, response amplitudes for each of the three event types (happy, fearful, and neutral expressions, each as compared to the fixation baseline) were estimated for each subject. These contrast images for the three emotional conditions were entered into the second-level random-effects model to estimate the main effect of group using an analysis of variance (ANOVA).
To investigate BD trait-related features, comparisons were performed between the BD group and the HC group. Comparisons were performed separately for each emotion face type (happy, fearful, and neutral). Signal extracted from hypothesized regions in which differences were observed was assessed by ANOVA for effects of mood state.
To test hypotheses regarding mood-state-related abnormalities, comparisons were performed between the elevated mood BD subgroup and the HC group, and the depressed BD subgroup compared to the HC group. Comparisons between the elevated and the depressed BD subgroups, each with the euthymic subgroup, were also explored.
Clusters with a height threshold of p < 0.005 and extent threshold of 20 voxels were defined as significant, which was consistent with previous publications (18, 42–45). Analyses were performed with small volume correction (SVC) for multiple comparisons (p < 0.05, corrected) to further confirm the findings for the hypothesized regions. Results are also presented with Bonferroni correction for the three emotional face types.
For both trait and state analyses, exploratory wholebrain analyses were performed to assess potential differences in regions not hypothesized a priori; findings were considered significant for p < 0.05 corrected for false discovery rate (FDR) and 20-voxel extent threshold.
Extracted values from regions of significant differences were also explored for potential effects of clinical factors by ANOVA, including presence or absence of history of rapid-cycling, and for medication status (on/off) at the time of scanning overall, and for each medication type (lithium salts, anticonvulsants, atypical antipsychotics, and antidepressants).
Results
No significant differences in age or sex were found among any of the four subgroups (HC, BD euthymic, BD depressed, BD elevated).
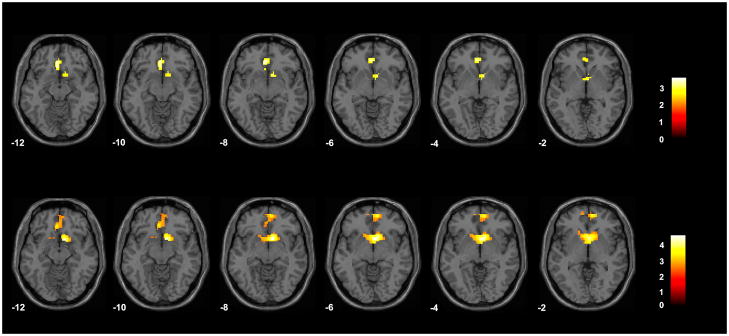
In the happy face condition in the overall BD group, compared to the HC group, activation was significantly reduced in the VACC and OFC [including Brodmann’s Areas (BA) 24/32 and 11] (maximum, in MNI coordinates, of x = 0 mm, y = 38 mm, z = −8 mm, cluster = 54 voxels) and right ventral striatum (x = 12 mm, y = 14 mm, z = −8 mm, cluster = 23 voxels) (Fig. 1). In the neutral face condition, activation was significantly reduced in the BD group, compared to the HC group, in VACC and OFC (BAs 24/32 and 11) (x = 9 mm, y = 56 mm, z = −2 mm, cluster = 160 voxels) and the bilateral ventral striatum (x = 12, y = 11, z = −8, cluster = 50 voxels) (Fig. 1). The findings in the VACC/OFC and ventral striatum for both the happy and neutral conditions survived SVC. At the Bonferroni significance threshold, correcting for the three emotional face type conditions, findings were reduced in spatial extent: for the happy condition for the VACC/OFC region (23 voxels) and ventral striatum (8 voxels), and for the neutral condition for the VACC/OFC region (49 voxels) and ventral striatum (43 voxels). Group differences were not detected in these regions for the fearful face condition. There were no significant effects of mood state (elevated, depressed, euthymic) on the VACC/OFC or ventral striatum findings for either the happy or neutral face conditions. There were no additional regional effects detected in the wholebrain exploratory analyses.
Fig. 1.
Regions of difference in activation between the group with bipolar disorder and the healthy comparison group in the happy and neutral face conditions. The axial-oblique images display the regions of significantly decreased activation in the ventral anterior cingulate and orbitofrontal cortices and ventral striatum in the group with bipolar disorder, compared to the healthy comparison group, for the happy face condition (top row) and the neutral face condition (bottom row). Results are displayed at a threshold of p < 0.005 and extent of 20 voxels. The numbers in the lower left corner of each image are the Montreal Neurological Institute atlas coordinates for the z-plane in mm. The right sides of the images show the right side of the brain. The color bars show the t-values.
