Abstract
Tax-interacting protein 1 (TIP-1, also known as Tax1bp3) inhibited proliferation of colon cancer cells through antagonizing the transcriptional activity of beta-catenin. However, in this study, elevated TIP-1 expression levels were detected in human invasive breast cancers. Studies with two human invasive breast cancer cell lines indicated that RNAi-mediated TIP-1 knockdown suppressed the cell adhesion, proliferation, migration and invasion in vitro, and inhibited tumor growth in mammary fat pads and pulmonary metastasis in athymic mice. Biochemical studies showed that TIP-1 knockdown had moderate and disruptive effects on the beta-catenin-regulated gene expression, but remarkably down regulated the genes for cell adhesion and motility in breast cancer cells. The decreased expression of integrins and paxillin was accompanied with reduced cell adhesion and focal adhesion formation on fibronectin-coated surface. In conclusion, this study revealed a novel oncogenic function of TIP-1 suggesting that TIP-1 holds potential as a prognostic biomarker and a therapeutic target in the treatment of human invasive breast cancers.
Keywords: Tax-interacting protein 1, breast cancer, beta-catenin, cell adhesion, migration, pulmonary metastasis
1. Introduction
Beta-catenin is a multifunctional protein that contributes fundamentally to embryonic development and many physiological or pathophysiological processes [1]. Nuclear and cytosolic accumulation of β-catenin or hyperactivation of beta-catenin signaling cascades has been implicated in many cancers including human breast cancer and associated with poor prognosis [2–4]. TIP-1 is a highly conserved protein across species [5]. One PSD-95/DlgA/ZO-1 (PDZ) domain (89 amino acids) is the only structural and functional unit in the small protein (total of 124 amino acids in human and mouse), distinguishing TIP-1 from other PDZ proteins that usually contain multiple structural and functional domains and serve as scaffolds in assembling large protein complex [6].
TIP-1 functions in a wide variety of biological events through selective interaction with intracellular proteins. TIP-1 interacts with rhotekin, a RhoA effector protein, to regulate cellular response to serum starvation [7]. Through selective association with glutaminase L [8], potassium channel Kir2.3 [9], or NMDA receptor [10], TIP-1 establishes and maintains cell polarity, or mediates neurotoxicity, respectively. TIP-1 is associated with several viral proteins including the Tax protein of human T-lymphotropic virus type I (HTLV-1) [11], E6 oncoprotein of human papillomavirus (HPV) [12] and NS1 protein of avian influenza [13], although more studies are needed to determine the biological relevance and respective mechanisms of such protein interactions. Interaction with TIP-1 antagonizes the transcriptional activity of beta-catenin and inhibits proliferation of colon cancer cells [14]. In contrast, TIP-1 knockdown in zebrafish embryo induced defects of cell filopodia growth and gastrulation movements [5], suggesting that TIP-1 might function as a tumor suppressor through modulating the functionality of beta-catenin.
In this study, we reported elevated TIP-1 expression levels in human invasive breast cancers. Using a combination of in vitro and in vivo model systems, it was demonstrated that TIP-1 knockdown suppressed cell proliferation, adhesion, migration and invasion of human breast cancer cells in vitro, and inhibited the tumor growth in mammary fat pads and pulmonary metastasis in athymic mice. With biochemical studies, this study revealed a novel oncogenic function of TIP-1 suggesting that TIP-1 holds potential as a prognostic biomarker and a therapeutic target in the treatment of human invasive breast cancers.
2. Materials and methods
2.1 Antibodies and reagents
TIP-1 polyclonal (rabbit) antibodies were produced in our lab and characterized as described [15]. Antibodies against integrin alpha-5 and paxillin were purchased from BD Biosciences (Rockville, MD). Anti-Ki67 antibody was obtained from Abcam (Cambridge, MA). Anti-beta-actin antibody was purchased from Sigma (St. Louis, MO). Anti-phospho-paxillin (Tyr118) antibody was purchased from Cell Signaling Technology (Danvers, MA). Alexa Fluor 594-labeled phallotoxins and all other Alexa Fluor dye-labeled secondary antibodies were obtained from Invitrogen (Grand Island, NY). Laminin was purchased from BD Biosciences, collagen IV from Millipore (Hayward, CA) and fibronectin was from Sigma.
