Abstract
Intestinal phagocytes transport oral antigens and promote immune tolerance, but their role in innate immune responses remains unclear. Here we report that intestinal phagocytes are anergic to Toll-like receptor ligands or commensals, but constitutively express pro-interleukin-1β (proIL-1β). Upon infection with pathogenic Salmonella or Pseudomonas, intestinal phagocytes produce mature IL-1β through the NLRC4 inflammasome, but not tumor necrosis factor or IL-6. Mice deficient in NLRC4 or IL-1 receptor on a Balb/c background were highly susceptible to orogastric but not intraperitoneal infection with Salmonella. Increased lethality was preceded by impaired expression of endothelial adhesion molecules, lower neutrophil recruitment, and poor intestinal pathogen clearance. Thus, NLRC4-dependent IL-1β production by intestinal phagocytes represents a specific response discriminating pathogenic from commensal bacteria and contributes to host defense in the intestine.
Keywords: NLRC4, inflammasome, intestinal phagocytes, host defense
Mammalian hosts deploy an arsenal of defense mechanisms to counter pathogenic microbes. Upon microbial invasion, sensing of pathogenic organisms is mediated by several classes of germline-encoded pattern recognition receptors (PRRs). These include membrane-bound Toll-like receptors (TLRs) and C-type lectin receptors as well as cytosolic Nod-like receptors (NLRs) and RIG-like helicases 1. PRRs recognize unique molecular motifs, often referred to as pathogen-associated molecular patterns (PAMPs) that are uniquely expressed by large classes of microbes. Although PAMPs are involved in the recognition of pathogens, they are also expressed by non-pathogenic microbes, and therefore the presence of PAMPs alone cannot explain the ability of the host immune system to discriminate between pathogenic and non-pathogenic microorganisms 2.
Upon activation, PRRs induce host defense signaling pathways that culminate in the production of pro-inflammatory and anti-microbial molecules 1. Caspase-1 activation by inflammasomes is a major signaling pathway mediated by certain NLRs in response to microbes 3, 4. To date, three NLR-containing inflammasomes, NLRP1, NLRP3 and NLRC4 have been identified 5. Inflammasomes are molecular platforms that drive the proteolytic activation of caspase-1, resulting in the release of mature, biologically active IL-1β and IL-18 5. Several stimuli including ATP, bacterial toxins and particulate matter activate the NLRP3 inflammasome 6, 7. In contrast, multiple Gram-negative bacteria, including Legionella pneumophila 8, Pseudomonas aeruginosa 9, 10, and the enteric pathogens, Salmonella enterica serovar Typhimurium (Salmonella) 11, 12 and Shigella flexneri 13 induce caspase-1 activation via the NLRC4 inflammasome 5. The activation of the NLRC4 inflammasome requires the presence of an intact type III (T3SS) or IV secretion system (T4SS) that mediate the translocation of bacterial virulence factors as well as small amounts of flagellin to induce NLRC4 activation 12, 14. In addition, the release of the T3SS PrgJ-like rod proteins into the cell cytosol can activate NLRC4 15. Both flagellin and rod proteins interact with specific Naip family members, which in turn, drive the assembly and activation of the NLRC4 inflammasome16, 17.
A central question in immunology is how the immune system discriminates between commensal and pathogenic bacteria. This problem is particularly important in the intestine where trillions of commensal microorganisms continually challenge the immune system without eliciting a proinflammatory response 18. Yet, in the presence of pathogenic bacteria, the immune system can elicit robust inflammation and protective host defense responses in the intestine 2, 19. Mononuclear phagocytic cells that normally reside in the intestinal lamina propria, such as macrophages and dendritic cells (DCs), are important in preventing harmful responses to commensal bacteria. In the intestine, resident macrophages and DCs are hyporesponsive to microbial stimulation which may be important for preventing inappropriate activation of inflammatory responses to the normal microflora 20–22. Yet, resident phagocytes maintain intestinal homeostasis through the induction of anti-inflammatory cytokines and the engulfment and degradation of commensal bacteria in intestinal macrophages 20–22. Given that resident macrophages and DCs are anergic to TLR ligands and commensal bacteria 22, 23, it remains unknown whether these phagocytic cells can actively promote host defense against pathogenic bacteria in the intestine. We find that mononuclear phagocytes that reside in the lamina propria do not produce tumor necrosis factor (TNF) or IL-6 when stimulated with TLR ligands, commensal or pathogenic bacteria, consistent with previous studies. Surprisingly, however, infection of resident phagocytes with Salmonella or P. aeruginosa, but not commensal bacteria, elicited robust amounts of mature IL-1β via the NLRC4 inflammasome, which was critical in the clearance of pathogenic bacteria in the intestine.
Results
iMP are unresponsive to TLR ligands but express proIL-1β
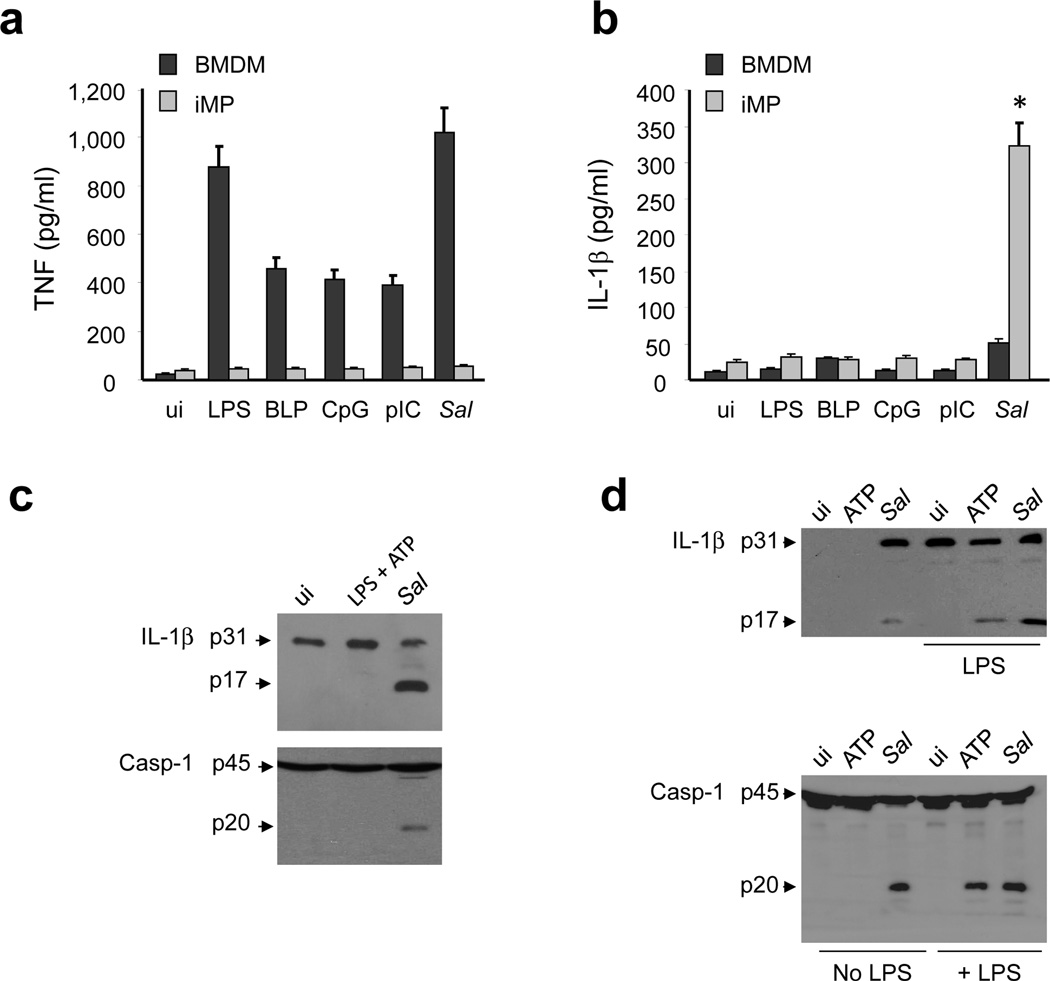
