Abstract
Summary
A therapeutic role for a cytoprotective peptide fragment of antrum mucosal protein (AMP)-18 was sought in three hamster models of radiation-induced oral mucositis. AMP peptide reduced mucosal erythema and ulceration and enhanced recovery, possibly by its antiapoptotic effects shown in cultures of endothelial and epithelial cells. AMP peptide did not ameliorate the antitumor effects of concomitant radiation in a lung xenograft model, thereby providing additional support for a new agent to treat oral mucositis.
Purpose
No effective agents currently exist to treat oral mucositis (OM) in patients receiving chemoradiation for the treatment of head-and-neck cancer. We identified a novel 21 –amino acid peptide derived from antrum mucosal protein-18 that is cytoprotective, mitogenic, and motogenic in tissue culture and animal models of gastrointestinal epithelial cell injury. We examined whether administration of antrum mucosal protein peptide (AMP-p) could protect against and/or speed recovery from OM.
Methods and Materials
OM was induced in established hamster models by a single dose of radiation, fractionated radiation, or fractionated radiation together with cisplatin to simulate conventional treatments of head-and-neck cancer.
Results
Daily subcutaneous administration of AMP-p reduced the occurrence of ulceration and accelerated mucosal recovery in all three models. A delay in the onset of erythema after irradiation was observed, suggesting that a protective effect exists even before injury to mucosal epithelial cells occurs. To test this hypothesis, the effects of AMP-p on tumor necrosis factor-α-induced apoptosis were studied in an endothelial cell line (human dermal microvascular endothelial cells) as well as an epithelial cell line (human adult low-calcium, high-temperature keratinocytes; HaCaT) used to model the oral mucosa. AMP-p treatment, either before or after cell monolayers were exposed to tumor necrosis factor-α, protected against development of apoptosis in both cell types when assessed by annexin V and propidium iodide staining followed by flow cytometry or ligase-mediated polymerase chain reaction.
Conclusions
These observations suggest that the ability of AMP-p to attenuate radiation-induced OM could be attributable, at least in part, to its antiapoptotic activity.
Keywords: Oral mucositis, Apoptosis, Endothelial cells, Epithelial cells, Gastrokine 1
Introduction
Oral mucositis (OM) is a common, dose-limiting toxicity of many radiation and chemotherapeutic antineoplastic regimens (1). It is characterized by breakdown of the oral mucosa and development of ulcerative lesions that result in pain, decreased quality of life, and increased health care resource use (2). In severe cases, OM leads to adverse modifications of antineoplastic treatment. There is currently no effective intervention for the prevention or treatment of OM.
We identified a 21 –amino acid peptide derived from the 18-kDa antrum mucosal protein (AMP-18) that exhibits protective, mitogenic, and motogenic properties on epithelial cells (3). AMP-18, also known as gastrokine 1, is constitutively expressed in epithelial cells of the gastric antrum (4), where it may protect barrier function and structure.
We have suggested that the protective effects of antrum mucosal protein peptide (AMP-p) may be attributable to its capacity to limit the loss of tight junction (TJ) proteins after injury (5). From the standpoint of OM, it seemed that an agent that targeted TJs, multiple proteins that bind together epithelial cells, might enhance the integrity of the oral mucosa and provide a novel interventional approach to preventing or treating OM (6). Consequently, we tested the effects of AMP-p on radiation-induced OM in three established models of the syndrome in hamsters (7, 8).
Methods and Materials
Materials
Human AMP-p (LDALVKEKKLQGKGPGGPPPK) and scrambled peptide (GKPLGQPGKVPKLDGKEPLAK) (3, 5) were synthesized by GenScript (Piscataway, NJ). Recombinant human tumor necrosis factor (TNF)-α and interferon gamma were purchased from PeproTech (Rocky Hill, NJ).
AMP-p treatment of radiation-induced OM in hamsters
LVG Syrian Golden hamsters (weight, approximately 92 g; all male) were anesthetized (ketamine and xylazine), and the left buccal pouch was everted, fixed, and isolated by use of a lead shield (7). OM was induced by radiation directed at the exposed cheek pouch. AMP-p or saline solution (vehicle) was administered by subcutaneous injection once daily. Similar daily subcutaneous dosing of peptide drugs (e.g., insulin) has been well tolerated in patients including the head-and-neck cancer population.