2.2. Cell culture and stable shRNA transfection
Human breast cancer cell line BT549 was a gift from Dr. Jennifer Pietenpol’s lab at Vanderbilt University, cell line MDA-MB-231 was purchased from ATCC (Manassa, VA). Both lines were maintained in RPMI1640 media (Invitrogen). Constructs for the validated TIP-1 targeted shRNA (clone TRCN0000159034 and TRCN0000162886) and a non-targeting negative control were purchased from Sigma. Preparation of recombinant lentivirus, trasfection, and selection of stable clones were conducted by following the manufacturer’s instructions. TIP-1 knockdown was assessed by western blot as described previously [15].
2.3. Migration and invasion assays
Cell migration and invasion assays were performed using 8-μm porous Boyden chambers (Corning Life Science, Lowell, MA) coated with or without Matrigel (BD Biosciences) according to the manufacturer’s recommendations. Briefly, cells were starved in serum-free media overnight prior to the migration and invasion assays. 20,000 (for migration) or 40,000 (for invasion) disaggregated cells were seeded into the upper chambers with serum-free media, while complete media with 10% serum were added to the bottom chamber. 12 hours later, cells stayed on top of the membrane were removed with cotton swabs, and cells migrated through the porous membrane were stained with DAPI for examination under fluorescence microscope.
2.4. Cell adhesion assay
96-well culture plates (Corning) were coated with 50 μl of 10 μg/ml of fibronectin, Collagen IV or laminin at room temperature for two hours (with cover open), followed by three washes with 100 μl of 0.2% (w/v) bovine serum albumin (BSA, from Sigma) solution in phosphate-buffered saline (PBS). 1 × 105 disaggregated cells resuspended in 50 μl of complete media were added into each well. After incubation at 37 ºC for 40 minutes, unattached cells were removed by shaking at 2000 rpm for 10~15 seconds followed by washing with 0.2 % BSA/PBS for 3 times. The cells adherent to the coated wells were fixed in 4% paraformaldehyde and stained with 0.05% (w/v) Crystal Violet. After washing with 200 μl of PBS for three times, the cell-associated dye was solubilized in 100 μl of 2 % (w/v) of sodium dodecyl sulfate (SDS) solution in PBS, and quantified upon spectrometer absorbance measurement (at 560 nm).
2.5. Immunofluorescence staining and imaging
As in the cell adhesion assay, cells were seeded onto the fibronectin-coated surface for incubation at 37ºC for 40 minutes before fixation in 4% (w/v) formaldehyde solution in PBS. Anti-phospho-paxillin, anti-actin and fluorescence dye-labeled secondary antibodies were used for immunofluorescence staining by following the manufacturer’s instructions. Images were acquired using a Zeiss LSM 510 inverted confocal microscope. Focal adhesion clusters were characterized as phospho-paxillin-positive and quantified upon digital images using the Image J software. At least 50 cells were measured in each of three independent experiments for statistic analysis.
2.6. Models of human breast cancer in athymic nude mice
Orthotopic model of human breast cancer in athymic mice was developed by injecting 5 × 105 MDA-MB-231 cells (in 50 μl of PBS) into mammary fat pads of female FoxN1-null nude mice (Harlan Laboratories, Prattville, AL) of 5-week old. Tumor size was measured with caliper in every three days and calculated as described [16]. At the end of the tumor growth study, lung was resected to check pulmonary metastasis. Pulmonary metastasis model was developed by injecting 2 × 105 MDA-MB-231 cells (in 100 μl of PBS) into tail veins of FoxN1-null nude mice. Tumor progression was tracked through daily monitoring animal hypomotility, difficulty breathing, and weight loss, and verified with histological assessment of the resected tissue. All the animal studies were conducted as approved by the Institutional Animal Care and Use Committee (IACUC) at Vanderbilt University.
2.7. Gene expression analysis
RNA transcripts from MDA-MB-231 cells (with or without TIP-1 knockdown) were isolated by the use of a RNAqueous Kit (Ambion, Austin, TX) as instructed by the manufacturer. The quality of the RNA was validated by agarose gel electrophoresis. One μg of total RNA was used for cDNA synthesis with a QuantiTect reverse Transcription Kit (QIAGEN, Valencia, CA). Affymetrix Exon/Gene arrays were used. Hybridization, scanning and image processing were performed as recommended by the manufacturer’ instructions and conducted at the Vanderbilt Functional Genomics Shared Resource (FGSR). Genes down- or up-regulated by more than 1.25-fold by TIP-1 knockdown were subjected to GoMiner analyses (http://discover.nci.nih.gov/gominer/index.jsp), the genes were classified into biological coherent categories and the significantly affected categories were identified. Clustering was based on Euclidean distance using the “average” cluster method. To determine the prognostic value of the TIP-1 protein expression in patients with invasive breast cancers, we reanalyzed published microarray datasets [17, 18] that contains clinically annotated human specimens including benign cystic lesions, invasive breast carcinomas, and adjacent normal mammary glands. Dataset from Minn et al.[19] was used to determine the prognostic value of TIP-1 expression in human invasive breast cancers. Patients with the top 40% of TIP-1 expression levels were defined as “high TIP-1 level” group, and those in the bottom 40% were defined as “low TIP-1 level” group.
