Abstract
We have previously identified differential effects of age on global brain gene expression profiles in subjects with schizophrenia compared to normal controls. Here, we have focused on age-related effects of genes associated with the arachidonic acid-related inflammation pathway. Linear correlation analysis of published microarray expression data reveal strong age- and cell-type specific-effects on the expression of genes related to the arachidonic acid signaling pathway, which differed in control subjects compared to those with schizophrenia. Using real-time qPCR analysis, we validated age- and disease-effects of arachidonic acid-related genes in a large cohort of subjects with schizophrenia and matched controls (n=76 subjects in total). We found that levels of prostaglandin-endoperoxide synthase 1 (PTGS1; aka COX-1) and prostaglandin-endoperoxide receptor 3 (PTGER3) mRNA are increased, and levels of prostaglandin-endoperoxide synthase 2 (PTGS2; aka COX-2) mRNA are decreased, in older subjects with schizophrenia (>40 years of age) compared to matched normal controls or younger subjects with schizophrenia (<40 years of age). These findings contribute to the accumulating evidence suggesting that inflammatory processes in the CNS contribute to pathophysiology of schizophrenia and further suggest that age may be an important factor in the potential use of anti-inflammatory therapies.
Keywords: psychiatric disorder, inflammation, prostaglandin, microarray, COX-2, Celecoxib
1. Introduction
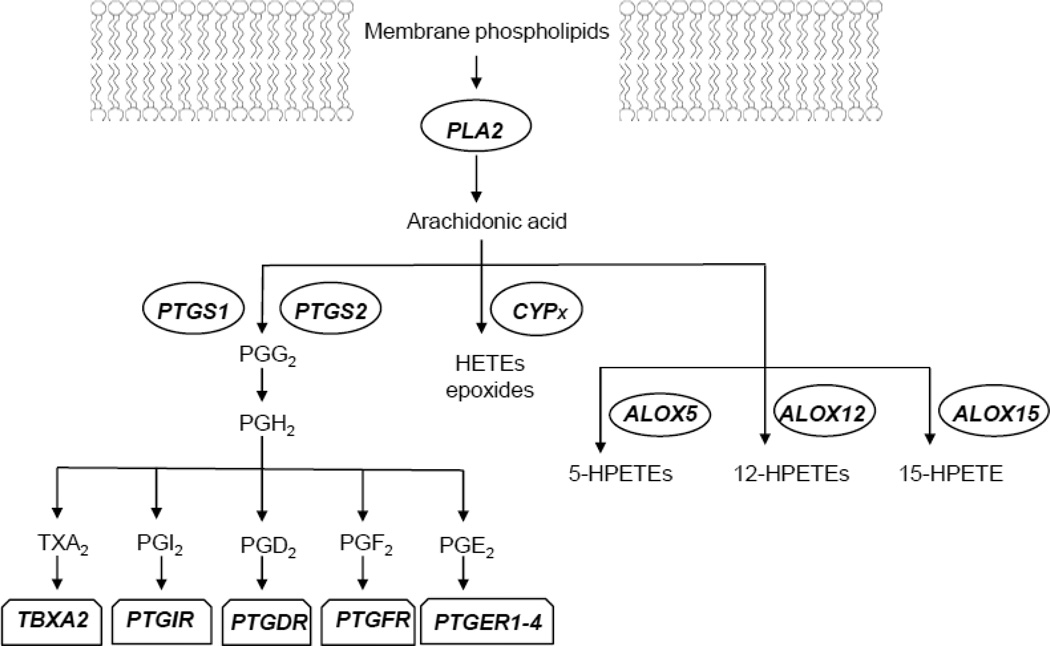
Arachidonic acid (AA) released from membrane phospholipids can be converted to a variety of biologically active metabolites, which are collectively referred to as eicosanoids, through the concerted reactions of phospholipase A2 (PLA2), prostaglandin-endoperoxide synthase (PTGS), lipooxygenase (ALOX) and cytochrome P450 family member (CYP) enzymes (Figure 1). Eicosanoids can mediate several pathophysiological processes, including regulation of nerve conduction, neurotransmitter release, inflammation, pain, immune responses and apoptosis (Phillis et al., 2006). In particular, the prostaglandin E2, (PGE2), which is generated by the actions of COX-1 and COX-2 enzymes, is an important component of the proinflammatory cascade. In addition to its actions via interactions with PGE2 receptors (PTGER1-4) to cause inflammation responses, PGE2 can stimulate the production of pro-inflammatory cytokines, such as IL-6 (Stolina et al., 2000).
Figure 1.
Schematic of depiction of arachidonic acid signaling pathway highlighting genes measured in this study. PTGS1, prostaglandin-endoperoxide synthase 1; PTGS2, prostaglandin-endoperoxide synthase 2; PLA2, phospholipase A2; CYPx, cytochrome P450 family member; ALOX5, arachidonate 5-lipoxygenase; ALOX12, arachidonate 12-lipoxygenase; ALOX15, arachidonate 15-lipoxygenase; TBXA2R, thromboxane A2 (TXA2) receptor; PTGIR, prostaglandin I2 (PGI2) receptor; PTGDR, prostaglandin D2 (PGD2) receptor; PTGFR, prostaglandin F2 (PGF2) receptor; PTGER1-4, prostaglandin E2 (PGE2) receptors 1–4.