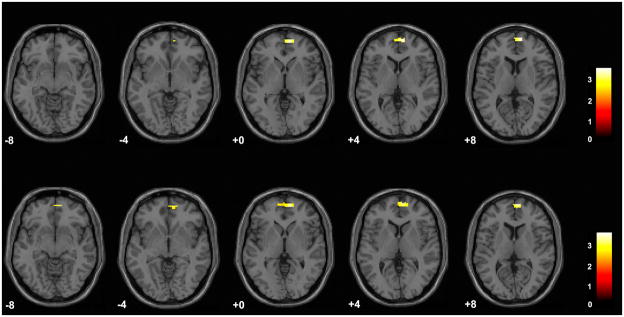
In addition to the trait-related abnormalities above, significant decreases in right RPFC (BA 10) were observed in the elevated mood BD subgroup, compared to the HC group, for the fear face condition (x = 9 mm, y = 59 mm, z = 4 mm, cluster = 41 voxels) and the neutral face condition (x = 6 mm, y = 62 mm, z = 7 mm, cluster = 84 voxels) (Fig. 2). After Bonferroni correction for the three face types, findings were reduced in spatial extent for the fear condition (12 voxels) and for the neutral condition (45 voxels).
Fig. 2.
Regions of difference between the bipolar disorder group in elevated mood states and the healthy comparison group in the fearful face and neutral face conditions. The axial-oblique images display regions of decreased activation in the right rostral prefrontal cortex in the subgroup with bipolar disorder experiencing elevated mood states during fearful face processing (top row) and neutral face processing (bottom row). Results are displayed at a threshold of p < 0.005 and extent of 20 voxels. The numbers in the lower left corner of each image are the Montreal Neurological Institute atlas coordinates for the z-plane in mm. The right sides of the images show the right side of the brain. The color bars show the t-values.
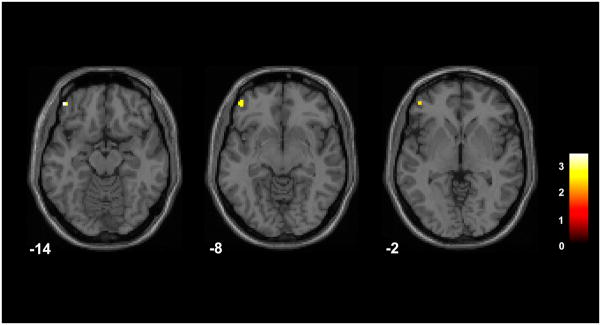
An additional regional abnormality was also observed in association with the depressed mood state. Left OFC (BA 11/47) activation was increased in the depressed BD subgroup, compared to the HC group, for the fearful face condition; however, this result did not survive the cluster threshold (x = −45 mm, y = 47 mm, z = −14 mm, cluster = 11 voxels) (Fig. 3). After Bonferroni correction for the three face types, the cluster size was 5 voxels.
Fig. 3.
Regions of difference between the depressed bipolar disorder group and the healthy comparison group in the fearful face condition. The axial-oblique images display regions of increases in activation during the fearful face condition in the left lateral orbitofrontal cortex in the subgroup with bipolar disorder experiencing depression, compared to the healthy comparison group. Results are displayed at a threshold of p < 0.005 and extent of 10 voxels. The numbers in the lower left corner of each image are the Montreal Neurological Institute atlas coordinates for the z-plane in mm. The right sides of the images show the right side of the brain. The color bar shows the t-values.
In performing comparisons between the elevated and depressed subgroups to the euthymic BD subgroup, the only additional significant finding was in the comparison between the depressed and euthymic BD groups. The finding of increased left OFC response to fearful faces in the depressed BD group versus the HC group was also observed in the comparison of the depressed BD group versus the euthymic group with the same maximum point of difference, but larger spatial extent (30 voxels).
Exploratory analyses within the BD group for potential effects of clinical factors did not reveal significant effects of rapid-cycling, or medications overall or for medication subclasses, on any of the regional differences above.