Mucositis was scored from 0 for normal to 4 for marked ulceration (7). Digital images of the mucosa were randomly numbered and then graded in blinded fashion by at least two trained evaluators. A score of 3 or more marks the development of mucosal ulceration, a clinically relevant and important outcome (9). This protocol was approved by the Animal Care and Use Committee and performed at Biomodels (Watertown, MA).
In the acute model (n = 16) a single dose of radiation (40 Gy) was given at a rate of 3.2 Gy/min. Subcutaneous dosing of AMP-p (40 mg/kg) began 5 days before radiation (Day −5) and continued until Day 15, excluding the day of radiation (Day 0). Vehicle (saline solution) was administered to control animals by use of the same schedule. Mucositis was evaluated starting on Day 6 and subsequently on alternate days.
For the fractionated radiation model, animals (n = 20) were each given eight doses of 7.5Gy on Days 0 to 3 and Days 7 to 10 at a rate of 3.3 Gy/min. AMP-p (40 mg/kg) or vehicle was administered subcutaneously on Day −5 to Day −1, Days 4 to 6, and Days 11 to 15.
For animals (n = 20) treated with fractionated radiation and cisplatin, eight radiation doses of 6.5 Gy were given on Days 0 to 3 and Days 6 to 9 and two cisplatin doses of 5 mg/kg were given on Days 0 and 6 as an intraperitoneal injection 2 h before radiation. AMP-p (40 mg/kg) was administered subcutaneously on Days −5 to −1, Day 4, and Day 5, and vehicle was administered to control animals by use of the same schedule.
Effect of AMP-p on radiation treatment of lung tumor cells in vivo
National Institutes of Health H460 human lung cancer cells (105) were implanted in the lower left flank of nude rats (n = 24). Focal radiation treatment (4 Gy on Days 0, 4, and 9) began when tumors reached a mean volume of approximately 100 mm3 (Day −5), or tumors were left untreated. One radiation-treated group received AMP-p (40 mg/kg) daily from Day −5 to Day 15, whereas the other group and the untreated group received vehicle (phosphate-buffered saline solution) on the same schedule. Tumor volume was measured on alternating days throughout the duration of the study. We evaluated the differences in tumor volume observed among the different treatment groups by calculating the individual area under the curve for tumor growth and comparing these values using a Mann-Whitney rank sum test.
Cell culture
Adult human dermal microvascular endothelial cells (HDMECs) (Applied Cell Biology Research Institute, Kirkland, WA) were grown in endothelial basal medium (EBM)-2 supplemented with 5% fetal bovine serum (FBS), microvascular endothelial cell growth medium-2 (EGM-2 MV) BulletKit (Lonza, Basel, Switzerland), and 100 U/mL of penicillin/streptomycin (Invitrogen, Paisley, Scotland). Human adult low-calcium, high-temperature keratinocyte (HaCaT) cells (10) (Cell Lines Service, Eppelheim, Germany) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 5% calf serum (Sigma-Aldrich, St Louis, MO).
Flow cytometry analysis of cell apoptosis
HDMECs (in DMEM and 5% FBS) and HaCaT cells (in DMEM and 0.5% FBS) were untreated (control), treated with TNF-α (50 ng/mL) only, pretreated with AMP-p (8 μg/mL) for 2 h before TNF-α was added, or treated with AMP-p (8 μg/mL) added 2 h after TNF-α. This dose of AMP-p was chosen as the lowest concentration that showed the most significant effect based on a doseeresponse curve (0 –10 μg/mL) in the presence of 50 ng/mL of TNF-α. At the end of the study period (8 h), cell apoptosis was determined by flow cytometry analysis (FACScanto; BD Biosciences, San Jose, CA) of annexin V– and propidium iodide (PI)– stained cells (Vybrant apoptosis assay kit; Invitrogen), which characterized early and late apoptosis, respectively.