To assess the role of intestinal mononuclear phagocytes (iMP) in intestinal homeostasis and host defense, we purified colonic iMP and bone marrow-derived macrophages (BMDM) from specific pathogen free (SPF) mice and germ-free mice (GF) devoid of commensal bacteria and compared their responses to TLR stimulation. BMDM, but not iMP, isolated from SPF mice produced TNF and IL-6 in response to TLR ligands (Fig. 1a and 1b). In addition, iMP from GF mice were also anergic to TLR stimulation, suggesting that exposure to commensal bacteria is not required for hyporesponsiveness to TLR ligands (Fig. 1a and 1b). We next assessed steady-state levels of IL-1β mRNA in peripheral blood monocytes, BMDM and iMP from SPF mice. Consistent with previous results, unstimulated monocytes and BMDM expressed undetectable or very low levels of proIL-1β mRNA as measured by quantitative RT-PCR (Fig. 1c). In contrast, unstimulated iMP and BMDM stimulated with LPS expressed higher levels of proIL-1β mRNA compared to untreated monocytes and BMDM (Fig. 1c). Consistently, pro-IL-1β protein was detected in iMP (Fig. 1d). Pro-IL-1β was also detected in iMP isolated from GF mice, but at reduced levels compared to that produced by iMP from SPF mice (Fig. 1d). Collectively, these results indicate that iMP are anergic to TLR simulation, but constitutively express pro-IL-1β.
Figure 1. iMP express high amounts of pro-IL-1β, but are hyporesponsive to TLR stimulation.
(a and b) BMDM, iMP isolated from specific pathogen free (SPF) or germ-free (GF) mice were stimulated (us) for 16 hrs with the indicated TLR agonists (LPS 10μg/ml; BLP 10μg/ml; CpG 10μg/ml; pIC10μg/ml). Cell-free supernatants were analyzed by ELISA for production of IL-β. Values represent mean ± s.d. of triplicate cultures. (c) Relative IL-1β levels in monocytes (Mo), BMDM, iMP and BMDM stimulated with LPS for 6 hrs as measured by quantitative RT-PCR. Relative expression was normalized to the expression of β-actin. Values represent mean ± s.d. of triplicate cultures. (d) IL-1β and Erk1-2 protein were measured by immunoblotting using protein extracts from iMP isolated from SPF or GF mice. (a-d) Results are representative of at least three separate experiments. * statistically significant; NS, not significant
Salmonella induces release of IL-1β by iMP
We next determined whether infection with Salmonella, a pathogenic enteric bacterium, can induce the production of mature IL-1β. BMDM and iMP from SPF mice were infected with Salmonella, and TNF and IL-1β production were measured. Both infection with Salmonella or stimulation with several TLR ligands induced the production of TNF in BMDM, but not in iMP (Fig. 2a). In contrast, Salmonella induced robust release of IL-1β in iMP, but not in unprimed BMDM (Fig. 2b). Furthermore, TLR stimulation did not induce significant IL-1β production in either BMDM or iMP (Fig. 2b). These results suggest that iMP are anergic to TLR stimulation, but can respond to a pathogenic bacterium by producing mature IL-1β rather than TNF. These results were confirmed by assessing the maturation of pro-IL-1β and caspase-1 activation. Upon infection with Salmonella, processing of pro-IL-1β (p31) into the mature p17 form and of pro-caspase-1 into the p20 subunit of active caspase-1 was evident in iMP (Fig. 2c). Consistent with the lack of responsiveness of iMP to TLR ligands, stimulation with LPS and ATP, two signals required for activation of the NLRP3 inflammasome 24, 25, did not induce maturation of pro-IL-1β and pro-caspase-1 processing in iMP (Fig. 2c). However, caspase-1 activation and pro-IL-1β maturation were induced by ATP in LPS-primed cells or after Salmonella infection in BMDM (Fig. 2d). These results indicate that mouse iMP respond to pathogenic bacteria through the production of IL-1β.
Figure 2. Salmonella infection induces caspase-1 activation and IL-1β production in iMP.
(a and b) BMDM and iMP were uninfected (ui) or stimulated with the indicated TLR agonists (LPS 10μg/ml; BLP 10μg/ml; CpG 10μg/ml; pIC 10μg/ml) or infected with Salmonella (Sal). After 16 hrs cell-free supernatants were analyzed for the production of TNF and IL-1β by ELISA. Values represent mean ± s.d. of triplicate cultures. (c and d) iMP (c) and BMDM (d) were uninfected (ui) or stimulated with LPS (1μg/ml) for 6 hrs , ATP (5 mM) for 30 minutes or infected with Sal. Cell extracts were immunoblotted with IL-1β antibody (arrows denote pro-IL-1β and the mature cytokine p17 subunit) or caspase-1 antibody (arrows denote procaspase-1 and its processed p20 subunit). Results are representative of at least three independent experiments. (e-f) Human peripheral blood (PB) monocytes and intestinal lamina propria (LP) macrophages were stimulated with LPS (100 ng/ml), commensal E. coli, wild-type Salmonella (WT) or Salmonella sipB-, flic-fljB-mutants for 2 hrs. Infection of monocytes and macrophages was performed at a cell/bacteria ratio of 1/10. The production of TNF and IL-1β in cell-free supernatants was measured by ELISA. Values represent mean ± s.d. of triplicate cultures of a representative individual. Cell extracts were immunoblotted with mature IL-1β antibody (p17 subunit) or caspase-1 antibody (arrows denote procaspase-1 and its processed p20 subunit). Results are representative of at least three independent experiments (a-d) and two experiments (e-f).* statistically significant, NS not significant.