Discussion
The main findings of this study were of decreases in responses of the VACC and OFC, as well as ventral striatum, to happy and neutral face stimuli in individuals with BD compared to healthy subjects, which did not differ across mood states. The presence of these findings across mood states, including euthymic, is consistent with these findings as trait abnormalities of the disorder. Mood-state-related features were also identified, as elevated mood states were associated with diminished responses in the right RPFC to fearful and neutral stimuli, while depressed mood states were associated with increased left OFC responses to fear stimuli.
The putative trait-related findings in the VACC and OFC in response to happy and neutral faces are consistent with previous reports of diminished responses to emotional stimuli in these regions in each of the three mood states of BD (2, 17, 22, 29, 37, 43, 46–48). Decreased ACC responses to happy face stimuli were reported in a recent meta-analysis of BD, although those findings were dorsal to those found here (49). As described earlier, Van der Schot et al. (37) also demonstrated decreased activation in an OFC region of interest; however, the region assessed was more lateral than the findings of the current study. Together, these studies suggest that ACC and OFC dysfunction may be a trait feature of BD. Although specific subregions have differed across studies, as findings have converged in the ACC and OFC across studies, we speculate that these cortices may be involved more generally in the disorder. Differences in the task design, subject selection, or imaging methods of the various studies may contribute to differences in the specific locations in which findings are detected in a particular study. There has been less previous evidence of trait abnormalities in ventral striatum in adults with BD, perhaps due to the fewer number of studies that focus on this region. Decreased ventral striatum metabolism in depression and alteration in ventral striatal responses to positive and negative emotional faces in depression and euthymia have been reported in BD (11, 28, 35).
The VACC and OFC share substantial interconnections, as well as reciprocal connections with the ventral striatum (50). Together, these structures comprise components of a neural system that coordinates processing of motivationally-salient stimuli and regulation of motivated behavior, and abnormalities in the connections among these structures have also been observed in BD (40). This system is implicated in both the coding of the motivational value of emotional stimuli and the integration of this information into adaptive decision making (51–56). The finding of trait effects for the happy face stimuli is consistent with theories that abnormalities in responses to positive emotional stimuli may be especially salient in BD (11, 22, 57). As this corticostriatal system also contributes to the resolution of conflict between stimuli associated with positive and negative reinforcers, we speculate that as neutral stimuli can also be seen as emotionally ambiguous, differences during the processing of neutral stimuli could reflect difficulties in resolving their reinforcing value. It is also possible that effects during the processing of happy faces are carried over into neutral face trials; however, this seems less likely as the findings during the processing of neutral faces were relatively robust. A recent meta-analysis (49) that included BD subjects in varying mood states showed decreases in ACC and ventral PFC regions in response to fearful faces in the BD subjects; however, these findings were not detected in association with the BD trait in our study. The reasons for the different findings are not clear, but it is possible that the proportion of BD subjects with particular symptoms in each study may have affected findings. We suggest that further study of the cortical and striatal components and their connections within this system in BD, especially in the processing of positive and emotionally ambiguous stimuli, may be fruitful in revealing abnormalities that underlie vulnerability to alterations in motivated behavior associated with BD.
The decreased right RPFC response in association with elevated mood states of BD is consistent with decreases in right RPFC activity in association with mania observed previously in a positron emission tomography study (3). Decreases in right RPFC activation in association with elevated mood states of BD and in response to fearful stimuli and neutral stimuli, as observed here, are consistent with a previous report of decreases in mania found in an overlapping region in association with response to negative and neutral emotional stimuli (7). The RPFC has been implicated in the integration of information from multiple emotional and cognitive domains, including the processing of internal mental states and the emotional states of others, and resolving conflicting information for adaptive control behavior (58–63). We speculate that right RPFC abnormalities in the processing of fearful or emotionally ambiguous stimuli during elevated mood states may reflect difficulties during these states in assessing the potential adverse consequences of situations and the need to inhibit approach behaviors, which leads to maladaptive, disinhibited behaviors.
We also observed overactivation of the left OFC in response to fearful faces in individuals with BD experiencing depression. Elevated ventral PFC activation to intensely fearful faces has been observed previously in depressed patients with BD, supporting the notion that this could represent a state-related feature of BD depression (11). We speculate that increased left ventral PFC activation may be consistent with mood-congruent biases towards negative emotional stimuli in depression, in which depressed individuals are more likely to perceive and exhibit exaggerated responses to negative emotional stimuli (64). Together with the findings in the elevated state, these findings in depression in association with fearful faces suggests that emotional stimuli of negative valence may especially elicit acute mood-state-related pathology, and that there may be a dissociation between the findings such that right frontal decreases in response to fearful stimuli are associated with elevated mood states whereas left increases are associated with depressed states.