Emerging data provide evidence that inflammatory processes in the brain contribute to the pathogenesis of schizophrenia (Yolken and Torrey, 1995; Strous and Shoenfeld, 2006; Potvin et al., 2008; Muller and Dursun, 2010; Dean, 2011). In particular, there is substantial data demonstrating abnormalities in AA and phospholipase A2 (PLA2) function in schizophrenia. Several studies have demonstrated marked depletions of AA in membranes of red blood cells, fibroblasts, and brain tissue in patients with schizophrenia, including those never-medicated (Yao et al., 2000; Arvindakshan et al., 2003; Skosnik and Yao, 2003; Reddy et al., 2004; Kemperman et al., 2006). These effects may be related to alterations in the activity of the enzyme PLA2, which is responsible for AA incorporation into and release from phospholipid membranes. Accordingly, elevations in the activity of PLA2 have been demonstrated in the serum and cortex of schizophrenic patients (Gattaz et al., 1987; Ross et al., 1997; Ross et al., 1999). Levels of prostaglandins in CNS of subjects with schizophrenia has not been studied, although, altered levels of PGE2 have been reported in the plasma of patients with schizophrenia (Kaiya et al., 1989).
In attempts to understand the molecular basis for altered AA, and subsequent PGE2, signaling in schizophrenia, we investigated disease-, age-, and cell-type specific- effects on the expression of genes related to the AA signaling pathway in schizophrenia. We interrogated previously published microarray data from our group (Narayan et al., 2008; Torkamani et al., 2010) followed by real-time qPCR analysis of selected genes in post-mortem cortical samples from n=76 subjects. We found significant disease and age effects for prostaglandin-endoperoxide synthase 1 (PTGS1; aka COX-1), prostaglandin-endoperoxide synthase 2 (PTGS2; aka COX-2), and prostaglandin-endoperoxide receptor 3 (PTGER3) in subjects with schizophrenia compared to normal controls. Further, we found that the expression of PTGS1 correlates with the expression of several microglial markers suggesting that microglia are the predominant cell type expressing PTGS1 in the brain.
2. Methods
2.1 Subjects
Approval was obtained from both the Ethics Committee of the Victorian Institute of Forensic Medicine and the Mental Health Research and Ethics Committee of Melbourne Health with all tissue being acquired from the Victorian Brain Bank Network. Prefrontal cortex, [Brodmann area (BA) 46], a region of the brain previously implicated in the pathology of schizophrenia (Goldman-Rakic and Seleman, 1997), was obtained at post-mortem from a large group of subjects: 38 subjects with schizophrenia and 38 subjects with no history of psychiatric illness, closely matched for gender, age, post-mortem interval (PMI) and tissue pH (Suppl Table 1). All schizophrenia subjects had prior history of treatment with a range of antipsychotic medications (see Suppl Table 1).
2.2 Linear correlation analysis
Microarray data generated in our previous study [see (Narayan et al., 2008)] and freely available on the NCBI GEO database (accession # GSE21138) were mined for expression differences due to schizophrenia. These data were generated using post-mortem human dorsolateral prefrontal cortex (Brodmann Area [BA] 46) from 29 people with schizophrenia (23M:6F) and 30 people with no history of psychiatric disorders (controls; 24M:6F) ranging from 19 to 81 years of age, using the Affymetrix Human Genome U133 platform.
Pearson Product Moment correlations were performed measuring linear relationships between age and the Log2 gene expression values for each subject as described previously (Tang et al., 2009). Before the Pearson’s correlations were run, the expression datasets were tested for a Gaussian distribution using the Kolmogorov-Smirnov method, which revealed that the expression data was normally distributed for all but two genes, in which cases Spearman correlation analyses were performed. Additional covariate analyses were performed to assess the effects of sample parameters (pH and PMI) on gene expression values in all subjects, and the effects of antipsychotic drug dose (in chlorpromazine equivalents) on gene expression in all subjects with schizophrenia. No significant effects of tissue pH or PMI or recorded drug doses on gene expression were found.
2.3 QPCR analysis
Real-time PCR experiments were performed on cDNA templates generated from 1 µg total RNA isolated from BA46 of all subjects. Real-time PCR assays were carried out as described previously (Desplats et al., 2006), using specific primers for each sequence of interest and against the housekeeping genes β-2-microglobulin (B2M) for human samples and hypoxanthine-guanine phosphoribosyltransferase (Hprt) for mouse samples (Suppl. Table 2). Our previous studies have shown that B2M gives the least variation in threshold cycle (Ct) among all samples and showed no significant differences in expression between control and schizophrenic subjects (Narayan et al., 2008). The amount of cDNA in each sample was calculated using SDS2.1 software by the comparative Ct method and expressed as 2exp(Ct). Significant differences in gene expression were determined using Student’s t test, unpaired, one-tailed (Prism GraphPad, San Diego, CA).