We next tested whether human macrophages purified from the colon respond to microbial stimuli. Consistent with previous results26, CD14+ macrophages isolated from healthy colonic tissue produced no or a low amount of TNF protein in response to LPS, Escherichia coli or Salmonella when compared to CD14+ human blood monocytes (Fig. 2e). In contrast, human CD14+ colonic macrophages produced robust amounts of IL-1β when infected with wild-type (WT) Salmonella (Fig. 2e). Previous studies showed that infection of mouse BMDM with Salmonella induces the activation of the NLRC4 inflammasome which requires the presence of a functional T3SS and flagellin 11, 12. Similarly, Salmonella mutants deficient in a functional T3SS (sipB-) or the two genes that encode for flagellin (flic-fljb-) were impaired in eliciting IL-1β production in human CD14+ colonic macrophages (Fig. 2e). Yet, WT and mutant Salmonella induced comparable amounts of TNF in CD14+ human blood monocytes (Fig. 2e). In accord with IL-1β production, infection of human CD14+ colonic macrophages with wild-type Salmonella, but not mutant Salmonella, LPS or E. coli, induced processing of pro-IL-1β into the p17 mature form and of procaspase-1 into its p20 active subunit (Fig. 2f). These results indicate that human CD14+ intestinal macrophages can respond to pathogenic Salmonella through the activation of caspase-1 and production of IL-1β.
Pathogenic bacteria induce NLRC4 activation in iMP
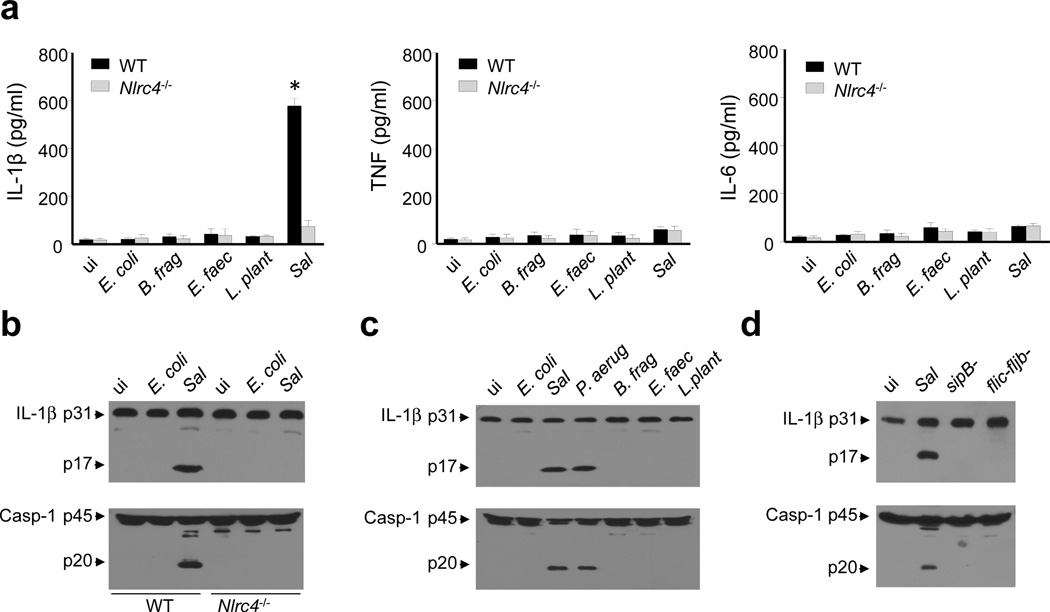
To gain insight into the molecular mechanism by which Salmonella induces the production of IL-1β in iMP, we infected iMP isolated from WT or Nlrc4−/− mice with the pathogenic bacterium or with a panel of intestinal commensal bacteria. We found that Salmonella, but none of the commensal bacteria tested, induced robust production of IL-1β in an NLRC4-dependent manner (Fig. 3a). In contrast, infection of iMP, with Salmonella or the intestinal commensals E. coli, Bacterioides fragilis, Enterococcus faecalis or Lactobacillus plantarum did not induce the production of either TNF or IL-6 (Fig. 3a). Addition of commensal bacteria or Salmonella to BMDM induced the secretion of TNF and IL-6 but little or no IL-1β (Supplementary Fig. 1). In iMP, maturation of IL-1β and caspase-1 activation induced by Salmonella were NLRC4-dependent (Fig. 3b). In addition, infection of iMP with P. aeruginosa, another pathogenic bacterium that activates NLRC4, induced maturation of pro-IL-1β and activation of caspase-1 (Fig. 3c). The maturation of IL-1β and activation of caspase-1 required bacterial T3SS and flagellin as Salmonella sipB- or flic-fljb- mutants were unable to elicit IL-1β maturation or caspase-1 activation in iMP (Fig. 3d). Furthermore, delivery of purified flagellin into the cytosol of iMP by transfection induced caspase-1 activation and pro-IL-1β processing in an NLRC4-dependent manner (Supplementary Fig. 2). Collectively, these results indicate that iMP can discriminate between pathogenic and commensal bacteria through the activation of the NLRC4 inflammasome and production of IL-1β.
Figure 3. Pathogenic, but not commensal bacteria, induce the activation of the NLRC4 - inflammasome in iMP.