Ligase-mediated polymerase chain reaction
Genomic DNA (0.5 μg) from HDMECs or HaCaT cells was annealed to primer targets via T4 DNA ligase. The ligated DNA template was amplified by polymerase chain reaction (PCR) for 26 cycles at 94°C (1 minute)/72°C (3 minutes). The reaction products were resolved on a 1.3% agarose gel, imaged after ethidium bromide staining, and quantified by densitometry by use of ImageJ (National Institutes of Health, Bethesda, MD). Standard PCR of the gene En-2 was performed to normalize the amount of DNA template used in each reaction.
Statistics
Data were analyzed with Minitab software (Minitab, State College, PA). Groups were compared by two-tailed t test or, for more than two groups, by analysis of variance. Pathologic scores were compared by Mann-Whitney rank sum analysis. We considered p ≤ .05 significant.
Results
AMP-p reduces severity of OM
The effect of AMP-p on radiation-induced mucositis was investigated in hamster models receiving a single dose of radiation, fractionated radiation, or fractionated radiation together with cisplatin (Fig. 1).
Fig. 1.
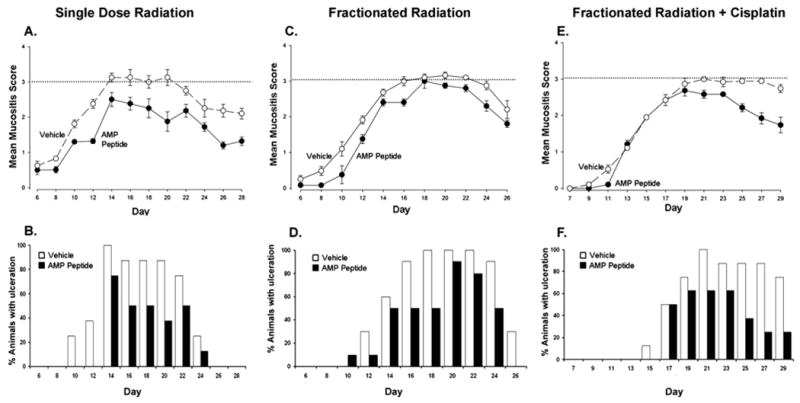
Administration of antrum mucosal protein peptide (AMP-p) reduces oral mucositis (OM) in three hamster models. OM was induced by radiation directed at the left buccal pouch of LVG Syrian Golden hamsters. (A and B) A single dose of radiation was given on Day 0. (C and D) Fractionated radiation was given on Days 0 to 3 and 7 to 10. (E and F) Fractionated radiation was given on Days 0 to 3 and Days 6 to 9, together with cisplatin on Days 0 and 6. AMP-p (40 mg/kg) or vehicle (saline solution) was administered subcutaneously on Days −5 to 15, excluding Day 0 (A and B); Days −5 to −1, Days 4 to 6, and Days 11 to 15 (C and D); or Days −5 to −1, Day 4, and Day 5 (E and F). Mucositis scores are presented in A, C, and E; the extent of mucosal ulceration is compared in B, D, and F A score of 3, shown as dotted lines on the graphs, indicates the development of mucosal ulceration that correlates with significant human OM. Each value is the mean ± standard error for 8 to 10 animals.
AMP-p was effective in attenuating the course and severity of OM in all three models. After acute radiation (Fig. 1A), the mean peak mucositis score was 3.13 on Days 14 to 20 in saline solution–treated control animals. After this peak, the mean score declined gradually to 2.06 by Day 28. In contrast, hamsters receiving AMP-p (n = 8) had a peak mean mucositis score of 2.44, on Day 14, which gradually resolved to a mean score of 1.31 on Day 28. We evaluated the clinical significance of differences between groups by calculating the number of days with a score of 3 or higher (mucosal ulceration). Whereas hamsters in the saline solution–treated control group had ulcerative mucositis (score ≥3) on 45.8% of the 192 animal days evaluated, ulcerative OM was only seen on 22.9% of days in AMP-p–treated animals, a 50% reduction (p < 0.001). Hamsters treated with AMP-p exhibited less erythema or ulceration than controls on Days 12 (p < 0.001), 16 (p < 0.05), 18 (p = 0.05), 20 (p = 0.002), 24 (p = 0.05), 26 (p < 0.001), and 28 (p = 0.003). In this analysis, 2 days with a significant reduction in the mucositis score, or a 25% to 30% reduction in the number of days with a mucositis score of 3 or more, is required for a meaningful beneficial effect (7). When the percent of animals with ulceration in each treatment group is compared by observation day (Fig. 1B), the effect of AMP-p in delaying the onset, as well as reducing OM severity, becomes apparent.