2.8. Statistics
All numerical data are expressed as mean ± SEM (standard error of the mean). Statistical analysis was performed by ANOVA. For survival assays, Kaplan-Meier survival analysis was used, and log rank test was performed using MedCalc to determine differences between survival curves. For all analyses, a p value less than 0.05 was considered significant.
3. Results
3.1. TIP-1 expression levels are elevated in human invasive breast cancers
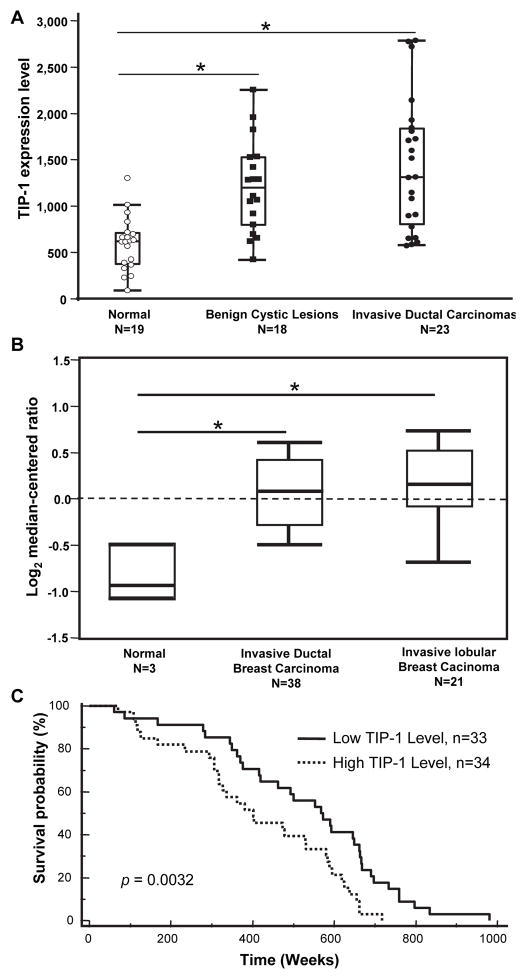
Two published microarray datasets [17, 18] covering a total of 100 clinical specimens of benign cystic lesions, invasive breast carcinomas and matched adjacent normal human mammy glands were analyzed. In both datasets, TIP-1 mRNA levels are significantly elevated (by ~ 2-fold) in invasive lobular or ductal breast carcinomas while compared to those in normal human mammary glands (Figs. 1A, B). It was also noted that TIP-1 levels are elevated in the benign cystic lesions of breast (Fig. 1A). Retrospective analysis of one dataset [19] revealed a significant correlation (p=0.0032) between TIP-1 expression levels and the prognostic outcomes of breast cancer patients (Fig.1C), patients with high TIP-1 levels had a reduced survival time (by ~180 weeks) than those patients with low TIP-1 levels after tumor diagnosis.
Fig. 1. TIP-1 expression levels are elevated in the invasive breast cancers.
(A, B) Relative TIP-1 expression levels in pathologically validated clinical specimens of benign cystic lesions, invasive ductal/lobular breast carcinomas or adjacent normal mammary glands from two independent datasets. The data distribution was presented as box plot. N represents case number for each subgroup, * p<0.05, ANOVA. C) Kaplan-Meier survival curve to show survival time of breast cancer patient after diagnosis is stratified by the TIP-1 expression levels (log-rank analysis, p=0.0032).