(a) iMP isolated from WT or Nlrc4−/− mice were uninfected (ui) or were infected with the indicated commensal bacteria or pathogenic Sal. Cell-free supernatants were analyzed by ELISA for the production of TNF, IL-6 and IL-1β. Values represent mean ± s.d. of triplicate cultures. (b-d) iMP isolated from WT or Nlrc4−/− mice were uninfected (ui) or were infected with E. coli, P. aeruginosa (P.aerug), B. fragilis (B. frag), E. faecalis (E. faec), L. plantarum (L. plant), Sal or Salmonella sipB- , flic-fljB- mutants as indicated in the figure. Cell extracts were immunoblotted with IL-1β antibody (arrows denote pro-IL-1β and the mature cytokine p17 subunit) or caspase-1 antibody (arrows denote procaspase-1 and its processed p20 subunit). Results are representative of at least three independent experiments (a-f). * statistically significant
iMP express functional NLRC4 but not NLRP3 inflammasomes
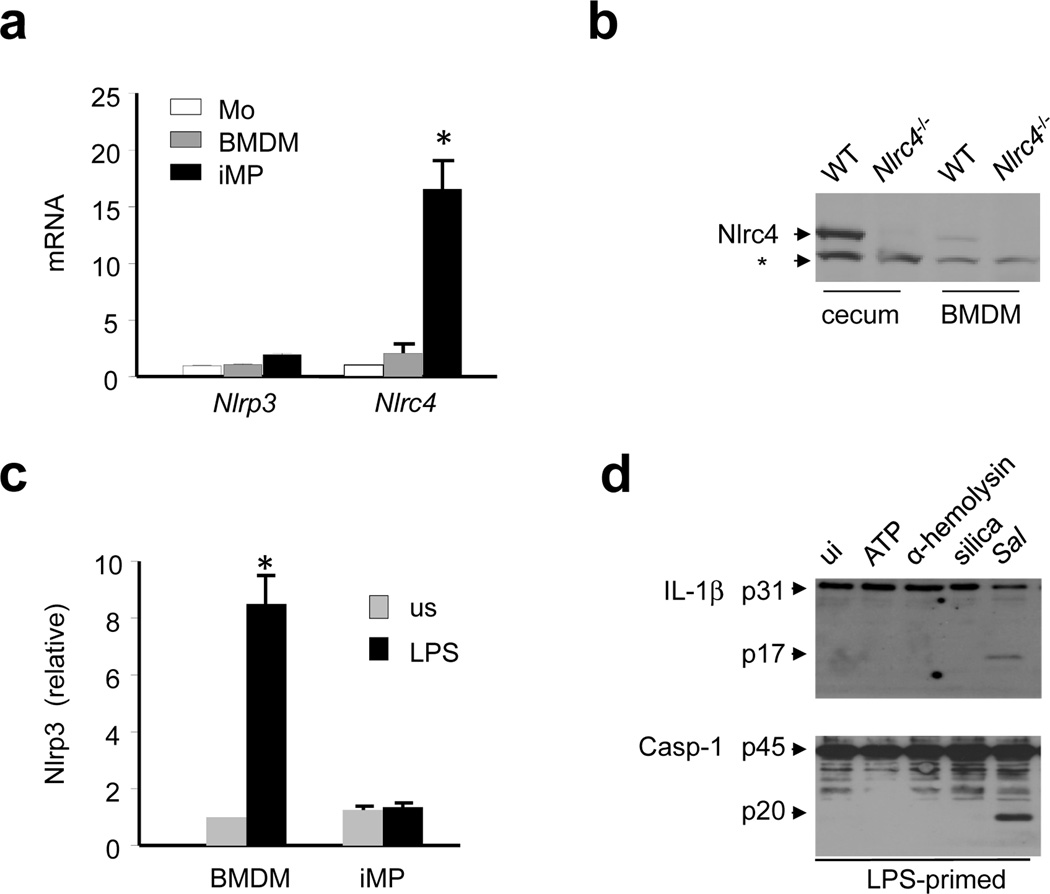
To understand the differential activity of NLRP3 and NLRC4 in iMP, we analyzed the expression of NLRC4 and NLRP3 in monocytes, BMDM, and iMP by qRT-PCR. Under resting conditions, NLRC4 expression was higher in iMP than in either monocytes or BMDM whereas NLRP3 expression was poorly expressed in all three cell populations (Fig. 4a). Endogenous NLRC4 protein was also detected at higher amounts in iMP than in BMDM from WT mice (Fig. 4b). The induction of NLRP3 expression in BMDM by TLR stimulation is a critical step in the activation of NLRP3 24. However, in iMP, NLRP3 expression was not upregulated by LPS unlike in BMDM (Fig. 4c), which is consistent with the anergic response of iMP to TLR ligands and commensals. Consistently, processing of pro-IL-1β and caspase-1 activation in iMP occurred after Salmonella infection, but not after stimulation of LPS-primed iMP with several activators of the NLRP3 inflammasome including ATP, Staphylococcus aureus α-hemolysin, or silica particles (Fig. 4d). These results indicate that the NLRC4, but not the NLRP3, inflammasome is expressed and functional in iMP.
Figure 4. Intestinal phagocytes express a functional NLRC4, but not NLRP3, inflammasome.
(a) Relative mRNA expression of NLRP3 and NLRC4, normalized to β-actin, was measured in monocytes (Mo), BMDM or iMP by quantitative RT-PCR. Values represent mean ± s.d. of duplicate cultures. (b) NLRC4 protein levels were measured by immunoblotting of cecal or BMDM extracts from WT or Nlrc4−/− mice. * denotes non-specific protein band (c) Relative NLRP3 mRNA expression in unstimulated (us) cells and cells stimulated with 1 µg/ml of LPS for 6 hrs. Relative gene expression was normalized to the expression of β-actin. Values represent mean ± s.d.. (d) iMP were primed with LPS (1μg/ml) for 4 hrs and stimulated with ATP (5 mM) for 30 minutes, α-hemolysin (1μg/ml) for 3 hrs, silica (500 μg/ml) for 6 hrs or live Salmonella (Sal) for 30 minutes. IL-1β production and caspase-1 activation were measured by immunoblotting. Arrows denote pro-IL-1β (p31) and the mature p17 subunit or procaspase-1 (p45) and its processed p20 subunit. Results are representative of at least three separate experiments (a-d). * statistically significant
Nlrc4−/− or Il1r−/− increases intestinal Salmonella susceptibility
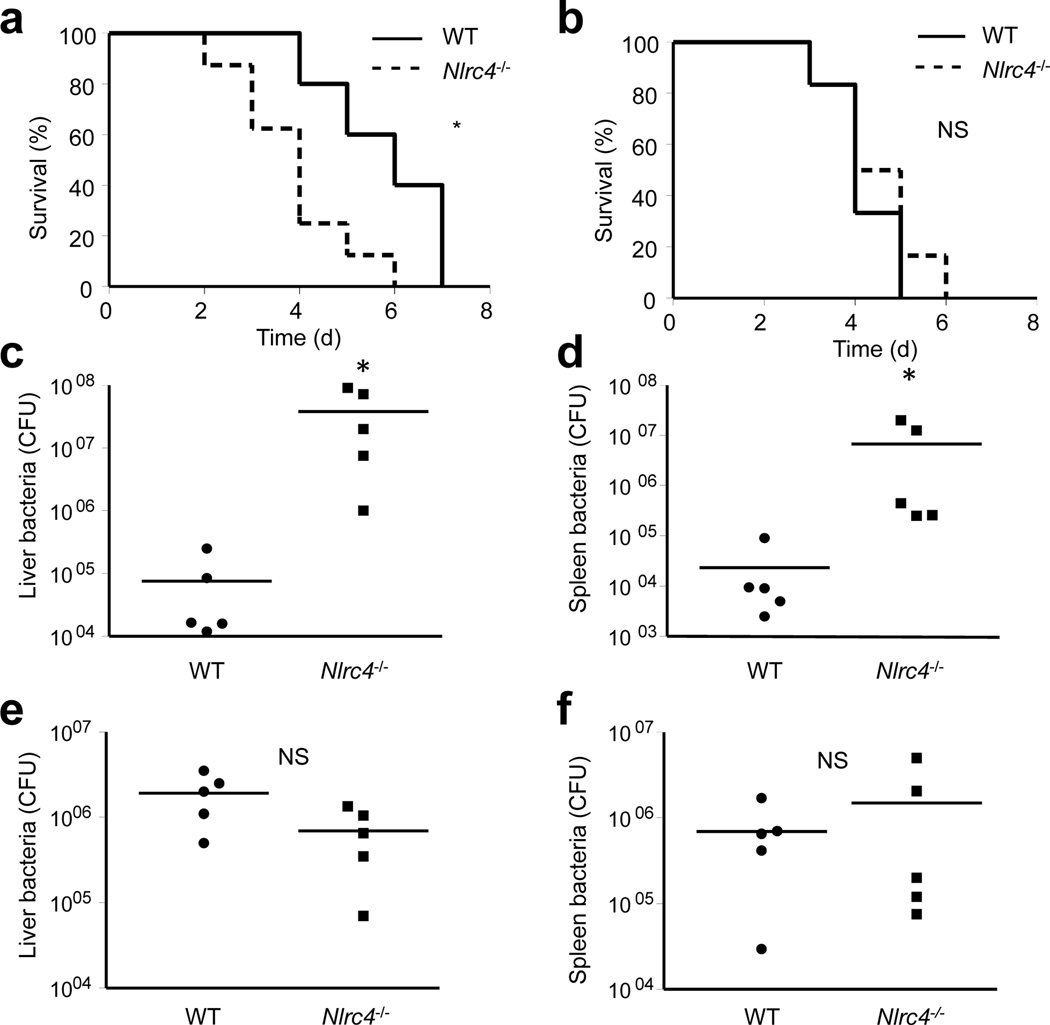
Infection of iMP with Salmonella drives robust production of IL-1β, but not of other inflammatory cytokines such as TNF or IL-6 (see above). To address the role of the NLRC4-IL-1β pathway in host defense, we performed in vivo experiments using Nlrc4−/− and IL-1 receptor-deficient (Il1r−/−) mice. Analysis of iMP revealed that WT, Nlrc4−/− and Il1r−/− have comparable percentages of CD11b+F4/80+CD11cloCD103− (macrophages), CD11b+F480+CD11chi (macrophage and DCs) and CD11b−F480−CD11chighCD103+ (DCs) in the colonic lamina propria (Supplementary Fig. 3). We next infected Nlrc4−/− and WT mice on either C57BL/6 or Balb/c backgrounds with Salmonella by oral gavage (o.g.) using the streptomycin colitis model 27 and assessed mouse survival. In agreement with published results 28, there was no difference in the susceptibility between WT and Nlrc4−/− mice on a C57BL/6 background to Salmonella (Supplementary Fig. 4). In contrast, Nlrc4−/− mice on a Balb/c background were more susceptible than WT mice in the streptomycin colitis model (Fig. 5a) and in the absence of pretreatment with streptomycin (Supplementary Fig. 5). We then infected mice by intraperitoneal (i.p.) injection of Salmonella and found no difference in survival between WT and Nlrc4−/− mice regardless of genetic background (Fig.5b and Supplementary Fig. 4). We next analyzed bacterial loads in the liver and spleen of WT and Nlrc4−/− Balb/c mice after infection with Salmonella via the o.g. or i.p. route. In agreement with the survival data, bacterial loads in the liver and spleen were ~150-fold higher in Nlrc4−/− than in WT mice after infection by o.g. (Fig. 5c, d) whereas no significant differences were observed when infection occurred by i.p. injection (Fig. 5e-f). These results indicate that NLRC4 is important in host defense against intestinal infection by Salmonella in Balb/c mice.