Whereas ulcerative OM was noted on 42.7% of study days among saline solution–treated controls subjected to fractionated radiation (Figs. 1C and 1D), ulcerative OM occurred only on 28.7% of the animal days among those treated with AMP-p (p < 0.001). Comparison of the scores for each group showed that the group treated with AMP-p had significantly lower scores than the saline solution–treated controls on Days 8 (p = 0.03), 10 (p < 0.001), 12 (p = 0.03), 16 (p < 0.03), and 24 (p < 0.03) (Fig. 1C). Ulceration (mucositis score ≥3) was substantially reduced after treatment with AMP-p on Days 12 to 18 compared with vehicle alone (Fig. 1D).
To model the human clinical situation, hamsters were subjected to both fractionated radiation and cisplatin (Figs. 1E and 1F). Consistent with the other models, AMP-p reduced ulceration days from 42.1% in controls to 22.0% (p < 0.001). AMP-p effectively attenuated mucositis in the three models tested, including the two in which higher cumulative doses of radiation were administered in a fractionated regimen. Fractionated dosing, especially with concomitant chemotherapy, is more damaging to mucosa than is acute exposure and results in more extensive and prolonged mucositis. This observation is consistent with the course of mucositis in patients.
No evidence of AMP-p–associated toxicity was noted in any of the three OM models studied (data not shown).
AMP-p does not impair therapeutic efficacy of radiation in lung tumor xenograft model
To determine whether AMP-p induced growth of tumor cells or inhibited the efficacy of radiation therapy in vivo, the peptide was studied in a human lung cancer xenograft model in which the tumors were irradiated (Fig. 2).
Fig. 2.
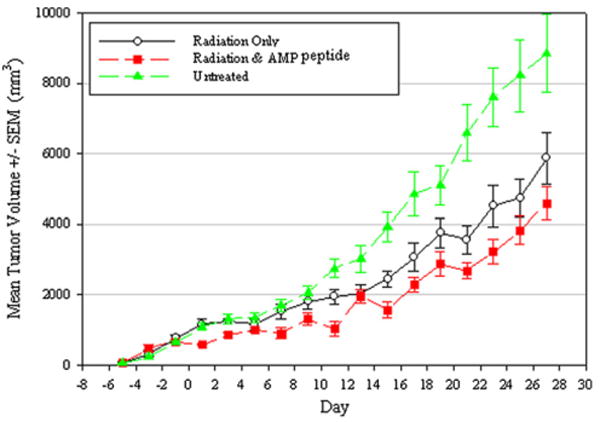
Antrum mucosal protein peptide (AMP-p) does not impair the therapeutic efficacy of radiation in a lung tumor xenograft model. Human lung cancer cells were implanted in the left flank of nude rats. Tumor volume was measured on alternating days in rats that received focal radiation treatment (4 Gy on Days 0, 4, and 9) with or without AMP-p (Day −5 to Day 15) or were left untreated as described in the “Methods and Materials” section. The volume of irradiated tumors was significantly less than untreated tumors in the presence or absence of AMP-p. Each value is the mean ± standard error for 8 animals.
Significant differences were observed between the group receiving radiation only (n = 8) and the untreated control group (n = 8) (p = 0.043), as well as nude rats given radiation plus AMP-p and the untreated controls (n = 8) (p < 0.001). Furthermore, administration of AMP-p did not diminish but slightly enhanced the capacity of radiation to reduce tumor size (p = 0.035).
AMP-p inhibits TNF-α—induced HDMEC apoptosis
Radiation therapy causes tissue damage through both direct (e.g., DNA lesions) and indirect (e.g., generation of proinflammatory cytokines) mechanisms. We focused on the indirect injurious effects and tested our hypothesis that the protective effects of AMP-p were mediated by an antiapoptotic effect on endothelial and/or epithelial cells by evaluating its impact on TNF-α. Because a beneficial effect of AMP-p was noted before ulceration, we evaluated the antiapoptotic effect of AMP-p on endothelial cells (1, 11, 12).