3.2. TIP-1 knockdown suppressed proliferation, migration and invasion of human breast cancer cells in vitro
Two established human breast cancer cell lines (BT549 and MDA-MB-231) were utilized to study the putative roles of TIP-1 expression in the breast cancer malignancy. TIP-1 expression in those cell lines were genetically manipulated with transfection of shRNA (Fig. 2A). Ki-67 staining assays (Fig. 2B) indicated that TIP-1 knockdown suppressed the cell proliferation of both cell lines. Cell motility was studied with Boyden chamber-based assays. The quantitative measurement indicated that TIP-1 knockdown significantly inhibited the cell’s capability to migrate (Fig. 2C) and invade through matrigel (Fig. 2D). In both of BT549 and MDA-MB-231 cell lines, TIP-1 knockdown reduced the cell migration or invasion rate by ~2 folds.
Fig. 2. TIP-1 knockdown suppressed proliferation, migration and invasion of human breast cancer cells in vitro.
(A) Western blot analyses of TIP-1 knockdown within BT549 (upper panel) and MDA-MB-231 (lower panel) cells with shRNAs. Actin was blotted as a loading control. (B) Cell proliferation assessed with Ki67 staining on monolayer cultures. The quantification was based on >500 cells for each group in three independent experiments. Cell migration (C) and invasion (D) were analyzed with the Boyden chamber-based system as described in the the Materials and Methods. Bar graph represents the number of cells that have migrated or invaded across the membrane. Results shown are mean ± SEM of three independent experiments. * p<0.05, the Student’s t-test.
3.3. TIP-1 knockdown suppressed the gene expression for cell adhesion and motility but had moderate and disruptive effects on the beta-catenin-regulated genes
Microarray profiling of gene expression in MDA-MB-231 cells indicated that TIP-1 knockdown had moderate and disruptive effects (< 1.3-fold change after TIP-1 knockdown) on the beta-catenin-regulated genes (Supplementary Fig. S1). Few genes, such as MYC and DKK1, were up-regulated by the TIP-1 knockdown, while majority of the beta-catenin downstream genes showed slight decrease at the gene transcription level after TIP-1 knockdown. These results suggest that TIP-1 might utilize other pathways as primary mechanism(s) to regulate the malignancy progression of human breast cancers. GoMiner analysis of the microarray data indicated that a panel of genes that were down regulated by TIP-1 knockdown in MDA-MB-231 cells is associated with cell adhesion and motility (Supplementary tables 1 & 2). Among those genes, paxillin and integrin alpha-5 (ITGA5) were further validated with western blot analyses by the use of specific antibodies (Fig. 3A), which showed that TIP-1 knockdown reduced the overall protein levels of paxillin and integrin alpha-5 in MDA-MB-231 cells. Flow cytometric analyses indicated that expression of integrin alpha-5 on the plasma membrane of MDA-MB-231 cells were also reduced by ~2-fold (from ~5.5 × 102 to ~2.8 × 102) (Fig. 3B).
Fig. 3. TIP-1 knockdown impaired the gene expression for focal adhesion formation and cell adhesion.
(A) Western blot and semi-quantification of paxillin and integrin alpha-5 (ITGA5) protein levels in MDA-MB-231 cells with or without TIP-1 knockdown. Data were normalized to the levels of actin. (B) Flow cytometry analysis of integrin alpha-5 expression on the cell plasma surface of MDA-MB-231 cells. A control primary antibody was included. (C) Adhesion of MDA-MB-231 cells to different extracellular matrices including fibronectin, collagen IV or laminin. (D) Numbers of focal adhesion formed in cells were quantified based on phosphor-paxillin immunofluorescent staining of MDA-MB-231 cells on fibronectin-coated surface. All statistic analyses were based on quantitative measurements from three independent experiments. * p<0.05, the Student’s t-test.
3.4. TIP-1 knockdown impaired the focal adhesion formation and cell adhesion on fibronectin- coated surface
Cell adhesion assays on extracellular matrices showed that TIP-1 primarily affected adhesion of MDA-MB-231 cells to fibronectin (Fig. 3C), suggesting the importance of TIP-1 regulated expression of integrin alpha-5 which mediates cell adhesion and breast cancer metastasis through binding to fibronectin [20]. Focal adhesion, in which integrins and paxillin are major components, is one fundamental event in cell adhesion, migration and invasion. Therefore, immunofluorescence staining of the phosphorylated paxillin (Tyr118) (Supplementary Fig. S2) was applied to study the putative impact of TIP-1 knockdown on the focal adhesion of MDA-MB-231 cells on fibronectin-coated surface. Quantitative measurement of the matured focal adhesion foci (Fig. 3D) showed that TIP-1 knockdown remarkably reduced the focal adhesion formation of MDA-MB-231 cells on the fibronectin-coated surface.