Structural neuroimaging and postmortem studies of BD suggest morphological and cellular abnormalities in these brain areas that may contribute to these findings. Decreases in cortical gray matter in VACC and OFC have been observed in BD by multiple research groups (65–78). Reductions in non-pyramidal cell density in perigenual ACC (79), and glial reductions in VACC and OFC (80, 81), have been reported in postmortem studies. Volume decreases in ventral striatum have been observed in youth with BD (82), although ventral striatum cellular decreases have not been reported to our knowledge. A postmortem study of BD did show decreased expression of glutamatergic signaling proteins in ventral striatum thought to reflect decreased innervation from the frontal cortex, suggesting a mechanism underlying the VACC-ventral striatum connectivity abnormalities noted above (83).
Limitations of the study include the cross-sectional design. Future studies with larger samples and longitudinal designs in which the same individuals are followed across mood states are needed. Mood-state-related findings were not as robust as the putative trait-related findings. This observation may relate to the size of the samples, as the euthymic sample was approximately twice the size of each of the acute mood state subgroups. We did not find group differences in activation to emotional stimuli of the amygdala. This might be related to aspects of the study design. For example, in a study of BD, Lawrence et al. (11) noted different effects depending on the intensity of the facial emotion. Future studies using stimuli with varying emotional intensity may better elicit amygdala differences. Another possible limitation was that one-quarter of the BD sample was unmedicated. The value of including subjects with BD who are taking medications in studies has been discussed previously (84); however, this may confound findings. Although we did not detect effects of medication on the regional findings, subjects were on various combinations of medications that limited the ability to control for the effects of any specific subclass of medication. Systematic studies of treatment effects are needed.
In summary, the results support trait- and state-related functional abnormalities during emotional processing in BD. The results also support trait-related abnormalities in VACC and OFC, point to a role for the ventral striatum in the BD trait, and suggest that positive—and perhaps emotionally ambiguous—stimuli elicit trait dysfunction. The acute mood states may be distinguished by additional abnormalities, including decreases in right RPFC responses to negative and ambiguous emotional stimuli in association with elevated mood state and increases in left OFC responses to negative emotional stimuli in association with depressive states. Further confirmation of the findings may provide information important to understanding neural system features associated with vulnerability to, and recovery from, acute mood episodes.
Acknowledgments
This work was supported by research grants from the National Institute of Health R01MH69747 (HPB), R01MH070902 (HPB), RC1MH088366 (HPB), K01MH086621 (FW), the Department of Veterans Affairs Research Enhancement Award Program (REAP) (FW, HPB), the National Alliance for Research on Schizophrenia and Depression (FW, HPB), the Attias Family Foundation (HPB), and Women’s Health Research at Yale–The Ethel F. Donaghue Women’s Health Investigator Program at Yale (HPB).
Footnotes
Disclosures
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Womer FY, Kalmar JH, Wang F, Blumberg HP. A ventral prefrontal-amygdala neural system in bipolar disorder: a view from neuroimaging research. Acta Neuropsychiatr. 2009;21:228–238. [PMC free article] [PubMed] [Google Scholar]
- 2.Altshuler LL, Bookheimer SY, Townsend J, et al. Blunted activation in orbitofrontal cortex during mania: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry. 1999;156:1986–1988. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg HP, Leung HC, Skudlarski P, et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 5.Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Rubinsztein JS, Fletcher PC, Rogers RD, et al. Decision-making in mania: a PET study. Brain. 2001;124:2550–2563. doi: 10.1093/brain/124.12.2550. [DOI] [PubMed] [Google Scholar]
- 7.Strakowski SM, Eliassen JC, Lamy M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sackeim HA, Greenberg MS, Weiman AL, Gur RC, Hungerbuhler JP, Geschwind N. Hemispheric asymmetry in the expression of positive and negative emotions. Neurologic evidence. Arch Neurol. 1982;39:210–218. doi: 10.1001/archneur.1982.00510160016003. [DOI] [PubMed] [Google Scholar]
- 9.Starkstein SE, Fedoroff P, Berthier ML, Robinson RG. Manic-depressive and pure manic states after brain lesions. Biol Psychiatry. 1991;29:149–158. doi: 10.1016/0006-3223(91)90043-l. [DOI] [PubMed] [Google Scholar]