Minimal HDMEC apoptosis was seen under control conditions, whereas TNF-α (50 ng/mL) markedly increased the number of annexin V-positive/PI-negative and annexin V-positive/PI-positive cells. Preincubation with AMP-p 2 h before TNF-α significantly decreased apoptosis, reducing annexin V-positive/PI-negative cells by approximately 26% (p < 0.001) and annexin V-positive/PI-positive cells by 51% (p < 0.001) (Figs. 3A and 3B), suggesting AMP-p–mediated protection of HDMECs from TNF-α–induced cellular injury. TNF-α–induced HDMEC apoptosis was also inhibited by AMP-p added 2 h after exposure to TNF-a, reducing annexin V-positive/PI-negative cells by approximately 39% (p < 0.001) and annexin V-positive/PI-positive cells by 50% (p < 0.01) (Fig. 3B). Exposure of HDMECs both before and after AMP-p reduced TNF-α–induced apoptosis to the basal level. Untreated control cells (3.6% ± 0.2%) (Fig. 3B) and 3.5% ± 0.3% of cells exposed to the scrambled peptide were annexin V positive/PI negative. Similarly, 1.6% ± 0.1% of untreated control cells (Fig. 3B) and 1.7% ± 0.2% of cells exposed to the scrambled peptide were annexin V positive/PI positive. Furthermore, treatment of HDMECs with scrambled peptide failed to significantly affect TNF-α–induced apoptosis (data not shown). Exposure of HDMECs to interferon-γ (10 ng/mL) under the same conditions induced a 2-fold increase in apoptosis when analyzed by annexin V and PI staining, which was completely reversed to the basal level by AMP-p (data not shown).
Fig. 3.
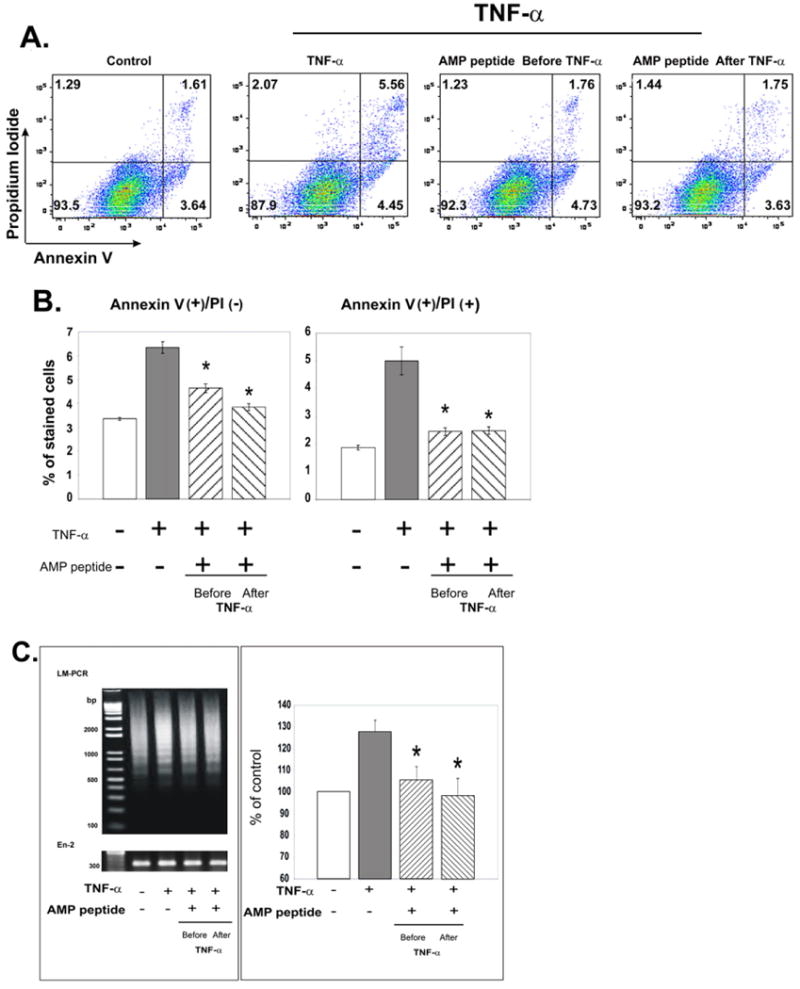
Antiapoptotic effects of antrum mucosal protein peptide (AMP-p) in human dermal microvascular endothelial cells (HDMECs) exposed to tumor necrosis factor (TNF) α. HDMECs in Dulbecco's modified Eagle's medium (DMEM) plus 5% fetal bovine serum (FBS) were exposed to TNF-α (50 ng/mL) in the presence or absence of AMP-p (8 μg/mL, 2 h before or after TNF-α). Control cells were untreated. Apoptosis was assessed by flow cytometry of annexin V– and propidium iodide (PI)–stained cells. A, Data from a single representative experiment. B, AMP-p inhibited the TNF-α–induced increase