2.4 Mouse drug treatments
Adult male mice (C57black/6J; 2–4 months of age) were individually housed in a temperature controlled environment and maintained on a normal 12-h light/dark cycle with lights on at 06:00 am. Food and water were available ad libitum. Groups of mice (n=4–6 per drug treatment and controls) received either haloperidol (4 mg/kg/day) or vehicle (0.1% acetic acid) for 4 weeks in solutions that replace drinking water as described previously (Narayan et al., 2006). 0.5% sucrose was added to haloperidol to enhance taste. New drug was given every 2–3 days. All procedures were in strict accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. At the end of the 1 month period mice were sacrificed and brains removed for gene expression analysis.
3. Results
3.1 Age effects on expression of inflammation-related genes
We have previously used two approaches to identify age-related effects on gene expression in schizophrenia using microarray data generated by our own group (Narayan et al., 2008), freely available on the NCBI GEO database (accession # GSE21138), and microarray data from the Harvard Brain Tissue Resource Center (www.brainbank.mclean.org) (Torkamani et al., 2010). Our approach was to identify age-related effects on gene expression (Tang et al., 2009) using Pearson’s correlation analysis and gene coexpression analysis using age as a specific covariate (Torkamani et al., 2010). Here, we characterized age-related genes from these previous studies using Ingenuity Pathways Analysis. Functional categorizing of age-related genes revealed highly significant associations of age to “inflammatory disease”, which encompasses several disease annotations, in controls subjects and those with schizophrenia (p=7.56E-10 to 4.47E-3; Table 1). Canonical pathways analysis demonstrated several distinct immune/inflammation-related pathways associated with age in control subjects, including IL-1 signaling, CXCR4 signaling and chemokine signaling (Suppl. Table 3). In subjects with schizophrenia, the Toll-like receptor signaling pathways was most significantly associated with age, in addition to IL-8 signaling, B cell receptor signaling and the AA signaling pathway (Suppl. Table 3).
Table 1.
Inflammation-related categories associated with age in affected and unaffected individuals. as determined by Ingenuity Systems Pathways Analysis.
| Normal aging | |||
|---|---|---|---|
| Category: | Functional Annotation: | p-Vatue: | # Genes: |
| Inflammatory Disease | inflammatory disorder | 7.56E-10 | 444 |
| Inflammatory Disease | rheumatoid arthritis | 8.48E-09 | 238 |
| Inflammatory Disease | arthritis | 1.83E-07 | 257 |
| Inflammatory Disease | rheumatic disease | 2.72E-07 | 264 |
| Inflammatory Disease | Crohn's disease | 1.34E-06 | 166 |
| Inflammatory Disease | inflammatory bowel disease | 1.27E-05 | 171 |
| Inflammatory Disease | inflammatory demyelinating disease | 3.59E-03 | 35 |
| Inflammatory Disease | Kawasaki's disease | 3.85E-03 | 4 |
| Inflammatory Disease | enterocolitis | 4.47E-03 | 6 |
| Aging in schizophrenia | |||
| Category: | Functional Annotation: | p-Value: | # Genes: |
| Inflammatory Disease | inflammatory disorder | 4.52E-05 | 359 |
| Inflammatory Disease | Crohn's disease | 7.61E-04 | 132 |