Figure 5. Nlrc4−/− mice are more susceptible to orogastric, but not intraperitoneal, Salmonella infection.
(a and b) WT and Nlrc4−/− mice in the Balb/c background were infected orogastrically (a) or intraperitoneally (b) with Salmonella, and survival was monitored over time. (a) P = 0.028 (b) P = 0.44 (c-f) WT and Nlrc4−/− Balb/c mice were infected orogastrically (c and d) or intraperitoneally (e and f) with Salmonella and total bacterial loads per indicated organ were determined on day 3 post infection. Each symbol represents an individual mouse. Results are representative of at least three independent experiments with at least five mice per genotype (a-f). * significant, NS not significant
To further address the role of IL-1β in host defense against Salmonella in the intestine, we infected WT or Il1r−/− mice with Salmonella either by o.g. or i.p. and assessed mouse survival and bacterial burden. As was observed in Nlrc4−/− mice, Il1r−/− on a Balb/c background, but not on a C57BL/6 background, were more susceptible to Salmonella infection via o.g., but not the i. p. route (Fig. 6a,b and Supplementary Fig. 6). Il1r−/− Balb/c mice also had higher Salmonella bacterial loads in the liver and spleen than WT mice after orogastric infection (Fig. 6c,d), but not after i.p. infection (Fig. 6e,f). Collectively, these results indicate that the NLRC4-IL-R1 axis is important for host defense against Salmonella in the intestine of Balb/c mice.
Figure 6. Il1r−/− mice are more susceptible to orogastric, but not intraperitoneal, Salmonella infection.
(a-d) WT and Il1r−/− mice in the Balb/c background were infected orally (a) or intraperitoneally (b) with Salmonella, and survival was monitored over time. (a) P = 0.012 (b) P = 0.54 (c-f) WT and Il1r−/− Balb/c mice were infected orogastrically (c and d) or intraperitoneally (e and f) with Salmonella and bacterial loads per indicated organ were determined on day 3 post infection. Each symbol represents an individual mouse. Results are representative of at least three separate experiments with at least five mice per genotype (a-f). * significant, NS not significant
NLRC4-mediated host defense via neutrophil recruitment
To understand the role of NLRC4 and IL-1R in host defense against Salmonella in the intestine we first examined macroscopic changes in the cecum after infection. The appearance of the ceca from uninfected WT, Nlrc4−/− and Il1r−/− was comparable. In infected WT mice, the ceca was shrunken and pale indicative of inflammation, which is consistent with previous reports 27 (data not shown). In contrast, the ceca of Nlrc4−/− and Il1r−/− mice infected with Salmonella appeared similar to that of uninfected mice with little macroscopic evidence of inflammation (data not shown). Consistently, microscopic examination of infected Nlrc4−/− and Il1r−/− mice revealed reduced pathological score (Fig. 7a), reflecting less submucosa edema and inflammatory cell infiltration within the lamina propria compared to WT mice (Fig. 7a and Supplementary Fig. 7a and b). To determine the cell types recruited to the intestine after infection, we purified lamina propria cells from the large intestine of Salmonella-infected WT, Nlrc4−/− and Il1r−/− mice and analyzed the expression of cellular surface markers by flow cytometry. There was a significant reduction in neutrophils (CD11b+Ly6G+) within the lamina propria of both Nlrc4−/− and Ilr1−/− mice, and only a modest reduction in CD11b+ in cells in Nlrc4−/−, but not in Il1r−/− mice when compared to WT mice (Fig. 7b and Supplementary Fig. 7c). Since IL-1β induces the upregulation of adhesion molecules within the endothelium to mediate recruitment of blood leukocytes to infection sites 29, we examined the expression of endothelial adhesion molecules including VCAM-1, ICAM-1, ICAM-2, E-selectin (Sele), and P-selectin (Ppsl)12 hrs after o.g. infection with Salmonella. Expression of VCAM-1, ICAM-2, E-selectin, and P-selectin, but not ICAM-1, were significantly reduced in the ceca of infected Nlrc4−/− and Il1r−/− mice when compared to WT animals (Fig. 7c).