in annexin V-positive/PI-negative cells (left) and annexin V-positive/PI-positive cells (right). Values are means ± standard errors for four independent experiments. Asterisk, p < 0.001. C, DNA laddering was assessed by ligase-mediated (LM) polymerase chain reaction (PCR) to evaluate apoptosis. Left, LM-PCR products were resolved on an agarose gel and imaged after ethidium bromide staining. DNA molecular weight markers (in base pairs) are shown in the left lane. Standard PCR of the gene En-2 was performed to normalize the amount of DNA template in each reaction. Right, LM-PCR results were quantified and normalized to the En-2 bands. Values are percent of control apoptosis (100%) presented as mean ± standard error for four experiments. Asterisk, p < 0.001.
The capacity of AMP-p to protect against TNF-α–induced HDMEC apoptosis was confirmed by use of ligase-mediated PCR to detect DNA fragmentation. Exposure of the cells to TNF-α for 2 h resulted in typical DNA laddering (Fig. 3C). The laddering was significantly reduced by a 2-h preincubation of the cells with AMP-p, as was the case when cells were treated with the peptide 2 h after TNF-α.
Effects of AMP-p on TNF-α—induced apoptosis in HaCaT cells
The effect of AMP-p was also assessed in TNF-α–treated HaCaT cells, which are keratinocytes used to model the oral mucosal squamous epithelium (10). HaCaT cells were exposed to TNF-a in the presence or absence of AMP-p or were left untreated. Minimal apoptosis was observed under control conditions, whereas TNF-a triggered a significant increase in apoptosis (p < 0.001) (Fig. 4). AMP-p prevented TNF-α–triggered apoptosis, either before or after cells were exposed to TNF-α. Pretreatment with AMP-p for 2 h reduced the number of TNF-α–triggered annexin V-positive/PI-negative cells by 48% (p < 0.001) and annexin V-positive/PI-positive cells by 73% (p < 0.001). AMP-p treatment after TNF-a reduced annexin V-positive/PI-negative cells by 43% (p < 0.001) and annexin V-positive/PI-positive cells by 67% (p < 0.01) (Fig. 4B). Ligase-mediated PCR confirmed the antiapoptotic effects of AMP-p (Fig. 4C).
Fig. 4.