3.5. TIP-1 knockdown inhibited tumor growth in mammary fat pads and pulmonary metastasis of human breast cancer cells in athymic mice
The putative roles of TIP-1 in tumor growth and pulmonary metastasis of human breast cancer were studied in athymic mice by using stable transfected MDA-MB-231 cells with or without TIP-1 knockdown. In an orthotopic xenograft model, TIP-1 knockdown moderately inhibited tumor growth in the mammary fat pads, and reduced pulmonary metastases from the primary tumors (Fig. 4A). The effect of TIP-1 knockdown on pulmonary metastasis was more dramatic when the tumor cells were injected into the tail veins. In six weeks after the tumor cell inoculation, it was observed that, compared to the vector control, tumor cells with TIP-1 knockdown formed less pulmonary metastases (Fig. 4B), number and diameter of the tumor nodules were significantly reduced (Fig. 4C).
Fig. 4. TIP-1 knockdown inhibited tumor growth in mammary fat pads and pulmonary metastasis of human breast cancer cells in athymic mice.
(A) Tumor growth curve (left) of MDA-MB-231 xenografts in mammary fat pads, and statistics (right) of pulmonary metastases from the primary xenograft tumors in FoxN1-null mice. n=5. (B) Representative light images of pulmonary metastases by six weeks after the intravenous injection of MDA-MB-231 cells. (C) Quantitative measurements of lung weight, number and diameter of tumor nodules of the pulmonary metastases. * p<0.05, n=9, the Student’s t-test.
4. Discussion
TIP-1 has demonstrated biological functions as a tumor suppressor in multiple models. It is critical in maintaining cell polarity of human epithelial cells [9, 21] and driving embryo development of zebrafish [5]. TIP-1 also antagonizes the transcriptional activity of beta-catenin and inhibits proliferation of colon cancer cells [14]. In the present study, we found elevated expression levels of TIP-1 in human invasive breast cancers, and the TIP-1 expression levels correlate to the prognostic outcomes of human breast cancers. Strikingly, a combination of in vitro and in vivo studies revealed that TIP-1 possesses oncogenic functions in human breast cancer. TIP-1 knockdown suppressed cell proliferation, adhesion, migration and invasion of human breast cancer cells in vitro, and inhibited the tumor growth and pulmonary metastasis in athymic mice. At molecular level, TIP-1 knockdown had moderate and disruptive effects on the beta-catenin-regulated gene expression, but a panel of genes involved in the cell adhesion and motility was modulated by TIP-1 knockdown. Collectively, this study has revealed a novel oncogenic function of TIP-1 in human breast cancer and provided clues for further investigation of the TIP-1-regulated biological functions.
TIP-1 knockdown slightly increased several beta-catenin downstream genes expression such as MYC and DKK1. However, majority of the beta-catenin downstream genes expression levels are decreased after TIP-1 knockdown. Compared to the inhibitory effects on beta-catenin-regulated gene expression and cell proliferation in colon cancer cells [14], this phenomenon suggests that the impact of TIP-1 on the beta-catenin’s functionality might depend on the intracellular context in different cell types. In human specimens, we also noted that TIP-1 expression levels are elevated in benign cystic lesions to a comparable level in invasive breast carcinomas. Since the cystic change lumps are smooth with defined edges, and are usually not invading into adjacent mammary gland structures, this result indicated that elevated TIP-1 expression is not able to promote the mammary epithelial cell migration and invasion by itself, it only predisposed the breast cancer cell to invasion and metastasis.