| Inflammatory Disease | inflammatory bowel disease | 2.44E-03 | 137 |
| Inflammatory Disease | dermatitis of organ | 2.50E-03 | 5 |
| Inflammatory Disease | rheumatic disease | 3.17E-03 | 205 |
| Inflammatory Disease | arthritis | 3.50E-03 | 198 |
| Inflammatory Disease | arthritis of mice | 4.37E-03 | 22 |
| Inflammatory Disease | onset of experimental autoimmune encephalomyelitis of mice | 4.60E-03 | 3 |
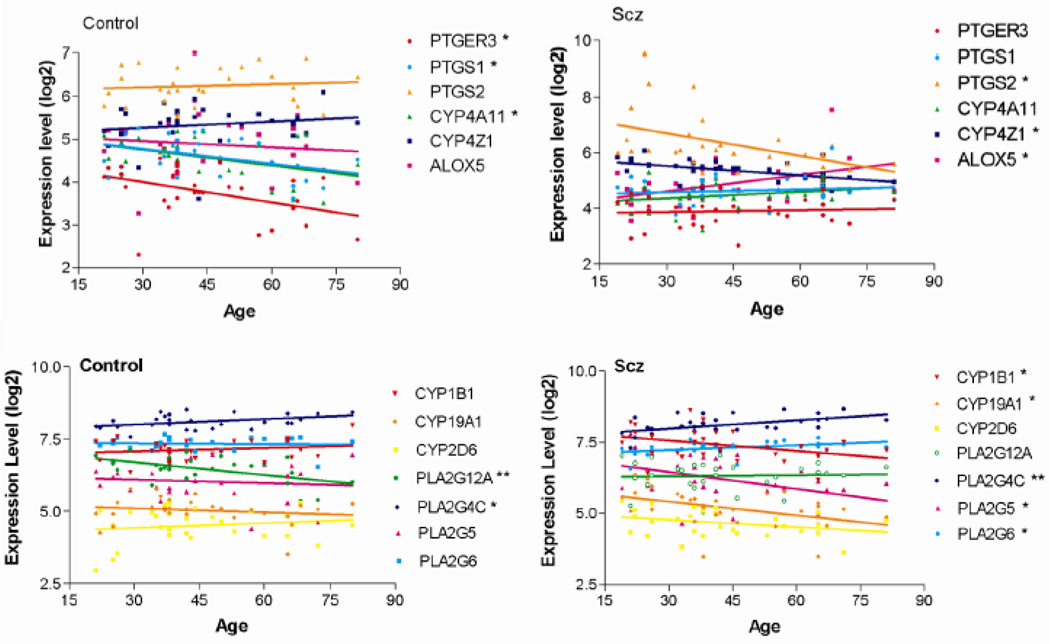
We next focused on genes in the AA signaling pathway in light of previous associations between AA and schizophrenia (Yao et al., 2000; Arvindakshan et al., 2003; Skosnik and Yao, 2003; Reddy et al., 2004; Kemperman et al., 2006) and the potential of this pathway for therapeutic intervention. Figure 2 shows linear correlations of the individual genes found to be altered with aging from these analyses in case and control subjects. Interestingly, we found that some genes are associated with age only in control subjects and some only in subjects with schizophrenia (Figure 2). Significant negative correlations in the expression of PTGS2, CYP4Z1, CYP1B1, CYP19A1, PLA2G5, PLA2G6 and PLA2G12A and a positive correlation in the expression of ALOX5 with age were detected in subjects with schizophrenia (Figure 2, Table 2). In contrast, significant negative correlations in the expression of PTGS1, PTGER3, CYP4A11, PLA2G12A and PLA2G4C, were detected with age in control subjects (Figure 2, Table 2).
Figure 2.
Correlated expression of the indicated genes with age in subjects with schizophrenia (Scz) and normal controls. Microarray expression data from individual subjects was plotted according to age in each group and subjected to Pearson correlation analysis. Each point represents a single subject. Asterisks indicated those genes showing a significant correlation with age in either controls subjects or those with schizophrenia. Linear correlation r values and p-values for these correlations are shown in Table 2.
Table 2.
Correlation of the expression levels of genes associated with arachidonic acid signaling with age in control subjects and those with schizophrenia.
| CONTROL | |||
|---|---|---|---|
| Gene: | r-value: | P-value: | Significance: |
| PTGER3 | −0.4351 | 0.0183 | * |
| PTGS1 | −0.4109 | 0.0268 | * |
| PTGS2 | 0.08788 | 0.6503 | ns |
| CYP4A11 | −0.4165 | 0.0246 | * |
| CYP4Z1 | 0.1564 | 0.4179 | ns |
| ALOX5 | −0.1104 | 0.5686 | ns |
| CYP1B1 | 0.172 | 0.372 | ns |
| CYP19A1 | −0.167 | 0.385 | ns |
| CYP2D6 | 0.159 | 0.409 | ns |
| PLA2G12A | −0.542 | 0.002 | ** |
| PLA2G4C | 0.397 | 0.032 | * |
| PLA2G5 | −0.096 | 0.619 | ns |
| PLA2G6 | −0.056 | 0.771 | ns |
| SCHIZOPHRENIA | |||
| Gene: | r-value: | P-value: | Significance: |
| PTGER3 | 0.06449 | 0.7349 | ns |
| PTGS1 | 0.1084 | 0.5684 | ns |
| PTGS2 | −0.5373 | 0.0092 | ** |
| CYP4A11 | 0.2902 | 0.1198 | ns |
| CYP4Z1 | −0.5155 | 0.0036 | ** |
| ALOX5 | 0.4281 | 0.0183 | * |
| CYP1B1 | −0.383 | 0.036 | * |
| CYP19A1 | −0.429 | 0.019 | * |
| CYP2D6 | −0.319 | 0.085 | ns |
| PLA2G12A | 0.048 | 0.799 | ns |
| PLA2G4C | 0.468 | 0.009 | ** |
| PLA2G5 | −0.427 | 0.018 | * |
| PLA2G6 | 0.419 | 0.022 | * |
Pearson or Spearman R values are shown along with the p-values for significance.