- 10.Wexler BE. Cerebral laterality and psychiatry: a review of the literature. Am J Psychiatry. 1980;137:279–291. doi: 10.1176/ajp.137.3.279. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Mah L, Zarate CA, Singh J, et al. Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biol Psychiatry. 2007;61:765–775. doi: 10.1016/j.biopsych.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Frangou S, Kington J, Raymont V, Shergill SS. Examining ventral and dorsal prefrontal function in bipolar disorder: a functional magnetic resonance imaging study. Eur Psychiatry. 2008;23:300–308. doi: 10.1016/j.eurpsy.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Kronhaus DM, Lawrence NS, Williams AM, et al. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 15.Kruger S, Alda M, Young LT, Goldapple K, Parikh S, Mayberg HS. Risk and resilience markers in bipolar disorder: brain responses to emotional challenge in bipolar patients and their healthy siblings. Am J Psychiatry. 2006;163:257–264. doi: 10.1176/appi.ajp.163.2.257. [DOI] [PubMed] [Google Scholar]
- 16.Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord. 2005;7 (Suppl 5):58–69. doi: 10.1111/j.1399-5618.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 17.Shah MP, Wang F, Kalmar JH, et al. Role of variation in the serotonin transporter protein gene (SLC6A4) in trait disturbances in the ventral anterior cingulate in bipolar disorder. Neuropsychopharmacol. 2009;34:1301–1310. doi: 10.1038/npp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary FMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacol. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- 19.Kruger S, Seminowicz D, Goldapple K, Kennedy SH, Mayberg HS. State and trait influences on mood regulation in bipolar disorder: Blood flow differences with an acute mood challenge. Biol Psychiatry. 2003;54:1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- 20.Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altshuler L, Bookheimer S, Proenza MA, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 22.Blumberg HP, Donegan NH, Sanislow CA, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacol. 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 23.Chang KD, Wagner C, Garrett A, Howe M, Reiss A. A preliminary functional magnetic resonance imaging study of prefrontal-amygdalar activation changes in adolescents with bipolar depression treated with lamotrigine. Bipolar Disord. 2008;10:426–431. doi: 10.1111/j.1399-5618.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 25.Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 26.Jogia J, Haldane M, Cobb A, Kumari V, Frangou S. Pilot investigation of the changes in cortical activation during facial affect recognition with lamotrigine monotherapy in bipolar disorder. Br J Psychiatry. 2008;192:197–201. doi: 10.1192/bjp.bp.107.037960. [DOI] [PubMed] [Google Scholar]
- 27.Kalmar JH, Wang F, Chepenik LG, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ketter TA, Kimbrell TA, George MS, et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- 29.Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychol Med. 2004;34:795–802. doi: 10.1017/s0033291704002557. [DOI] [PubMed] [Google Scholar]
- 30.Malhi GS, Lagopoulos J, Ward PB, et al. Cognitive generation of affect in bipolar depression: an fMRI study. Eur J Neurosci. 2004;19:741–754. doi: 10.1111/j.0953-816x.2003.03159.x. [DOI] [PubMed] [Google Scholar]
- 31.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WDS, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- 34.Foland-Ross LC, Bookheimer SY, Lieberman MD, et al. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. Neuroimage. 2012;59:738–744. doi: 10.1016/j.neuroimage.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooks JO, 3rd, Wang PW, Bonner JC, et al. Decreased prefrontal, anterior cingulate, insula, and ventral striatal metabolism in medication-free depressed outpatients with bipolar disorder. J Psychiatr Res. 2009;43:181–188. doi: 10.1016/j.jpsychires.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blumberg HP, Martin A, Kaufman J, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- 37.Van der Schot A, Kahn R, Ramsey N, Nolen W, Vink M. Trait and state dependent functional impairments in bipolar disorder. Psychiatry Res. 2010;184:135–142. doi: 10.1016/j.pscychresns.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 38.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I & II Disorders (Version 2.0) New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 39.Lish JD, Weissman MM, Adams PB, Hoven CW, Bird H. Family psychiatric screening instruments for epidemiologic studies: pilot testing and validation. Psychiatry Res. 1995;57:169–180. doi: 10.1016/0165-1781(95)02632-7. [DOI] [PubMed] [Google Scholar]
- 40.Wang F, Kalmar JH, He Y, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ekman P, Friesen WV. Pictures of facial affect. Palo Alto: Consulting Psychologists Press; 1976. [Google Scholar]