Integrins are the major cellular components involved in cell adhesion and motility, abnormal integrin expression or localization has been documented in malignancy progression of several tumor types [22]. In this study, TIP-1 knockdown decreased the transcription levels of multiple integrin isoforms, and also reduced the overall protein level of integrin alpha-5 and its presentation on the cell plasma membrane surface of breast cancer cells. The reduced integrin expression and presentation on cell plasma membrane coincide with the decreased cell adhesion to fibronectin-coated surface and the impaired focal adhesion formation. Considering the relatively moderate impact of TIP-1 on the gene expression levels of integrins and paxillin, we hypothesized that TIP-1 probably does not directly regulate the transcription of those genes. However, TIP-1 might regulate cell adhesion, migration, and invasion through modulating intracellular trafficking or modifications of the molecules involved in these biological events. Microarray profiling indicated TIP-1 knockdown also affected the transcription of a group of genes that are involved in protein phosphorylation (Supplementary table 1). Recent studies revealed that TIP-1 can interact with Rho guanine nucleotide exchange factors (RhoGEFs) [23], which regulate intracellular protein modification and trafficking through the downstream kinases [24], such as the p21 activated-kinase (PAK) and the Rho-associated serine/threonine kinase (ROCK) [25], which are in the top ranked function categories affected by TIP-1 knockdown in our analysis (Supplementary table 2). In addition, since TIP-1 binds to beta-catenin with a high affinity [26], we still can not exclude one possibility that TIP-1 modulates adhesion, migration and invasion through beta-catenin/Cadherin signaling cascade [27]. Further investigations are needed to have a definitive conclusion.
In conclusion, this study revealed oncogenic functions of TIP-1 in human invasive breast cancer. RNAi-mediated TIP-1 knockdown in human breast cancer cell lines suppressed the cell proliferation, adhesion, migration and invasion, as well as the tumor growth in mammary fat pads and pulmonary metastasis in athymic mice. Since pulmonary metastasis is one major cause of breast cancer-related casualty, this study suggests more studies are needed to further evaluate the potential of TIP-1 as a prognostic marker and a therapeutic target of human invasive breast cancer.
Supplementary Material
This study has revealed novel oncogenic functions of TIP-1 in human invasive breast cancer.
Elevated TIP-1 expression levels in human breast cancers correlate to the disease prognosis.
TIP-1 knockdown suppressed the cell migration and pulmonary metastasis of human breast cancer cells.
TIP-1 knockdown suppressed the expression and functionality of motility-related genes.
Acknowledgments
This study was supported in part by NIH grants R01CA127482 (Z Han), P50 CA128323 (J Gore) and the Vanderbilt CTSA grant RR024975-01 from NCRR/NIH. We thank Dr. Jennifer Pietenpol at Vanderbilt-Ingram Cancer Center for the kind gift of human breast cancer cell line BT549. We appreciate the technical assistance and advices from Dr. Ling Geng (Pathology, Vanderbilt University) in the animal studies, and the excellent technical services at the Cell Imaging, Histology, and Genomics Cores at the Vanderbilt-Ingram Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polakis P. Wnt Signaling in Cancer. Cold Spring Harb Perspect Biol. 2012 doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, Natrajan R, Reis-Filho JS. beta-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 2011;24:209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Goode EL, Fredericksen ZS, Vierkant RA, Pankratz VS, Liu-Mares W, Rider DN, Vachon CM, Cerhan JR, Olson JE, Couch FJ. Association of genetic variation in genes implicated in the beta-catenin destruction complex with risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2101–2108. doi: 10.1158/1055-9965.EPI-08-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besser J, Leito JT, van der Meer DL, Bagowski CP. Tip-1 induces filopodia growth and is important for gastrulation movements during zebrafish development. Dev Growth Differ. 2007;49:205–214. doi: 10.1111/j.1440-169X.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- 6.Dev KK. Making protein interactions druggable: targeting PDZ domains. Nat Rev Drug Discov. 2004;3:1047–1056. doi: 10.1038/nrd1578. [DOI] [PubMed] [Google Scholar]