3.2 Disease effects on expression of prostaglandin-related genes
We next determined expression differences of genes related to the COX1/2 pathway, given their prominent role in generating pro-inflammatory prostaglandins (Figure 2). We performed real-time qPCR analysis to determine levels of mRNA for four genes, PTGS1 (COX-1), PTGS2 (COX-2), PTGER3 and CYP4Z1 in tissue from subjects with schizophrenia and age-matched controls. When subjects of all ages (18–81 years) were analyzed together, no significant differences in the expression of these genes were detected. However, when subjects were separated into younger (<40 yrs) and older (>40 yrs) groups, significant differences in gene expression emerged. Increases in the expression levels of PTGS1 and PTGER3 in were detected in older subjects with schizophrenia compared to their age-matched controls (Figure 3), while no significant differences were detected in younger subjects with the disease, although a trend towards a decrease was detected for PTGER3. In contrast, a decreased expression of PTGS2 was detected in older subjects with schizophrenia compared to matched controls and a trend towards an increase in younger affected subjects (Figure 3). No changes in the expression of CYP4Z1 were detected in affected subjects at any age (Figure 3).
Figure 3.
Real-time PCR analysis on the indicated genes in subjects with schizophrenia and age- and sex-matched controls. Real-time PCR data reflect expression levels from n=38 subjects with schizophrenia and n=38 age-matched controls. The relative abundance of each gene expression was normalized by beta-2 microglobulin (B2M). Asterisks denote significant decreases in expression using Student’s t test, *, p<0.05; +, p<0.07.
3.3 Antipsychotic drug effects
A confounding factor in post-mortem research on schizophrenia is the unknown effect of antipsychotic drugs, which are known to alter gene expression [25]. Given that older subjects with chronic schizophrenia were associated with a longer lifetime exposure to antipsychotic drugs, we tested whether their expression levels of PTGS1, PTGS2 and PTGER3 were altered in the brains of mice that were treated with haloperidol (2 mg/kg; 4 weeks), the main drug with which most of the subjects with schizophrenia were treated. No changes in expression of any genes were found in response to drug treatment (Suppl. Figure 1). Additionally, we did not find a correlation between the recorded drug doses and the expression of any gene in the subjects with schizophrenia. These findings suggest that antipsychotic drug exposure does not significantly contribute to our observed gene expression changes, although this possibility cannot be completely excluded.
3.4 CNS cell-type correlations
Our gene expression data was generated from prefrontal cortex, which represents a heterogeneous collection of different cell types. Hence, we attempted to identify cell-type associations to gene expression by comparing the expression levels of AA-related genes to those genes encoding a panel of markers that distinguish neurons and the three types of glial cells, astrocytes, oligodendrocytes and microglia (all from microarray data NCBI GEO database accession # GSE21138). We first correlated the expression levels of all markers for a given cell type to ascertain the ability of the chosen markers to correlate to each other. We found excellent correlation in the expression of markers within an individual cell type in both normal subjects and those with schizophrenia (Suppl. Figure 2). The correlation among oligodendrocyte markers was the highest, with the expression of all markers correlating to one another with an average Pearson’s r value = 0.887 for controls and 0.875 for schizophrenia (both P<0.0001). The correlation in expression among the microglia markers was the lowest with an average correlation r value of 0.388 in normal subjects; however, the average r value increased to 0.555 in subjects with schizophrenia. As many of these markers are specific for activated microglia, this finding could implicate microglial activation in schizophrenia on a molecular level. We next correlated the expression levels of selected genes with all cell-type specific markers. We found that the expression levels of PTGS1 and ALOX5 were significantly correlated with 5 of the 7 microglia markers tested in subjects with schizophrenia, but not in control subjects (Table 3). This suggests that PTGS1 and ALOX5 expression in schizophrenia occur is microglial driven. The expression levels of the other genes did not show significant correlation with any single cell-specific marker, suggesting that these genes are expressed in multiple CNS cell types.
Table 3.
Correlation of AA/PG-related genes with the expression of genes encoding microglia
| Microglial markers - Control: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pearson R values: | ||||||||
| PTGS1 | PTGS2 | PTGER3 | ALOX5 | CYP4A11 | CYP4Z1 | PTGS1 | PTGS2 | |