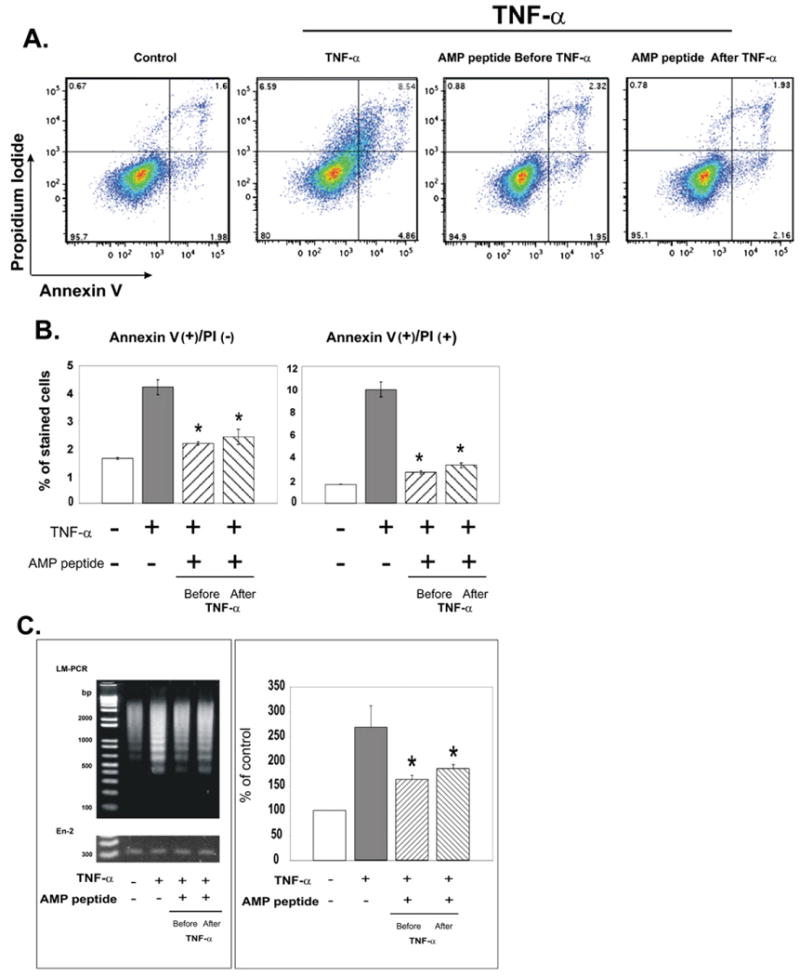
Antiapoptotic effects of antrum mucosal protein peptide (AMP-p) in human adult low-calcium, high-temperature keratinocyte (HaCaT) cells exposed to tumor necrosis factor (TNF) α. HaCaT cells in Dulbecco's modified Eagle's medium (DMEM) plus 0.5% fetal bovine serum (FBS) were exposed to TNF-α (50 ng/mL) in the presence or absence of AMP-p (8 μg/mL, 2 h before or after TNF-α). Control cells were untreated. Apoptosis was assessed by flow cytometry of annexin V– and propidium iodide (PI)–stained cells. A, Data from a single representative experiment. B, AMP-p inhibited the TNF-α–induced increase in annexin V-positive/PI-negative cells (left) and annexin V-positive/PI-positive cells (right). Values are means ± standard errors for four independent experiments. Asterisk, p < 0.001. C, DNA laddering was assessed by ligase-mediated (LM) polymerase chain reaction (PCR) to evaluate apoptosis as described in Fig. 3C. Left, LM-PCR products were resolved on agarose gel. Right, LM-PCR results were quantified and normalized to the En-2 bands. Values are percent of control apoptosis (100%) shown as mean ± standard error for four experiments. Asterisk, p < 0.001.
Discussion
In three hamster models of OM, administration of AMP-p slowed development and reduced the extent of erythema, prevented ulcer formation, and accelerated recovery (Fig. 1). These effects were significant even before the development of mucosal ulceration on Day 14 or thereafter. No evidence of AMP-p—associated toxicity was noted in any of the three OM models studied (data not shown), and AMP-p did not induce growth of tumor cells or inhibit the efficacy of radiation therapy in a human lung cancer xenograft model (Fig. 2).
To illuminate mechanism(s) by which AMP-p reduced erythema during development of OM (Fig. 1), we were guided by previous observations that endothelial cell damage could be a triggering event for radiation-induced mucosal injury (1, 12). Similarly, endothelium appears to be the initial target of radiation injury in other tissues such as gut, kidney, and brain (11, 13, 14). In this study the trajectory of OM severity in hamsters treated with radiation (Fig. 1) suggested that AMP-p had an early, protective effect that led us to assess the effect of AMP-p on endothelial cell apoptosis in culture (Fig. 3). We subsequently looked for and found that AMP-p is also antiapoptotic for epithelial cells (Fig. 4), because injury of each cell type has been suggested as a determinant of the radiation gastrointestinal syndrome (11, 15, 16). The antiapoptotic effect of AMP-p could also reduce cytokine production, thereby limiting injury in subsequent stages of OM (12). Furthermore, our earlier studies show that AMP-p can enhance TJ formation and reduce epithelial cell monolayer permeability (5). Importantly, TJ stabilization may contribute to the antiapoptotic effect of AMP-p (17, 18).