Figure 7. The NLRC4 inflammasome promotes host defense through neutrophil recruitment in the intestine.
(a) WT, Nlrc4−/− and Il1r−/− mice were infected with Salmonella orogastrically and 20 hrs later intestinal tissue was harvested. Cecum sections were stained with hematoxylin and eosin and scored for the indicated pathological changes. Results are representative of three separate experiments with five mice for each group. (b) Lamina propria mononuclear cells were isolated from the large intestine of WT, Nlrc4−/− and Il1r−/− 20 hrs after orogastric Salmonella (Sal) infection, labeled with antibodies for indicated surface markers and analyzed by flow cytometry. The percentage of neutrophils (CD11b+Ly6G+ positive cells) from 4 different experiments was plotted. (c) Expression of indicated adhesion molecules by quantitative RT-PCR in cecal tissue 12 hrs after Salmonella infection. Gene expression was normalized to the expression of β-actin, and expressed as fold induction compared to uninfected mice. Values represent mean ± s.d. n=6 . (d and e). Mice were pretreated with anti Ly-6G antibody, or control rat IgG, and then infected orogastrically with 1 × 105 Salmonella. Bacterial burden in the cecum was determined on day 3 post infection (d) and survival was monitored over time (e). (f and g) WT, Nlrc4−/− and Il1r−/− mice were infected orogastrically with Salmonella and the bacterial burden in the cecum was determined on day 2 (f) and on day 3 (g) post infection. Results are representative of three independent experiments. * statistically significant NS not significant.
Neutrophils are important for controlling systemic Salmonella infection 30, but their role during the intestinal phase of the infection is largely unknown. To address the role of neutrophils in host defense against Salmonella in the intestine, we depleted neutrophils in mice by administration of anti-Ly6G, an antibody specific to neutrophils (Supplementary Fig. 8) and assessed mouse survival and bacterial loads in intestinal tissue after o. g. infection. Depletion of neutrophils was associated with increased Salmonella loads in the cecum and enhanced mortality in Balb/c and C57BL/6 mice (Fig. 7d,e and Supplementary Fig. 9). To further investigate the role of the NLRC4 inflammasome in the innate immune response to Salmonella, we examined intestinal bacterial loads in the intestine on days 2 and 3 after o.g. infection. There were increased Salmonella loads in the ceca of Nlrc4−/− and Il1r−/− mice compared to WT mice (Fig. 7f,g). Altogether, these results indicate that the NLRC4-IL-1R axis is critical for upregulation of endothelial adhesion molecules, neutrophil recruitment and control of Salmonella burden in the intestine.
Discussion
The innate immune system represents the first line of defense and plays a critical role in activating host defense pathways that ultimately control microbial invasion. In most tissues, the activation of PRRs in macrophages and dendritic cells, by microbes leads to robust inflammatory responses that lead to the recruitment of professional phagocytes and activation of adaptive immune responses 1, 31. However, within the intestinal tract, there are large numbers of non-pathogenic microbes, which requires a poorly understood mechanism to curb harmful inflammatory responses to commensals while maintaining activity against invading pathogens 2, 19. Key cells in maintaining gut homeostasis are resident iMP that comprise different populations of intestinal macrophages and DCs 32. Because colonic iMP produce anti-inflammatory molecules and are hyporesponsive to microbial stimulation, they are presumed to play primarily a regulatory role by promoting tolerization to oral antigens and commensal bacteria 32, 33. Consistent with previous work 22, 23, 34, we found that resident iMP do not produce TNF and IL-6 in response to TLR ligands and commensal bacteria. In addition, infection with pathogenic Salmonella did not elicit significant amounts of TNF and IL-6 in iMP when compared to BMDM. The hyporesponsiveness of iMP to commensals and pathogenic bacteria can be explained, at least in part, by the absence or low expression of TLRs and key components of PRR signaling pathways in iMP 35. However, unlike TNF and IL-6, IL-1β is produced in significant amounts by iMP via the NLRC4 inflammasome after infection with pathogenic, but not with commensal, bacteria. IL-1β production in iMP is facilitated by their constitutive expression of pro-IL-1β and NLRC4. Thus, the release of mature IL-1β in iMP requires only the activation of the NLRC4 inflammasome which is not dependent on TLR-signaling 25. The ability of Salmonella or P. aeruginosa, but not commensal bacteria, to induce mature IL-1β production in iMP is further explained by the fact that activation of the NLRC4 inflammasome requires a functional T3SS or T4SS, an activity that is unique to certain pathogenic bacteria such as Salmonella. Infection with Salmonella, however, does not result in TNF or IL-6 production, which typically involves TLR signaling, which is impaired in iMP 22, 23, 34. In contrast to the sensing of cytosolic flagellin by NLRC4, there is evidence that stimulation of TLR5 by extracellular flagellin is deleterious after oral infection with Salmonella 35. Although the mechanism involved remains poorly understood, a subpopulation of CD11c+ DCs in the small intestine express TLR5, respond to flagellin and promote the transport of Salmonella to mesenteric lymph nodes 35. However, comparable DC populations from the large intestine did not respond to flagellin 36. Thus, unlike cytosolic recognition of flagellin via the NLRC4 inflammasome, there is no evidence for extracellular sensing of flagellin by colonic iMP. Collectively, these results indicate that NLRC4-mediated production of mature IL-1β in iMP represents a specific innate immune response that can discriminate pathogenic from commensal bacteria in the large intestine.
The signals that program iMP to become anergic to TLR ligands and commensals are still poorly understood, although there is evidence that production of IL-10 by iMP and transforming growth factor-β (TGFβ) by stromal cells might be important 37, 38. It has been suggested that commensal bacteria tolerize iMP to microbial stimulation through continuous exposure of PAMPs to iMP. However, we found that the microbiota are not required for the hyporesponsiveness of iMP to TLR ligands and commensal bacteria. Notably, iMP from GF mice expressed reduced levels of pro-IL-1β. The mechanism by which the microbiota promotes pro-IL-1β expression is unclear. One possibility is that commensals stimulate epithelial cells or stromal cells to induce molecules that regulate pro-IL-1β in resident iMP. This possibility is consistent with the observation that the microbiota induces expression of anti-microbial molecules in intestinal epithelial cells 39. Similarly, intestinal stromal cells can produce pro-inflammatory molecules in response to TLR ligands and could contribute to the induction of pro-IL-1β in iMP 40. Alternatively, commensal microorganisms may produce factors that act directly on iMP to induce pro-IL-1β. Whatever the mechanism involved, the induction of pro-IL-1β by the microbiota is beneficial to the host in that it promotes the production of IL-1β in iMP that is required for clearance of enteric pathogens such as Salmonella.