| AIF1 | 0.285 | 0.014 | 0.132 | 0.702 | −0.105 | 0.040 | 0.134 | 0.941 |
| CD53 | 0.404 | 0.075 | −0.078 | 0.334 | 0.029 | 0.428 | 0.030 | 0.697 |
| CX3CR1 | 0.253 | −0.148 | −0.006 | 0.347 | 0.120 | −0.205 | 0.185 | 0.443 |
| FTL | 0.288 | 0.090 | −0.242 | −0.093 | 0.524 | 0.328 | 0.130 | 0.641 |
| ITGAM | 0.085 | 0.070 | 0.209 | 0.595 | −0.095 | 0.154 | 0.661 | 0.717 |
| ITGB2 | 0.133 | 0.094 | 0.166 | 0.632 | 0.083 | 0.064 | 0.491 | 0.627 |
| KRAS | −0.159 | 0.338 | 0.302 | 0.153 | 0.038 | 0.202 | 0.410 | 0.073 |
| MKI67 | −0.131 | −0.174 | 0.108 | −0.128 | 0.032 | 0.093 | 0.498 | 0.367 |
| PTPRC | −0.094 | 0.252 | −0.076 | 0.458 | 0.100 | −0.060 | 0.626 | 0.188 |
| Microglial markers - Schiz: | ||||||||
| Pearson R values: | ||||||||
| PTGS1 | PTGS2 | PTGER3 | ALOX5 | CYP4A11 | CYP4Z1 | PTGS1 | PTGS2 | |
| AIF1 | 0.545 | −0.236 | −0.151 | 0.714 | −0.250 | −0.299 | 0.002 | 0.209 |
| CD53 | 0.447 | −0.270 | −0.174 | 0.372 | −0.206 | −0.084 | 0.006 | 0.660 |
| CX3CR1 | 0.445 | −0.330 | −0.055 | 0.655 | −0.380 | −0.098 | 0.014 | 0.075 |
| FTL | −0.406 | −0.195 | −0.217 | −0.342 | 0.499 | 0.220 | 0.026 | 0.691 |
| ITGAM | −0.042 | −0.065 | −0.304 | 0.298 | 0.006 | −0.171 | 0.481 | 0.421 |
| ITGB2 | 0.491 | 0.170 | 0.115 | 0.728 | −0.115 | −0.271 | 0.013 | 0.148 |
| KRAS | −0.289 | −0.153 | −0.087 | 0.061 | 0.135 | −0.115 | 0.121 | 0.369 |
| MKI67 | −0.067 | 0.143 | −0.283 | −0.357 | 0.104 | 0.440 | 0.325 | 0.301 |
| PTPRC | 0.364 | −0.180 | −0.234 | 0.583 | −0.017 | −0.257 | 0.048 | 0.341 |
Correlation (R) values were determined by Pearson correlation analysis, with p-values reflecting the significance of Genes listed are designated by their official UniGene symbols.
4. Discussion
In this study, we report significant differences in the effects of age on the expression of AA and prostaglandin-related genes in subjects with schizophrenia compared to normal controls. Notably, PTGS2 expression levels showed a decreased correlation with age in subjects with schizophrenia, but not control subjects, while PTGS1 and PTGER3 expression levels were significantly decreased with age in controls subjects but not those with schizophrenia. These differential aging effects likely contribute to the expression differences observed for these genes in our real-time qPCR studies when subjects with schizophrenia were compared to their age-matched controls (see Figure 3). The expression of genes encoding ALOX, PLA2 and CYP enzyme variants also showed differential age correlations in control versus affected subjects. These changes imply that different branches of eicosanoid signaling play different roles in the pathology of schizophrenia depending on the age of patient.
The study of prostaglandin production in schizophrenia has high relevance given that anti-inflammatory therapy has been suggested to have beneficial effects in schizophrenia. In particular, recent studies have explored the use of COX-2 inhibitors as a possible adjunctive therapeutic approach, along with antipsychotic drugs for treatment of schizophrenia (Chakraborti et al.; Muller et al., 2002; Muller et al., 2004; Rapaport et al., 2005; Riedel et al., 2005; Akhondzadeh et al., 2007). COX-2 inhibitors prevent the production of prostaglandins (Figure 1); however they have also been reported to rebalance Th1 and Th2 T-cell responses (Muller et al., 1999), regulate cytokine production (Nakanishi et al.; Riedel et al., 2005), and down-regulate NMDA receptor-mediated neurotoxicity (Nogawa et al., 1997; Yermakova and O'Banion, 2000). In two clinical trials, it was reported that the addition of the COX-2 inhibitor, Celecoxib, to risperidone-treatment had a significant effect on the mean improvement in total positive and negative syndrome scale (PANSS) score, as compared to risperidone monotherapy (Muller et al., 2004; Akhondzadeh et al., 2007). Additional studies found that beneficial effects of Celecoxib were observed only in patients with recent onset schizophrenia (>7 years) (Muller et al., 2004; Riedel et al., 2005). Consistent with this finding, was another report that Celecoxib adjunct therapy did not show clinical improvement in chronically ill patients (Rapaport et al., 2005). Our data showing that PTGS2 expression decreases with age in schizophrenia is highly relevant in light of these clinical results. That is, COX-2 inhibitors are likely to have the most benefit early in illness, when the PTGS2 expression levels, and presumed COX-2 enzyme activity, are the highest. However, if PTGS2 expression levels are already low in chronic illness or in elderly patients, inhibition of the enzyme levels by a COX-2 inhibitor would be unlikely to show further benefit. In addition, we found that subjects with schizophrenia do not show a normal decrease in the expression of PTGS1 with age, thereby leading to abnormally high expression levels of this gene in patients with chronic schizophrenia. This could potentially suggest the therapeutic targeting of COX-1 in chronic illness.