Conclusion
AMP-p reduced the extent of mucosal erythema and ulceration and enhanced recovery in three hamster models of OM. The peptide was found to be antiapoptotic for endothelial and epithelial cells. Together with its ability to increase accumulation of TJ proteins, these observations suggest that AMP-p may have therapeutic potential for OM.
Acknowledgments
The authors thank Margaret M. Walsh-Reitz and Patrick Cunningham for valuable discussions.
Supported by National Institutes of Health grant R21 DE018811 to F.G. Toback.
Footnotes
Conflict of interest: The animal experiments of this study were funded by NephRx Corporation, of which F.G. Toback is a founder. S.T. Sonis is a partner in Biomodels, at which the animal studies were performed.
References
- 1.Sonis ST, Fey EG. Oral complications of cancer therapy. Oncology (Williston Park) 2002;16:680–686. discussion 686, 691–682, 695. [PubMed] [Google Scholar]
- 2.Peterson DE. Research advances in oral mucositis. Curr Opin Oncol. 1999;11:261–266. doi: 10.1097/00001622-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Toback FG, Walsh-Reitz MM, Musch MW, et al. Peptide fragments of AMP-18, a novel secreted gastric antrum mucosal protein, are mitogenic and motogenic. Am J Physiol Gastrointest Liver Physiol. 2003;285:G344–G353. doi: 10.1152/ajpgi.00455.2002. [DOI] [PubMed] [Google Scholar]
- 4.Martin TE, Powell CT, Wang Z, et al. A novel mitogenic protein that is highly expressed in cells of the gastric antrum mucosa. Am J Physiol Gastrointest Liver Physiol. 2003;285:G332–G343. doi: 10.1152/ajpgi.00453.2002. [DOI] [PubMed] [Google Scholar]
- 5.Walsh-Reitz MM, Huang EF, Musch MW, et al. AMP-18 protects barrier function of colonic epithelial cells: Role of tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2005;289:G163–G171. doi: 10.1152/ajpgi.00013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madara JL, Stafford J, Barenberg D, et al. Functional coupling of tight junctions and microfilaments in T84 monolayers. Am J Physiol. 1988;254:G416–G423. doi: 10.1152/ajpgi.1988.254.3.G416. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez E, Fey EG, Valax P, et al. Preclinical characterization of CG53135 (FGF-20) in radiation and concomitant chemotherapy/radiation-induced oral mucositis. Clin Cancer Res. 2003;9:3454–3461. [PubMed] [Google Scholar]
- 8.Sonis ST, Peterson RL, Edwards LJ, et al. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000;36:373–381. doi: 10.1016/s1368-8375(00)00012-9. [DOI] [PubMed] [Google Scholar]
- 9.Murphy CK, Fey EG, Watkins BA, et al. Efficacy of superoxide dismutase mimetic M40403 in attenuating radiation-induced oral mucositis in hamsters. Clin Cancer Res. 2008;14:4292–4297. doi: 10.1158/1078-0432.CCR-07-4669. [DOI] [PubMed] [Google Scholar]
- 10.Boukamp P, Petrussevska RT, Breitkreutz D, et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 12.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 13.Jaenke RS, Robbins ME, Bywaters T, et al. Capillary endothelium. Target site of renal radiation injury. Lab Invest. 1993;68:396–405. [PubMed] [Google Scholar]
- 14.Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: Protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000;60:321–327. [PubMed] [Google Scholar]
- 15.Brown M. What causes the radiation gastrointestinal syndrome?: Overview. Int J Radiat Oncol Biol Phys. 2008;70:799–800. doi: 10.1016/j.ijrobp.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuller BW, Rogers AB, Cormier KS, et al. No significant endothelial apoptosis in the radiation-induced gastrointestinal syndrome. Int J Radiat Oncol Biol Phys. 2007;68:205–210. doi: 10.1016/j.ijrobp.2006.12.069. [DOI] [PubMed] [Google Scholar]
- 17.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010;20:142–149. doi: 10.1016/j.tcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via down regulation of occludin. J Cell Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]