- 7.Reynaud C, Fabre S, Jalinot P. The PDZ protein TIP-1 interacts with the Rho effector rhotekin and is involved in Rho signaling to the serum response element. J Biol Chem. 2000;275:33962–33968. doi: 10.1074/jbc.M000465200. [DOI] [PubMed] [Google Scholar]
- 8.Olalla L, Aledo JC, Bannenberg G, Marquez J. The C-terminus of human glutaminase L mediates association with PDZ domain-containing proteins. FEBS letters. 2001;488:116–122. doi: 10.1016/s0014-5793(00)02373-5. [DOI] [PubMed] [Google Scholar]
- 9.Alewine C, Olsen O, Wade JB, Welling PA. TIP-1 Has PDZ Scaffold Antagonist Activity. Mol Biol Cell. 2006;17:4200–4211. doi: 10.1091/mbc.E06-02-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui H, Hayashi A, Sun H-S, Belmares MP, Cobey C, Phan T, Schweizer J, Salter MW, Wang YT, Tasker RA, Garman D, Rabinowitz J, Lu PS, Tymianski M. PDZ Protein Interactions Underlying NMDA Receptor-Mediated Excitotoxicity and Neuroprotection by PSD-95 Inhibitors. J Neurosci. 2007;27:9901–9915. doi: 10.1523/JNEUROSCI.1464-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rousset R, Fabre S, Desbois C, Bantignies F, Jalinot P. The C-terminus of the HTLV-1 Tax oncoprotein mediates interaction with the PDZ domain of cellular proteins. Oncogene. 1998;16:643–654. doi: 10.1038/sj.onc.1201567. [DOI] [PubMed] [Google Scholar]
- 12.Hampson L, Li C, Oliver AW, Kitchener HC, Hampson IN. The PDZ protein Tip-1 is a gain of function target of the HPV16 E6 oncoprotein. Int J Oncol. 2004;25:1249–1256. [PubMed] [Google Scholar]
- 13.Obenauer JC, Denson J, Mehta PK, Su X, Mukatira S, Finkelstein DB, Xu X, Wang J, Ma J, Fan Y, Rakestraw KM, Webster RG, Hoffmann E, Krauss S, Zheng J, Zhang Z, Naeve CW. Large-scale sequence analysis of avian influenza isolates. Science. 2006;311:1576–1580. doi: 10.1126/science.1121586. [DOI] [PubMed] [Google Scholar]
- 14.Kanamori M, Sandy P, Marzinotto S, Benetti R, Kai C, Hayashizaki Y, Schneider C, Suzuki H. The PDZ protein tax-interacting protein-1 inhibits beta-catenin transcriptional activity and growth of colorectal cancer cells. J Biol Chem. 2003;278:38758–38764. doi: 10.1074/jbc.M306324200. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Yan H, Fu A, Han M, Hallahan D, Han Z. TIP-1 translocation onto the cell plasma membrane is a molecular biomarker of tumor response to ionizing radiation. PloS one. 2010;5:e12051. doi: 10.1371/journal.pone.0012051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Z, Fu A, Wang H, Diaz R, Geng L, Onishko H, Hallahan DE. Noninvasive assessment of cancer response to therapy. Nat Med. 2008;14:343–349. doi: 10.1038/nm1691. [DOI] [PubMed] [Google Scholar]
- 17.Cheng AS, Culhane AC, Chan MW, Venkataramu CR, Ehrich M, Nasir A, Rodriguez BA, Liu J, Yan PS, Quackenbush J, Nephew KP, Yeatman TJ, Huang TH. Epithelial progeny of estrogen-exposed breast progenitor cells display a cancer-like methylome. Cancer Res. 2008;68:1786–1796. doi: 10.1158/0008-5472.CAN-07-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H, Langerod A, Ji Y, Nowels KW, Nesland JM, Tibshirani R, Bukholm IK, Karesen R, Botstein D, Borresen-Dale AL, Jeffrey SS. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 2004;15:2523–2536. doi: 10.1091/mbc.E03-11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Shenouda S, Baranwal S, Rathinam R, Jain P, Bao L, Hazari S, Dash S, Alahari SK. Integrin subunits alpha5 and alpha6 regulate cell cycle by modulating the chk1 and Rb/E2F pathways to affect breast cancer metastasis. Molecular cancer. 2011;10:84. doi: 10.1186/1476-4598-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alewine C, Kim BY, Hegde V, Welling PA. Lin-7 targets the Kir 2.3 channel on the basolateral membrane via a L27 domain interaction with CASK. Am J Physiol Cell Physiol. 2007;293:C1733–1741. doi: 10.1152/ajpcell.00323.2007. [DOI] [PubMed] [Google Scholar]
- 22.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliver AW, He X, Borthwick K, Donne AJ, Hampson L, Hampson IN. The HPV16 E6 binding protein Tip-1 interacts with ARHGEF16, which activates Cdc42. Br J Cancer. 2011;104:324–331. doi: 10.1038/sj.bjc.6606026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Yoo Y, Fan H, Kim E, Guan KL, Guan JL. Regulation of Integrin beta 1 recycling to lipid rafts by Rab1a to promote cell migration. J Biol Chem. 2010;285:29398–29405. doi: 10.1074/jbc.M110.141440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Yan X, Shi C, Yang X, Guo Y, Tian C, Long J, Shen Y. Structural basis of beta-catenin recognition by Tax-interacting protein-1. J Mol Biol. 2008;384:255–263. doi: 10.1016/j.jmb.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 27.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.