- 42.Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–549. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 43.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal fMRI brain activation in euthymic bipolar disorder patients during a counting stroop interference task. Am J Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- 45.Xiong J, Gao JH, Lancaster JL, Fox PT. Assessment and optimization of functional MRI analyses. Hum Brain Mapp. 1996;4:153–167. doi: 10.1002/(SICI)1097-0193(1996)4:3<153::AID-HBM1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Altshuler L, Bookheimer S, Townsend J, et al. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jogia J, Haldane M, Cobb A, Kumari V, Frangou S. Pilot investigation of the changes in cortical activation during facial affect recognition with lamotrigine monotherapy in bipolar disorder. Br J Psychiatry. 2008;192:197–201. doi: 10.1192/bjp.bp.107.037960. [DOI] [PubMed] [Google Scholar]
- 48.Malhi GS, Lagopoulos J, Owen AM, Ivanovski B, Shnier R, Sachdev P. Reduced activation to implicit affect induction in euthymic bipolar patients: an fMRI study. J Affect Disord. 2007;97:109–122. doi: 10.1016/j.jad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Delvecchio G, Fossati P, Boyer P, et al. Common and distinct neural correlates of emotional processing in Bipolar Disorder and Major Depressive Disorder: A voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol. 2012;22:100–113. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 51.Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- 52.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 53.Monk CS, Klein RG, Telzer EH, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 54.O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 56.Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leibenluft E, Charney DS, Pine DS. Researching the pathophysiology of pediatric bipolar disorder. Biol Psychiatry. 2003;53:1009–1020. doi: 10.1016/s0006-3223(03)00069-6. [DOI] [PubMed] [Google Scholar]
- 58.Bishop S, Duncan J, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 59.Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- 60.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 61.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 62.Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 63.Gilbert SJ, Spengler S, Simons JS, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- 64.Murphy FC, Sahakian BJ, Rubinsztein JS, et al. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 65.Blumberg HP, Krystal JH, Bansal R, et al. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 66.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 67.Lyoo IK, Sung YH, Dager SR, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 68.Doris A, Belton E, Ebmeier KP, Glabus MF, Marshall I. Reduction of cingulate gray matter density in poor outcome bipolar illness. Psychiatry Res. 2004;130:153–159. doi: 10.1016/j.pscychresns.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Foland-Ross LC, Thompson PM, Sugar CA, et al. Investigation of cortical thickness abnormalities in lithium-free adults with bipolar I disorder using cortical pattern matching. Am J Psychiatry. 2011;168:530–539. doi: 10.1176/appi.ajp.2010.10060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frazier JA, Breeze JL, Makris N, et al. Cortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorder. Bipolar Disord. 2005;7:555–569. doi: 10.1111/j.1399-5618.2005.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalmar JH, Wang F, Spencer L, et al. Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. J Int Neuropsychol So. 2009;15:476–481. doi: 10.1017/S1355617709090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaur S, Sassi RB, Axelson D, et al. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry. 2005;162:1637–1643. doi: 10.1176/appi.ajp.162.9.1637. [DOI] [PubMed] [Google Scholar]
- 73.Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- 74.Lyoo IK, Kim MJ, Stoll AL, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 75.Najt P, Nicoletti M, Chen HH, et al. Anatomical measurements of the orbitofrontal cortex in child and adolescent patients with bipolar disorder. Neurosci Lett. 2007;413:183–186. doi: 10.1016/j.neulet.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nugent AC, Milham MP, Bain E, et al. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30:485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 77.Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 2004;131:57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Wang F, Kalmar JH, Womer FY, et al. Olfactocentric paralimbic cortex morphology in adolescents with bipolar disorder. Brain. 2011;134:2005–12. doi: 10.1093/brain/awr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry. 2001;50:395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- 80.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rajkowska G. Cell pathology in mood disorders. Seminars Clin Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- 82.Dickstein DP, Milham MP, Nugent AC, et al. Frontotemporal alterations in pediatric bipolar disorder - Results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 83.Kristiansen LV, Meador-Woodruff JH. Abnormal striatal expression of transcripts encoding NMDA interacting PSD proteins in schizophrenia, bipolar disorder and major depression. Schizophr Res. 2005;78:87–93. doi: 10.1016/j.schres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 84.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]