While schizophrenia is not typically considered to be a neurodegenerative disorder, the neuropathology of schizophrenia has recently been reported to be associated with microglial activation (Monji et al., 2009), which is commonly seen in neurodegenerative disorders, such as Parkinson's disease and Alzheimer's disease (Block and Hong, 2005). Despite the fact that microglia comprise <10% of the total brain cells, microglia respond rapidly to even minor pathological changes in the brain and may contribute directly to the neuronal degeneration by producing various pro-inflammatory cytokines and free radicals. Prolonged microglial hyperactivity can lead to neuronal apoptosis and brain damage. Several studies have reported altered expression of microglia-related surface markers in schizophrenia, at least in subpopulations of individuals with schizophrenia (Bayer et al., 1999; Radewicz et al., 2000; Steiner et al., 2006). More recent approaches to measure microglial activation in vivo using (R)-[11C]PK11195 and positron emission tomography have reported increased microglial activation in the gray matter of patients with schizophrenia (van Berckel et al., 2008). On a gene expression level, our studies showed increased correlation in the expression of microglial markers with one another in subjects with schizophrenia. As many of these markers are specific for activated microglia, this finding could implicate microglial activation in schizophrenia on a molecular level. Given the caveat of antipsychotic drug exposure in subjects with schizophrenia, it is possible that antipsychotic medications affect the expression of microglial genes. However, it has been reported that most antipsychotic drugs reduce microglial inflammatory reactions (Monji et al., 2009), hence it is more likely to reflect an ongoing pathology in schizophrenia. Hence, our findings suggest that microglialcells are the primary source of PTGS1 and ALOX5 expression in schizophrenic subjects.
Overall, our results support the hypothesis that inflammatory mechanisms contribute to the pathophysiology of schizophrenia, but that different aspects of eicosanoid signaling, especially prostaglandins, are active in young versus old patients. With regards to adjunct treatment with anti-inflammatory medications in schizophrenia, our data suggest that the age of the patient be considered.
Supplementary Material
The effects of haloperidol treatment (2 mg/kg; 4 weeks) on the expression of PTGS1, PTGS2 and PTGER3 in the cortex and striatum of mice. The expression of PTGER3 in the striatum was too long to measure with confidence. All expression values were normalized to Hprt.
Correlation among the expression of CNS cell-type-specific markers in control subjects and those with schizophrenia.
Acknowledgements
This study was funded by grants from the National Institutes of Health (NS44169 and MH069696 to E.A.T.). BD is an NH&MRC Senior Research Fellow (# APP1002240).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophrenia Research. 2007;90:179–185. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Arvindakshan M, Sitasawad S, Debsikdar V, Ghate M, Evans D, Horrobin DF, Bennett C, Ranjekar PK, Mahadik SP. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biological Psychiatry. 2003;53:56–64. doi: 10.1016/s0006-3223(02)01443-9. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Buslei R, Havas L, Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neuroscience Letters. 1999;271:126–128. doi: 10.1016/s0304-3940(99)00545-5. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Progress in Neurobiology. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Chakraborti AK, Garg SK, Kumar R, Motiwala HF, Jadhavar PS. Progress in COX-2 inhibitors: a journey so far. Current Medicinal Chemistry. 17:1563–1593. doi: 10.2174/092986710790979980. [DOI] [PubMed] [Google Scholar]
- Dean B. Understanding the role of inflammatory-related pathways in the pathophysiology and treatment of psychiatric disorders: evidence from human peripheral studies and CNS studies. International Journal of Neuropsychopharmacology. 2011;14:997–1012. doi: 10.1017/S1461145710001410. [DOI] [PubMed] [Google Scholar]
- Desplats PA, Kass KE, Gilmartin T, Stanwood GD, Woodward EL, Head SR, Sutcliffe JG, Thomas EA. Selective deficits in the expression of striatal-enriched mRNAs in Huntington's disease. Journal of Neurochemistry. 2006;96:743–757. doi: 10.1111/j.1471-4159.2005.03588.x. [DOI] [PubMed] [Google Scholar]
- Gattaz WF, Kollisch M, Thuren T, Virtanen JA, Kinnunen PKJ. Increased plasma phospholipase-A2 activity in schizophrenic patients: reduction after neuroleptic therapy. Biological Psychiatry. 1987;22:421–426. doi: 10.1016/0006-3223(87)90164-8. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Seleman LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophrenia Bulletin. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- Kaiya H, Uematsu M, Ofuji M, Nishida A, Takeuchi K, Nozaki M, Idaka E. Elevated plasma prostaglandin E2 levels in schizophrenia. Journal of Neural Transmission. 1989;77:39–46. doi: 10.1007/BF01255817. [DOI] [PubMed] [Google Scholar]
- Kemperman RF, Veurink M, van der Wal T, Knegtering H, Bruggeman R, Fokkema MR, Kema IP, Korf J, Muskiet FA. Low essential fatty acid and B-vitamin status in a subgroup of patients with schizophrenia and its response to dietary supplementation. Prostaglandins Leukotrienes and Essential Fatty Acids. 2006;74:75–85. doi: 10.1016/j.plefa.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry and Clinical Neurosciences. 2009;63:257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- Muller N, Dursun SM. Schizophrenia genes, epigenetics and psychoneuroimmunology therapeutics: all make sense now? Journal of Psychopharmacology. 2010 doi: 10.1177/0269881110364268. [DOI] [PubMed] [Google Scholar]