The susceptibility to pathogens and pro-inflammatory stimuli is a complex biological trait controlled by multiple host genes that can differ in mice with different genetic backgrounds 41. In the current work, we found that Nlrc4−/− and Il1r−/− mice in the Balb/c, but not C57BL/6 background, were highly susceptible to orogastric infection with Salmonella. Il1r−/− and Nlrc4−/− mice in the Balb/c background showed comparable survival and histopathology, but Il1r−/− mice exhibited slightly lower pathogen loads than Nlrc4−/− mice after oral infection. Although the reason for this minor difference is unclear, it may be explained by a role of IL-18 in intestinal Salmonella infection. Our results in the C57BL/6 background are consistent with previous studies that showed no role or a modest contribution for NLRC4, caspase-1 and IL-1β in Salmonella infection via the oral route in mice pretreated or not with streptomycin 42–44. These background-dependent differences could not be explained by differential expression of Nramp1 because genotyping of the mice revealed that the mutant mice in both genetic backgrounds were homozygous for the susceptibility allele Nramp1s/s. The latter is consistent with our findings that Nlrc4−/− and Il1r−/− mice in both genetic backgrounds were equally susceptible to intraperitoneal Salmonella infection. We also found that purified iMP from Balb/c and C57BL/6 mice showed comparable NLRC4 activation and IL-1β secretion in response to Salmonella (data not shown) In addition, similar to what was observed with Balb/c mice, iMP from C57BL/6 mice were hyporesponsive to TLR ligands, commensals, and pathogenic bacteria with the exception of IL-1β release that was triggered by Salmonella, but not commensal bacteria (data not shown). Thus, iMP can discriminate between pathogenic and commensal bacteria through the activation of the NLRC4 inflammasome and production of IL-1β in both Balb/c and C57BL/6 mice as well as in humans. Profound differences in the inflammatory response elicited in Balb/c and C57BL/6 mice are well known. One example is the contrasting inflammatory response between C57BL/6 and Balb/c mice to ovalbumin in the lung 41. Other examples include the different susceptibility of C57BL/6 and Balb/c mice to colitis induced by the hapten trinitrobenzene or arthritis that develop in IL-1R antagonist-deficient mice 41. We do not have as yet an explanation to account for the differential susceptibility of Nlrc4−/− and Il1r−/− mice to orogastric infection with Salmonella. Our results indicate that the IL-1 pathway might be more important for the intestinal innate response against Salmonella in Balb/c than in C57Bl/6 mice. One possibility is that there is functional redundancy between the NLRC4 inflammasome and other innate signaling pathways that is of greater importance in the intestine of C57Bl/6 mice. Alternatively the IL-1 pathway might be more important for the intestinal innate response against Salmonella in Balb/c than in C57Bl/6 mice. The differences in phenotype between C57BL/6 and Balb/c mice raise questions about the most appropriate genetic background to translate findings in mouse models to human disease 41. Importantly, discrimination between pathogenic and commensal bacteria occur through the activation of the NLRC4 inflammasome and the release of IL-1β in human iMP.
NLRP3 and NLRC4 are the most studied and understood of the inflammasomes to-date. While NLRP3 is activated by multiple signals such as ATP, bacterial toxins, and particulate matter, activation of the NLRC4 inflammasome is induced by bacterial flagellin and PrgJ-like rod proteins that are presumably leaked into the host cytosol via T3SS or T4SS 3, 45. Salmonella encodes two T3SS, SPI-1 promotes intestinal invasion while SPI-2 is important for the pathogen systemic spread 46. During the intestinal phase of infection, Salmonella expresses SPI-1 and flagellin 46. In line with these observations, we provide evidence that a functional T3SS and flagellin are critical for the activation of NLRC4 in iMP infected with Salmonella. Consistently, we show that the NLRC4 inflammasome is important for the clearance of Salmonella in the intestine, but not during systemic infection. The latter observation could be partly explained by the fact that expression of flagellin is downregulated during the systemic phase of Salmonella infection 30. Consistently, constitutive expression of flagellin in a mutant Salmonella strain promotes the clearance of the bacterium through NLRC4 and caspase-1-induced macrophage pyroptosis during systemic infection 30. NLRP3 and NLRC4 act redundantly to promote the clearance of Salmonella after oral infection in the absence of pre-treatment with streptomycin in the C57BL/6 background 44. However, it is unclear if NLRP3 and NLRC4 function redundantly in the intestine or systemically because this issue was not assessed by the authors44. Unlike NLRC4, the NLRP3 inflammasome, which is major conduit for caspase-1 activation as well as IL-1β and IL-18 release by BMDM and DCs, is non functional in iMP likely due to its low constitutive expression and lack of induction by TLR ligands. Some, but not all, investigators have shown that Nlrp3−/− mice have increased susceptibility to colitis induced by dextran sodium sulphate (DSS), which is largely mediated by the influx of commensals through the injured epithelium 47. However, NLRC4 -deficiency has not been associated with increased susceptibility to DSS-induced colitis 48. After infection and injury, blood monocytes are recruited to local intestinal sites and these cells can respond to stimuli via TLRs and NLRs including NLRP3 40. In human monocytes, stimulation with TLR ligands alone induces the release of IL-1β which might be explained by the release of endogenous ATP and subsequent activation of the purinergic receptor P2X7 49, 50. Collectively, these results indicate a complex role for the different inflammasomes in chemical-induced and pathogen-driven inflammation. Our results reveal a role for the NLRC4 inflammasome in the host defense against infection by the enteric pathogen Salmonella through the production of IL-1β by resident iMP.
Methods
Mice
Balb/c and C57BL/6 mice were originally purchased from Jackson Laboratories, and bred in-house. Nlrc4−/− and Il1r−/− mice in the C57BL/6 background have been previously described 12. Nlrc4−/− were backcrossed to the Balb/c background for 6 generations. Il1r−/− Balb/c mice were obtained from H.L. Rosenzweig, Oregon Health & Science University. All mice used in this study were homozygous for the Nramp1 susceptible allele (Nramp1s/s). All the experiments were performed on Balb/c mice unless otherwise stated. GF mice were maintained in flexible film isolators and were checked weekly for germ-free status by aerobic and anaerobic culture. The absence of microbiota was verified by microscopic analysis of stained cecal contents to detect unculturable contamination. The animal studies were conducted under protocols approved by the University of Michigan Committee on Use and Care of Animals.
Nramp1 genotyping
A 514-bp fragment of the nramp1 gene was amplified by PCR using primers 5′-AAGTGACATCTCGCCATAGGTGCC-3′ and 5′-TTCTCTCACCATAGTTATCCAAG AAG-3′ (forward and reverse, respectively). The purified PCR product was then sequenced using the primer 5′-CCCCCATCTATGTTATCACCC-3′.