- Muller N, Riedel M, Ackenheil M, Schwarz MJ. The role of immune function in schizophrenia: an overview. European Archives of Psychiatry and Clinical Neuroscience. 1999;249(Suppl 4):62–68. doi: 10.1007/pl00014187. [DOI] [PubMed] [Google Scholar]
- Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, Ulmschneider M, Engel RR, Moller HJ, Schwarz MJ. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. American Journal of Psychiatry. 2002;159:1029–1034. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- Muller N, Ulmschneider M, Scheppach C, Schwarz MJ, Ackenheil M, Moller HJ, Gruber R, Riedel M. COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:14–22. doi: 10.1007/s00406-004-0478-1. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Nakatsuji M, Seno H, Ishizu S, Akitake-Kawano R, Kanda K, Ueo T, Komekado H, Kawada M, Minami M, Chiba T. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis. doi: 10.1093/carcin/bgr128. In press. [DOI] [PubMed] [Google Scholar]
- Narayan S, Kass K, Thomas EA. Chronic haloperidol treatment results in a decrease in the expression of myelin/oligodendrocyte-related genes in the mouse brain. Journal of Neuroscience Research. 2006;85:757–765. doi: 10.1002/jnr.21161. [DOI] [PubMed] [Google Scholar]
- Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B, Thomas EA. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Research. 2008;1239:235–248. doi: 10.1016/j.brainres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. Journal of Neuroscience. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW, Horrocks LA, Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Research Reviews. 2006;52:201–243. doi: 10.1016/j.brainresrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biological Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. Journal of Neuropathology and Experimental Neurology. 2000;59:137–150. doi: 10.1093/jnen/59.2.137. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D. Celecoxib augmentation of continuously ill patients with schizophrenia. Biological Psychiatry. 2005;57:1594–1596. doi: 10.1016/j.biopsych.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Reddy RD, Keshavan MS, Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophrenia Bulletin. 2004;30:901–911. doi: 10.1093/oxfordjournals.schbul.a007140. [DOI] [PubMed] [Google Scholar]
- Riedel M, Strassnig M, Schwarz MJ, Muller N. COX-2 inhibitors as adjunctive therapy in schizophrenia: rationale for use and evidence to date. CNS Drugs. 2005;19:805–819. doi: 10.2165/00023210-200519100-00001. [DOI] [PubMed] [Google Scholar]
- Ross BM, Hudson C, Erlich J, Warsh JJ, Kish SJ. Increased phospholipid breakdown in schizophrenia. Evidence for the involvement of a calcuim-independent phospholipase A2. Archives of General Psychiatry. 1997;54:487–494. doi: 10.1001/archpsyc.1997.01830170113015. [DOI] [PubMed] [Google Scholar]
- Ross BM, Turenne S, Moszczynska A, Warsh JJ, Kish SJ. Differential alteration of phospholipase A2 activities in brain of patients with schizophrenia. Brain Research. 1999;821:407–413. doi: 10.1016/s0006-8993(99)01123-3. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Yao JK. From membrane phospholipid defects to altered neurotransmission: is arachidonic acid a nexus in the pathophysiology of schizophrenia? Prostaglandins Leukotrienes and Essential Fatty Acids. 2003;69:367–384. doi: 10.1016/j.plefa.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Steiner J, Mawrin C, Ziegeler A, Bielau H, Ullrich O, Bernstein HG, Bogerts B. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathologica. 2006;112:305–316. doi: 10.1007/s00401-006-0090-8. [DOI] [PubMed] [Google Scholar]
- Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, Lee JC, Dubinett SM. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. Journal of Immunology. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- Strous RD, Shoenfeld Y. Schizophrenia, autoimmunity and immune system dysregulation: a comprehensive model updated and revisited. Journal of Autoimmunity. 2006;27:71–80. doi: 10.1016/j.jaut.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Tang B, Chang WL, Lanigan CM, Dean B, Sutcliffe JG, Thomas EA. Normal human aging and early-stage schizophrenia share common molecular profiles. Aging Cell. 2009;8:339–342. doi: 10.1111/j.1474-9726.2009.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkamani A, Dean B, Schork NJ, Thomas EA. Coexpression network analysis of neural tissue reveals perturbations in developmental processes in schizophrenia. Genome Research. 2010;20:403–412. doi: 10.1101/gr.101956.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biological Psychiatry. 2008;64:820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophrenia Research. 2000;42:7–17. doi: 10.1016/s0920-9964(99)00095-x. [DOI] [PubMed] [Google Scholar]
- Yermakova A, O'Banion MK. Cyclooxygenases in the central nervous system: implications for treatment of neurological disorders. Current Pharmaceutical Design. 2000;6:1755–1776. doi: 10.2174/1381612003398672. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF. Viruses, schizophrenia, and bipolar disorder. Clinical Microbiology Reviews. 1995;8:131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effects of haloperidol treatment (2 mg/kg; 4 weeks) on the expression of PTGS1, PTGS2 and PTGER3 in the cortex and striatum of mice. The expression of PTGER3 in the striatum was too long to measure with confidence. All expression values were normalized to Hprt.
Correlation among the expression of CNS cell-type-specific markers in control subjects and those with schizophrenia.