Preparation of macrophage and intestinal phagocytes
BMDM were prepared as described 12. All the experiments on iMFs were performed using mice on Balb/c background unless otherwise stated. To purify iMFs, the cecum and colon were removed and washed with PBS. The tissues were cut into small pieces and washed 3 times with calcium and magnesium-free Hanks’ balanced salt solution (HBSS; Sigma-Aldrich, St Louis, MO) containing 2.5% heat-inactivated fetal bovine serum (BioSource, Camarillo, CA). After washing, tissues were incubated in HBSS containing 1 mM dithiothretol (Sigma-Aldrich) for 15 min at room temperature to remove mucus, and then incubated twice in HBSS containing 1 mM EDTA (Sigma-Aldrich) for 45 min at 37°C to remove epithelial cells. After washing with HBSS three times, tissues were collected and incubated in HBSS containing 400 U/ml collagenase type 3 and 0.1 mg/ml DNase I (Worthington Biochemical, Freehold, NJ) for 90 min at 37°C. The digested samples were pelleted and resuspended in a 40% Percoll solution (Amersham Biosciences), layered onto 75% Percoll and centrifuged at 2000 rpm for 20 min at room temperature. Viable LPMCs were recovered from the 40–75% layer interface. Intestinal phagocytic cells purified from the colon lamina propria (LP) were positively selected using CD11b microbeads by MACS according to the manufacturer instructions (Miltenyi Biotec Inc.). Purified iMP were ≥ 80% CD11b and F4/80 positive, and the majority of cells (≥ 70%) displayed typical macrophage morphology characterized by an abundant vacuolar cytoplasm and peripheral nuclei.
Preparation of human peripheral blood monocytes and intestinal lamina propria macrophages
Peripheral CD14+ monocytes were isolated from peripheral blood mononuclear cells using EasySep Human CD14+ (StemCell Technologies Inc., Vancouver, Canada) according to the manufacturer’s instructions. The percentage of monocytes isolated using this method was evaluated by flow cytometry and was routinely >98%. Lamina propria mononuclear cells (LPMCs) were isolated from disease-free areas of colon specimens as previously described 26. Lamina propria (LP) CD14+ macrophages were isolated from LPMCs using EasySep Human CD14+. The percentage of each subset of cells isolated using this method was evaluated by flow cytometry and was routinely >95%. All experiments using human tissues were approved by the Institutional Review Board of the Tissue Procurement Core of the University of Michigan Comprehensive Cancer Center, and written informed consent was obtained.
Statistical analysis
We performed statistical analysis using the unpaired Student’s T test. For survival assays and in vivo bacterial burden analysis, comparisons were made with the Mann-Whitney U test. A value of p ≤ 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank G.Y. Chen (University of Michigan) for critically reading the manuscript, P. Vandenabeele for generously providing purified caspase-1, and the University of Michigan Flow Cytometry, Immune Monitoring Core and Tissue Procurement Service for assistance. This work was supported by National Institute of Health Grant (NIH) R01 DK61707 and the University of Michigan’s Cancer Center Support Grant (5 P30 CA46592). L. F. and N. K. were supported by a Research Career Development Award and Postdoctoral Fellowship, respectively, from the Crohn's and Colitis Foundation of America. M.H.S. was supported by Lung Immunopathology Training Grant T32-HL007517 from the NIH. Y-G.K. was supported by training funds from the University of Michigan Comprehensive Cancer Center. A.B. was supported by A.B. was supported by a Predoctoral Fellowship Training Program in Organogenesis T32 HD007505 from the University of Michigan. This study is supported in part by the Tissue Procurement Core of the University of Michigan Comprehensive Cancer Center, Grant #CA46952
Footnotes
Author Contributions
L.F., N.K. and G. N. conceived the study. L.F. and N.K. designed and performed most of the experiments. P.K. produced antibodies. S.S. and A. B. helped with experiments, P.K., S.S., A.B. M.S., Y.-G. K., helped in the design of several experiments and provided critical advice. G.N. supervised all aspects of this study. L.F. and G.N. wrote the manuscript with contributions from all authors.
References
- 1.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franchi L, Munoz-Planillo R, Reimer T, Eigenbrod T, Nunez G. Inflammasomes as microbial sensors. Eur J Immunol. 2010;40:611–615. doi: 10.1002/eji.200940180. [DOI] [PubMed] [Google Scholar]
- 4.Franchi L, et al. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. doi: 10.1111/j.1462-5822.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 5.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauernfeind F, et al. Inflammasomes: current understanding and open questions. Cell Mol Life Sci. 2011;68:765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amer A, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 9.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci U S A. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franchi L, et al. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 11.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 12.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchi L. Role of inflammasomes in salmonella infection. Front Microbiol. 2011;2:8. doi: 10.3389/fmicb.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao EA, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 17.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald TT, Monteleone I, Fantini MC, Monteleone G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology. 2011;140:1768–1775. doi: 10.1053/j.gastro.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 20.Manicassamy S, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 22.Smythies LE, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotz M, et al. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-{alpha} Mediates Sensitization to ATP and Silica via the NLRP3 Inflammasome in the Absence of Microbial Stimulation. J Immunol. 2009 doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamada N, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barthel M, et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lara-Tejero M, et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinarello CA. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 30.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi O, Akira S. Pattern Recognition Receptors and Inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith PD, et al. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011;4:31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith PD, et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol. 2001;167:2651–2656. doi: 10.4049/jimmunol.167.5.2651. [DOI] [PubMed] [Google Scholar]
- 35.Uematsu S, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 36.Monteleone I, Platt AM, Jaensson E, Agace WW, Mowat AM. IL-10-dependent partial refractoriness to Toll-like receptor stimulation modulates gut mucosal dendritic cell function. Eur J Immunol. 2008;38:1533–1547. doi: 10.1002/eji.200737909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smythies LE, et al. Inflammation anergy in human intestinal macrophages is due to Smad-induced I{kappa}B{alpha} expression and NF-{kappa}B inactivation. J Biol Chem. 2010 doi: 10.1074/jbc.M109.069955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandl K, et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YG, et al. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity. 2011;34:769–780. doi: 10.1016/j.immuni.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivera J, Tessarollo L. Genetic background and the dilemma of translating mouse studies to humans. Immunity. 2008;28:1–4. doi: 10.1016/j.immuni.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Lara-Tejero M, Galan JE. Salmonella enterica serovar typhimurium pathogenicity island 1-encoded type III secretion system translocases mediate intimate attachment to nonphagocytic cells. Infect Immun. 2009;77:2635–2642. doi: 10.1128/IAI.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raupach B, Peuschel SK, Monack DM, Zychlinsky A. Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006;74:4922–4926. doi: 10.1128/IAI.00417-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broz P, et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grassl GA, Finlay BB. Pathogenesis of enteric Salmonella infections. Curr Opin Gastroenterol. 2008;24:22–26. doi: 10.1097/MOG.0b013e3282f21388. [DOI] [PubMed] [Google Scholar]
- 47.Chen GY, Nunez G. Inflammasomes in Intestinal Inflammation and Cancer. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu B, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A. 2010;107:21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Netea MG, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piccini A, et al. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.